Abstract
Batrachochytrium dendrobatidis (Bd) is the causative agent of chytridiomycosis, a fungal skin disease in amphibians and driver of worldwide amphibian declines.
We focussed on the early stages of infection by Bd in 3 amphibian species with a differential susceptibility to chytridiomycosis. Skin explants of Alytes muletensis, Litoria caerulea and Xenopus leavis were exposed to Bd in an Ussing chamber for 3 to 5 days. Early interactions of Bd with amphibian skin were observed using light microscopy and transmission electron microscopy. To validate the observations in vitro, comparison was made with skin from experimentally infected frogs. Additional in vitro experiments were performed to elucidate the process of intracellular colonization in L. caerulea.
Early interactions of Bd with amphibian skin are: attachment of zoospores to host skin, zoospore germination, germ tube development, penetration into skin cells, invasive growth in the host skin, resulting in the loss of host cell cytoplasm. Inoculation of A. muletensis and L. caerulea skin was followed within 24 h by endobiotic development, with sporangia located intracellularly in the skin. Evidence is provided of how intracellular colonization is established and how colonization by Bd proceeds to deeper skin layers. Older thalli develop rhizoid-like structures that spread to deeper skin layers, form a swelling inside the host cell to finally give rise to a new thallus.
In X. laevis, interaction of Bd with skin was limited to an epibiotic state, with sporangia developing upon the skin. Only the superficial epidermis was affected. Epidermal cells seemed to be used as a nutrient source without development of intracellular thalli. The in vitro data agreed with the results obtained after experimental infection of the studied frog species. These data suggest that the colonization strategy of B. dendrobatidis is host dependent, with the extent of colonization most likely determined by inherent characteristics of the host epidermis.
Introduction
Chytridiomycosis is a lethal skin disease in amphibians caused by the fungal pathogen Batrachochytrium dendrobatidis (Bd). Causing widespread amphibian declines, this disease constitutes a major threat to amphibian biodiversity and conservation [1]–[4].
Bd colonizes the keratinized layers (stratum corneum) of amphibian skin or larval mouthparts [5]–[7]. Clinical infection is characterized by epidermal hyperplasia, hyperkeratosis and excessive shedding of the epidermis [5], [8]. Extensive colonization gives rise to a series of physiological effects such as disruption of the osmoregulatory function of the skin, leading to dehydration, electrolyte imbalance and death due to cardiac arrest [5], [9]–[13].
The lifecycle of Bd in culture and the pathology in skin from diseased animals are well documented [5], [8], [14]. Infection is established by zoospores, the motile flagellated stage of Bd [8], [14]. Zoospores display chemotactic responses in search of a suitable host to infect [15]. Upon colonization of the host epidermis, the zoospores encyst [8]. The flagellum is absorbed and a cell wall is formed [8]. Based on observations in infected Litoria gracilenta, an intracellular development of Bd was described by Berger et al. [8]. As such, the fungus proliferates within the epidermal cells and has its cycle tuned to the maturation of the epidermal cells [8]. Immature fungal bodies, termed thalli or sporangia are carried to the skin surface by differentiating epidermal cells [8]. Mature sporangia containing zoospores finally occur in the sloughing stratum corneum [8].
Early stages of infection have hitherto been poorly studied [16]. As such it is still not clear how host cell entry is achieved. In analogy with other pathogenic fungi e.g. Candida albicans [17] and dermatophytes [18], most probably a range of digestive enzymes capable of degrading skin components enable penetration of Bd into the host cells [16], [19], [20].
The main objective of this study was to find out how Bd infection is established. The early interaction between Bd and anuran skin was characterized using an in vitro infection model. Amphibian skin explants were inoculated with Bd and incubated in an Ussing chamber. To determine how Bd infection is established and to what extent infection strategies of Bd are host dependent, host-pathogen interactions were evaluated in 3 species with a differential susceptibility to Bd: the African Clawed Frog (Xenopus laevis), the Mallorcan Midwife Toad (Alytes muletensis) and the Green Tree Frog (Litoria caerulea). X. laevis generally does not show clinical signs associated with chytridiomycosis, nor have population declines due to chytridiomycosis been reported [21]. A. muletensis is a vulnerable European species restricted to Mallorca (Balearic Islands, Spain) [22]. Since Bd has been detected in reintroduced captive-bred populations this species is currently threatened by decline [22]–[23]. L. caerulea is a common Australasian species [22], but has proven to be highly susceptible to chytridiomycosis in the wild [22], [24] as well as under laboratory conditions [25], [26].
