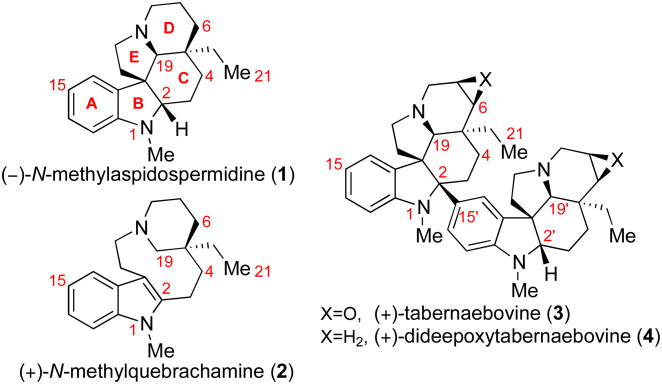
The monoterpene indole alkaloids represent the largest family of alkaloid natural products whose more than 2,000 members display a broad range of chemical diversity and potent biological activity.1 The structural challenges presented by this family have long been a source of interest, resulting in the development of a variety of inventive synthetic strategies to access various family members. The biogenetically related natural alkaloids N-methylaspidospermidine (1), N-methylquebrachamine (2), and tabernaebovine (3) represent the aspidosperma subfamily of monoterpene indole alkaloids (Figure 1).2-5 The dimeric alkaloid 3, isolated from Tabernaemontana bovina in 1998,2e has a fascinating molecular constitution that exhibits a unique C2–C15′ linkage between two pentacyclic aspidosperma skeletons. While elegant strategies for synthesis of other dimeric monoterpene indole alkaloids have been reported,5 no synthetic solution to the distinctive C2–C15′ union present in 3 exists. As an outgrowth of our laboratory's studies concerning electrophilic amide activation,6 we report a concise and convergent strategy for the enantioselective synthesis of alkaloids (–)-1, (+)-2, and dimeric (+)-dideepoxytabernaebovine (4).
Figure 1.
Representative aspidosperma alkaloids.
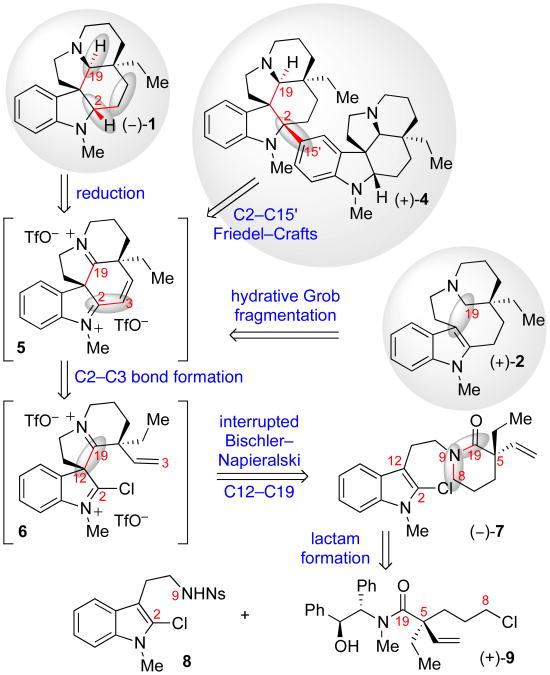
Inspired by precedence in biogenetically relevant dimerizations of other monoterpene indole alkaloids,1,5 we posited the biogenesis of (+)-tabernaebovine (3) to involve late-stage union of two aspidosperma fragments at the C2–C15′ linkage. This retrobiosynthetic analysis7 prompted the development of a regio- and diastereoselective C2-arylation of pentacycle 5 (Scheme 1) en route to decacycle (+)-4. We envisioned that a highly electrophilic diiminium ion 5 would allow for stereo- and regioselective transformations that provide divergent access to dimeric decacycle (+)-4 as well as monomeric aspidosperma alkaloids such as (–)-1 and (+)-2 (Scheme 1). We expected reduction of the diiminium ion 5 would afford (–)-1, whereas hydrative Grob fragmentation followed by reduction would afford (+)-2. We hypothesized that the diiminium ion 5 could be generated from lactam (–)-7 via a novel, stereoselective double cyclization cascade. We envisioned a transformation involving spirocyclization of an electrophilically activated lactam intermediate onto the 2-chloroindole and subsequent interruption of the Bischler–Napieralski reaction by cyclization of an unactivated C3–C4 vinyl group onto the C2 position of the putative 2-chlorospiroindoleninium intermediate 6. The overall stereochemical outcome of the process would be secured from the resident stereochemistry of the C5 quaternary center. The requisite lactam (–)-7 could be simplified via N-acylation and N-alkylation transforms to 2-chlorotryptamine sulfonamide 8 and α-quaternary amide (+)-9, the latter of which could be synthesized diastereoselectively via alkylative quaternization of an amide enolate.
