Abstract
Armed antibody-based targeted molecular therapies offer the possibility of effective tumor control with a minimum of side effects. Photoimmunotherapy (PIT) employs a monoclonal antibody-phototoxic phthalocyanine dye, IR700 conjugate that is activated by focal near infrared (NIR) light irradiation after antibody binding to the targeted tumor cell surface leading to rapid necrotic cell death. Therapy by single NIR light irradiation was effective without significant side-effects, however, recurrences were seen in most of treated mice probably because of inhomogeneous distribution of panitumumab-IR700 immuno-conjugate in the tumor, leading to ineffective PIT. We describe here an optimized regimen of effective PIT method for the same HER1-overexpressing tumor model (A431) with fractionated administration of panitumumab-IR700 conjugate followed by systematic repeated NIR light irradiation to the tumor based on timing of antibody redistribution into the remnant tumor under the guidance of IR700 fluorescence signal. Eighty percents of the A431 tumors were eradicated with repeated PIT without apparent side effects and survived with tumor free more than 120 days even after stoping therapy at the day 30. Therapeutic effects were monitored using IR700 fluorescent signal. PIT is a promising highly selective and clinically feasible theranostics for the treatment of MAb-binding tumors with minimal off target effects.
Keywords: photoimmunotherapy, theranostics, epidermal growth factor receptor, molecular targeting, monoclonal antibody
INTRODUCTION
Targeted cancer therapies offer the promise of more effective tumor control with fewer side effects than conventional cancer therapies. The class of molecular therapies based on monoclonal antibodies (MAb) is highly selective for tumors expressing particular antigens, however, with few exceptions, patient outcomes have been only modestly improved when the naked MAb is used as monotherapy or in combination with other therapies.1–3 The primary mechanisms of action of MAb therapy are thought to be antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) and/or receptor signaling blockade.4, 5 Another way in which MAbs can be used in therapy is as vectors for the delivery of other therapies, such as toxins or radioisotopes,6, 7 however, the toxicity of such immunotoxins or radioimmunotherapeutics are often dose limiting due to off target effects to normal organs.
We recently reported a new form of MAb based immunotherapy termed “photoimmunotherapy” (PIT) that utilizes an MAb conjugated with a near infrared (NIR) phthalocyanine dye, IRDye700DX (IR700). IR700 was originally designed as a fluorescent agent and its therapeutic properties were only recently discovered. As a consequence, IR700 is not only phototoxic but also fluorescent and thus can be used as an imaging agent. Additionally, when exposed to more intense levels of NIR light, the conjugate becomes lethal but only to those cells to which the conjugate is bound.8 NIR light exposure leads to rapid, target-selective necrotic cell death in vitro. In addition, effective tumor shrinkage was obtained even with a single administration of MAb-IR700 followed by a single exposure to NIR light in vivo. Although single administration of the therapy (agent + NIR light) was highly effective, recurrences were seen in treated mice, because inhomogeneous MAb-IR700 distribution in the targeted tumor could lead not to kill a small fraction of cancer cells, to which MAb-IR700 did not bind, with NIR light. Unlike non-targeting small molecule photosensitizers, MAb-IR700 conjugate stays longer in the blood circulation that allows unbound MAb-IR700 to redistribute into the remnant target tumor after the first NIR light irradiation. This suggested that repeated administrations of the therapy (agent + NIR light) could theoretically lead to even better tumor control. In this study, we investigated the optimization of treatment regimens by fractionated dosing of MAb-IR700 conjugate and NIR light irradiation in order to enhance the effectiveness and safety of PIT employing an anti-HER1 MAb, panitumumab-IR700 (Pan-IR700) conjugate in a xenograft tumor model.
EXPERIMENTAL PROCEDURES
Reagents
A water soluble, silicon-phthalocyanine derivative, IRDye 700DX NHS ester (IR700; C74H96N12Na4O27S6Si3, molecular weight of 1954.22) was obtained from LI-COR Bioscience (Lincoln, NE). Panitumumab, a fully humanized IgG2 MAb directed against the human epidermal growth factor receptor 1 (HER1), was purchased from Amgen (Thousand Oaks, CA). All other chemicals used were of reagent grade.
