Abstract
Growing evidence suggests that the presence of a subpopulation of hypoxic non-replicating, phenotypically drug-tolerant mycobacteria is responsible for the prolonged duration of tuberculosis treatment. The discovery of new antitubercular agents active against this subpopulation may help in developing new strategies to shorten the time of tuberculosis therapy. Recently, the maintenance of a low level of bacterial respiration was shown to be a point of metabolic vulnerability in Mycobacterium tuberculosis. Here, we describe the development of a hypoxic model to identify compounds targeting mycobacterial respiratory functions and ATP homeostasis in whole mycobacteria. The model was adapted to 1,536-well plate format and successfully used to screen over 600,000 compounds. Approximately 800 compounds were confirmed to reduce intracellular ATP levels in a dose-dependent manner in Mycobacterium bovis BCG. One hundred and forty non-cytotoxic compounds with activity against hypoxic non-replicating M. tuberculosis were further validated. The resulting collection of compounds that disrupt ATP homeostasis in M. tuberculosis represents a valuable resource to decipher the biology of persistent mycobacteria.
Tuberculosis (TB) claims 1.8 million lives each year, and it is estimated that one-third of the global population is latently infected by the bacilli.1 Despite the implementation and success of Directly Observed Treatment Short course (DOTS), there is a steady increase in the number of patients infected with multi- (MDR) and extensively (XDR) mycobacterial drug-resistant strains.2 Reducing the time required for TB therapy represents one of the main objectives in antituberculosis drug discovery since it would simplify treatment and improve compliance.3,4 The long duration of tuberculosis treatment is thought to be due to a subpopulation of bacilli persisting in a hypometabolic state. These quiescent mycobacteria, often referred to as dormant, are phenotypically tolerant to most antituberculosis agents.5 Hypoxia is one of the environmental signals that triggers mycobacteria to enter into a non-replicating persistence phase.6−8 Indeed, hypoxic lesions have been documented in the rat, guinea pig, rabbit, and non-human primate TB models.9,10 Importantly, bacilli residing within rabbit lesions acquire sensitivity to metronidazole, an antibiotic that has potent bactericidal activity only under very low-oxygen culture conditions.10Mycobacterium tuberculosis responds to hypoxia by upregulating a set of 50 genes known as the dosR regulon.11−13 This regulon initiates metabolic alterations essential for M. tuberculosis to survive in the absence of oxygen.14 More recently, a second gene cluster consisting of 230 genes was identified that was required for long-term survival under hypoxic conditions.15
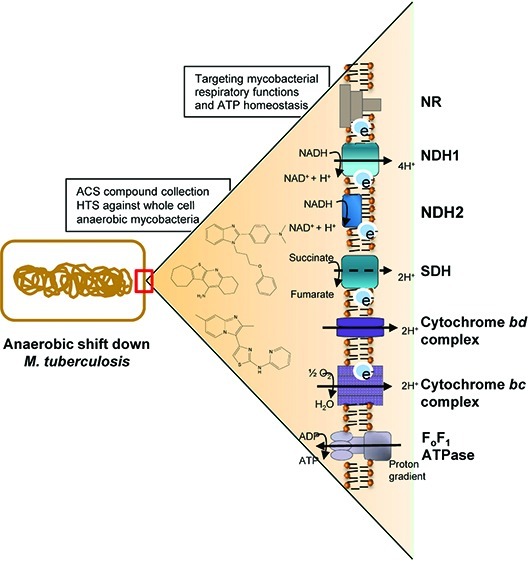
We have previously demonstrated that hypoxic nongrowing M. tuberculosis maintains a reduced but significant pool of ATP when compared to growing bacilli, making them exquisitely sensitive to further ATP depletion.16 A key feature of maintaining viability in the hypoxic quiescent state lies in the ability to maintain an energized membrane and in the process of synthesizing ATP through the F0F1 ATP synthase. In addition, M. tuberculosis can maintain a proton gradient in the absence of respiration by reductively operating the TCA cycle and secreting succinate, a fermentative process essential to allow ATP synthesis without a terminal electron acceptor.17 The clinical candidate TMC207, a F0F1 ATP synthase inhibitor, was recently shown to be active against M. tuberculosis in phase II studies in humans.18 TMC207 kills hypoxic non-replicating M. tuberculosis by directly inhibiting the F0F1 ATP synthase, thereby depleting the ATP pool.19 The success of TMC207 is an important proof of concept that targeting the maintenance of the proton motive force and ATP homeostasis represents an attractive approach to kill non-replicating M. tuberculosis.
In this study, we report the development of a phenotypic screen designed to identify compounds that target the process of ATP synthesis and maintenance under hypoxic conditions in M. tuberculosis. Upon adaptation to a 1,536-well plate format, this assay was shown to be suitable for high-throughput screening. A collection of nontoxic chemical entities that lower ATP content in hypoxic non-replicating mycobacteria was identified. These results demonstrate the feasibility of large scale screening against hypoxic non-replicating mycobacteria.
Results and Discussion
Development of a DosR-Dependent Hypoxic Shift Assay
The classical model used to study the physiology of hypoxic non-replicating mycobacteria is the defined headspace model described by Wayne and colleagues.20−22 In this model, mycobacteria are grown in stirred, airtight tubes with a defined headspace and require an extended incubation period to consume the headspace oxygen, enter hypoxia, and cease replication. The extended incubation and variable timing of hypoxic adaptation precluded the use of this model for preparing inocula for high-throughput screening. We therefore adopted an alternative rapid method to shift growing cells into hypoxic conditions.23Mycobacterium bovis BCG was used as a surrogate avirulent mycobacteria to safely handle a primary screen of over 600,000 compounds. Early log phase M. bovis BCG cultures were placed within a hypoxic atmosphere. The oxygen dissolved in the culture medium was gradually depleted by bacteria, as demonstrated by decolorization of the oxygen-sensitive probe methylene blue (Figure 1a). Culture medium without bacteria did not decolorize, even after a period of 5 days of incubation. Concurrent with this shift to anaerobiosis, the intracellular ATP concentration progressively reduced as the oxygen tension dropped and stabilized by about 5 days (Figure 1b). M. bovis BCG ceased replication but did not experience any loss of viability and CFU remained constant for at least 10 days (Figure 1c), suggesting that the kinetic of oxygen depletion allowed sufficient time for adaptation to anaerobiosis, as described in the original Wayne model.24
Figure 1.