Scheme 1.
Retrosynthetic analysis of the aspidosperma alkaloids.
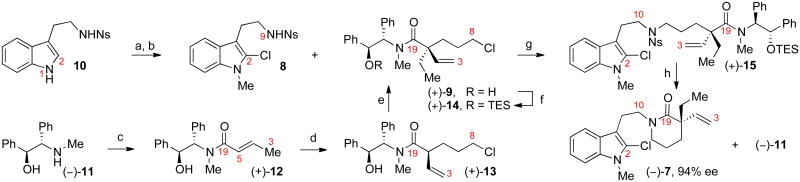
The concise enantioselective synthesis of the key tryptamine-lactam (–)-7 is illustrated in Scheme 2. Regioselective methylation of sulfonamide 108 via its disodium dianion provided the requisite N1-methylated derivative in 91% yield. Subsequent treatment with N-chlorosuccinimide afforded C2-chlorotryptamine 8 in 76% yield. The C5-quaternary stereogenic center that we envisioned would enable stereocontrolled introduction of all stereocenters found in alkaloids (–)-1, (+)-2, and (+)-4 was secured by successive diastereoselective a-alkylations of crotonamide (+)-12. Acylation of (–)-pseudoephenamine (11)10 with E-crotonyl chloride gave the enamide (+)-12 in 97% yield. Chemoselective γ–deprotonation of enamide (+)-12 with lithium 2,2,6,6-tetramethylpiperidide in the presence of lithium chloride, followed by electrophilic trapping of the resulting enolate with 3-chloro-1-iodopropane afforded α-vinyl amide (+)-13 as a single diastereomer in 83% yield. Inspired by Myers' alkylative quaternizations of pseudoephedrine amides and precedent for (α)-alkylation of (α)-methyl crotonimides, we reasoned that deprotonation of (α)-vinyl amide (+)-13 would afford the corresponding enolate with the sterically less demanding vinyl group cis to the amide nitrogen. Alkylation from the less sterically shielded face of the enolate10,11 would secure the desired C5 quaternary stereocenter. Gratifyingly, deprotonation of amide (+)-13 with lithium diisopropylamide in the presence of lithium chloride, followed by electrophilic trapping with iodoethane at −50 °C in the presence of N,N′-dimethylpropylene urea provided the α-quaternary amide (+)-9 in 72% yield with an excellent level of stereo selection (>29:1 dr).9,14,15
Scheme 2.
Enantioselective synthesis of lactam (–)-7: a) NaH, DMF, 23 °C; MeI, 0→23 °C, 91%. b) N-chlorosuccinimide, MeCN, 23 °C, 76%. c) E-crotonyl chloride, Et3N, THF, −30→23 °C, 97%. d) lithium 2,2,6,6-tetramethylpiperidide, LiCl, THF, 0→−78 °C; 3-chloro-1-iodopropane, −78→0 °C, 83%. e) lithium diisopropylamide, LiCl, THF, −78→0 °C; N,N′-dimethylpropylene urea, −40 °C; EtI, −50 °C, 72%, >29:1 dr. f) triethylsilyl triflate, 2,6-lutidine, CH2Cl2, 23 °C, 100%. g) (+)-14, 8, KH, n-Bu4NI, DMF, 100 °C, 86%. h) PhSH, K2CO3, DMSO, 23 °C; KOEt, Et3N•3HF, EtOH, 85 °C, 95%, 94% ee, 99% recovery of (–)-11. DMF = N,N′-dimethylformamide, Ns = 2-nitrobenzenesulfonyl, TES = triethylsilyl.
