Abstract
This study examined whether polymorphisms in the serotonin transporter (SLC6A4, 5-HTTLPR) and brain derived neurotropic factor (BDNF Val66Met, rs6265) genes moderate the relationship between life stress and rumination. Participants were a large homogenous group of healthy, unmedicated, never depressed individuals with few current symptoms of depression (N = 273). Results indicate that individuals with two short (S) alleles of the 5-HTTLPR polymorphism or two Met alleles of the BDNF Val66Met polymorphism ruminate more under conditions of life stress, compared to the other genotypes. Moreover, the accumulation of risk alleles (i.e., S and Met alleles) across genes is associated with significantly greater rumination in the context of life stress. These results suggest that both 5-HTTLPR and BDNF Val66Met moderate the relationship between life stress and rumination. These findings support the notion that variation in these genes is associated with biological sensitivity to the negative effects of stress.
Keywords: Rumination, Genes, Biological Sensitivity, 5-HTTLPR, BDNF Val66Met, Life Stress
INTRODUCTION
A polymorphism in the serotonin transporter gene (5-HTT, SLC6A4) has been associated with individual differences in response to stress. Individuals carrying one or two copies of the relatively low-expressing short (S) allele of the serotonin transporter linked polymorphic region (5-HTTLPR) demonstrate more aversive responses to stressful events in field and laboratory studies (Caspi et al., 2010 for review) and are at greater risk for developing depression than long allele homozygotes (e.g., Karg, Burmeister, Shedden, & Sen, 2011; although see, Risch et al., 2009).
One way susceptibility to stress may increase vulnerability to depression is through intermediary phenotypes. Rumination, or the tendency to perseverate on problems and negative feelings, represents an important cognitive vulnerability for depression (Nolen-Hoeksema, 2000; Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008). Ruminative thinking predicts the onset of depression, prolongs episodes of negative mood, hinders cognitive and behavioral efforts to improve mood, and is associated with diminished social support (e.g., Beevers, Rohde, Stice, & Nolen-Hoeksema, 2007; Just & Alloy, 1997; Lyubomirsky & Nolen-Hoeksema, 1993; Nolen-Hoeksema & Davis, 1999).
People who ruminate tend to believe that ruminative thinking helps them understand and solve problems (Papageorgiou & Wells, 2001; Papageorgiou & Wells, 2003). Thus, adverse events may provide the fodder for rumination and increases in adverse events are likely to increase rumination. In a small study (N = 21), Canli and colleagues (2006) found that S allele carriers reported higher levels of rumination than L allele homozygotes but only when they experienced current life stress (Canli et al., 2006). Similarly, individuals with two S alleles who experienced higher levels of emotional abuse in childhood report higher levels of rumination in adulthood than individuals carrying at least one copy of the high-expressing long (L) allele (Antypa & Van der Does, 2010). These findings are consistent with the idea that the 5-HTTLPR polymorphism moderates the effect of current life stress on rumination.
Variation in a gene regulating brain derived neruotropic factor (BDNF) may also influence the effect of current adverse events on rumination. Brain derived neurotropic factor (BDNF) is a protein involved in neuronal and synaptic development. An amino acid substitution (valine to methionine) at codon 66 (Val66Met, rs6265) of the BDNF gene results in two alleles: Val and Met. We recently reported that the Met allele was associated with increased levels of rumination in a sample of non-depressed adults (Beevers et al., 2009). Similar findings have been reported among women with adult onset depression (Hilt, Sander, Nolen-Hoeksema, & Simen, 2007). It remains unclear whether BDNF Val66Met variation interacts with life stress to predict differences in rumination, although a growing animal literature indicates that Met homozygotes display more aversive responses to stress than animals carrying at least one Val allele (e.g., Chen et al., 2006; Spencer et al., 2010; Yu et al., 2009).
In summary, we hypothesized that individuals with genotypes associated with stress sensitivity (S 5-HTTLPR carriers or Met BDNF homozygotes) would report higher levels of rumination than L 5-HTTLPR and Val BFND homozygotes when they experienced recent adverse events. Exploratory analyses investigated the aggregate effect of risk alleles across genes (i.e., S and Met alleles) on the relationship between life stress and rumination.
METHOD
Participants
Participants were 273 undergraduate students recruited from introductory psychology courses at the University of Texas at Austin (see Table 1 for demographic information). Participants were 57% Caucasian, 6% Black/African American, 15% Asian, 0.01% Hawaiian/Pacific Islander, 0.003% Native American/American Indian, 7% Multiple Ethnicities, and 15% did not endorse an ethnicity. Across these categories, 21% of the sample was Hispanic. Participants received one research credit for participating in this study. During the study, participants completed self-report measures of rumination, current adverse events, current depressive symptoms, past depressive episodes, current psychiatric medication use, and demographic information. They also provided a sample of buccal cells used to extract DNA for genotyping. Inclusion criteria included no history of major depressive disorder and no current use of any psychiatric medications.