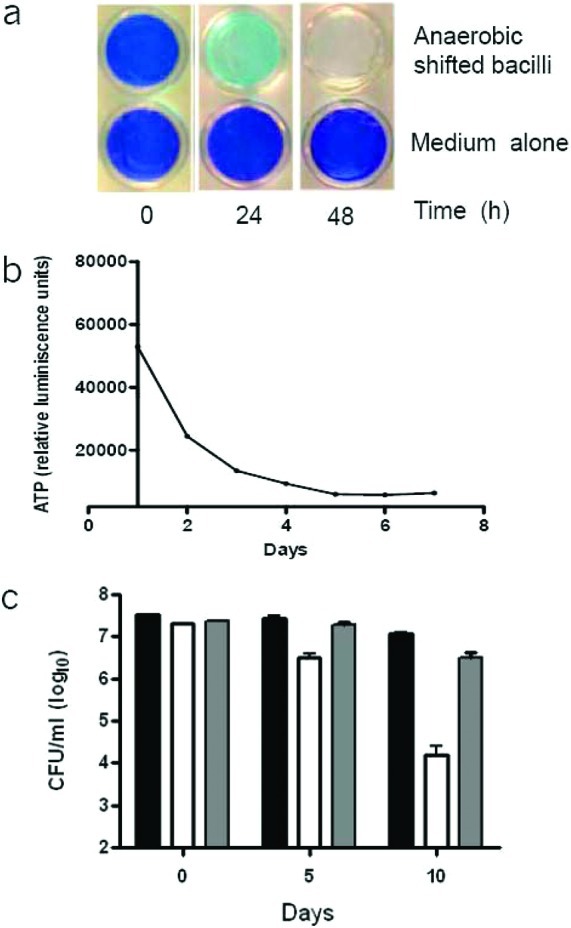
Viability and intracellular ATP levels of M. bovis BCG during the hypoxic shift down assay. (a) Upper row: A hypoxic M. bovis culture was inoculated at an OD600 of 0.2 in a 24-well plate and shifted into a hypoxic atmosphere. The presence of oxygen left in the culture was monitored by decolorization of methylene blue. Methylene blue decolorized after 2 days. Lower row: Control wells with methylene blue, but without bacilli, in a hypoxic atmosphere. (b) ATP levels (RLU, relative luminescence units) were monitored daily for a period of 7 days. (c) Survival of M. bovis BCG and M. bovis BCG:ΔdosR in the hypoxic shift down model. Viability (CFU/mL) of M. bovis BCG parental (black bar), M. bovis BCG:ΔdosR (white bar), and complemented mutant (gray bar) was determined at each time point in the hypoxic shift down model. The experiment was carried out three times in triplicate, and results are given as means ± SD.
The metabolic response to hypoxia is initially coordinated by the DosR regulon.11 A deletion of the dosR/dosT two-component system results in a dramatic loss in cell viability in the Wayne model, both in M. bovis BCG and in M. tuberculosis.11,25−28 To verify if the survival of our rapid hypoxic shift down model was DosR-dependent, an M. bovis BCG strain deficient for the expression of DosR (BCG ΔdosR) was exposed to the hypoxic shift down assay, and the viability was followed over a period of 10 days. The BCG ΔdosR strain was highly attenuated for survival in this model, with 1.5- and 3.5-log CFU reduction observed at day 5 and day 10, respectively. This phenotype could be complemented with the reintroduction of a wild-type copy of dosR. The results shows that survival of mycobacteria in the hypoxic shift model is DosR-dependent (Figure 1c), suggesting that the metabolic state of these cells is similar to that observed in the Wayne model.24
Known Inhibitors of the Electron Transport Chain Reduce the ATP Pool in the Hypoxic Shift down Assay
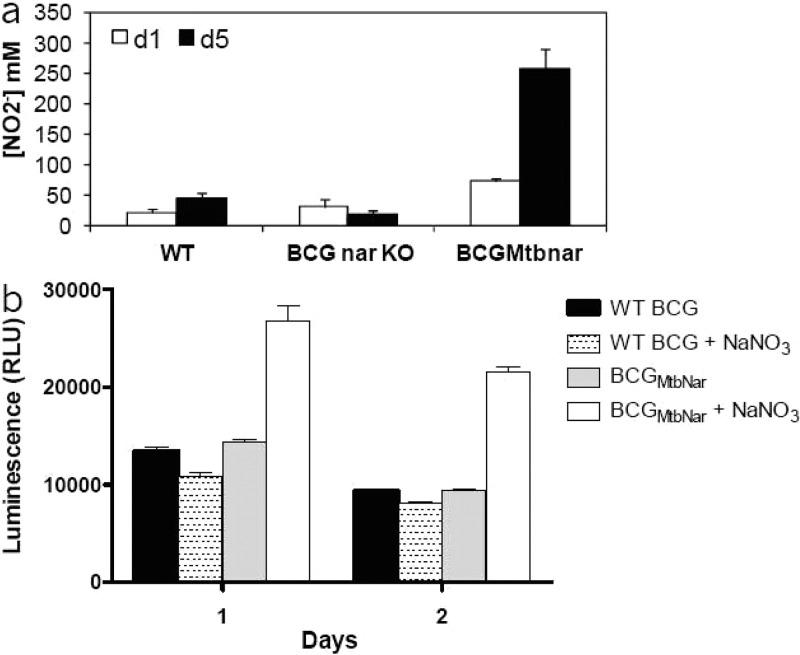
The hypoxic shift down assay was developed to identify inhibitors of the electron transport chain in a cell-based screen. Since the intracellular ATP pool is strongly affected by the proton gradient established by the respiratory chain, we used ATP levels as readout for selecting active compounds. However, our initial attempts to develop a robust assay were hampered by the extremely low levels of ATP in hypoxic, non-replicating mycobacteria. Therefore, we sought to use nitrate, an efficient exogenous electron acceptor, to stimulate the electron transport chain and ATP synthesis. M. bovis BCG is not able to use nitrate as efficiently as M. tuberculosis because of a point mutation in the promoter region of nitrate reductase.29 Hence, the M. tuberculosis narGHJI locus was introduced into M. bovis BCG as previously described.23 As expected a significant increase in the nitrate reductase activity of M. bovis BCGMtbNar compared to wild type strain was observed (Figure 2a). The recombinant M. bovis BCGMtbNar survived the rapid hypoxic shift down assay similar to the parental strain (Supplementary Figure 3). Importantly, the ATP pool was increased and maintained at a level ∼2-fold higher in the presence of nitrate, showing that the presence of an efficient electron acceptor stimulated ATP synthesis in this model (Figure 2b).
Figure 2.
Complementation of M. bovis BCG with narGHJI cluster gene from M. tuberculosis enhanced nitrate reductase activity and ATP levels in the hypoxic shift down model. (a) Production of nitrite in culture by M. bovis BCG wild type (WT), M. bovis BCG narGHJI KO strain (BCGnar-KO), and M. bovis BCG complemented with M. tuberculosis narGHJI (BCGMtbNar) at days 1 and 5. (b) Intracellular ATP production measured for M. bovis BCG WT and M. bovis BCGMtbNar complemented strains at day 1 and 2 upon methylene blue decolorization in the absence or presence of 20 mM sodium nitrate (NaNO3). Results are expressed as the means ± SD of triplicates.
