Abstract
Objective
To determine the prevalence of the use of prenatal corticosteroids in women who delivered prematurely in 3 Latin American counties and to evaluate the maternal characteristics associated with use.
Methods
A multicenter, prospective, descriptive study was conducted in 4 hospitals in Ecuador, 5 in Uruguay, and 3 in El Salvador between 2004 and 2008. Women who had delivered between 24 and 34 weeks of pregnancy responded to a questionnaire assessing sociodemographic characteristics, obstetric history, prenatal care, women's attitudes to health services and knowledge of preterm risk factors, prenatal corticosteroid administration, and characteristics of the delivery and neonate. The association between the prenatal corticosteroid use and the study variables was evaluated through a logistic regression analysis based on a hierarchical model.
Results
A total of 1062 women who had a preterm birth were included in the study. Prenatal corticosteroid use was 34.8% (95% CI, 29.9%–39.9%) in Ecuador, 54.6% (95% CI, 49.6%–59.6%) in El Salvador, and 71.0% (95% CI, 65.3%–76.2%) in Uruguay. Hospital admission-to-delivery time was associated with the use of prenatal corticosteroids in all 3 countries.
Conclusion
The study revealed a varied pattern of use of prenatal corticosteroids across the 3 countries, and a diversity of influencing factors.
Keywords: Prenatal corticosteroids, Preterm birth, Respiratory distress syndrome/prevention
1. Introduction
Preterm birth (PTB) is the primary cause of 4 million neonatal deaths worldwide each year, with 99% of these deaths occurring in low- and middle-income countries [1].
Prenatal corticosteroids administered to women at high risk for PTB have been shown to reduce the risk of respiratory distress syndrome, intraventricular hemorrhage, and neonatal mortality by 50% or greater [2-5]. Despite the documented efficacy, the mothers of less than 10% of preterm newborns in low- and middle-income countries receive prenatal corticosteroids, while in high-income countries their use surpasses 70% [6-7]. In Latin America, hospital-based studies of preterm births have shown that use of prenatal corticosteroids ranges from 4%–37% [8-11].
The aim of the present study was to determine the prevalence of the use of prenatal corticosteroids and the maternal characteristics associated with their use in women who delivered prematurely in public maternity hospitals in 3 Latin American countries.
2. Materials and methods
The study was a multicenter, prospective, descriptive study conducted between July 2004 and June 2008 in 4 public hospitals in Ecuador (Guayaquil, Cuenca, Loja, and Quito); 5 public hospitals in Uruguay (2 in Montevideo, Paysandú, Salto, and Tacuarembó); and 3 public and 1 social security hospital in El Salvador (2 in San Salvador, San Miguel, and Santa Ana). The countries were selected to geographically represent the Andean region, the South Cone, and Central America. The study was approved by the following Institutional Review Boards: Universidad de la Republica del Uruguay, Universidad Central del Ecuador, Facultad de Ciencias Medicas del El Salvador, and Hospital Nacional Rosales de Ecuador.
Participants were women who had delivered prematurely between 24 and 34 weeks plus 6 days of pregnancy at a participating hospital, or who had been referred to a participating hospital. Women with mental or physical impairments that prevented them from completing the study questionnaire and women who had been diagnosed with a stillbirth at admission were excluded.
Eligible women were identified from the delivery ward logbooks and the neonatal intensive care unit (NICU) admission registries. Each day, in-hospital data collectors checked both registries and reported all live births that had been delivered under 37 weeks and all preterm neonates under 37 weeks that had been admitted to the NICU. Eligible women were invited to participate and provided written informed consent.
A questionnaire was designed that included 65 items grouped into 2 sections: a survey for the mother and a clinical data form. The maternal survey included questions concerning sociodemographic characteristics, obstetric history, prenatal care, attitudes regarding health services, and knowledge about risk factors for PTB. The clinical data form included items related to the administration of prenatal corticosteroids and to events surrounding the delivery and the health of the newborn; the clinical data form was completed using data extracted from the clinical records. The questionnaire was administered by interviewers (nurses, midwives, and residents) who had been similarly trained in the 3 countries; it was piloted before the start of the study in one participating hospital in each country to ensure consistency. All interviewers signed a confidentiality agreement. A random sample of 8%–10% of the participating women was taken and the women were either re-interviewed or their records were reviewed for data quality assurance.
In the maternal survey, any request for care owing to pregnancy complications was considered positive if the mother reported having attended a health facility to seek care without an appointment. Previous admission to any hospital during the pregnancy was considered positive if the mother was hospitalized during the index pregnancy. Type of PTB was classified into 1 of 3 categories by the principal investigator: (1) PTB associated with preterm premature rupture of membranes (PPROM); (2) medically indicated PTB; or (3) spontaneous preterm birth (sPTB) (idiopathic). Preterm births were assigned to a category by reviewing the cause of admission in the clinical records. Time from admission to delivery was defined as the number of hours between a woman's admission to hospital and delivery. Accessibility to hospital was defined as the time it took a mother to reach the health facility from her home.