Synthesis of IR700-conjugated panitumumab
Panitumumab (1 mg, 6.8 nmol) was incubated with IR700 (66.8 μg, 34.2 nmol, 5 mmol l-1 in DMSO) in 0.1 mol l−1 Na2HPO4 (pH 8.5) at room temperature for 2 h. The mixture was purified with a Sephadex G50 column (PD-10; GE Healthcare). The protein concentration was determined with Coomassie Plus protein assay kit (Thermo Fisher Scientific Inc, Rockford, IL) by measuring the absorption at 595 nm with spectroscopy (8453 Value System; Agilent Technologies, Santa Clara, CA). The concentration of IR700 was measured by absorption with spectroscopy to confirm the number of fluorophore molecules conjugated to each MAb molecule.
Cells
HER1-expressing A431 cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in tissue culture flasks in a humidified incubator at 37 °C in an atmosphere of 95% air and 5% carbon dioxide.
In vivo photoimmunotherapy for A431 tumors with Pan-IR700
All in vivo procedures were conducted in compliance with the Guide for the Care and Use of Laboratory Animal Resources (1996), US National Research Council, and approved by the local Animal Care and Use Committee. Six- to eight-week-old female homozygote athymic nude mice were purchased from Charles River (NCI-Frederick). During the procedure, mice were anesthetized with isoflurane. One million A431 cells were injected subcutaneously in the right dorsum of the mice. In order to determine tumor volume, the greatest longitudinal diameter (length) and the greatest transverse diameter (width) were determined with external caliper. Tumor volume based on caliper measurements were calculated by the following formula; tumor volume = length × width2 × 0.5.9 Tumors reaching approximately 40 mm3 in volume were selected for the study. Selected mice were randomized into 7 groups of at least 10 animals per group for the following treatments: (1) no treatment; (2) 100 μg of panitumumab i.p.every week, no NIR light exposure; (3) 100 μg of Pan-IR700 i.p. every week, no NIR light exposure; (4) PBS i.p. every week, NIR light was administered at 50 J cm−2 on day 1 after injection and 100 J cm−2 on day 2 after injection; (5) 100 μg of Pan-IR700 i.p. every week, NIR light was administered at 50 J cm−2 on day 1 after injection (6) 100μg of Pan-IR700 i.p. every week, NIR light was administered at 100 J cm−2 on day 2 after injection ; (6) 100 μg of Pan-IR700 i.p. every week, NIR light was administered at 50 J cm−2 on day 1 after injection and 100 J cm−2 on day 2 after injection. These therapies were performed every week for up to 4 weeks until mice had to be euthanized due to tumor growth > 500 mm3. Mice were monitored daily, and tumor volume was measured three times a week until the tumor volume reached 500 mm3, at which time mice were euthanized with carbon dioxide. An LED light source (L690-66-60, Marubeni America Co., Santa Clara, CA) was used for NIR irradiation of PIT at 670 to 710 nm (peak at 690 nm). Fluorescence images, as well as white light images, were obtained using a Pearl Imager (LI-COR Biosciences) with a 700 nm fluorescence channel with a 685 nm laser excitation. For analyzing fluorescence intensities, tumors of the same size were compared and regions of interest (ROI) were placed over the entire tumor. Average fluorescence intensity of each ROI was calculated.
Histrogical analysis
To evaluate serial histological changes after PIT and micro-distribution of Pan-IR700, light and fluorescence microscopy study was performed (BX51, Olympus America). A431 tumors were harvested in 10% formalin 1h and 1d after 50J/cm2 of PIT. Serial 10-μm slice sections were fixed on 2 slide grasses without staining or with H-E staining. The filter set to detect IR700 fluorescence consisted of a 590–650 nm excitation filter, a 665–740 nm band pass emission filter.