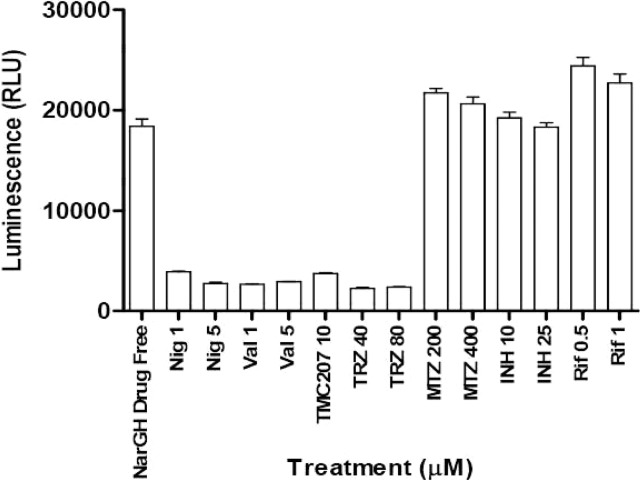
The effect of standard anti-TB drugs and compounds known to interfere with the proton motive force were tested in this assay. Phenothiazines, ionophores, and the known ATP synthase inhibitor TMC207 were all able to significantly reduce the ATP pool (Figure 3). In contrast, ATP levels were not affected by transcription cell-wall synthesis inhibitors, nor were they affected by metronidazole whose mode of action against mycobacteria remains largely unknown (Figure 3). These data are in agreement with findings obtained with other dormancy models16 and showed that this assay was highly specific for compounds that interfere with the maintenance of proton motive force and with ATP homeostasis.
Figure 3.
Effect of proton motive force inhibitors, phenothiazines, and standard anti-TB drugs on ATP levels of hypoxic shifted M. bovis BCGMtbNar. Reference compounds at indicated concentrations were tested on hypoxic adapted M. bovis BCGMtbNar complemented strain in 384-well microwell plates (40 μL/well). Hypoxic shift down bacilli were added together with nitrate upon methylene blue decolorization in control plates. After 2 days of drug exposure plates were treated with 40 μL of BTG reagent. ATP levels (RLU) were quantified 10 min after incubation. The experiment was carried out three times in triplicates and results are given as means ± SD. Nig: nigericine; Val: valinomycin; TRZ: thioridazine; MTZ: metronidazole; INH: isoniazid; Rif: rifampicin.
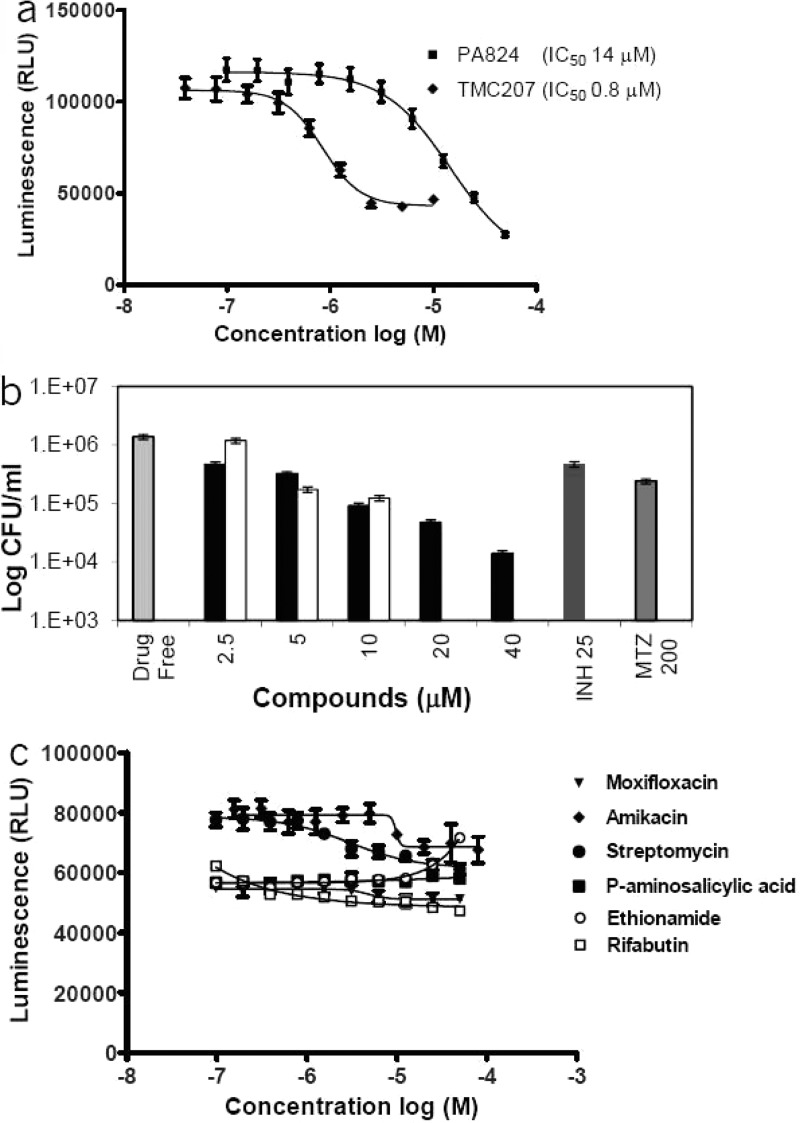
We further ensured that the observation made with M. bovis BCG could be translated to M. tuberculosis. M. tuberculosis H37Rv cells were adapted under similar culture conditions and treated with several classes of antituberculars (Figure 4). The results showed a reduction in ATP levels when M. tuberculosis was treated with TMC20716 and PA-824,30 two clinical-stage compounds known to interfere with mycobacterial respiration. ATP depletion was dose-dependent and correlated with reduction in bacterial viability (Figure 4a and b). As observed for M. bovis BCGMtbNar, ATP levels were not affected by transcription, translation, gyrase, antifolates, or cell-wall synthesis inhibitors (Figure 4c). Overall these results demonstrate that our cell-based screening system was selective for compounds that interfere with the processes of ATP homeostasis.
Figure 4.
Effects of various anti-mycobacterials on ATP levels and viability of hypoxic shift down M. tuberculosis. (a) TMC207 and PA-824 compounds at indicated concentrations were tested on hypoxic shift down M. tuberculosis. ATP levels (RLU) were quantified by using the BTG Assay Kit. (b) The number of viable cells after treatment with different concentrations of PA-824 (black bars) and TMC207 (white bars) over a period of 5 days was determined by CFU counts on 7H11 agar. Results are expressed as the means ± SD of triplicates. (c) Moxifloxacin, amikacin, streptomycin, p-amino salicylic acid, ethionamide, and rifabutin ranging from 40 to 0.02 μM were tested on hypoxic shift down M. tuberculosis. After 2 days of drug exposure plates were treated with 40 μL of BTG reagent. Luminescence was recorded 10 min after incubation. Values reported are average of duplicate measurements.