Table 1.
Demographics as a function of BDNF Val66Met and 5-HTTLPR allele group.
| 5-HTTLPR | BDNF Val66Met | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| L’L’ (n = 52) |
L’S’ (n = 139) |
S’S’ (n = 77) |
Val/Val (n = 161) |
Val/Met (n = 92) |
Met/Met (n = 20) |
|
| Age (years) | 18.96 (0.99) | 18.80 (0.97) | 19.01 (2.16) | 18.84 (1.00) | 18.87 (0.86) | 19.55 (3.95) |
| % Gender (M/F) | 54/46 | 54/46 | 52/48 | 55/45 | 46/54 | 75/25 |
| % Caucasian/Other | 67/33 | 64/36 | 35/65 | 60/40 | 58/42 | 25/75 |
| Depressive Symptoms | 3.30 (2.82) | 3.46 (3.57) | 3.64 (3.26) | 3.72 (3.45) | 2.96 (3.20) | 3.86 (2.68) |
| Adverse Events | 2.83 (1.42) | 2.77 (1.64) | 3.05 (1.73) | 2.88 (1.65) | 2.78 (1.60) | 2.9 (1.52) |
| Rumination | 9.89 (4.96) | 9.29 (5.17) | 9.44 (5.46) | 8.86 (4.60) | 10.26 (5.78) | 9.95 (6.69) |
Note: Within both BDNF Val66Met and 5-HTTLPR allele groups there were significantly different distributions for ethnicity (Caucasian/Non-Caucasian). Therefore, this variable was tested as a moderator for all genetic analysis. Rumination scores were significantly different between Val/Val and Val/Met groups. There were no other significant differences between allele groups.
Materials
Genetic Sample
Genomic DNA was isolated from buccal cells using a modification of published methods (e.g., Freeman et al., 1997). The cheeks and gums are rubbed for 20 seconds with three sterile, cotton-tipped wooden swabs. The swabs are placed in a 50-ml capped polyethylene tube containing lysis bugger (500 μl of 1 M Tris-HCl; 200 mM disodium ethylene diaminetetracetic acid (EDTA), pH 8.0; 500 μl of 10% sodium docecyl sulfate; and 100 μl of 5 M sodium chloride). The subjects then rinse out the mouth vigorously with 10 ml of distilled water for 20 seconds and this was added to the 50-ml tube. The tubes were stored at 4°C until the DNA was extracted.
Serotonin transporter promoter region polymorphism (5-HTTLPR)
The 5-HTTLPR gene, which maps to 17q11.1-17q12, contains a 43 bp insertion/deletion in the 5′ regulatory region of the gene (Heils et al., 1996). This polymorphism in the promoter appears to be associated with variations in transcriptional activity: the long variant has approximately three times the basal activity of the shorter promoter with the deletion (Lesch et al., 1996). The assay is a modification of the method of Lesch and colleagues (1996). The primer sequences are: forward, 5′-GGCGTTGCCGCTCTGAATGC-3′ (fluorescently labeled), and reverse, 5′-GAGGGACTGAGCTGGACAACCAC-3′. These primer sequences yield products of 484 or 528 bp. Allele sizes are scored by two investigators independently and inconsistencies were reviewed and rerun when necessary.
The 5-HTTLPR long allele (L) has two variants (LA and LG). The LG variant mirrors the S allele in terms of transcriptional activity (Wendland et al., 2006): Two copies of the LA variant are associated with significantly greater synaptic serotonin reuptake, compared to one or two copies of the S or LG alleles (Hu et al., 2005; Lesch et al., 1996).To distinguish between the S, LA, and LG fragments, the PCR fragment was digested with MspI according to the methods reported by Wigg and colleagues (2006). The resulting polymorphic fragments were separated using an ABI 3130xl DNA sequencer (S: 297, 127, 62 bp; LA: 340, 127, 62 bp; LG: 174, 166, 127, and 62 bp). Consistent with previous research, the low expressing S and LG alleles were designated S’ and the higher expressing LA allele was designated L’. We therefore formed three groups: (1) S’S’ (i.e., SS: n = 58; SLG: n = 17; LGLG: n = 2); (2) S’L’ (i.e., SLA: n = 113; LGLA: n = 26); and (3) L’L’ (i.e. LALA: n = 52). Results of an exact test for Hardy Weinberg proportions using Markov chain–Monte Carlo implementation (Guo and Thompson, 1992) indicate that our observed genotype frequencies do not differ from Hardy Weinberg equilibrium (p= 0.525). We were unable to genotype 5-HTTLPR among three individuals for whom we had BDNF genotypes.