Statistical Analysis
Data are expressed as mean ± s.e.m. Statistical analyses were carried out using a statistics program (GraphPad Prism; GraphPad Software Inc, La Jolla, CA). The Student’s t test was used to compare the treatment effects with controls. The cumulative probability of survival (based on the failure of the tumor to reach 500 mm3), were estimated in each group with the use of the Kaplan–Meier survival curve analysis, and the results were compared with use of the log-rank test with Bonferroni's correction for multiple comparisons. P < 0.05 was used to determine statistical significance.
RESULTS
Repeated irradiation of NIR light can minimize dose of Pan-IR700 conjugate without compromising therapeutic effect of PIT
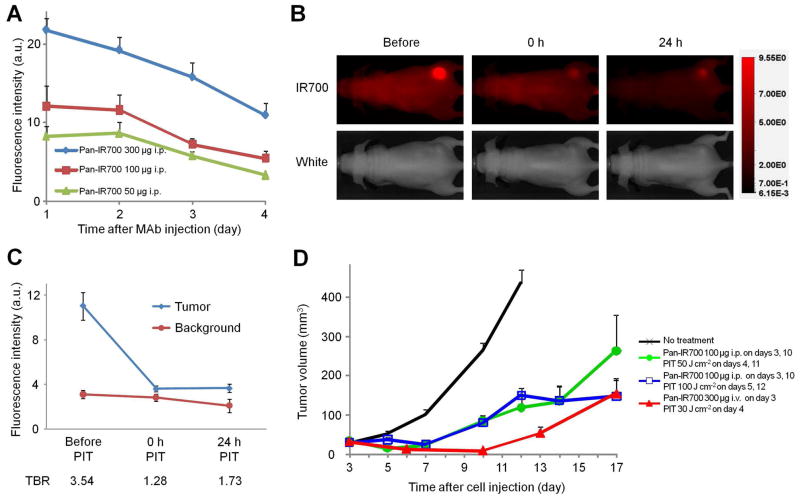
In prior studies we have used 300 μg as the dose of Pan-IR700. We examined the effect of the lower dose (100 μg) of Pan-IR700 used in this study. As expected, highest IR700 signals were obtained at 1 day after injection in each group and the tumor based fluorescence was lower with the decreased dose of Pan-IR700 (Figure 1a). Interestingly, the target-to-background ratio (TBR) of IR700 fluorescence intensity in A431 tumor decreased to background level just after NIR light irradiation that ensured sufficient therapeutic effects to all cancer cells to which Pan-IR700 bound (Figure 1b, c). Circulating Pan-IR700 re-accumulated in the remnant tumor mass within a day, leading to an increase in TBR on fluorescence imaging (Figure 1b, c). This indicated that Pan-IR700 was still accumulating in the survived cancer cells even after initial treatment with NIR light and therefore the second dose of NIR light irradiation at 100 J cm−2 was effective in treating the tumors (2 days after Pan-IR700 administration). Therefore, the reduced dose of Pan-IR700 (100 μg) did not compromise PIT efficacy and still enabled visualization with IR700 fluorescence (Figure. 1d).
Figure 1. Effectiveness of fractionated Pan-IR700 with multiple NIR light exposures as detected by IR700 fluoresence.
Pan-IR700 was administered to A431 tumor-bearing mice which were then exposed to NIR light and imaged with a fluorescent camera. (a) IR700 fluorescence of A431 tumors over time (n = 3 mice in each group). (b) Images of A431 tumor bearing mice before (left), immediately after (center) and 24 h after (right) PIT with 50 J cm-2 NIR light irradiation. IR700 fluorescence signal in the tumor decreased to background level immediately after PIT (center). Signal within the tumor increased 24 h after PIT indicating reaccumulation of Pan-IR700 due to circulating Pan-IR700 (right). (c) IR700 fluorescence of A431 tumor before and after NIR light irradiation. Data are mean ± s.e.m. (n = 4 mice). (d) Tumor volume reduction in response to Pan-IR700 PIT in A431 xenograft tumor (n≥3 10 mice in each treatment group).