Assay Development for High-Throughput Screen (HTS)
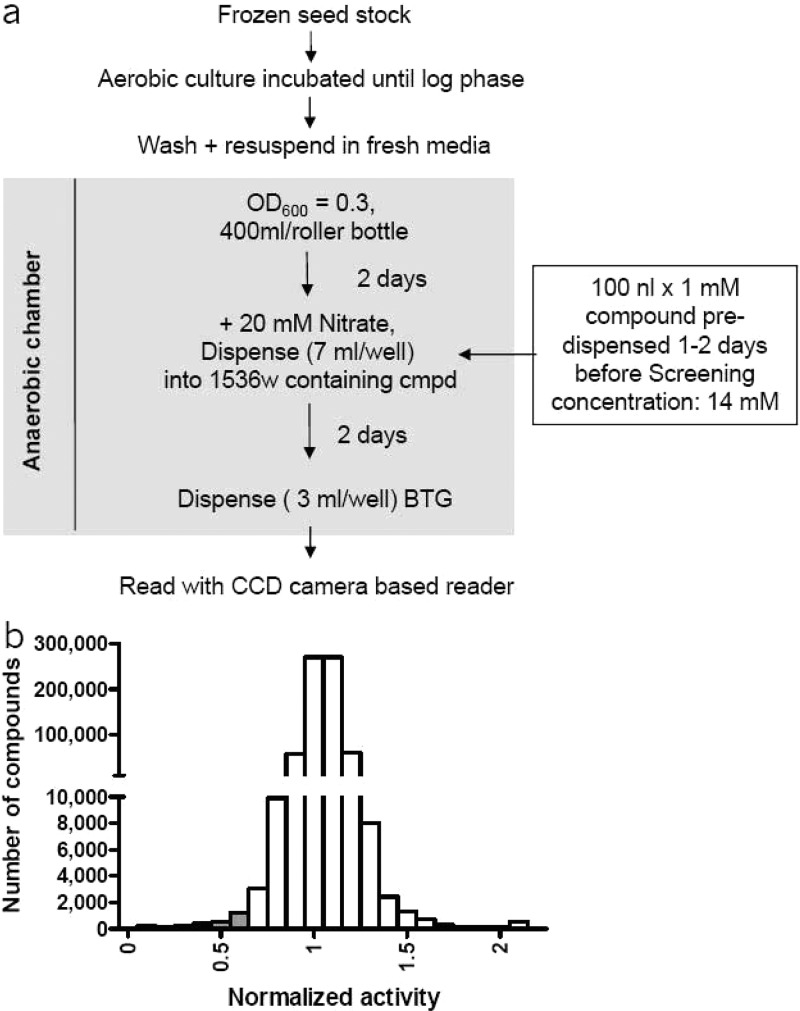
In order to screen large numbers of compounds, the assay was miniaturized and adapted to 1,536-well format (Supporting Information). To validate the HTS assay, a pilot screen was carried out with hypoxic adapted M. bovis BCGMtbNar, and 11,480 arbitrarily selected compounds were screened at 14.3 μM in 1,536-well plate format. The screen was performed in triplicate with compounds incubated for 48 h with cells before addition of detection reagent and plate reading (Figure 5a). An average Z′ value of 0.507 and CV of 11% was observed. The screen had a hit rate of about 0.1%, but given the small number of arbitrarily selected compounds this was not likely to be predictive for the hit rate in a larger collection. It was noted, however, that the majority of the hits reconfirmed in all three replicates, further supporting the robustness of the assay.
Figure 5.
High-throughput screening of the chemical compound library on hypoxic M. bovis BCGMtbNar. (a) Schematic representation of the M. bovis BCGMtbNar hypoxic shift down HTS process. (b) Histogram showing the distribution of compounds versus activity for the HTS. Plate median activity was normalized to an arbitrary value of 1, and the putative hits were identified from those of activity <0.7 (gray bars).
This assay was used to conduct an HTS against 600,000 compounds collected from various commercial vendors. Over the course of the HTS, the average assay window was maintained at ∼2-fold and CV at ∼12%. The hit rate using a stringent cutoff of 2-fold inhibition (activity of <0.5) was 0.05%. Since we were capable of handling a much larger number of compounds in confirmation stages, we relaxed the cutoff threshold to <0.7 yielding 2,900 hits (Figure 5b).
Dose–Response, Cytotoxicity, and Validation of the Hits in M. tuberculosis
Compounds showing significant ATP reduction at 14.3 μM were selected for dose–response determination. A total of 2,900 compounds were tested, and 866 hits were confirmed to reduce intracellular ATP levels in a dose-dependent manner in M. bovis BCGMtbNar (Figure 6a).
Figure 6.
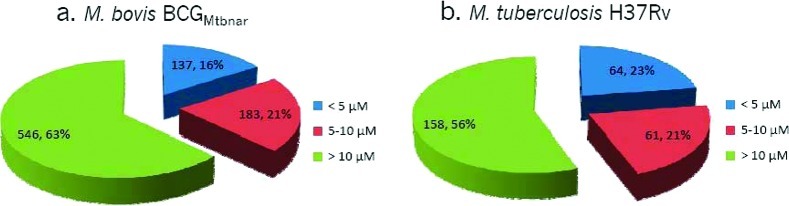
Reconfirmation of hypoxic ATP IC50 activity of HTS hits in hypoxic non-replicating mycobacteria. Ability to reduce the intracellular ATP levels of HTS hits was evaluated using various concentrations of compounds. A total of 866 hits showed activity against hypoxic M. bovis BCGMtbNar (a), and 283 hits showed activity against M. tuberculosis (b). The notation >10 μM in panel a corresponds to compounds that showed at least 20% ATP reduction at 14.1 μM, and >10 μM in panel b corresponds to compounds that showed at least 20% ATP reduction at 10 μM. The number of compounds belonging to each group and percentage of hits are depicted on the pieces in the pie chart.
Out of the 866 compounds, 790 were obtained from stored liquid stocks for reconfirmation against hypoxic shift down M. tuberculosis H37Rv. Two hundred and eighty three compounds (35.8%) were reconfirmed in a M. tuberculosis hypoxic ATP IC50 assay (Figure 6b). Among the compounds that reduced ATP levels in hypoxic non-replicating M. tuberculosis, 17 were also active against aerobic replicating M. tuberculosis (Supplementary Figure 1). Comparative analysis of the ATP inhibitors between M. bovis BCGMtbNar and M. tuberculosis H37Rv showed a good correlation for compounds with IC50 less than 10 μM (Supplementary Figure 2). The relatively low rate of reconfirmation between M. bovis BCG and M. tuberculosis could be explained by alteration in the expression network between these species. There are more than 2,400 single-nucleotide polymorphisms (SNPs) between M. bovis and M. tuberculosis that may be responsible for distinct characteristics and differential drug susceptibility.31 For example, a point mutation in the pncA gene in M. bovis confers resistance to the first line antitubercular pyrazinamide.32,33 Thus, we may have missed selectively active compounds against M. tuberculosis.
Confirmed hits were further tested for cytotoxicity against 6 different cell lines (HEK293T, CHO, Ba/Fe, HEp2, HeLa, and Huh7). Hits having cytotoxicity (CC50) greater than 10 μM in at least 4 cell lines were considered non-cytotoxic. This preliminary assessment allowed us to rapidly discriminate 140 attractive non-cytotoxic molecules. Importantly, cytotoxicity did not correlate with hypoxic ATP IC50 (Table 1 in Supporting Information).