Brain derived neurotrophic factor (BDNF)
The Val66Met polymorphism (rs6265) was genotyped using Taqman assay C___11592758_10 (Applied Biosystems) using an ABI 7900HT Real time PCR system. The frequency of the BDNF genotypes (Val/Val, n = 161; Val/Met, n = 92; Met/Met, n = 20) did not differ from the Hardy-Weinberg equilibrium (χ2 = 1.78, p = 0.18).
Adverse Events Questionnaire (AEQ)
The AEQ (Carver, 1998) was developed to measure current adverse events in the life of undergraduate students. Two items measure adverse events in the domains of academics and relationships, one item measures adverse events in any other domain, and one item measures the impact of accumulated minor negative events. Participants respond using a scale from 0 to 3 indicating the frequency of adverse events in each domain (i.e., 0 = No, 1 = Yes, this happened to me once, 2 = Yes, this happened to me twice, 3 = Yes, this happened to me more than twice). This measure of life stress has previously been found to interact with a cognitive vulnerability in the prospective prediction of dysphoria (Beevers & Carver, 2003; Carver, 1998).
Ruminative Responses Scale (RRS)
The RRS (Treynor, Gonzalez, & Nolen-Hoeksema, 2003) is a 10-item scale that measures an individual’s tendency to ruminate, or repetitive and passive thinking about problems, negative events, and negative feelings. Participants respond using a scale from 1 (almost never) to 4 (almost always) indicating the frequency of with which they endorse rumination items. The RRS provides a measure of rumination that is not confounded with depression symptoms. Previous reports indicate good internal reliability and predictive validity (Treynor et al., 2003).
Beck Depression Inventory – II (BDI-II)
The BDI-II (Beck, Steer, & Brown, 1996) is commonly used in research and clinical settings to assess depression symptoms and their severity. The inventory consists of 21 items sampling depressive symptoms across cognitive, motivational, affective and somatic domains. Previous reports indicate adequate test-retest reliability and validity among undergraduate student populations (Beck et al., 1996). BDI-II scores were included as a covariate in all analyses.
The Inventory for Diagnosing Depression – Lifetime Version (IDD-L)
The IDD-L (Zimmerman & Coryell, 1987) is a self-report questionnaire used to diagnose lifetime major depressive disorder. It has been shown to have similar sensitivity and specificity as the Diagnostic Interview Schedule, and good construct validity and test-retest reliability (Zimmerman & Caryell, 1987). People who endorsed the presence of five of nine symptoms for a two week period or greater were classified as having a history of depression. Participants were required not to have a past history of depression to be included in this study.
Demographic Questionnaire
Participants completed a demographic questionnaire that included age, gender, race/ethnicity, and medication use (see Table 1). Participants were required to not be taking any psychiatric medications to be included in this study. To test moderating effects of ethnicity, we collapsed minority groups and created an ethnicity variable reflecting Caucasians vs. non-Caucasians (see Beevers et al., 2009).
Procedure
Participants were initially recruited based on having low scores on the short form of the Beck Depression Inventory, completed during a mass pre-testing session at the beginning of the academic semester. We chose to examine study hypotheses in a sample of non-depressed healthy adults so that we could isolate genetic and environmental effects on rumination while simultaneously eliminating confounds like depression. For this reason, we also used a measure of rumination that is not confounded by depression symptoms (see above) and controlled for depressive symptoms in our sample at each stage of analysis (see below). Individuals with low scores (<4) were invited to participate in the current study. All participants provided informed consent, completed the self-report questionnaires described above, completed additional assessments not relevant to this research, and provided buccal cells via a cheek swab for genotyping. Upon completion of these procedures, participants were debriefed and assigned course credit for their participation. The institutional review board (IRB) at the University of Texas at Austin approved all study procedures.
RESULTS
Sample Characteristics
Descriptive statistics for the sample are presented in Table 1 and organized by BDNF and 5-HTTLPR allele groups (Val/Val, Val/Met, Met/Met; L’L’, S’L’, S’S’). To test whether the distribution of allele groups differed based on ethnicity we collapsed all Non-Caucasian individuals into one group and compared this group to the Caucasian group. There were significantly different distributions of this reduced ethnicity variable (Caucasian/Non-Caucasian) across BDNF (Fisher’s exact = 0.013) and 5-HTTLPR allele groups (χ2 < 0.0001). Therefore, we tested whether this reduced ethnicity variable (hereafter called “ethnicity”) moderated the interaction between genes and life events in all subsequent analysis. No other significant demographic differences were observed between groups.