HER1-specific tumor shrinkage in response to Pan-IR700 mediated repeated photoimmunotherapy
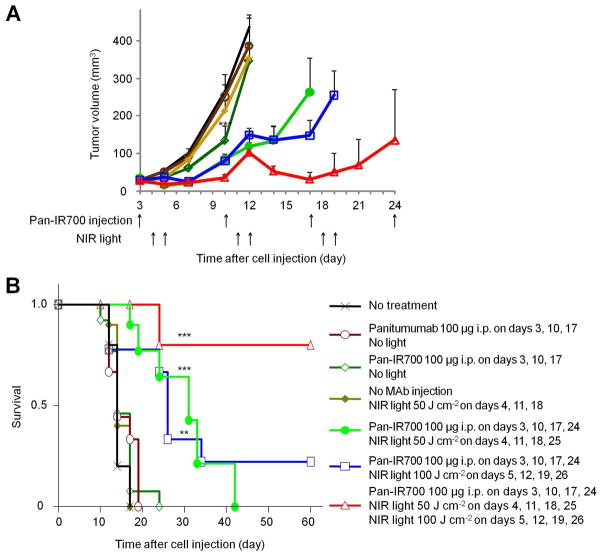
The efficacy of repeated NIR irradiation regimen was studied in 7 groups of A431 tumor bearing mice (n = 10 mice in each group). All treated tumors were less than 500 mm3 in accordance with our institution’s animal care and use guidelines. After 100 μg of Pan-IR700 injection, which was administered every week for up to 4 weeks, A431 tumors were treated with or without NIR light on day 1 (50 J cm−2), day 2 (100 J cm−2) or both days. Tumor volume was significantly reduced in A431 tumors treated with Pan-IR700 and NIR light compared with untreated control mice. Mice injected with panitumumab but not exposed to NIR light showed no significant treatment effect (each PIT group; P < 0.001 vs. untreated control mice analyzed at 9 days after first Pan-IR700 injection). Similarly, mice exposed to NIR light alone had no treatment effect. However, multiple NIR light irradiations following Pan-IR700 led to more effective tumor reduction (Figure 2a). In survival analysis, multiple Pan-IR700 injection followed by multiple NIR light irradiation contributed to prolonged survival in the PIT treatment groups. Notably 80% of A431 tumors were eradicated and the mice survived for 120 days in the mice treated with Pan-IR700 injection followed by NIR light irradiation on day 1 (50 J cm−2) and day 2 (100 J cm−2) (Figure 2b) vs. control mice that required euthanasia by 24 days.
Figure 2. An optimized regimen of PIT resulted in complete pathologic response in mice with A431 xenograft tumors.
(a) Repeated Pan-IR700 mediated PIT lead to more effective tumor volume reduction (n = 10 mice in each treatment group; *** P < 0.001 vs. non treatment control mice). Note that data were analyzed and shown only when each group of tumors were not exceeded 500 mm3. (b) Repeated Pan-IR700 mediated PIT led to prolonged survival. (n = 10 mice in each treatment group; *** P < 0.001, ** P < 0.01 vs. non treatment control mice).
Detection and monitoring in response to photoimmunotherapy with NIR fluorescence
We have shown previously that IR700 fluorescence can be used to monitor Pan-IR700 in tumors. One day after Pan-IR700 injection, intratumoral fluorescence was detectable. Mice receiving 100 μg of Pan-IR700 but no NIR light served as controls in this case. In the group not receiving NIR light, increased IR700 fluorescence was observed, however, reductions in fluorescence were observed in tumors receiving Pan-IR700 and NIR light. When injected Pan-IR700 for the next PIT and IR700 fluorescence was down to the background level at the tumor site, no tumor recurrence was observed, however when IR700 fluorescence was higher than the background level, regrowth was observed within 8 days (Figure 3).