The questions concerning women's satisfaction with health care and perception of the quality of care received were adapted from a previous survey conducted in Uruguay [12]. We assumed that a woman was satisfied with her health care when she answered “yes” to the questions “did you feel respected?” and “did you feel well or very well treated?” and “no” to the question “did you feel ashamed?” Any other answer was considered to represent dissatisfaction with the health care that the woman had received. We assumed that a woman perceived the quality of care to be good when she answered “yes” to the questions “would you recommend this health service?” and “would you return to seek health care?” and answered “total and great trust” to the question “how much trust do you have in the health care facility?” Any other answer was considered to represent a perception of poor quality of health care. We classified each woman's knowledge of 5 risk factors for PTB in 3 categories: poor when none or 1 risk factor was known; fair when 2–3 risks factors were known; and good when 4–5 risks factors were known. The risk factors were painful and periodic uterine contractions; vaginal bleeding; leakage of amniotic fluid; history of PTB; and excessive work load.
After collecting the completed data forms, data managers from each country took digital pictures of each form and transmitted the files to special email accounts at the study data center using the SSL (Secure Sockets Layer) protocol, with a hosting provider digital certificate. Data were entered in a secure data management system, which had been specifically designed for the study and was fully compliant with good clinical practice. Double data entry was performed for a random sample of 15% of the data forms to assess the quality of data entry. Data queries were resolved by the country data managers by email, using the same email accounts. To preserve the confidentiality of the participants, personal identifiers were not included in the data forms nor were they transmitted to the data center.
A sample size of 365 women per country was calculated assuming a proportion of prenatal corticosteroid use of 0.40, and a 95% confidence interval of 0.35–0.45. Consecutive eligible women who agreed to participate were included in the study until the estimated sample size was reached.
A descriptive analysis of the participants’ characteristics was performed for each country. Prevalence of the use of prenatal corticosteroids and the 95% confidence interval (CI) were estimated. Crude odds ratios (ORs) and 95% CI were computed as measures of association between the use of prenatal corticosteroids and the women's characteristics in the bivariate and multivariate analysis.
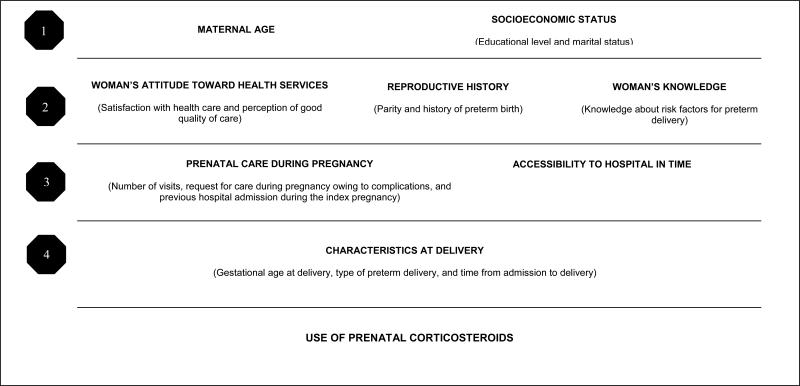
The relationship between prenatal corticosteroid use and the study variables was conceptually based on a hierarchical model designed by the study investigators (Figure 1) [13-14]. According to this model, maternal age and socioeconomic variables may directly or indirectly determine all the other factors under study. The next hierarchical level comprises reproductive history, women's attitudes toward health services, and knowledge about risk factors, which can be partially explained by socioeconomic factors and maternal age. The third level includes prenatal care and accessibility to hospital facilities. Finally, characteristics of PTB such as gestational age at delivery, clinical type of PTB, and time from admission to delivery may be affected by the preceding variables, and directly influence the use of prenatal corticosteroids.
Figure 1.
Hierarchical model explaining the relationship between the study variables and use of prenatal corticosteroids
We ran separate multivariate logistic regression analyses for each country. We considered determinants of prenatal corticosteroid use to be those variables that showed a statistically significant association (5% level) in each respective level of the hierarchical model. In the first step, maternal age and all socioeconomic variables were entered. The variables in the second level were then added, keeping all the statistically significant variables from the first level. A similar procedure was repeated for the variables for the other levels. The reported ORs were those corresponding to the level in which the risk factor of interest was first entered, and not from the final full model with all the variables. This prevents the mediating variables removing some of the explanatory associations of the more distal determinants. For example, if it were to exist, part of the effect of maternal education on receiving or not receiving prenatal corticosteroids may be mediated through attending prenatal care, or how quickly the mother can reach the hospital in case of an emergency. To avoid underestimating its role, the overall effect of maternal education should be analyzed in a model in which prenatal care variables were not included.