In a first experiment adhesion, invasion and the development of Bd in skin of A. muletensis, L. caerulea and X. laevis were studied during 3 to 5 consecutive days of in vitro infection using light microscopy (LM) and transmission electron microscopy (TEM). In parallel, A. muletensis, L. caerulea and X. laevis frogs were experimentally infected, to assess the validity of the observations
In a second experiment, skin of L. caerulea was exposed to Bd for 1, 2, 4, 8, 16 and 24 hours to further characterize the process of intracellular colonization. The morphology of the infecting fungal elements during invasion of the skin was followed by LM and TEM. The time-points of exposure found most critical for intracellular colonization were repeated in triplicate.
Results
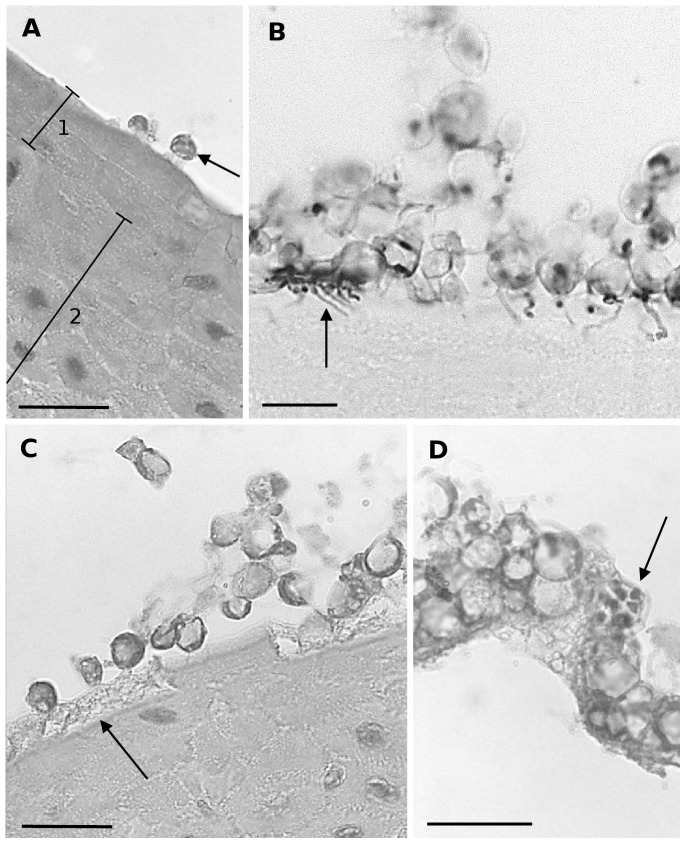
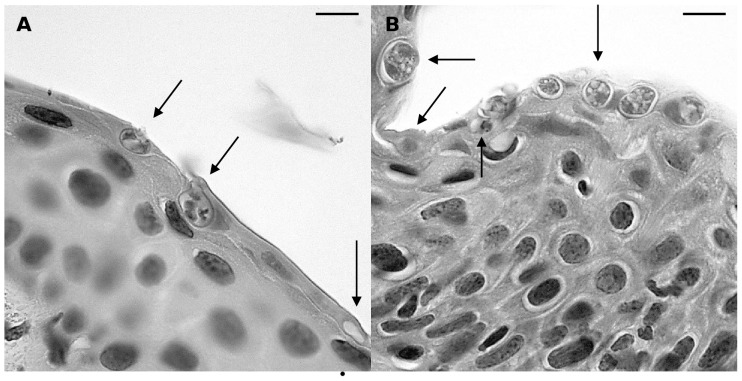
An overview of the early pathogenesis in X. laevis skin as observed by light microscopy is given in Figure 1 . At 1 day post infection (dpi) numerous encysted zoospores had settled in clusters upon the epidermis or were situated in glandular pores ( Fig. 1A ). Zoospore cysts were spherical and had doubled in size (n = 30, (5)–6.1–(7.5) µm diameter) when compared to zoospores (n = 10, (2.0)–2.35–(3.5) µm diameter). From 1 dpi on, zoospore cysts germinated (termed germlings) and developed a short tubular structure of (0.5)–0.58–(0.86) µm diameter, further called germ tube ( Fig. 1B ). Germ tubes had elongated over the epidermal surface or had protruded into the cells of the stratum corneum. In heavily colonized cells the germ tubes growed into a profusely branched, fuzzy mesh work of rhizoids that spread out in the entire cell and was most clearly demonstrated by Gomori's methenamine silver stain (GMS) ( Fig. 1B ). At 2 dpi germlings had increased in size (n = 30, (5)–8.8–(13.2) µm diam.) and were developing into maturing zoosporangia. From 2 dpi on, invasion of the host cells of the stratum corneum resulted in loss of their cytoplasm and only their cell membrane persisted ( Fig. 1C ). Both at 3 and 4 dpi, the germlings upon the epidermal surface had matured into zoosporangia, containing zoospores. Several post-discharge zoosporangia were observed upon the epidermal skin surface. Sporangia were shed together with the affected upper layer of the stratum corneum ( Fig. 1D ).