Attempts to hydrolyze amide (+)-9 to the corresponding carboxylic acid were unsatisfactory due to competitive lactone formation under either basic or acidic conditions. Initial efforts to couple (α)-quaternary amide (+)-9 with sulfonamide 8 via nucleophilic displacement of the C8-chloride proved inefficient; fast N→O acyl transfer of amide (+)-9 led to intramolecular N-alkylation. This propensity of amide (+)-9, however, could be used to our advantage for the synthesis of lactam (–)-7 and recovery of the chiral auxiliary. Sequential treatment of sulfonamide 8 with potassium hydride and O-silyl derivative (+)-14 in DMF followed by heating to 100 °C afforded N-alkylated sulfonamide (+)-15 in 86% yield. Desulfonylation16 of (+)-15 to the corresponding secondary amine and in situ desilylation and heating in ethanol afforded the key lactam (–)-7 in 95% yield and 94% ee.9 This single-step transformation, which occurs via a desilylation/N→O acyl transfer/lactam cyclization cascade, also leads to efficient recovery of the chiral auxiliary (–)-11 in 99% yield (Scheme 2).17
We next focused on the development of a unified strategy to access a versatile intermediate en route to alkaloids (–)-1, (+)-2, and (+)-4. Electrophilic activation of lactam (–)-7 with trifluoro-methanesulfonic anhydride6,18 initiated a double cyclization cascade leading to the versatile diiminium ion 5 (Scheme 3). Guided by our prior studies of azaheterocycle synthesis employing electrophilic amide activation,6 we recognized the optimal conditions for conversion of lactam (–)-7 to diiminium ion 5 involve the use of mildly basic additive 3-cyanopyridine in acetonitrile followed by warming. This transformation relies on C19-electrophilic activation of lactam (–)-7 and rapid C12-nucleophilic spirocyclization affording the putative 2-chlorospiroindoleninium intermediate 6 (Scheme 1) that undergoes C2-addition by the vinyl group and loss of hydrogen chloride. The ability to employ an unactivated C3–C4 olefin as the nucleophile in the second cyclization is likely due to the enhanced C2-electrophilicity of intermediate 6 imparted by the chlorine atom,19 together with a high resilience of 6 toward an undesired Pictet–Spengler rearrangement.20,21 Consistent with the sensitivity of this double cyclization step, the use of less basic 2-chloropyridine or more nucleophilic pyridine as the additive gave the desired diiminium ion 5 with reduced efficiency as evidenced by the presence of singly cyclized side-products, recovered starting material, and significant decomposition.22 The synthetic versatility of diiminium ion 5 is illustrated by its conversion to alkaloids (–)-1, (+)-2, and (–)-17 (Scheme 3). In situ reduction of intermediate 5 with sodium cyanoborohydride furnished (–)-Nmethyldehydroaspidospermidine (16) in 50% yield as a single diastereomer. Catalytic hydrogenation of cis-alkene (–)-16 with a carbon-supported platinum catalyst afforded (–)-N-methylaspidospermidine (1) {[α]24D = −23 (c 0.17, CHCl3); lit. [α]25D= −23 (c 1.1, CHCl3);3d for (+)-1, [α]20D= +24 (c 1.25, CHCl3)2c} in quantitative yield (Scheme 3). All spectroscopic data for our synthetic (–)-1 was consistent with those reported in the literature.3d The concise enantioselective synthesis of lactam (–)-7 combined with the double cyclization strategy described above enables rapid access to useful intermediates with highly reactive C2-and C19-iminium functions.
Scheme 3.