Figure 3. Monitoring accumulation of Pan-IR700 into tumors using NIR fluorescence imaging.
Monitoring PIT with Pan-IR700 fluorescence. Images were obtained 1 day after Pan-IR700 injection just before NIR light irradiation. Tumor growth or regression in response to Pan-IR700 mediated PIT was observable with IR700 fluorescence.
Histological changes and micro-distribution of Pan-IR700
Few fluorescent cells in diffuse necrotic cells with micro-hemorrhage are shown 1 h after PIT (Fig. 4, Right upper images). Scattered fluorescent micro-clusters of survived tumor cells are shown 1 d after PIT (Fig. 4, Left lower images). All cancer cells in clusters are fluoresced that indicate Pan-IR700 homogeneously distributed in survived and proliferated cancer cells. This result supports the effective PIT at the 2nd exposure of NIR light.
Figure 4. Serial histology pictures and micro-distribution of IR700 fluorescence in the tumor 1 h and 1 d after first PIT are shown together with a schema for explaining effectiveness of repeated PIT. Few fluorescent cells in diffuse necrotic cells with micro-hemorrhage are shown 1 h after PIT (Right upper images). Scattered fluorescent micro-clusters of survived tumor cells are shown 1 d after PIT (Left lower images). All cancer cells in clusters (black arrows) are fluoresced (white arrows) that indicate Pan-IR700 homogeneously distributed in survived and proliferated cancer cells.
DISCUSSION
We have recently reported that HER1 target-specific cell killing was achieved in response to a single dose of Pan-IR700 and NIR ligth both in vitro and in vivo.8 Pan-IR700 localized specifically in HER1 positive A431 tumors in vivo, and tumor shrinkage was confirmed after only one dose of Pan-IR700 (300 μg) followed by one exposure to NIR light irradiation (30 J cm−2). However in that study, tumor regrowth was eventually seen. In the current study, we demonstrate that repeated doses of smaller amounts of Pan-IR700 and repeated exposures of NIR light resulted in complete responses in A431 tumors. This was in marked contrast to controls that received Pan-IR700 but no light or light exposure without Pan-IR700. Thus, this study suggests that complete pathologic responses in solid tumors are possible with PIT by using fractionated doses of the MAb-IR700 conjugate and repeated light exposures of NIR.
Preferable distribution of therapeutic MAb in the tumor is required for the effective target-specific tumor cell killing, however, generally MAb distribution is inhomogeneous in most tumors, especially in peripheral localization where xenografted tumors receive good blood supply, leading to require multiple high-dose MAb administration.8, 10–12 Similar to unlabeled MAb, efficacy of MAb-IR700 mediated PIT is partly determined with MAb-IR700 micro-distribution in the targeted tumor.
We used a similar cumulative dose of Pan-IR700 but fractionated into four doses to cover four weeks to safely perform PIT with more effective results (Figure 2a, b).8 Since the maximum concentration of IR700 in A431 tumors became lower using repeated 100 μg doses vs. a single dose of 300 μg,(Figure. 1a), prudent increasing NIR light exposure from 30 J cm−2 to 50 J cm−2 should be considered. Additionally, lowering the dose leads to distribute the MAb-IR700 conjugate peripherally within the xenografted tumor. Therefore, PIT with 100 μg of Pan-IR700 showed reduced cell killing in the center of the tumor as shown in this study.8, 13 To overcome this problem, the Pan-IR700 conjugate was allowed to re-accumulate in the treated tumor 1 day after immediate killing of most peripheral cancer cells near tumor vasculatures with the first NIR light irradiation because massive crashing of cells near by the tumor vessels facilitated to deliver MAb-IR700 conjugate deep away from the tumor vessel into the center part of tumor. Since NIR light irradiation does not have a dose limitation, a repeated NIR light exposure at a day after the first therapy successfully operated killing in the central part of the tumor (Figure 1b). Therefore, additional NIR irradiation 1 day after the first therapy (2 days after Pan-IR700 administration) greatly enhanced the therapeutic effect of PIT (Figure 4). With this therapeutic regimen, fractionating the Pan-IR700 injections followed with multiple NIR irradiation, complete tumor eradication was observed in more than half the treated mice, while most of the mice treated with one shot irradiation regimen of PIT died due to tumor re-growth (Figure 1d).