Compound Clustering and Hit Characterization
We evaluated whether the 140 non-cytotoxic hits followed Lipinski’s rule of 5 by calculating their molecular weight (MW), cLogP (measure of hydrophobicity), and hydrogen bond acceptors and donors (HBA and HBD). We found that 137 hits had MW less than 500, 120 hits had cLogP of less than 5, 135 hits had less than 10 HBA, and all 140 hits had less than 5 HBD (Table 1 in Supporting Information). Most of the hits followed Lipinsky’s rule of 5.
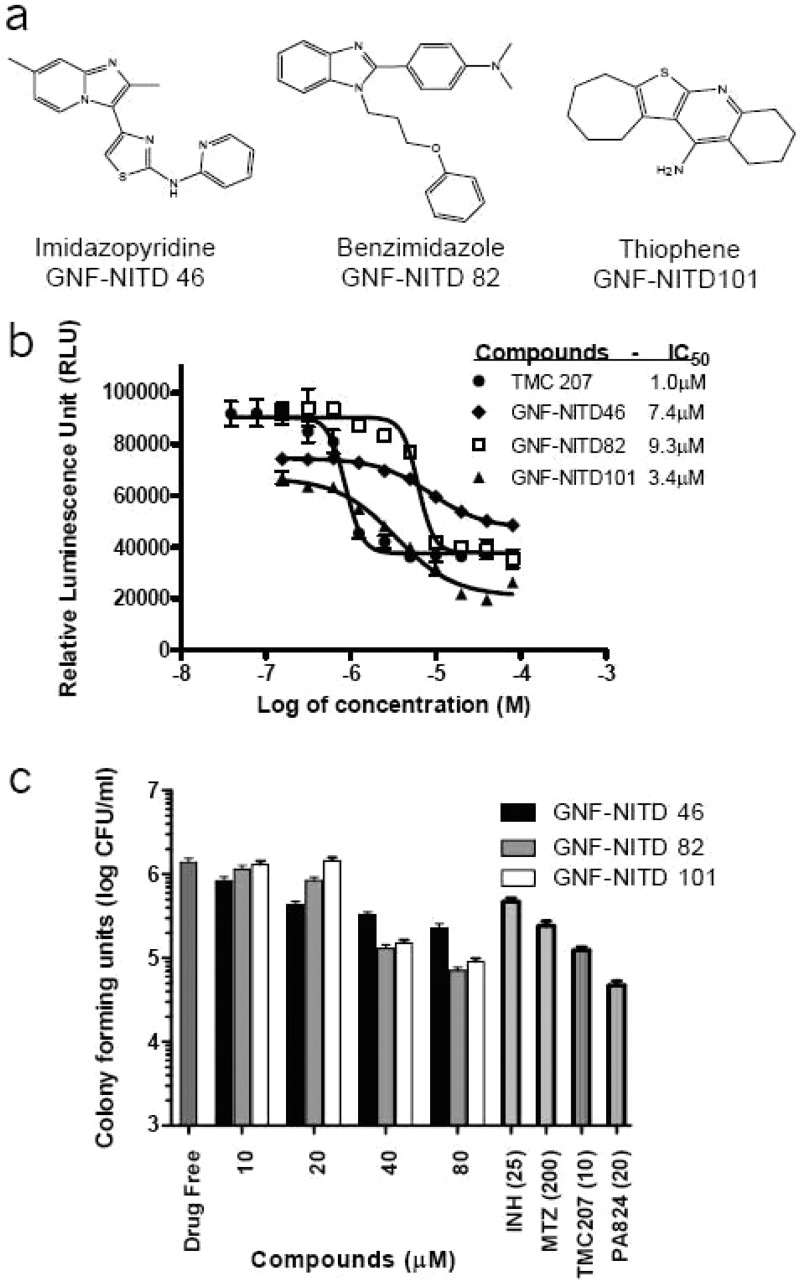
The 140 hits were further clustered with MedChem Studio34 to identify common structure elements among this diverse set of structures. Compounds were grouped into 32 clusters and 8 singletons. Table 1 in Supporting Information summarizes the in vitro anti-TB activity of the hits in hypoxic condition (ATP IC50), actively multiplying M. tuberculosis (MIC50), and cellular toxicity (IC50). Three clusters, benzimidazole (BZ), thiophene (TH), and imidazopyridines (IP), were selected for further studies as only few reports described antimycobacterial effects for these particular chemical structures.
Benzimidazoles
Nine compounds belonging to the BZ class were identified. The compounds had hypoxic ATP IC50 values ranging from 3.3 to >10 μM (Table 1 in Supporting Information). These 9 hits had slightly higher MW compared to IPs and were also moderately lipophilics with cLogP values greater than 3. One of the compounds (GNF-NITD 82) was resynthesized and reconfirmed to reduce ATP levels in hypoxic shifted cells (ATP IC50 = 9.3 μM). In addition, GNF-NITD 82 was cidal against hypoxic non-replicating M. tuberculosis (HCC90 of 40 μM) and growth-inhibitory for replicating M. tuberculosis (MIC50 = 27.0 μM) (Figure 7b and c). Substitutions in positions 1 and 2 were well tolerated, but all of the molecules showed aerobic MIC50 >10 μM. Recently, a BZ class of compounds were shown to be moderately active against replicating and non-replicating mycobacteria.35 However, Pieroni and co-workers synthesized pyrido-benzimidazoles with submicromolar activity against drug resistant replicating mycobacteria.36
Figure 7.
Comparative efficacy of selected hypoxic hits against M. tuberculosis. (a) Structure of benzimidazole (BZ), imidazopyridines (IPs), and thiophene compound class. (b) Compounds were obtained as pure solids and tested against hypoxic shift down M. tuberculosis for ATP activity (ATP IC50). (c) Hypoxic cidal activity (HCC90).
Thiophene
This potent singleton was selected for its small molecular weight (MW) of 272 and high potency. The resynthesized compound (GNF-NITD 101), showed a hypoxic ATP IC50 of 3.4 μM, aerobic MIC50 of 1.7 μM, and a hypoxic cidal HCC90 of 20 μM (Figure 7b and c). Interestingly, no literature reports showing antitubercular activity has been reported for this class.
Imidazopyridines
Thirteen hits belonging to the IP class were identified. Their hypoxic ATP IC50 values ranged from 2.68 to >10 μM (Table 1 in Supporting Information). Most of the compounds were non-cytotoxic in 6 different cell lines. Preliminary SAR suggested that a few substituents at the 3-position are tolerated: short linkers (amine, amide, and thiazole), followed by aromatic/hydrophobic group (phenyl, pyridyl, and benzodioxole). One of the compounds (GNF-NITD 46) was resynthesized and showed a hypoxic ATP IC50 of 7.4 μM, moderate anaerobic cidal activity HCC90 greater than 80 μM, and aerobic MIC50 of 1.7 μM. The low solubility of this compound at concentrations above IC50 could have affected its anaerobic activity (Figure 7b and c). The other inhibitors from this series were highly lipophilics with a cLogP greater than 3.0 despite an average MW of ∼300. Further medicinal chemistry is required to increase potency and improve the physicochemical properties.