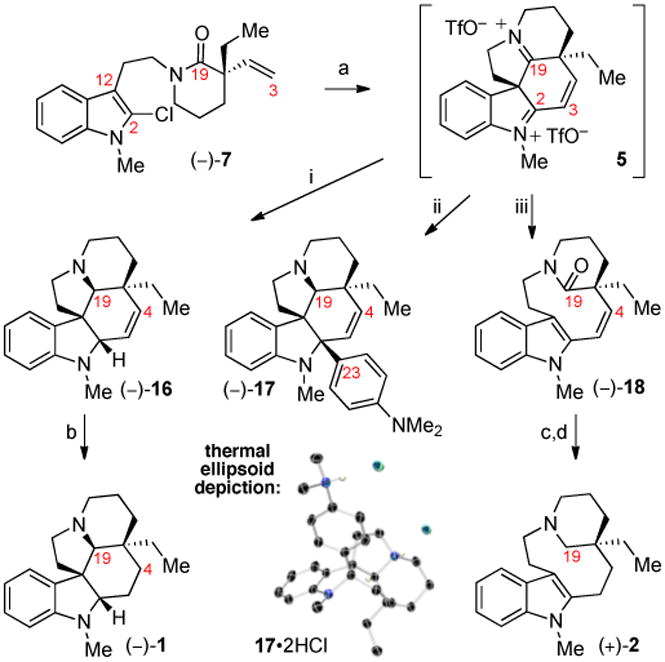
Synthesis of aspidosperma alkaloids by interception of diiminium ion 5: a) Tf2O, 3-cyanopyridine, MeCN, 85 °C. i) NaBH3CN, THF, 50%. ii) 4-(Me2N)-C6H4MgBr, −40 °C; Red-Al, 40%. iii) trifluoroacetic acid, sodium trifluoroacetate, H2O, 70 °C, 57%. b) H2, Pt/C, THF, 100%. c) H2, Pt/C, THF. d) LiAlH4, THF, 65 °C, 82% (two steps). Tf2O = trifluoromethanesulfonic anhydride.
The unique reactivity of diiminium ion 5 is demonstrated by its utility in a C2-arylation reminiscent of the C2–C15′ bond adjoining the two halves of the complex natural alkaloid (+)-tabernaebovine (3, Figure 1). We reasoned that the vicinal C19 iminium ion of intermediate5would enhance the electrophilicity of the C2 iminium ion both inductively and by reducing the steric environment through the flattening of the DE–ring system. Gratifyingly, treatment of in situ generated diiminium ion 5 with 4-(N,N-dimethylamino) phenyl magnesium bromide at −40 °C for 30 seconds followed by addition of Red-Al afforded hexacyclic C2-aniline adduct (–)-17 in 40% yield as a single diastereomer (Scheme 3).9 The steric congestion about C2 in adduct (–)-17 is evidenced by the ∼20 kcal/mol barrier to rotation9 about the C2–C23 σ-bond as measured through NMR coalescence temperature experiments. Additionally, heating an acidic aqueous solution of diiminium ion 5 to 70 °C effected Grob fragmentation to give the tetracyclic lactam (–)-1823 in 57% yield in a single step from lactam (–)-7. Platinum catalyzed hydrogenation24 of the C3–C4 olefin and subsequent C19-carbonyl reduction with lithium aluminium hydride at 60 °C provided (+)-N-methylquebrachamine (2) {[α]24D= +102 (c 0.22, CHCl3); lit. [α]24D= +110 (CHCl3)2a} in 82% yield over two steps.9
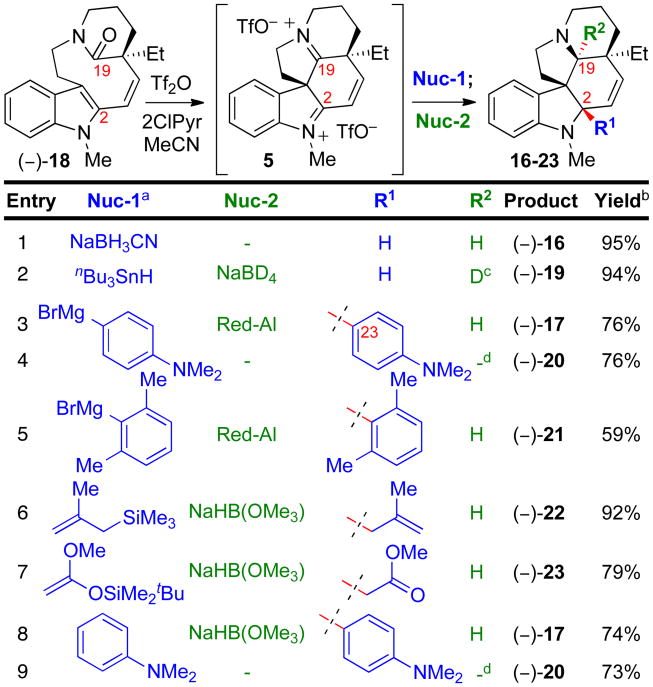
With particular interest in evaluating this chemistry as a general entry to the synthesis of complex aspidosperma alkaloids, we investigated a series of C2-addition reactions of relevance in synthetic planning. Importantly, lactam (–)-18, requiring mild activation conditions, proved to be an excellent precursor to diiminium ion 5. Treatment of lactam (–)-18 with Tf2O–2-chloropyridine reagent combination6 in acetonitrile (23 °C, 10 min) resulted in rapid stereo- and regioselective electrophilic transannular spirocyclization to 5 en route to various C2-adducts 16–23 (Table 1). Introduction of sodium cyanoborohydride afforded (–)-N-methyldehydroaspidospermidine (16) in 95% yield (Table 1, entry 1) consistent with efficient generation of the same electrophilic intermediate 5 accessed from lactam (–)-7 (Scheme 3). The greater reactivity at C2 compared to C19 of diiminium ion 5can be used for regioselective addition of the first nucleophile at the former. For example, treatment of 5 with tributylstannane, followed by introduction of sodium borodeuteride, afforded C19-deuterated pentacycle (–)-19 in 94% yield with no deuterium enrichment at C2 and 93% deuterium incorporation at C19 (Table 1, entry 2). Notably, the C2-arylated product (–)-17 could be prepared efficiently from lactam (–)-18 using 4-(N,N-dimethylamino) phenyl magnesium bromide as the first nucleophile followed by in situ C19 reduction (Table 1, entry 3, 76% yield). Alternatively, hexacyclic iminium triflate (–)-20 could be isolated then reduced with sodium cyanoborohydride to (–)-17 in a subsequent step (Table 1, entries 4 and 9). That this C19 reduction of pentacycle (–)-20 occurs in the absence of an acidic additive is consistent with its spectroscopic data revealing its iminium ion structure.9 It is notable that the ∼12 kcal/mol barrier to rotation9 about the C2–C23 σ-bond in iminium ion (–)-20 is significantly lower than in the reduced product (–)-17 (vide supra), consistent with the aforementioned structural flattening effect of the C19 iminium ion. The high electrophilicity of diiminium ion 5 allows for C–C bond formation at C2 with highly hindered and mildly nucleophilic species. Treatment of intermediate 5 with 2,6-dimethylphenyl magnesium bromide followed by hydride reduction afforded the highly congested xylene adduct (–)-21 in 59% yield (Table 1, entry 5). The high degree of steric congestion about C2 in (–)-21 is evidenced by the complete lack of observable C2–C23 σ -bond rotation on the 1H NMR timescale, even at 140 °C. Reaction of 5 with 2-methallyltrimethylsilane or 1-(tert-butyldimethylsilyloxy)-1-methoxyethene and subsequent hydride reduction afforded methallyl adduct (–)-22 (Table 1, entry 6, 92% yield) or methyl acetate adduct (–)-23 (Table 1, entry 7, 79% yield), respectively. The utility of this strategy to access C2-arylated derivatives is highlighted by a Friedel–Crafts reaction of 5 with N,N-dimethylaniline (23 °C, 90 min) and either in situ C19 reduction to provide C2-arylated amine (–)-17 or isolation of the pentacyclic C19-iminium salt (–)-20 (Table 1, entries 8 and 9, 74% and 73% yield, respectively).
Table 1.
Regio- and stereoselective transformations of lactam (–)-18.
|
Grignard reagents at −40 °C. Other nucleophiles at 23 °C.
Isolated yield of single diastereomer.
93% Deuterium incorporation at C19.
Isolated as C19 iminium triflate. 2-ClPyr = 2-chloropyridine.