Statistical Analysis
All analyses were preformed in STATA 11 (StataCorp: College Station, Texas, USA). RRS scores were linearly transformed with a square root transformation to help normalize the distribution and correct for heterscedasticity in the regression analyses. For each analysis we implemented multiple regression with a factors approach. This approach requires setting a reference group and then comparing groups of interest to this reference group using a dummy code structure (e.g., two dummy codes are examined for the 5-HTTLPR variable: LL vs. SL, LL vs. SS). One advantage of this approach is that we can directly assess differences between specific genetic groups and their interaction with adverse events to the reference group in the initial model. Therefore, follow-up tests are unnecessary. We visually inspected residual plots at each stage of analysis to check for violations in the assumptions underlying linear regression.
5-HTTLPR
In order to test whether 5-HTTLPR genotype predicts rumination scores, while controlling for current depression symptoms, we performed a multiple regression analysis with 5-HTTLPR group (allele: L’L’, S’L’, and S’S’) as the predictor variable, RRS scores as the dependent variable, and BDI-II scores as a covariate. We treated the L’L’ genotype as a reference group in the analysis. Neither the S’L’ group, t (266) = −0.85, p = 0.40, nor the S’S’ group, t (266) = −0.77, p =0.44, differed from the L’L’ group. Consistent with our previous findings (Beevers et al., 2009), there was no 5-HTTLPR genotype main effect for the prediction of rumination. Ethnicity did not moderate these effects, S’L’: t (256) = 0.67, p = 0.50; S’S’: t (256) = 0.59, p = 0.56.
5-HTTLPR × Adverse Events
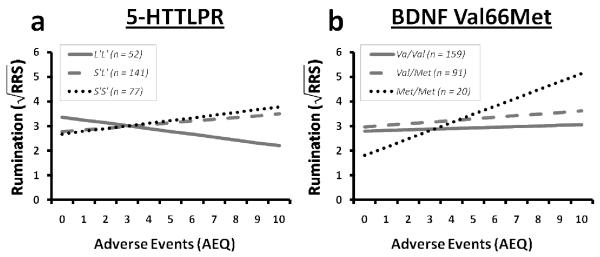
In order to test whether 5-HTTLPR genotype interacts with current adverse events to predict rumination we performed a multiple regression analysis with AEQ and 5-HTTLPR genotype as predictor variables and BDI-II as a covariate. High-expressing long allele homozygotes (LL) were treated as a reference group in the regression model. Results indicate a significant S’S’ × life stress interaction, t (263) = 2.11, p = 0.036, effect size r = 0.13 (see Figure 1a). The S’L’ × life stress interaction fell just short of statistical significance, t (263) = 1.93, p = 0.054, effect size r = 0.12 (see Figure 1a). Relative to the L’L’ group, S-carriers ruminated more as number of adverse events increased. Ethnicity did not moderate these effects, S’L’ × AEQ × ethnicity, t (250) = 0.44, p = 0.66; S’S’ × AEQ × ethnicity, t (250) = −0.31, p = 0.75.
Figure 1.
Rumination as a function of life stress and candidate gene (a – 5-HTTLPR, b – BDNF Val66Met)
BDNF Val66Met
In order to test whether BDNF genotype predicts rumination scores, while controlling for depressive symptoms, we performed a multiple regression analysis with BDNF group (allele: Val/Val, Val/Met, and Met/Met) as the predictor variable, RRS scores as the outcome variable, and BDI-II scores as a covariate. The Val/Val group was treated as a reference group in the regression model. Results revealed a significant term for the Val/Met homozygote group, t (269) = 2.08, p = 0.039, effect size r = 0.13. Consistent with our previous findings, Val/Met individuals report higher levels of rumination than the Val/Val group (Beevers et al., 2009). Rumination for the Met homozygote group was not significantly different from the Val/Val group, t (269) = 0.41, p = 0.68 (see Table 1). Ethnicity did not moderate these effects, Val/Met × ethnicity, t (259) = 0.38, p = 0.70; Met/Met × ethnicity, t (259) = −0.22, p = 0.82.
BDNF Val66Met × Adverse Events
In order to test whether BDNF Val66Met genotype interacts with current adverse events to predict rumination we performed a multiple regression analysis with AEQ and BDNF status as predictor variables and BDI-II as a covariate. The Val/Val group was treated as a reference group in the regression model. Results indicate a significant interaction term for Met/Met × AEQ, t (266) = 2.62, p = 0.009, effect size r = 0.16 (see Figure 1b), but not for Val/Met × AEQ, t (266) = 0.42, p = 0.68. Relative to Val homozygotes, Met homozygous individuals ruminate more as adverse events increase. The effect for adverse events on rumination did not differ for the Val/Met versus the Val/Val groups. Ethnicity did not moderate these effects, Val/Met × AEQ × ethnicity, t (253) = 0.76, p = 0.45; Met/Met × AEQ × ethnicity, t (253) = 0.33, p = 0.74.