3. Results
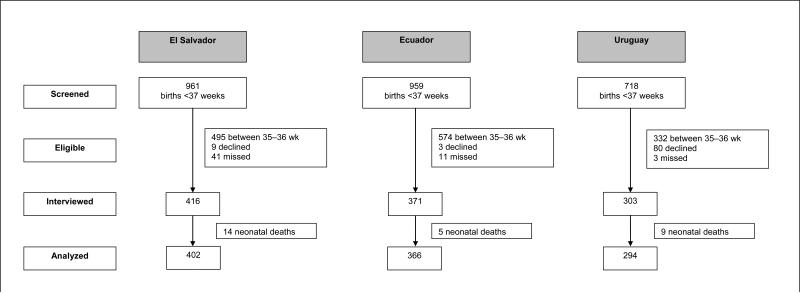
A total of 1062 women who had delivered prematurely agreed to participate in the study and were interviewed at the 13 participating hospitals: 366 women in Ecuador, 402 in El Salvador, and 294 in Uruguay. Figure 2 shows the number of women screened and the reasons for exclusion in each country. Table 1 shows the characteristics of the participants in each country. In general, these characteristics were similar among the 3 countries, with the exception of the level of education and the time from admission to delivery, which was longer in Uruguay.
Figure 2.
Flow chart showing recruitment process by country.
Table 1.
Sociodemographic characteristics, current and previous pregnancy/delivery characteristics, and women's knowledge and attitudesa
| Maternal characteristics | El Salvador No. (%) (n = 402) | Ecuador No. (%) (n = 366) | Uruguay No. (%) (n = 294) | |||
|---|---|---|---|---|---|---|
| Sociodemographic | ||||||
| Age, y | ||||||
| ≤15 | 15 | 3.7% | 14 | 3.8% | 16 | 5.4% |
| 16–34 | 349 | 86.8% | 314 | 85.8% | 252 | 85.7% |
| >34 | 38 | 9.5% | 38 | 10.4% | 26 | 8.8% |
| Educational level, y | ||||||
| <6 | 114 | 29.1% | 31 | 8.6% | 39 | 13.3% |
| 6–11 | 125 | 31.9% | 211 | 58.8% | 237 | 80.6% |
| >12 | 153 | 39.0% | 117 | 32.6% | 18 | 6.1% |
| Marital status | ||||||
| With stable partner | 318 | 79.1% | 319 | 87.4% | 224 | 76.5% |
| Without stable partner | 84 | 20.9% | 46 | 12.6% | 69 | 23.5% |
| History of previous pregnancies | ||||||
| Parity | ||||||
| 0 | 178 | 44.3% | 127 | 34.7% | 99 | 35.5% |
| 1–4 | 203 | 50.5% | 215 | 58.7% | 156 | 55.9% |
| >4 | 21 | 5.2% | 24 | 6.6% | 24 | 8.6% |
| History of preterm birthb | ||||||
| No | 143 | 69.1% | 162 | 73.0% | 92 | 55.1% |
| Yes | 64 | 30.9% | 60 | 27.0% | 75 | 44.9% |
| Women's attitudes toward health services | ||||||
| Satisfied with health care | ||||||
| No | 56 | 14.7% | 69 | 19.7% | 40 | 14.7% |
| Yes | 325 | 85.3% | 281 | 80.3% | 232 | 85.3% |
| Perceived good quality of care | ||||||
| No | 143 | 37.4% | 159 | 45.4% | 132 | 48.4% |
| Yes | 239 | 62.6% | 191 | 54.6% | 141 | 51.6% |
| Women's knowledge | ||||||
| Knowledge of risk factors of preterm delivery | ||||||
| Poor (≤1) | 18 | 4.5% | 26 | 7.1% | 23 | 8.2% |
| Fair (2–3) | 91 | 22.7% | 120 | 32.9% | 93 | 33.3% |
| Good (4–5) | 292 | 72.8% | 219 | 60.0% | 163 | 58.4% |
| Prenatal care during pregnancy and accessibility | ||||||
| Number of prenatal visits | ||||||
| 0 | 18 | 4.6% | 25 | 6.9% | 15 | 5.6% |
| 1+4 | 81 | 20.9% | 89 | 24.6% | 88 | 33.0% |
| ≥5 | 289 | 74.5% | 248 | 68.5% | 164 | 61.4% |
| Request for care during pregnancy owing to complications | ||||||
| No | 20 | 5.0% | 15 | 4.1% | 7 | 2.5% |
| Yes | 382 | 95.0% | 351 | 95.9% | 273 | 97.5% |
| Previous hospital admission in this pregnancy | ||||||
| No | 334 | 83.1% | 295 | 81.0% | 190 | 69.9% |
| Yes | 68 | 16.9% | 69 | 19.0% | 82 | 30.1% |
| Accessibility to hospital (in time), minc | ||||||
| <60 | 362 | 91.0% | 272 | 89.5% | 247 | 92.5% |
| ≥60 | 36 | 9.0% | 32 | 10.5% | 20 | 7.5% |
| Characteristics at delivery | ||||||
| Gestational age, wk | ||||||
| <28 | 46 | 11.4% | 31 | 8.5% | 41 | 13.9% |
| 28-31 | 114 | 28.4% | 99 | 27.0% | 105 | 35.7% |