Interestingly, IPs have been reported to be active against replicating mycobacteria.37,38 Moreover, transcriptional profiles of M. tuberculosis treated with IPs resulted in the up-regulation of the cytochrome bd oxidase, which is the high oxygen-affinity respiratory enzyme. IPs also up-regulated phosphoenolpyruvate carboxykinase, which plays an important role in modulating carbon flow during cellular energy restriction and has previously been observed to be up-regulated by stresses such as hypoxia, valinomycin, nigericin, PA-824, cyanide, and the ATP inhibitor dicyclohexylcarboxydiimide, which limit energy generation through respiration.38
Collectively the analysis of three compound clusters confirmed that reduction of cellular ATP levels correlated with cell death, validating that the screen did, in fact, identify compounds cidal for hypoxic non-replicating bacilli.
The hits described in this paper were made available through the collaborative drug discovery database (CDD) (https://www.collaborativedrug.com), a repository of public access data relevant to drug discovery from leading research groups around the world. This work was done in the context of the Grand Challenge in Global Health initiative GCHG11 (http://www.gatesfoundation.org/tuberculosis/Pages/default.aspx), and the compounds identified in this study were transferred to the GCGH11 consortium for further progression and lead characterization.
Conclusions
We have developed a hypoxic ATP-depletion model to carry out a HTS campaign to identify compounds that target the maintenance of ATP homeostasis in hypoxic non-replicating mycobacteria. Using a M. bovis BCGMtbNar recombinant strain, we could stimulate anaerobic respiration, increasing the assay window to a range compatible with high-throughput screening. In this model, mycobacteria were rapidly adapted to hypoxic conditions, and chemical ATP depletion resulted in cell death. Importantly, two of the most promising sterilizing drug candidates for tuberculosis, PA-824 and TMC207, were active in our assay. Although the screen is biased toward the identification of compounds interfering with the maintenance of the proton motive force, such as ionophores or uncouplers that may display mitochondrial toxicity, a large number of non-cytotoxic molecules that reduced mycobacterial ATP levels were identified. The discovery of three classes of compounds, imidazopyridines, benzimidazoles, and thiophenes, with activity against non-replicating and replicating mycobacteria and non-cytotoxicity further validated our approach. Further studies are focusing on validating the target mechanism of these molecules and optimizing ADME through further analogue development. For example, the effect of these molecules on proton motive force and ATP synthesis in inverted membrane vesicles39 from M. smegmatis is being tested by the GCGH11 consortium. Additional in vitro evaluations including generation of spontaneous resistant mutants followed by whole genome sequencing are in progress. Overall, the current study has generated several tool compounds that could be useful in understanding the basic biology of the hypoxic non-replicating mycobacteria.
Methods
Bacterial Strains, Culture Media, and Chemicals
M. tuberculosis H37Rv (ATCC 27294), M. bovis BCG Pasteur (ATCC 35734), derived mutant, and complemented strain, were maintained in Dubos broth (Difco) supplemented with 0.05% (v/v) Tween 80 and 10% Dubos Medium Albumin (Difco). When appropriate, hygromycin and kanamycin were added at 80 μg mL–1 and 20 μg mL–1, respectively. Enumeration of bacteria was performed by plating on Middlebrook 7H11 Agar plates (Difco) containing 0.5% (v/v) glycerol. Sodium nitrate (cat. no. S5506), isoniazid (cat. no. I3377), rifampicin (cat. no. R3501), streptomycin (cat. no. S9137), N,N′-dicyclohexylcarbodiimide (DCCD) (cat. no. 36650), metronidazole (cat. no. M3761), rifabutin (cat. no. R3530), and ethionamide (cat. no. E6005) were obtained from Sigma-Aldrich. TMC207 and PA-824 were synthesized in-house. Stock solutions of the compounds were prepared in DMSO 90%. BacTiter-Glo Microbial Cell Viability Assay (BTG) (cat. no. G8232) was obtained from Promega. Oxoid gas sachet was obtained from Oxoid (cat. no. AN0035A). The chemical library of 600,000 compounds was collected from various commercial vendors.
Generation of M. bovis BCG Mutants
M. bovis BCG ΔdosR::kan mutant and complemented strains are described elsewhere.25 The narGHJI mutants was generated in M. bovis BCG background by homologous recombination using pYUB854 as previously describe.23,40 Regions flanking the target gene were amplified by PCR and cloned into pYUB854 containing the PacI-digested lacZ/sacB inserts from pGOAL17.41M. bovis BCG bacteria was electroporated with 1 μg of the UV-irradiated plasmid solutions as described previously.16 Complementation of M. bovis BCG narGHJI mutant was performed by introducing the narGHJI operon from M. tuberculosis cloned under control of its own promoter into the integrative plasmid pMV306.42 The resulting strain was designated M. bovis BCGMtbNar.
Nitrate Reductase Assay
Intracellular nitrite production was quantified using the Griess reagent kit (Molecular Probes) following the manufacturer’s instructions. Briefly, 130 μL of water, 150 μL of bacterial cell lysate, and 20 μL of Griess reagent were combined in each well of a 96-well tissue culture plate, and the reaction was incubated for 30 min before absorbance was read at 548 nm. Absorbance readings were extrapolated to absolute nitrite concentrations by reference to a nitrite standard curve (ranging from 12.5 to 100 μM).
Hypoxic Shift down Assay
Aerated precultures in Dubos complete medium were harvested at midlog phase, washed twice in phosphate-buffered saline (PBS) supplemented with 0.05% Tween-80, and resuspended in Dubos complete medium at a final OD600 of 0.2. The bacterial suspension was then distributed in 24-well tissue culture plates (1 mL/well). Methylene blue (1.5 mg/mL) was added as an indicator of oxygen depletion in control wells. The plates were incubated in airtight anaerobic jars (BioMerieux) with anaerogen gas packs (Oxoid), to generate hypoxic atmospheric conditions. Atmospheric oxygen depletion was indicated by an anaerobic indicator strip (BD Diagnostics). Hypoxia was typically achieved within 48 h after incubation under hypoxic conditions, as witnessed by the complete decolorization of the oxygen sensor methylene blue. ATP levels were quantified using the BTG kit (Promega). Survival was monitored by plating on Middlebrook 7H11 agar to determine the colony forming units (CFU) up to 10 days after methylene decolorization. Each experimental sample consisted of triplicate wells. When high volume of hypoxic downshift cultures was required, 400 mL of Dubos completed medium at a final OD600 of 0.2 was placed in vented culture flasks (T75 NUNC), and the hypoxic shift down was performed as describe above.
Hypoxic ATP IC50 Assay
Intracellular ATP production was measured using the BTG assay kit as described before,16 with few modifications. Addition of hypoxic cells to the compound-spotted plates was carried out in an anaerobic chamber (Coy Laboratory, oxygen concentration less than 0.16%) filled with a mixture of 10% hydrogen (H2), 10% carbon dioxide (CO2), and 80% nitrogen (N2). Hypoxic mycobacteria suspensions were adjusted to an OD600 of 0.2 and added to the compound test plate containing serial diluted test compounds. The plates were placed in an airtight container (Lock and Lock container, capacity of 2.7 L) with an anaerobic gas sachet and a wet tissue paper to minimize evaporation and incubated at 37 °C for 2 days. An anaerobic indicator strip was placed inside the containers to visually confirm anaerobiosis. The ATP level of hypoxic cells was determined by adding a volume of BTG reagent equal to the volume of cell culture and incubating for 10 min at RT outside of the anaerobic chamber. The plates were read using a luminescence reader (Tecan Infinite M1000). IC50 values, inhibitory concentrations of the compound that reduced ATP levels after 2 days by 50%, were determined using Graph Pad PRISM software. Mycobacterial viability was determined by CFU counts on Middlebrook 7H11 agar.