Figure 1. Light microscopical overview of the development of Bd in skin explants of Xenopus laevis.
(A) adhesion of encysted zoospores (arrow) to the host epidermis at 1 dpi; (1) stratum corneum, (2) stratum spinosum; haematoxylin and eosin (HE) stain; scale bar = 20 µm; (B) at 1 dpi Bd germlings have developed germ tubes, that penetrate the stratum corneum and develop into a branched mesh work of rhizoids (arrow) in heavily infected epidermis; Gomori methenamine silver stain; scale bar = 10 µm; (C) at 2 dpi the infected host cells have lost their cytoplasm (arrow) subsequent to invasion by Bd, only the cell membrane remains; HE stain; scale bar = 20 µm; (D) at 4 dpi germlings have developed into mature zoosporangia (arrow), the upper layer of the stratum corneum is shed; HE stain; scale bar = 20 µm.
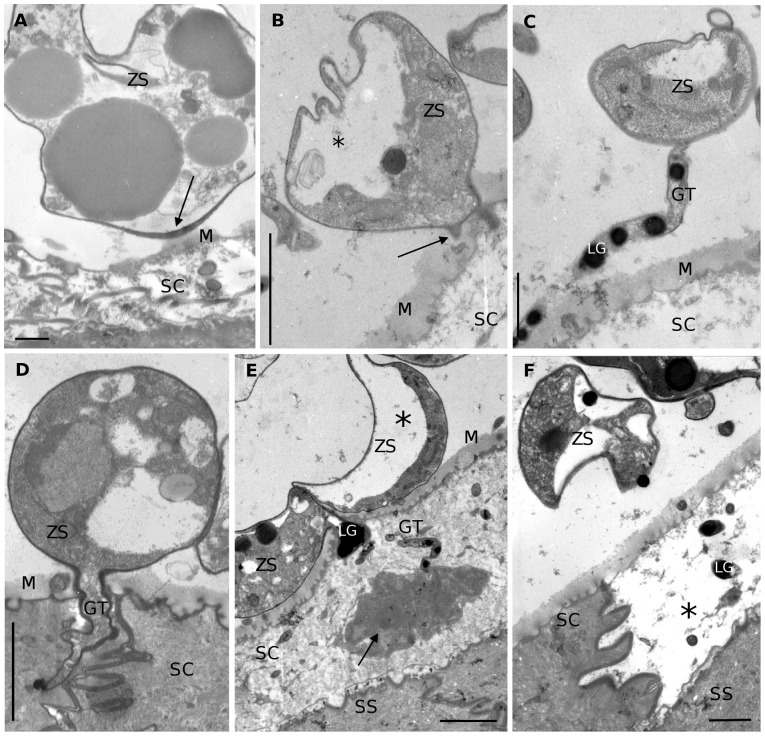
TEM provided more detailed information on ultrastructural changes ( Fig. 2 ). At 1 dpi, encysted zoospores were attached to a thin residual superficial mucus layer on top of the stratum corneum and adhesion to this layer was characterized by a conspicuous thickening of the fungal cell wall (a 3 to 6 fold increase, from 0.05–0.1 µm to 0.2–0.3 µm) ( Fig. 2A ). The initiation of a germ tube, as shown in Figure 2B , started as a pointed outgrowth of the thickened cell wall. Cross-sections of a germ tube by TEM show prominent osmiophilic rounded structures inside the germ tube ( Fig. 2C ). These structures without a membrane and of variable size resembled the lipid globules in the zoospores of Bd. Analogous structures were seen in the cytoplasm of the affected keratinocytes ( Figs. 2E,F ). No mitochondria or nuclei could be discerned in these germ tubes. Figure 2D shows a growing germ tube that had protruded in the stratum corneum. The affected epidermal cells seemed to have partially or completely lost their cytoplasm and only their cell membrane persisted ( Figs. 2E,F ). Remnants of the host cell cytoplasm were observed at the tip of an invading germ tube ( Fig. 2E ). In un-inoculated skin samples, incubated during 5 days under the same conditions as described above, the stratum corneum was still intact and no altered keratinocytes were observed (Fig. S1).