| 32-33 | 131 | 32.6% | 147 | 40.2% | 89 | 30.3% |
| 34 | 111 | 27.6% | 89 | 24.3% | 59 | 20.1% |
| Type of preterm birth | ||||||
| Spontaneous preterm birth (unknown cause) | 169 | 42.3% | 144 | 39.3% | 112 | 38.5% |
| Preterm birth with PPROMd | 102 | 25.5% | 96 | 26.2% | 95 | 32.6% |
| Medically indicated preterm birth | 129 | 32.3% | 126 | 34.4% | 84 | 28.9% |
| Time from admission to delivery, h | ||||||
| < 3 | 123 | 31.0% | 142 | 39.3% | 73 | 26.8% |
| 3–11.59 | 104 | 26.2% | 88 | 24.4% | 46 | 16.9% |
| 12–23.59 | 62 | 15.6% | 28 | 7.8% | 22 | 8.1% |
| ≥24 | 108 | 27.2% | 103 | 28.5% | 131 | 48.2% |
| Type of delivery | ||||||
| Vaginal | 192 | 47.8% | 146 | 39.9% | 137 | 46.6% |
| Cesarean | 210 | 52.2% | 220 | 60.1% | 157 | 53.4% |
Missing values were excluded from the analysis.
Only multiparous women were considered.
Time taken to travel from the woman's house to the health facility.
PPROM, preterm premature rupture of membranes.
Use of prenatal corticosteroids was heterogeneous among the 3 countries: 34.8% (95% CI, 29.9%–39.9%) in Ecuador; 54.6% (95% CI, 49.6%–59.6%) in El Salvador; and 71.0% (95% CI, 65.3%–76.2%) in Uruguay (Table 2).
Table 2.
Use of prenatal corticosteroids according to sociodemographic characteristics, current and previous pregnancy/delivery characteristics, and women's knowledge and attitudes
| Maternal characteristics | Received prenatal corticosteroids | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| El Salvador (n=402) |
Ecuador (n=366) |
Uruguay (n=294) |
|||||||
| n/N | % | OR (95% CI) | n/N | % | OR (95% CI) | n/N | % | OR (95% CI) | |
| All |
219 / 401 |
54.6 |
(49.6–59.6) |
127 / 365 |
34.8 |
(29.9–39.9) |
203 / 286 |
71.0% |
(65.3–76.2) |
|
Sociodemographic
| |||||||||
| Age, y | |||||||||
| ≤15 | 6 / 15 | 40.0 | - | 1 / 14 | 7.1 | - | 9 / 16 | 56.3 | - |
| 16–34 | 186 / 348 | 53.4 | 1.72 (0.60–4.94) | 108 / 313 | 34.5 | 6.85 (0.88–53.06) | 174 / 244 | 71.3 | 1.93 (0.69–5.39) |
| >34 |
27 / 38 |
71.1 |
3.68 (1.06–12.8) |
18 / 38 |
47.4 |
11.70 (1.39–98.58) |
20 / 26 |
76.9 |
2.59 (0.68–9.95) |
| Educational level | |||||||||
| <6 | 70 / 114 | 61.4 | - | 12 / 31 | 38.7 | - | 25 / 38 | 65.8 | - |
| 6–11 | 59 / 124 | 47.6 | 0.57 (0.34–0.96) | 65 / 211 | 30.8 | 0.71 (0.32–1.54) | 167 / 231 | 72.3 | 1.36 (0.65–2.81) |
| >12 |
82 / 153 |
53.6 |
0.73 (0.44–1.19) |
50 / 117 |
42.7 |
1.18 (0.53–2.66) |
11 / 17 |
64.7 |
0.95 (0.29–3.16) |
| Marital status | |||||||||
| Without stable partner | 44 / 84 | 52.4 | - | 17 / 45 | 37.8 | - | 47 / 67 | 70.1 | - |
| With stable partner |
175 / 317 |
55.2 |
0.89 (0.55–1.45) |
110 / 319 |
34.5 |
1.15 (0.61–2.20) |
155 / 218 |
71.1 |
0.96 (0.52–1.74) |
|
History of previous pregnancies
| |||||||||
| Parity | |||||||||
| 0 | 94 / 178 | 52.8 | - | 33 / 126 | 26.2 | - | 70 / 95 | 73.7 | - |
| 1–4 | 110 / 202 | 54.5 | 1.07 (0.71–1.60) | 84 / 215 | 39.1 | 1.81 (1.12–2.93) | 102 / 153 | 66.7 | 0.71 (0.41–1.26) |
| >4 |
15 / 21 |
71.4 |
2.23 (0.83–6.02) |
10 / 24 |
41.7 |
2.01 (0.82–4.97) |
20 / 24 |
83.3 |
1.79 (0.56–5.73) |
| History of preterm birtha | |||||||||
| No | 81 / 142 | 57.0 | - | 65 / 162 | 40.1 | - | 64 / 91 | 70.3 | - |
| Yes |
37 / 64 |
57.8 |
1.03 (0.57–1.88) |