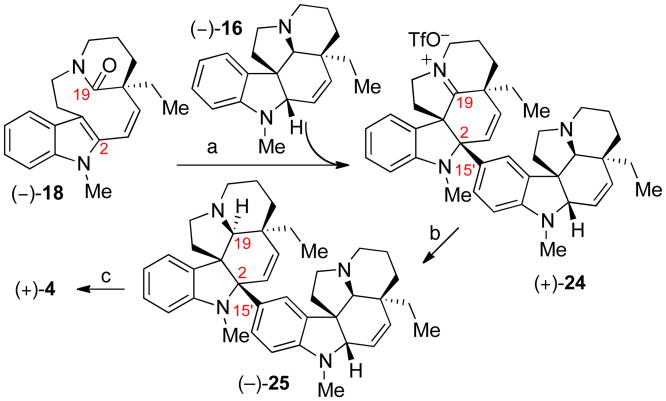
With insight gained from these studies, in particular entries 8 and 9 of Table 1, we sought to implement this chemistry in effecting the dimerization of two pentacyclic aspidosperma type molecular frameworks at the challenging C2–C15′ linkage (Scheme 5). In the event, electrophilic activation of tetracyclic lactam (–)-18 followed by treatment with equimolar (–)-N-methyldehydroaspidospermidine (16) and heating to 85 °C afforded the decacyclic iminium triflate (+)-24 in 80% yield. Subsequent C19-reduction of (+)-24 gave (–)-didehydrodideepoxytabernaebovine (25), which upon hydrogenation provided (+)-dideepoxytabernaebovine(4) in 64% yield over two steps (Scheme 5). Alternatively, in situ C19 reduction of dimeric iminium ion (+)-24, formed through the union of lactam (–)-18 with (–)-N-methyldehydroaspidospermidine(16) as described above, with sodium cyanoborohydride directly afforded product (–)-25 from (–)-18 in 73% yield.9 Apart from increasing the electrophilicity of the vicinal C2 iminium ion, the C19 iminium ion may be responsible for reducing the nucleophilicity of the dimeric intermediate (+)-24, as no oligomerized products could be observed even when only one equivalent of (–)-16 was employed as nucleophile.
Scheme 5.
Synthesis of (+)-dideepoxytabernaebovine(4): a) Tf2O, 2-ClPyr, MeCN, 23 °C; (–)-16 (1.0 equiv), 85 °C, 80%. b) Red-Al, 0 °C, 76%. c) H2, Pt/C, THF, 84%.
We have developed a concise synthetic strategy to access the aspidosperma type molecular framework employing a double cyclization cascade that sets up to three contiguous stereogenic centers and forms up to three carbon-carbon bonds with complete regio- and stereochemical control in a single step. The use of the chiral auxiliary (–)-1110 was critical in enabling our concise and enantioselective synthesis of the key intermediate (–)-7. The ability to use an unactivated olefin as a pendant nucleophile minimizes the need for functional group removal and allows for concise and convergent access to complex aspidosperma alkaloids. We have shown putative diiminium ion 5 to be a highly reactive and versatile intermediate, allowing the rapid enantioselective total syntheses of (–)-N-methylaspidospermidine (1) and (+)-N-methylquebrachamine (2) in 8 and 9 steps, respectively, from E-crotonyl chloride and (–)-pseudoephenamine (11), as well as the unprecedented C-C bond formations onto the highly congested C2 position of the aspidosperma skeleton. The power of this synthetic strategy has been demonstrated in the first example of a C2-C15′ dimerization of two aspidosperma type systems, a complex assembly drawing on biogenetic considerations of (+)-3, in the synthesis of (+)-dideepoxytabernaebovine (4).
Footnotes
We are grateful for financial support by NIH-NIGMS, (GM074825). We thank Mr. Justin Kim, Mr. Owen Fenton and Dr. Peter Müller for X-ray crystal structure analysis of 17•2HCl.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author. CCDC 862060.