Risk Alleles
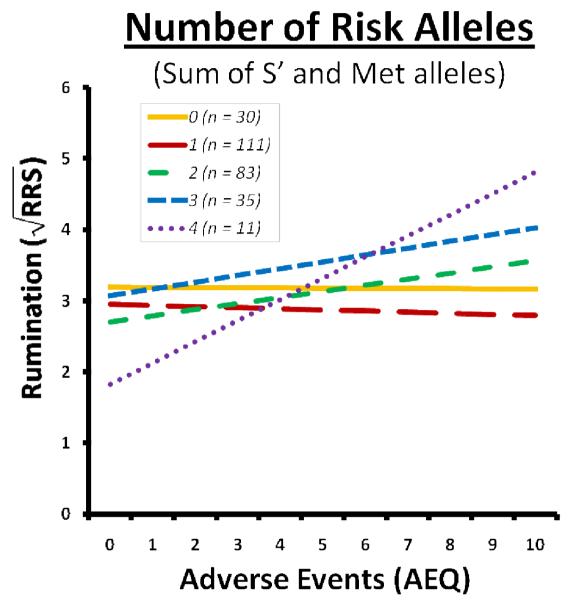
In order to assess whether the current adverse events interact with number of risk alleles to predict rumination we conducted a regression analysis with AEQ and risk alleles as the predictor variables, RRS scores as the outcome variable, and BDI-II scores as a covariate. Given that we are examining two polymorphisms, number of risk alleles could range from 0 to 4 (M=1.57, SD=0.99). Number of risk alleles was used as a continuous variable, so there is no reference group for these analyses. The interaction term was significant, t (265) = 2.49, p = 0.013, effect size r = 0.15. The association between adverse events and rumination varied as a function of number of risk alleles across genes, with a higher number of risk alleles predicting a stronger association between adverse events and rumination (see Figure 2). Ethnicity did not moderate this effect, t (254) = −0.14, p = 0.89.
Figure 2.
Rumination as a function of life stress and number of combine risk alleles.
DISCUSSION
The goal of this study was to examine whether 5-HTT and BDNF genes moderate the relationship between life stress and rumination. We tested the hypothesis that individual differences in 5-HTTLPR and BDNF Val66Met polymorphisms predict differences in the relationship between adverse events and ruminative thinking. Results suggest that individuals with two S’ alleles (5-HTTLPR) and individuals with two Met alleles (BDNF Val66Met) tend to ruminate more when they experience more adverse life events. Individuals with one S’ allele showed a similar pattern (although this effect fell just short of statistical significance, p = 0.054). Individuals with two L’ 5-HTTLPR alleles and individuals with at least one Val BDNF allele do not show increased rumination in the context of life stress. These results support the hypothesis that 5-HTT and BDNF genes moderate the relationship between life stress and rumination.
These findings are consistent with a growing body of research that indicating that the 5-HTTLPR polymorphism is associated with susceptibility to the negative effects of stress (Caspi et al., 2010). They are also consistent with evidence that 5-HTTLPR variation moderates the relationship between early life stress and rumination (Antypa & Van der Does, 2010). Taken together, these results suggest individual differences in 5-HTTLPR genotype interact with life stress occurring across the life span to predict differences in ruminative thinking. Rumination represents an important cognitive vulnerability for depression (e.g., Nolen-Hoeksema et al., 2008); these results support the idea that rumination also represents an intermediary phenotype for depression vulnerability among S’ allele homozygotes who experience life stress.
Moreover, these results provide the first evidence that the BDNF Val66Met polymorphism interacts with life stress to predict rumination. Previous studies showed that the Met allele is associated with direct differences in ruminative thinking (Beevers et al., 2009; Hilt et al., 2007); however, these studies did not (a) investigate the influence of life stress and (b) did not include Met homozygous individuals as a separate group in the analysis. A growing literature suggests that Met homozygotes exhibit susceptibility to the negative effects of life stress (e.g., Gatt et al., 2009; Schule et al., 2006; Vinberg et al., 2009). Results from this study are consistent with this evidence, as Met homozygotes were the only BDNF Val66Met genotype to demonstrate increased rumination when they experienced increased adverse events. These results highlight the importance of including this less common genotype in analyses, a demand that requires sampling a large number of participants in BDNF Val66Met association studies.