23 / 60 |
38.3 |
0.93 (0.51–1.70) |
51 / 73 |
69.9 |
0.98 (0.50–1.92) |
|
Women's attitudes towards health services
| |||||||||
| Satisfied with health care | |||||||||
| No | 25 / 56 | 44.6 | - | 26 / 68 | 38.2 | - | 30 / 39 | 76.9 | - |
| Yes |
181 / 324 |
55.9 |
1.57 (0.89–2.78) |
96 / 281 |
34.2 |
0.84 (0.49–1.45) |
158 / 227 |
69.6 |
0.69 (0.31–1.52) |
| Perceived good quality of care | |||||||||
| No | 76 / 143 | 53.1 | - | 52 / 158 | 32.9 | - | 91 / 130 | 70.0 | - |
| Yes |
130 / 238 |
54.6 |
1.06 (0.70–1.61) |
70 / 191 |
36.6 |
1.18 (0.76–1.84) |
98 / 137 |
71.5 |
1.08 (0.64–1.83) |
|
Women's knowledge
| |||||||||
| Knowledge of risk factors of preterm delivery | |||||||||
| Poor (≤1) | 12 / 18 | 66.7 | - | 8 / 26 | 30.8 | - | 14 / 23 | 60.9 | - |
| Fair (2–3) | 45 / 91 | 49.5 | 0.49 (0.17–1.42) | 44 / 120 | 36.7 | 1.30 (0.52–3.24) | 62 / 92 | 67.4 | 1.33 (0.52–3.42) |
| Good (4–5) |
162 / 291 |
55.7 |
0.63 (0.23–1.72) |
75 / 218 |
34.4 |
1.18 (0.49–2.84) |
115 / 157 |
73.2 |
1.76 (0.71–4.39) |
|
Prenatal care during pregnancy and accessibility
| |||||||||
| Number of prenatal visits | |||||||||
| 0 | 10 / 18 | 55.6 | - | 7 / 25 | 28.0 | - | 6 / 14 | 42.9 | - |
| 1–3 | 38 / 80 | 47.5 | 0.72 (0.26–2.02) | 26 / 88 | 29.5 | 1.08 (0.40–2.89) | 59 / 85 | 69.4 | 3.03 (0.95–9.60) |
| ≥4 |
167 / 289 |
57.8 |
1.10 (0.42–2.86) |
94 / 248 |
37.9 |
1.57 (0.63–3.90) |
117 / 162 |
72.2 |
3.47 (1.14–10.55) |
| Request for care during pregnancy owing to complications | |||||||||
| No | 13 / 20 | 65.0 | - | 5 / 15 | 33.3 | - | 3 / 6 | 50.0 | - |
| Yes |
206 / 381 |
54.1 |
0.63 (0.25–1.62) |
122 / 350 |
34.9 |
1.07 (0.36–3.20) |
189 / 267 |
70.8 |
2.42 (0.48–12.27) |
| Previous hospital admission during the index pregnancy | |||||||||
| No | 181 / 334 | 54.2 | - | 84 / 294 | 28.6 | - | 120 / 185 | 64.9 | - |
| Yes |
38 / 67 |
56.7 |
1.11 (0.65–1.88) |
43 / 69 |
62.3 |
4.14 (2.39–7.16) |
68 / 81 |
84.0 |
2.83 (1.46–5.51) |
| Accessibility to hospital (in time), minb | |||||||||
| <60 | 22 / 36 | 61.1 | 0.74 (0.37–1.49) | 9 / 32 | 28.1 | 1.44 (0.64–3.23) | 11 / 19 | 57.9 | 1.81 (0.70–4.70) |
| ≥60 |
194 / 361 |
53.7 |
- |
98 / 272 |
36.0 |
- |
172 / 241 |
71.4 |
- |
|
Characteristics at delivery
| |||||||||
| Gestational age at delivery, wk | |||||||||
| <28 | 22/ 46 | 47.8 | - | 4 / 31 | 12.9 | - | 25 / 41 | 61.0 | - |
| 28–31 | 72 / 113 | 63.7 | 1.92 (0.96–3.84) | 43 / 99 | 43.4 | 5.18 (1.69–15.93) | 75 / 101 | 74.3 | 1.85 (0.86–3.99) |
| 32–33 | 83 / 131 | 63.4 | 1.89 (0.96–3.72) | 60 / 146 | 41.1 | 4.71 (1.57–14.16) | 63 / 85 | 74.1 | 1.83 (0.83–4.05) |
| 34 |
42 / 111 |
37.8 |
0.66 (0.33–1.33) |
20 / 89 |
22.5 |
1.96 (0.61–6.25) |
40 / 59 |
67.8 |
1.35 (0.59–3.10) |
| Type of preterm birth | |||||||||
| Spontaneous preterm birth (unknown cause) | 78 / 168 | 46.4 | - | 31 / 144 | 21.5 | - | 60 / 107 | 56.1 | - |
| Preterm birth with PPROMc | 60 / 102 | 58.8 | 1.64 (1.01–2.71) | 45 / 95 | 47.4 | 3.28 (1.86–5.78) | 82 / 93 | 88.2 | 5.84 (2.80–12.19) |
| Medically indicated preterm birth |
79 / 129 |
61.2 |
1.82 (1.14–2.91) |
51 / 126 |
40.5 |
2.48 (1.45–4.23) |
60 / 84 |
71.4 |
1.96 (1.07–3.60) |
| Time from admission to delivery, h | |||||||||