References
- 1.a) Saxton JE. The Alkaloids, Chem and Biol. 1998;51:1. [Google Scholar]; b) Dewick PM. Medicinal Natural Products: A Biosynthetic Approach. 2nd. John Wiley & Sons Ltd; Chichester: 2001. p. 350. [Google Scholar]; c) O'Connor SE, McCoy E. Rec Adv Phytochem. 2006;40:1. [Google Scholar]; d) O'Connor SE. In: Comprehensive Natural Products II. Mander L, Liu HW, editors. Vol. 1. Elsevier; Amsterdam: 2010. p. 977. [Google Scholar]
- 2.a) Mokry J, Kompis I, Dubravkova L, Sefcovic P. Tetrahedron Lett. 1962;25:1185. [Google Scholar]; b) Mokry J, Kompis I. Lloydia. 1964;27:428. [Google Scholar]; c) Mokry J, Kompis I, Spiteller G. Collect Czech Chem Commun. 1967;32:2523. [Google Scholar]; d) Zèches-Hanrot M, Nuzillard JM, Richard B, Schaller H, Hadi HA, Sévenet T, Le Men-Olivier L. Phytochemistry. 1995;40:587. [Google Scholar]; e) Lien TP, Kamperdick C, Sung TV, Adam G, Ripperger H. Phytochemistry. 1998;49:1797. doi: 10.1016/s0031-9422(98)00227-1. [DOI] [PubMed] [Google Scholar]; f) Merzweiler K, Lien TP, Sung TV, Ripperger H, Adam G. J Prakt Chem. 1999;341:69. [Google Scholar]
- 3.For syntheses related to 1, see: Benchekroun-Mounir N, Dugat D, Gramain JC, Husson HP. J Org Chem. 1993;58:6457.Kozmin SA, Iwama T, Huang Y, Rawal VH. J Am Chem Soc. 2002;124:4628. doi: 10.1021/ja017863s.Marino JP, Rubio MB, Cao GF, de Dios A. J Am Chem Soc. 2002;124:13398. doi: 10.1021/ja026357f.Ishikawa H, Elliot GI, Velcicky J, Choi Y, Boger DL. J Am Chem Soc. 2006;128:10596. doi: 10.1021/ja061256t.Sasaki Y, Kato D, Boger DL. J Am Chem Soc. 2010;132:13533. doi: 10.1021/ja106284s.Jones SB, Simmons B, Mastracchio A, MacMillan DWC. Nature. 2011;475:183. doi: 10.1038/nature10232. For syntheses related to 2, see ref. 3b and Takano S, Yonaga M, Ogasawara K. J Chem Soc Chem Commun. 1981:1153.; h) Node M, Nagasawa H, Fuji K. J Am Chem Soc. 1987;109:7901. [Google Scholar]; i) Temme O, Taj SA, Andersson PG. J Org Chem. 1998;63:6007. doi: 10.1021/jo9807417. [DOI] [PubMed] [Google Scholar]; j) Amat M, Lozano O, Escolano C, Molins E, Bosch J. J Org Chem. 2007;72:4431. doi: 10.1021/jo070397q. [DOI] [PubMed] [Google Scholar]; k) Malcolmson SJ, Meek SJ, Satterly ES, Schrock RR, Hoveyda AH. Nature. 2008;456:933. doi: 10.1038/nature07594. [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Satterly ES, Meek SJ, Malcolmson SJ, Schrock RR, Hoveyda AH. J Am Chem Soc. 2009;131:943. doi: 10.1021/ja8084934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For a review, see Hajicek J. Collect Czech Chem Commun. 2004;69:1681. For related syntheses, see ref. 5 and: Kuehne ME, Podhorez DE, Mulamba T, Bornmann WG. J Org Chem. 1987;52:347.Cardwell K, Hewitt B, Ladlow M, Magnus P. J Am Chem Soc. 1988;110:2242.Kobayashi S, Peng G, Fukuyama T. Tetrahedron Lett. 1999;40:1519.He F, Bo Y, Altom JD, Corey EJ. J Am Chem Soc. 1999;121:6771.Kobayashi S, Ueda T, Fukuyama T. Synlett. 2000:883.
- 5.a) Mangeney P, Andriamialisoa RZ, Langlois N, Langlois Y, Potier P. J Am Chem Soc. 1979;101:2243. doi: 10.1021/jo01336a006. [DOI] [PubMed] [Google Scholar]; b) Kutney JP, Choi LSL, Nakano J, Tsukamoto HH, McHugh M, Boulet CA. Heterocycles. 1988;27:1845. [Google Scholar]; c) Kuehne ME, Matson PA, Bornmann WG. J Org Chem. 1991;56:513. [Google Scholar]; d) Magnus P, Mendoza JS, Stamford A, Ladlow M, Willis P. J Am Chem Soc. 1992;114:10232. [Google Scholar]; e) Yokoshima S, Ueda T, Kobayashi S, Sato A, Kuboyama T, Tokuyama H, Fukuyama T. J Am Chem Soc. 2002;124:2137. doi: 10.1021/ja0177049. [DOI] [PubMed] [Google Scholar]; f) Ishikawa H, Colby DA, Boger DL. J Am Chem Soc. 2008;130:420. doi: 10.1021/ja078192m. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Ueda H, Satoh H, Matsumoto K, Sugimoto K, Fukuyama T, Tokuyama H. Angew Chem. 2009;121:7736. doi: 10.1002/anie.200902192. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:7600. [Google Scholar]; h) Nicolaou KC, Dalby SM, Li S, Suzuki T, Chen DYK. Angew Chem. 2009;121:7752. doi: 10.1002/anie.200904588. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:7616. [Google Scholar]
- 6.a) Movassaghi M, Hill MD, Ahmad OK. J Am Chem Soc. 2007;129:10096. doi: 10.1021/ja073912a. [DOI] [PubMed] [Google Scholar]; b) Movassaghi M, Hill MD. Org Lett. 2008;10:3485. doi: 10.1021/ol801264u. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Medley JW, Movassaghi M. J Org Chem. 2009;74:1341. doi: 10.1021/jo802355d. [DOI] [PubMed] [Google Scholar]
- 7.Movassaghi M, Siegel DS, Han S. Chem Sci. 2010;1:561. doi: 10.1039/c0sc00351d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuda Y, Kitajima M, Takayama H. Org Lett. 2008;10:125. doi: 10.1021/ol702637r. [DOI] [PubMed] [Google Scholar]
- 9.See the Supporting Information for details.