High-throughput screening of over 600,000 compounds from different vendors was carried out using the above assay adapted to the 1,536 well-plate format. A detailed description of the process is given in Supporting Information.
Hypoxic Cidal Assay (HCC90 Determination)
Compounds dissolved in 90% DMSO were 2-fold serial diluted and spotted with a mosquito HTS liquid handler (TPP LabTech) to 96-well plates, resulting in 10 dilutions of each compound. Then 200 μL of hypoxic shift down M. tuberculosis H37Rv cells prepared as described above was added to each well, and plates were incubated at 37 °C under hypoxic conditions for 5 days. Viability was determined by plating on 7H11-agar plates and CFU determination. A concentration at which 90% of the bacteria were killed after 5 days of incubation was defined as the HCC90.
Mammalian Cellular Toxicity Assays
Cytotoxicity was tested on HEK293T, Ba/F3, CHO, Hela, HEp2, and Huh7 cells in 1,536-well plate format. A total of 125–375 cells were distributed per well in a final volume of 8 μL and incubated overnight at 37 °C. Next day, the cells were treated with test compounds at 12.5 μM and incubated for 3 days. Cell viability was assessed by adding CellTiter-Glo reagent (Promega) to each well at 2 μM. Hits having cytotoxicity (CC50) greater than 10 μM in at least 4 cell lines were considered non-cytotoxic.
Aerobic Growth Inhibition Assay (MIC50 Determination)
Compounds dissolved in 90% DMSO were 2-fold serial diluted and spotted with a mosquito HTS liquid handler (TPP LabTech) to 384-well plates, resulting in 10 dilutions of each compound. Then, 50 μL of M. tuberculosis at an OD600 of 0.02 was added to each well, and the assay plates were incubated for 5 days at 37 °C. OD600 values were recorded using a SpectraMax M2 spectrophotometer, and MIC50 curves were plotted using GraphPad Prism 5 software.
Acknowledgments
This study was funded by a grant from the Bill and Melinda Gates Foundation (37882) and the Wellcome Trust (077381) through the Grand Challenges in Global Health initiative. This study was also funded (in part) by the Division of Intramural Research of NIAID, NIH. We thank D. Young, B. Robertson, D. Schnappinger, S. Ehrt, and D. Sherman for scientific discussions and GNF colleagues for Project Management and Advanced Automations and Compound Management. We would also like to thank M. Ujjini, M. Herve, and C. Bodenreider for critically reviewing the manuscript and helpful suggestions.
Supporting Information Available
This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Author Contributions
□ These authors contributed equally to this work.
Author Present Address
∥ Dept. of Infection, Immunity and Biochemistry, Henry Welcome Building, Cardiff University, Heath Park, Cardiff, Wales, U.K. CF14 4XN.
Author Present Address
⊥ Experimental Therapeutics Centre, A*STAR, Singapore 138669.
Author Present Address
¶ Department of Microbiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117597.
Author Present Address
# Antibacterial Drug Discovery Unit, Institute Pasteur Korea, Sampyeong-dong 696, Bundang-gu, Seongnam-si, Korea 463-400.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Dye C.; Scheele S.; Dolin P.; Pathania V.; Raviglione M. C. (1999) Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. J. Am. Med. Assoc. 282, 677–686. [DOI] [PubMed] [Google Scholar]
- Mahmoudi A.; Iseman M. D. (1993) Pitfalls in the care of patients with tuberculosis. Common errors and their association with the acquisition of drug resistance. J. Am. Med. Assoc. 270, 65–68. [PubMed] [Google Scholar]
- Duncan K.; Barry C. E. III (2004) Prospects for new antitubercular drugs. Curr. Opin. Microbiol. 7, 460–465. [DOI] [PubMed] [Google Scholar]
- Young D. B.; Perkins M. D.; Duncan K.; Barry C. E. III (2008) Confronting the scientific obstacles to global control of tuberculosis. J. Clin. Invest. 118, 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez J. E.; McKinney J. D. M. (2004) Tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinburgh) 84, 29–44. [DOI] [PubMed] [Google Scholar]
- Dick T. (2001) Dormant tubercle bacilli: the key to more effective TB chemotherapy?. J. Antimicrob. Chemother. 47, 117–118. [DOI] [PubMed] [Google Scholar]
- Boshoff H. I.; Barry C. E. III (2005) Tuberculosis - metabolism and respiration in the absence of growth. Nat. Rev. Microbiol. 3, 70–80. [DOI] [PubMed] [Google Scholar]
- Wayne L. G.; Sohaskey C. D. (2001) Nonreplicating persistence of mycobacterium tuberculosis. Annu. Rev. Microbiol. 55, 139–163. [DOI] [PubMed] [Google Scholar]
- Heng Y.; Seah P. G.; Siew J. Y.; Tay H. C.; Singhal A.; Mathys V.; Kiass M.; Bifani P.; Dartois V.; Herve M. (2011) Mycobacterium tuberculosis infection induces hypoxic lung lesions in the rat. Tuberculosis (Edinburgh) 91, 339–341. [DOI] [PubMed] [Google Scholar]
- Via L. E.; Lin P. L.; Ray S. M.; Carrillo J.; Allen S. S.; Eum S. Y.; Taylor K.; Klein E.; Manjunatha U.; Gonzales J.; Lee E. G.; Park S. K.; Raleigh J. A.; Cho S. N.; McMurray D. N.; Flynn J. L.; Barry C. E. III (2008) Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 76, 2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. D.; Guinn K. M.; Harrell M. I.; Liao R.; Voskuil M. I.; Tompa M.; Schoolnik G. K.; Sherman D. R. (2003) Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 48, 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muttucumaru D. G.; Roberts G.; Hinds J.; Stabler R. A.; Parish T. (2004) Gene expression profile of Mycobacterium tuberculosis in a non-replicating state. Tuberculosis (Edinburgh) 84, 239–246. [DOI] [PubMed] [Google Scholar]
- Schnappinger D.; Ehrt S.; Voskuil M. I.; Liu Y.; Mangan J. A.; Monahan I. M.; Dolganov G.; Efron B.; Butcher P. D.; Nathan C.; Schoolnik G. K. (2003) Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: Insights into the phagosomal environment. J. Exp. Med. 198, 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leistikow R. L.; Morton R. A.; Bartek I. L.; Frimpong I.; Wagner K.; Voskuil M. I. (2010) The Mycobacterium tuberculosis DosR regulon assists in metabolic homeostasis and enables rapid recovery from nonrespiring dormancy. J. Bacteriol. 192, 1662–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustad T. R.; Harrell M. I.; Liao R.; Sherman D. R. (2008) The enduring hypoxic response of Mycobacterium tuberculosis. PLoS One 3, e1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S. P.; Alonso S.; Rand L.; Dick T.; Pethe K. (2008) The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 105, 11945–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S.; Zimmermann M.; Goodwin M. B.; Sauer U.; Barry C. E.; Boshoff I. I. I.; Fumarate Reductase H. I. (2011) Activity maintains an energized membrane in anaerobic Mycobacterium tuberculosis. PLoS Pathog. 7, e1002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacon A. H.; Pym A.; Grobusch M.; Patientia R.; Rustomjee R.; Page-Shipp L.; Pistorius C.; Krause R.; Bogoshi M.; Churchyard G.; Venter A.; Allen J.; Palomino J. C.; De M. T.; van Heeswijk R. P.; Lounis N.; Meyvisch P.; Verbeeck J.; Parys W.; de B. K.; Andries K.; Mc Neeley D. F. (2009) The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 360, 2397–2405. [DOI] [PubMed] [Google Scholar]