| <3 | 45 / 122 | 36.9 | - | 20 / 141 | 14.2 | - | 23 / 67 | 34.3 | - |
| 3–11.59 | 60 / 104 | 57.7 | 2.34 (1.37–3.99) | 18 / 88 | 20.5 | 1.56 (0.77–3.14) | 36 / 46 | 78.3 | 6.89 (2.90–16.33) |
| 12–23.59 | 33 / 62 | 53.2 | 1.95 (1.05–3.62) | 12 / 28 | 42.9 | 4.54 (1.87–11.00) | 19 / 22 | 86.4 | 12.12 (3.24–45.26) |
| ≥24 | 80 / 108 | 74.1 | 4.89 (2.78-8.61) | 77 / 103 | 74.8 | 17.92 (9.36–34.29) | 115 / 131 | 87.8 | 13.75 (6.65–28.43) |
Only multiparous women were considered.
Time taken to travel from the woman's house to the health facility.
PPROM, preterm premature rupture of membranes.
In the bivariate analysis, the variables that were significantly associated with the use of prenatal corticosteroids in any of the 3 countries were age, parity, number of prenatal visits, previous hospital admission during the pregnancy, gestational age at delivery, type of PTB, and time from admission to delivery (Table 2). In Ecuador, women older than 16 years were more likely to receive prenatal corticosteroids compared with young adolescents. Previous admission to the hospital during the index pregnancy was associated with greater use of prenatal corticosteroids in Ecuador and Uruguay. In Uruguay, women with 4 or more prenatal visits were more likely to receive corticosteroids. The statistical associations with parity and gestational age were observed only in Ecuador. Type of preterm birth and time from admission to delivery were associated in all 3 countries. Medically indicated preterm births and those associated with PPROM showed a greater use of corticosteroids compared with spontaneous preterm births. Women who were admitted more than 3 hours before the delivery were more likely to receive prenatal corticosteroids compared with those who were admitted less than 3 hours prior to delivery. There was a positive trend in the use of prenatal corticosteroids as time from admission to delivery increased in all countries (P<0.001).
The multivariate analysis showed that in Ecuador, maternal age was the only significant variable in the most distal level of the hierarchical model (Table 3). This effect may be mediated through women's reproductive history, knowledge or attitudes toward health care, as the observed association disappeared after including the variables for the second hierarchical level. Women with a history of previous admission to a hospital during the index pregnancy were 3–4 times more likely to receive prenatal corticosteroids in Ecuador and Uruguay. Type of preterm birth remained significant only in Uruguay, where women with PPROM were 4 times more likely to receive corticosteroids compared with women with spontaneous or medically indicated preterm birth. In Ecuador, women who gave birth at 28–33 weeks were nearly 5 times more likely to receive the intervention compared with those delivering at less than 28 weeks; in El Salvador, twice as many women received corticosteroids at 28–31 weeks than at less than 28 weeks. The positive trend in the use of corticosteroids as time from admission to delivery increases remained significant in the 3 countries after the adjustment (P<0.001). It should be noted that confidence intervals were very wide for most of the estimates in all variables.
Table 3.