- 10.Morales MR, Mellem KT, Myers AG. Angew Chem Int Ed. submitted We are grateful to Professor Myers for providing us with a generous sample of (–)-pseudoephenamine (11) [Google Scholar]
- 11.a) Myers AG, Yang BH, Chen H, McKinstry L, Kopecky DJ, Gleason JL. J Am Chem Soc. 1997;119:6496. [Google Scholar]; b) Yang BH. PhD thesis. Harvard University; 1997. [Google Scholar]
- 12.Kummer DA, Chain WJ, Morales MR, Quiroga O, Myers AG. J Am Chem Soc. 2008;130:13231. doi: 10.1021/ja806021y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Hosokawa S, Sekiguchi K, Enemoto M, Kobayashi S. Tetrahedron Lett. 2000;41:6429. [Google Scholar]; b) Hosokawa S, Kobayashi S. J Synth Org Chem Jpn. 2001;59:1103. [Google Scholar]; c) Abe T, Suzuki T, Sekiguchi K, Hosokawa S, Kobayashi S. Tetrahedron Lett. 2003;44:9303. [Google Scholar]; d) Haesler J, Schindelholz I, Riguet E, Bochet CG. Nature. 2007;446:526. doi: 10.1038/nature05653. [DOI] [PubMed] [Google Scholar]
- 14.Chain WJ, Myers AG. Org Lett. 2007;9:355. doi: 10.1021/ol0628762. [DOI] [PubMed] [Google Scholar]
- 15.A parallel strategy using (+)-pseudoephedrine as chiral auxiliary did not provide products with higher than 6:1 dr.
- 16.Fukuyama T, Jow CK, Cheung M. Tetrahedron Lett. 1995;36:6373. [Google Scholar]
- 17.At partial conversion the uncyclized secondary C9-amine retains the triethylsilyl group consistent with O-desilylation preceding cyclization.
- 18.a) Charette AB, Grenon M. Can J Chem. 2001;79:1694. [Google Scholar]; b) Charette AB, Mathieu S, Martel J. Org Lett. 2005;7:5401. doi: 10.1021/ol052069n. [DOI] [PubMed] [Google Scholar]
- 19.Use of C2-H derivatives of substrate 7 gave no double cyclization products.
- 20.a) Desmaelee D, Mekouar K, d'Angelo J. J Org Chem. 1997;62:3890. [Google Scholar]; b) Yasui Y, Takeda H, Takemoto Y. Chem Pharm Bull. 2008;56:1567. doi: 10.1248/cpb.56.1567. [DOI] [PubMed] [Google Scholar]
- 21.An elegant report involving a functional pendant nucleophile (ref. 4e) requires additional steps for synthetic simplification.
- 22.The use of less electrophilic dehydrating agents (e.g., trifluoroacetic anhydride, POCl3, POBr3, the Hendrickson reagent or the Burgess reagent) provided none of the desired cyclization products.
- 23.For semisynthesis of a similar tetracyclic lactam, see Yates P, MacLachlan FN, Rae ID. Can J Chem. 1978;56:1052.
- 24.Hydrogenation at 400 psi; the C3–C4 olefin in 3,4-dehydroquebrachamine systems is resilient to hydrogenation, see Ziegler FE, Kloek JA, Zoretic PA. J Am Chem Soc. 1969;91:2342.