- Andries K.; Verhasselt P.; Guillemont J.; Gohlmann H. W.; Neefs J. M.; Winkler H.; Van Gestel J.; Timmerman P.; Zhu M.; Lee E.; Williams P.; de Chaffoy D.; Huitric E.; Hoffner S.; Cambau E.; Truffot-Pernot C.; Lounis N.; Jarlier V. (2005) A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307, 223–227. [DOI] [PubMed] [Google Scholar]
- Wayne L. G. (1977) Synchronized replication of Mycobacterium tuberculosis. Infect. Immun. 17, 528–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G.; Lin K. Y. (1982) Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect. Immun. 37, 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G.; Hayes L. G. (1996) An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64, 2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M. P.; Sequeira P.; Lin W. W.; Phong W. Y.; Cliff P.; Ng S. H.; Lee B. H.; Camacho L.; Schnappinger D.; Ehrt S.; Dick T.; Pethe K.; Alonso S. (2010) Nitrate respiration protects hypoxic Mycobacterium tuberculosis against acid- and reactive nitrogen species stresses. PLoS One 5, e13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G.; Hayes L. G. (1996) An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64, 2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon C.; Dick T. (2002) Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J. Bacteriol. 184, 6760–6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V.; Sharma D.; Ramanathan V. D.; Shakila H.; Saini D. K.; Chakravorty S.; Das T. K.; Li Q.; Silver R. F.; Narayanan P. R.; Tyagi J. S. (2004) Disruption of response regulator gene, devR, leads to attenuation in virulence of Mycobacterium tuberculosis. FEMS Microbiol. Lett. 231, 237–245. [DOI] [PubMed] [Google Scholar]
- Roberts D. M.; Liao R. P.; Wisedchaisri G.; Hol W. G.; Sherman D. R. (2004) Two sensor kinases contribute to the hypoxic response of Mycobacterium tuberculosis. J. Biol. Chem. 279, 23082–23087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D. R.; Voskuil M.; Schnappinger D.; Liao R.; Harrell M. I.; Schoolnik G. K. (2001) Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha -Crystallin. Proc. Natl. Acad. Sci. U.S.A. 98, 7534–7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stermann M.; Bohrssen A.; Diephaus C.; Maass S.; Bange F. C. (2003) Polymorphic nucleotide within the promoter of nitrate reductase (NarGHJI) is specific for Mycobacterium tuberculosis. J. Clin. Microbiol. 41, 3252–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunatha U.; Boshoff H. I.; Barry C. E. (2009) The mechanism of action of PA-824: Novel insights from transcriptional profiling. Commun. Integr. Biol 2, 215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier T.; Eiglmeier K.; Camus J. C.; Medina N.; Mansoor H.; Pryor M.; Duthoy S.; Grondin S.; Lacroix C.; Monsempe C.; Simon S.; Harris B.; Atkin R.; Doggett J.; Mayes R.; Keating L.; Wheeler P. R.; Parkhill J.; Barrell B. G.; Cole S. T.; Gordon S. V.; Hewinson R. G. (2003) The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. U.S.A. 100, 7877–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshoff H. I.; Mizrahi V.; Barry C. E. III (2002) Effects of pyrazinamide on fatty acid synthesis by whole mycobacterial cells and purified fatty acid synthase I. J. Bacteriol. 184, 2167–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorpio A.; Zhang Y. (1996) Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 2, 662–667. [DOI] [PubMed] [Google Scholar]
- MedChem Studio, 2.0.0.1 (2011) Simulations Plus, Inc., Lancaster, CA.
- Khoje A. D.; Charnock C.; Wan B.; Franzblau S.; Gundersen L. L. (2011) Synthesis and antimycobacterial activities of non-purine analogs of 6-aryl-9-benzylpurines: Imidazopyridines, pyrrolopyridines, benzimidazoles, and indoles. Bioorg. Med. Chem. 19, 3483–3491. [DOI] [PubMed] [Google Scholar]
- Pieroni M.; Lilienkampf A.; Wan B.; Wang Y.; Franzblau S. G.; Kozikowski A. P. (2009) Synthesis, biological evaluation, and structure-activity relationships for 5-[(E)-2-arylethenyl]-3-isoxazolecarboxylic acid alkyl ester derivatives as valuable antitubercular chemotypes. J. Med. Chem. 52, 6287–6296. [DOI] [PubMed] [Google Scholar]
- Maddry J. A.; Ananthan S.; Goldman R. C.; Hobrath J. V.; Kwong C. D.; Maddox C.; Rasmussen L.; Reynolds R. C.; Secrist J. A. III; Sosa M. I.; White E. L.; Zhang W. (2009) Antituberculosis activity of the molecular libraries screening center network library. Tuberculosis (Edinburgh) 89, 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraski G. C.; Markley L. D.; Hipskind P. A.; Boshoff H.; Cho S.; Franzblau S. G.; Miller M. J. (2011) Advent of imidazo[1,2-a]pyridine-3-carboxamides with potent multi- and extended drug resistant antituberculosis activity. ACS Med. Chem. Lett. 2, 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul A.; Dendouga N.; Vergauwen K.; Molenberghs B.; Vranckx L.; Willebrords R.; Ristic Z.; Lill H.; Dorange I.; Guillemont J.; Bald D.; Andries K. (2007) Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat. Chem. Biol. 3, 323–324. [DOI] [PubMed] [Google Scholar]
- Bardarov S.; Bardarov S Jr; Pavelka M. S. J. Jr; Sambandamurthy V.; Larsen M.; Tufariello J.; Chan J.; Hatfull G.; Jacobs W. R. J. Jr (2002) Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148, 3007–3017. [DOI] [PubMed] [Google Scholar]
- Parish T.; Stoker N. G. (2000) Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology 146(Pt 8), 1969–1975. [DOI] [PubMed] [Google Scholar]
- Stover C. K.; de la Cruz V. F.; Fuerst T. R.; Burlein J. E.; Benson L. A.; Bennett L. T.; Bansal G. P.; Young J. F.; Lee M. H.; Hatfull G. F. (1991) New use of BCG for recombinant vaccines. Nature 351, 456–460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.