It is interesting to note that Val/Met individuals reported higher levels for rumination than Val homozygotes in this study, independent of the effects of life stress. This result is consistent with our previous research (Beevers et al., 2009). BDNF Val66Met variation, therefore, appears to influence rumination via two pathways: (1) moderating stable differences in the tendency to ruminate among Val/Met individuals and (2) moderating susceptibility to rumination under conditions of stress among Met homozygous individuals. More work is needed to replicate these initial findings. However, the notion that a gene can influence vulnerability for rumination via different mechanisms has important implications for understanding the etiology of rumination.
Consistent with this idea, future work should also examine whether these genotypes influence sensitivity to both positive and negative environmental contexts (Ellis & Boyce, 2008). This model, which is routed in evolutionary-developmental biology, suggests that selection pressures favor adaptive phenotypic plasticity—the capacity for a genotype to flexibly influence behavior depending on environmental context (Boyce & Ellis, 2005). The 5-HTTLPR appears to fit this model (Belsky & Pluess, 2009). In the current study, Met/Met carriers and individuals with 4 risk alleles reported the lowest levels of rumination in the absence of life stress. This may be evidence for differential susceptibility to context—the BDNF genotypes may increase vulnerability to rumination in stressful environments but lead to lowered rumination in low stress (or more supportive) environments. Future should measure directly positive and negative environmental contexts to test this intriguing possibility.
Finally, our results suggest that susceptibility accumulates across polymorphisms associated with rumination. Previous research has documented evidence of epistatic interactions between 5-HTTLPR and BDNF Val66Met polymorphisms predicting differences in response to adversity (Dourghty et al., 2010) and mood challenges (Wells et al., 2010). This is the first study, to our knowledge, that shows that the accumulation of risk alleles (i.e., S’ and Met alleles) across these polymorphisms is associated with greater susceptibility for ruminative thinking in the context of adverse events. These findings require replication; however, they suggest that susceptibility to stress is not only moderated by epistatic interactions, but can accumulate across combinations of risk alleles from different genes. Future work should consider including a larger number of SNPs that might influence rumination in the aggregate genetic risk score (e.g., De Jager et al., 2009). Doing so may increase the power of genetic models to predict individual differences in rumination.
There are two important limitations to this study. First, the correlational research design prevents conclusions about causal relationships. We assume that adverse events are capable of causing increases in rumination among individuals with certain genetic profiles. Creating adverse events in the laboratory and measuring rumination across genetic profiles would allow us to better understand these relationships. Longitudinal studies would allow us to better explore the temporal relations between stress and rumination among different genetic profiles across the life span. This is particularly important given ambiguity about the nature of BDNF Val66Met associations with rumination at different stages of development (Hilt et al., 2007). Hilt and colleagues (2007) have reported that an association between the Val/Val genotype and rumination in daughters of women with adult onset depression; but among their mothers, there was an association between the Met allele and rumination. Future longitudinal research, including measures of life stress, may help to better characterize these associations across stages of development.
Second, this research does not identify mediating mechanisms. It is likely that genetic effects on biological systems underlying stress response, affect, and cognition mediate genetic influences on the relationship between life stress and rumination. There is growing evidence that both 5-HTTLPR and BDNF polymorphisms are associated with individual differences in stress reactivity, neural function, and brain development (e.g., Alexander et al., 2009; Canli et al., 2005; Dougherty et al., 2010; Hariri & Holmes, 2006; Lau et al., 2010; Montag et al., 2008). Future work integrating functional and structural neuroimaging, for example, may help to identify mechanisms that mediate observed genetic differences in the relationship between life stress and rumination (see Canli et al., 2006; Canli & Lesch, 2007).
One of these mechanisms may include cognitive control. There is emerging evidence that 5-HTTLPR variation moderates inhibition of emotional stimuli following a laboratory stressor (Markus & De Raedt, 2011). Deficits in cognitive control have been associated with rumination (e.g. Joormann, 2006; Lissynder et al., 2010; see also Koster et al., 2011). Together, these results suggest that cognitive control represents an important mediating mechanism for the relationship between genes and rumination in the context of life stress. Future work is required to further examine this hypothesis and explore these relationships in other candidate genes (e.g., BDNF).
Despite these limitations, this research provides important insight into who is most likely to ruminate and when rumination is most likely to occur. Individual differences in the 5-HTT and BDNF genes moderate the relationship between adverse events and rumination among healthy adults. Individuals with two S’ alleles of the 5-HTTLPR polymorphism or two Met alleles of the BDNF Val66Met polymorphism reported higher levels of rumination as adverse events increased. This susceptibility to rumination accumulates across genes, as individuals with the greatest number of summed risk alleles (i.e., S’ and Met alleles) demonstrated the strongest relationship between adverse events and rumination. More work is needed to examine the causal nature of these relationships and to uncover mediating mechanisms that link genetic variation to broad cognitive thinking styles like rumination. This work requires an integrated approach across levels of analyses (i.e., genetic, neural, cognitive, environmental) that will facilitate more comprehensive models of how genes confer susceptibility to the negative effects of stress.