The Pan-IR700 conjugate can also be used to monitor the effects of PIT due to the fluorescence emitted by the IR700 (Figure 3). However, the fluorescence of IR700 is relatively weak and is best for shallow targets, whereas, the PIT effects can extend several centimeters beneath the skin surface. Nevertheless, for endoscopic or surgical uses of PIT the fluorescent features of MAb-IR700 can be considered an advantage. Next step of this study is to compare this method with recently developed approaches of the photodynamic therapy 14, 15 by using orthotopic tumor models, which will be superior models for human cancer16 , employing fluorescence protein as an internal tumor marker.17
In conclusion, by fractionating the administration of Pan-IR700 and applying repeated NIR light irradiation, highly effective tumor treatments based on the effective tumor redistribution of Pan-IR700, including complete pathologic responses were achieved, with no apparent side effects in a majority of A431 bearing mice. Thus, PIT using MAb-IR700 conjugates is a promising theranostic method for highly selective cancer treatment and monitoring.
Acknowledgments
Grant Support: This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waldmann TA. Immunotherapy: past, present and future. Nat Med. 2003;9:269–277. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- 3.Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol. 2005;23:1073–1078. doi: 10.1038/nbt0905-1073. [DOI] [PubMed] [Google Scholar]
- 4.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 5.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, Newman RA, Hanna N, Anderson DR. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- 6.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg DM, Sharkey RM, Paganelli G, Barbet J, Chatal JF. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J Clin Oncol. 2006;24:823–834. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 8.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685–1691. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Euhus DM, Hudd C, LaRegina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1986;31:229–234. doi: 10.1002/jso.2930310402. [DOI] [PubMed] [Google Scholar]
- 10.Kosaka N, Ogawa M, Paik DS, Paik CH, Choyke PL, Kobayashi H. Microdistribution of fluorescently-labeled monoclonal antibody in a peritoneal dissemination model of ovarian cancer. Proc SPIE. 2010;7576:7576041–7576049. doi: 10.1111/j.1349-7006.2009.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Mehren M, Adams GP, Weiner LM. Monoclonal antibody therapy for cancer. Annu Rev Med. 2003;54:343–369. doi: 10.1146/annurev.med.54.101601.152442. [DOI] [PubMed] [Google Scholar]
- 12.Jain RK, Baxter LT. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 1988;48:7022–7032. [PubMed] [Google Scholar]
- 13.Kosaka N, Ogawa M, Paik DS, Paik CH, Choyke PL, Kobayashi H. Semiquantitative assessment of the microdistribution of fluorescence-labeled monoclonal antibody in small peritoneal disseminations of ovarian cancer. Cancer Sci. 2010;101:820–825. doi: 10.1111/j.1349-7006.2009.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura H, Lee C, Hayashi K, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Bouvet M, Hoffman RM. UV light killing efficacy of fluorescent protein-expressing cancer cells in vitro and in vivo. J Cell Biochem. 2010;110:1439–1446. doi: 10.1002/jcb.22693. [DOI] [PubMed] [Google Scholar]
- 15.Tsai MH, Aki R, Amoh Y, Hoffman RM, Katsuoka K, Kimura H, Lee C, Chang CH. GFP-fluorescence-guided UVC irradiation inhibits melanoma growth and angiogenesis in nude mice. Anticancer Res. 2010;30:3291–3294. [PubMed] [Google Scholar]
- 16.Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17:343–359. doi: 10.1023/a:1006326203858. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer. 2005;5:796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]