Women's characteristics associated with the use of prenatal corticosteroids in the multivariate model
| Characteristics |
El Salvador |
Ecuador |
Uruguay |
|||
|---|---|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| Age, y | ||||||
| ≤15a | 1.00d | - | ||||
| 16–34 | 6.85 | (0.88–53.06) | ||||
| >34 |
|
|
11.70 |
(1.39–98.58) |
|
|
| Previous hospital admission during the index pregnancy | ||||||
| Noa | 1.00e | - | 1.00g | - | ||
| Yes |
|
|
3.91 |
(2.25–6.78) |
2.83 |
(1.46–5.51) |
| Type of preterm birth | ||||||
| Spontaneous preterm birth (unknown cause)a | 1.00h | - | ||||
| Preterm birth with PPROM** | 4.23 | (1.69–10.63) | ||||
| Medically indicated preterm birth |
|
|
|
|
1.00 |
(0.45–2.23) |
| Gestational age at delivery, wk | ||||||
| <28a | 1.00c | - | 1.00f | - | ||
| 28–31 | 2.22 | (1.05–4.66) | 5.03 | (1.39–18.27) | ||
| 32–33 | 1.93 | (0.94–3.99) | 5.21 | (1.48–18.28) | ||
| 34 |
0.60 |
(0.28–1.25) |
1.45 |
(0.38–5.55) |
|
|
| Time from admission to delivery, h | ||||||
| <3a | 1.00c | - | 1.00f | - | 1.00h | - |
| 3–11.59 | 2.45 | (1.40–4.28) | 1.97 | (0.92–4.26) | 5.73 | (2.17–15.12) |
| 12–23.59 | 2.22 | (1.16–4.24) | 6.41 | (2.37–17.33) | 10.30 | (2.58–41.12) |
| ≥24 | 5.96 | (3.28–10.84) | 22.85 | (10.83–48.15) | 12.37 | (5.36–28.59) |
Reference group.
PPROM, preterm premature rupture of membranes.
Model 1, El Salvador: Gestational age at delivery and time from admission to delivery.
Model 1, Ecuador: Age
Model 2, Ecuador: Model 1 plus previous hospital admission during the index pregnancy.
Model 3, Ecuador: Model 2 plus gestational age at delivery and time admission to delivery.
Model 1, Uruguay: Previous hospital admission during the index pregnancy.
Model 2, Uruguay: Model 1 plus spontaneous preterm birth and time from admission to delivery.
4. Discussion
The study shows a varied pattern of use of prenatal corticosteroids among women delivering prematurely between 24 to 34 weeks and 6 days of pregnancy in hospitals in 3 Latin American countries. Ecuador had the lowest use (34.8%), followed by El Salvador (54.6%), and Uruguay (71%).
Rates in Uruguay are similar to those reported in high-income countries [15], whereas in El Salvador and Ecuador prenatal corticosteroids are underused. However, figures in Uruguay are not homogeneous. Although most hospitals showed rates above 70%, hospitals in the north of the country showed less than 60% use (data not shown). Maternal sociodemographic characteristics do not appear to explain the differences observed among the countries, since the distributions of age, civil status, and parity were similar among the participating women.
The multivariate analysis showed that the time from admission to delivery was the only variable that was statistically associated with the use of prenatal corticosteroids in all 3 countries. Use of corticosteroids increased as the time from admission to delivery increased. Whether this was due to a timely maternal request for care or a provider's attitude toward admission of women with an imminent preterm birth could not be disentangled.
In Ecuador and El Salvador, women delivering after 28 weeks of pregnancy were more likely to receive corticosteroids. This finding has also been reported in a study conducted in Mexico [16]. Lack of understanding about the benefits of corticosteroids in the early stages of pregnancy by health providers is a potential explanation [16].
Women who had been admitted to hospital during the pregnancy were more likely to receive corticosteroids in Uruguay and Ecuador. Better identification of the potential risk for preterm birth by healthcare providers may have contributed to the increased use of prenatal corticosteroids. In addition, the women may have been more aware of the risks in their pregnancy, enabling them to seek care.
Even though the participating countries were selected to represent 3 main Latin American subregions, and the participating hospitals are reference institutions in their countries, because they were not randomly selected we cannot infer that the observed pattern of use is representative of other hospitals in those countries. However, because we selected influential reference hospitals in the countries’ capital cities, we believe that this might increase the likelihood that the use of corticosteroids in other hospitals may be similar.
The study did not include women who were at high risk for preterm birth, but did not actually have a preterm birth. Thus, the reported rates of prenatal corticosteroid use do not reflect the overall proportion of pregnant women who received the intervention irrespective of the gestational age at delivery. The study was limited to women who had a preterm birth and therefore might have benefited from receiving prenatal corticosteroids.
Uruguay showed a higher rate of women who declined to participate in the study. A possible explanation is that most of the women included were from a large teaching hospital in which women are exposed to many ongoing studies. Sample size in Uruguay was lower than expected. However, since the use of corticosteroids was higher than expected, the precision of the estimates was between the expected margins.