ACKNOWLEDGEMENTS
The authors thank the research assistants from the Mood Disorders Laboratory at the University of Texas for their help with data collection. Preparation of this article was supported by grant R01MH076897 from the National Institute of Mental Health to Christopher Beevers. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Contributor Information
Valerie S. Knopik, Division of Behavioral Genetics, Rhode Island Hospital and Department of Psychiatry and Human Behavior, Brown University
John E. McGeary, Providence Veterans Affairs Medical Center and Center for Alcohol and Addiction Studies, Brown University
Christopher G. Beevers, The University of Texas at Austin
WORKS CITED
- Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, Hennig J. Gene-environment interactions predict cortisol responses after acute stress: implications for the etiology of depression. Psychoneuroendocrinology. 2009;34:1294–1303. doi: 10.1016/j.psyneuen.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Antypa N, Van der Does AJW. Serotonin transporter gene, childhood emotional abuse and cognitive vulnerability to depression. Genes Brain Behav. 2010;9:615–620. doi: 10.1111/j.1601-183X.2010.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Beevers CG, Carver CS. Attentional bias and mood persistence as prospective predictors of dysphoria. Cognitive Therapy and Research. 2003;27:619–637. [Google Scholar]
- Beevers CG, Rohde P, Stice E, Nolen-Hoeksema S. Recovery from major depressive disorder among female adolescents: a prospective test of the scar hypothesis. J Consult Clin Psychol. 2007;75:888–900. doi: 10.1037/0022-006X.75.6.888. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, McGeary JE. The BDNF Val66Met polymorphism is associated with rumination in healthy adults. Emotion. 2009;9:579–584. doi: 10.1037/a0016189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc. Natl. Acad. Sci. U.S.A. 2005;102:12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, Herrmann MJ, et al. Neural correlates of epigenesis. Proc. Natl. Acad. Sci. U.S.A. 2006;103:16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS. Generalization, adverse events, and development of depressive symptoms. J Pers. 1998;66:607–619. doi: 10.1111/1467-6494.00026. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chen Z-Y, Jing D, Bath KG, Ieraci A, Khan T, Siao C-J, Herrera DG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager PL, Chibnik LB, Cui J, Reischl J, Lehr S, Simon KC, Aubin C, et al. Integration of genetic risk factors into a clinical algorithm for multiple sclerosis susceptibility: a weighted genetic risk score. Lancet Neurol. 2009;8:1111–1119. doi: 10.1016/S1474-4422(09)70275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lissnyder E, Koster EHW, Derakshan N, De Raedt R. The association between depressive symptoms and executive control impairments in response to emotional and non-emotional information. Cognition and Emotion. 2010;24:264–280. [Google Scholar]
- Dougherty LR, Klein DN, Congdon E, Canli T, Hayden EP. Interaction between 5-HTTLPR and BDNF Val66Met polymorphisms on HPA axis reactivity in preschoolers. Biol Psychol. 2010;83:93–100. doi: 10.1016/j.biopsycho.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Craig I, Plomin R. DNA by mail: an inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behav. Genet. 1997;27:251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- Frodl T, Schüle C, Schmitt G, Born C, Baghai T, Zill P, Bottlender R, et al. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch. Gen. Psychiatry. 2007;64:410–416. doi: 10.1001/archpsyc.64.4.410. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol. Psychiatry. 2009;14:681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics. 1992;48:361–372. [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn. Sci. (Regul. Ed.) 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J. Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hilt LM, Sander LC, Nolen-Hoeksema S, Simen AA. The BDNF Val66Met polymorphism predicts rumination and depression differently in young adolescent girls and their mothers. Neurosci. Lett. 2007;429:12–16. doi: 10.1016/j.neulet.2007.09.053. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol. Clin. Exp. Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Joormann J. The relation of rumination and inhibition: Evidence from a negative priming task. Cognitive Therapy and Research. 2006;30:149–160. [Google Scholar]
- Just N, Alloy LB. The response styles theory of depression: tests and an extension of the theory. J Abnorm Psychol. 1997;106:221–229. doi: 10.1037//0021-843x.106.2.221. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. [Accessed April 21, 2011];The Serotonin Transporter Promoter Variant (5-HTTLPR), Stress, and Depression Meta-analysis Revisited: Evidence of Genetic Moderation. Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2010.189. http://www.ncbi.nlm.nih.gov/pubmed/21199959. [DOI] [PMC free article] [PubMed]