The present study provides useful information on the use of prenatal corticosteroids in preterm births. It is essential that health providers are educated to understand that, regardless of gestational age and time from admission to delivery, prenatal corticosteroids are beneficial. In addition, improving early identification and admission to hospital of women with spontaneous preterm labor, which made up the largest PTB group, may also contribute to an increase in the use of corticosteroids. However, such initiatives should be carefully evaluated to determine efficacy and cost-effectiveness before they are promoted.
Qualitative studies focused on the attitudes of healthcare providers and pregnant women are needed to obtain more information to design interventions targeted at improving the use of prenatal corticosteroids [17].
Acknowledgments
The USA National Institute of Health funded the study (NIH/GRIP grant # 1R01 TW006970-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Synopsis: Use of prenatal corticosteroids varied among El Salvador, Uruguay, and Ecuador, with a range of factors influencing their use.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.MacDorman MF, Martin JA, Mathews MS, Hoyert DL. Explaining the 2001-02 Infant Mortality Increase: Data from the linked birth/infant data set. [September 4, 2009];National Vital Statistics Reports. 53(12) Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr53/nvsr53_12.pdf. [PubMed]
- 2.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–25. [PubMed] [Google Scholar]
- 3.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel. JAMA. 1995;273(5):413–8. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 5.Antenatal corticosteroids revisited: repeat courses. NIH Consens Statement. 2000;17(2):1–18. [PubMed] [Google Scholar]
- 6.Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L, Lancet Neonatal Survival Steering Team Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365(9463):977–88. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 7.Jones G, Steketee R, Black RE, Bhutta ZA, Morris SS. How many child deaths can we prevent this year? Lancet. 2003;362(9377):65–71. doi: 10.1016/S0140-6736(03)13811-1. [DOI] [PubMed] [Google Scholar]
- 8.Forteza C, Díaz Rossello JL, Matijasevich A, Barros F. Morbidity and mortality of very low birth weight (VLBW) infants in Montevideo, Uruguay.. Pediatr Res; Abstracts from the XXXIX Annual Meeting of the Latin American Society for Pediatric Research.; November 2001; Colonia del Sacramento, Uruguay: 2002. p. 467. [Google Scholar]
- 9.Krauss Silva L, Pinheiro T, Franklin R, Oliveira N. Assessment of quality of obstetric care and corticoid use in preterm labor. Cadernos de Salude Publica. 1999;15(4):1–23. doi: 10.1590/s0102-311x1999000400016. [DOI] [PubMed] [Google Scholar]
- 10.Vallejo Valdivieso N, Chinga Sampedro J, Sánchez Macías M, Tumbaco García R. Epidemiology of the childbirth preterm and its repercussion in neonatal morbi-mortality registered in hospital Dr Verdi Cevallos. Medicina (Guayaquil) 2002;8(1):36–41. [Google Scholar]
- 11.Colomar M, Belizán M, Cafferata ML, Labandera A, Tomasso G, Althabe F, et al. Practices of maternal and perinatal care performed in public hospitals in Uruguay [in Spanish]. Ginecol Osbstet Mex. 2004;72:55–65. [PubMed] [Google Scholar]
- 12.López Gómez A, Benia W, Contera M, Güida C. From the maternal-infant focus to the reproductive health focus: tensions, obstacles and perspectives. Cátedra Libre en Salud Reproductiva, Sexualidad y Género. Facultad de Psicología, UDELAR, Montevideo. 2003.
- 13.Victora C, Fuchs S, Flores JA, Fonseca W, Kirkwood B. Risk factors for pneumonia among children in a Brazilian metropolitan area. Pediatrics. 1994;93(6 Pt 1):977–85. [PubMed] [Google Scholar]
- 14.Victora CG, Huttly SR, Fuchs SC, Olinto MT. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epidemiol. 1997;26(1):224–7. doi: 10.1093/ije/26.1.224. [DOI] [PubMed] [Google Scholar]
- 15.Bronstein JM, Goldenberg RL. Practice variation in the use of corticosteroids: a comparison of eight datasets. Am J Obstet Gynecol. 1995;173(1):296–8. doi: 10.1016/0002-9378(95)90217-1. [DOI] [PubMed] [Google Scholar]
- 16.Vargas-Origel A, Leon Ramirez D, Zamora-Orozco J, Vargas-Nieto MA. Prenatal corticosteroids. Use and attitudes of the gynecology-obstetrics medical staff [in Spanish]. Ginecol Obstet Mex. 2000;68:291–5. [PubMed] [Google Scholar]
- 17.Thorsen T, Mäkelä M, editors. Changing Professional Practice Theory and Practice of Clinical Guidelines Implementation. Danish Institute for Health Services Research and Development; Copenhagen: 1999. [Google Scholar]