- Koster EHW, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from an affective neuroscience perspective: The impaired disengagement hypothesis. Clin Psychol Rev. 2011;31:138–145. doi: 10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Lau JYF, Goldman D, Buzas B, Hodgkinson C, Leibenluft E, Nelson E, Sankin L, et al. BDNF gene polymorphism (Val66Met) predicts amygdala and anterior hippocampus responses to emotional faces in anxious and depressed adolescents. Neuroimage. 2010;53:952–961. doi: 10.1016/j.neuroimage.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Nolen-Hoeksema S. Self-perpetuating properties of dysphoric rumination. J Pers Soc Psychol. 1993;65:339–349. doi: 10.1037//0022-3514.65.2.339. [DOI] [PubMed] [Google Scholar]
- Markus R, De Raedt R. Differential effects of 5-HTTLPR genotypes on inhibition of negative emotional information following acute stress exposure and tryptophan challenge. Neuropsychopharmacology. 2011;36:819–826. doi: 10.1038/npp.2010.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C, Reuter M, Newport B, Elger C, Weber B. The BDNF Val66Met polymorphism affects amygdala activity in response to emotional stimuli: evidence from a genetic imaging study. Neuroimage. 2008;42:1554–1559. doi: 10.1016/j.neuroimage.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Nemoto K, Ohnishi T, Mori T, Moriguchi Y, Hashimoto R, Asada T, Kunugi H. The Val66Met polymorphism of the brain-derived neurotrophic factor gene affects age-related brain morphology. Neurosci. Lett. 2006;397:25–29. doi: 10.1016/j.neulet.2005.11.067. [DOI] [PubMed] [Google Scholar]
- Neves-Pereira M, Cheung JK, Pasdar A, Zhang F, Breen G, Yates P, Sinclair M, et al. BDNF gene is a risk factor for schizophrenia in a Scottish population. Mol. Psychiatry. 2005;10:208–212. doi: 10.1038/sj.mp.4001575. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol. 2000;109:504–511. [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Davis CG. “Thanks for sharing that”: ruminators and their social support networks. J Pers Soc Psychol. 1999;77:801–814. doi: 10.1037//0022-3514.77.4.801. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco B, Lyubomirsky S. Rethinking Rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Papageorgiou C, Wells A. An empirical test of a clinical metacognitive model of rumination and depression. Cognitive Therapy and Research. 2001;27:261–273. [Google Scholar]
- Papageorgiou C, Wells A. Metacognitive beliefs about rumination in refcurrent major depression. Cognitive and Behavioral Practice. 2003;8:160–164. [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K-Y, Eaves L, Hoh J, Griem A, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Skibinska M, Szczepankiewicz A, Kapelski P, Leszczynska-Rodziewicz A, Czerski PM, et al. Prefrontal cognition in schizophrenia and bipolar illness in relation to Val66Met polymorphism of the brain-derived neurotrophic factor gene. Psychiatry Clin. Neurosci. 2006;60:70–76. doi: 10.1111/j.1440-1819.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- Schüle C, Zill P, Baghai TC, Eser D, Zwanzger P, Wenig N, Rupprecht R, et al. Brain-derived neurotrophic factor Val66Met polymorphism and dexamethasone/CRH test results in depressed patients. Psychoneuroendocrinology. 2006;31:1019–1025. doi: 10.1016/j.psyneuen.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, Lee FS, McEwen BS. BDNF variant Val66Met interacts with estrous cycle in the control of hippocampal function. Proc. Natl. Acad. Sci. U.S.A. 2010;107:4395–4400. doi: 10.1073/pnas.0915105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27:247–259. [Google Scholar]
- Vinberg M, Trajkovska V, Bennike B, Knorr U, Knudsen GM, Kessing LV. The BDNF Val66Met polymorphism: relation to familiar risk of affective disorder, BDNF levels and salivary cortisol. Psychoneuroendocrinology. 2009;34:1380–1389. doi: 10.1016/j.psyneuen.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Wells TT, Beevers CG, McGeary JE. Serotonin transporter and BDNF genetic variants interact to predict cognitive reactivity in healthy adults. J Affect Disord. 2010;126:223–229. doi: 10.1016/j.jad.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch K-P, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol. Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Wigg KG, Takhar A, Ickowicz A, Tannock R, Kennedy JL, Pathare T, Malone M, et al. Gene for the serotonin transporter and ADHD: no association with two functional polymorphisms. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006;141B:566–570. doi: 10.1002/ajmg.b.30247. [DOI] [PubMed] [Google Scholar]
- Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, Bath KG, et al. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J. Neurosci. 2009;29:4056–4064. doi: 10.1523/JNEUROSCI.5539-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Coryell W. The inventory to diagnose depression, lifetime version. Acta Psychiatr Scand. 1987;75:495–499. doi: 10.1111/j.1600-0447.1987.tb02824.x. [DOI] [PubMed] [Google Scholar]