Figure 2. TEM overview of the development of Bd in skin explants of Xenopus laevis.
(A) adhesion of an encysted zoospore (ZS) to the superficial mucus layer (M) on top of the stratum corneum (SC); at the site where adhesion occurs the cell wall of the encysted zoospore is remarkably thickened (arrow); scale bar = 500 nm; (B) initiation of germ tube development (arrow); note the polarisation of the cell cytoplasm (*); scale bar = 2 µm; (C) germ tube (GT) elongating upon the epidermis of X. laevis, with the presence of numerous lipid globules (LG) in the germ tube; scale bar = 1 µm; (D) a growing germ tube protruding the stratum corneum; scale bar = 2 µm; (E) invasion of a host cell resulting in the loss of cell cytoplasm; remnants of the host cell cytoplasm (arrow) are seen at the tip of a protruded germ tube; note the presence of a collapsed sporangium (ZS) due to cell polarisation (*); (SS): stratum spinosum; scale bar = 2 µm; (F) infected epidermal cell with digested cell content (*) alternated by an uninfected normal epidermal cell; note the presence of lipid globules in the infected host cell; scale bar = 1 µm.
Another striking feature seen from 1 dpi onwards was the presence of collapsed sporangia. This was observed in histological and TEM preparations ( Fig. 2E ). A polarisation of the sporangial cytoplasm was observed. The cytoplasm was concentrated at one side of the sporangium, lined by an empty space most probably to be considered as a vacuole.
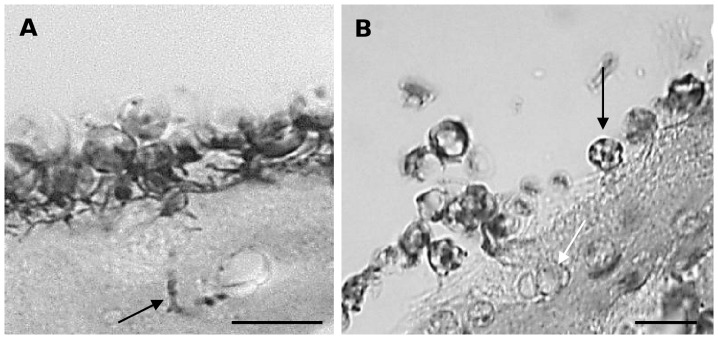
Figure 3 illustrates the development of Bd in skin explants of A. muletensis and L. caerulea. Compared to X. laevis, a similar initial infection process was seen in A. muletensis and L caerulea. Likewise, zoospore cysts adhered to the stratum corneum, host cells were invaded by germ tubes that developed into rhizoidal axes spreading out in the entire cell ( Figs. 3A,B ). Invasion of the keratinocytes by germ tubes and loss of the cellular cytoplasm was most obvious by TEM ( Figs. 4A,B ). From 2 dpi on, maturing sporangia were observed upon the infected skin surface. However, the development of Bd in A. muletensis and L. caerulea was clearly distinct in the respect that besides superficial colonization, intracellular chytrid thalli were observed in superficial and deeper layers of the epidermis. Within 24 hours after inoculation, marked intracellular colonization was seen in L. caerulea by LM (Fig. 3B ) and TEM ( Fig. 4B ) and occasional intracellular colonization in A. muletensis.
Figure 3. Light microscopical overview of the development of Bd in skin explants of Alytes muletensis and Litoria caerulea.
(A) at 1 day post infection (dpi) germlings have developed germ tubes (arrow) that invade the epidermis of A. muletensis; Gomori methenamine silver (GMS) stain; scale bar = 10 µm; (B) at 1 dpi both Bd germlings (black arrow) attached upon the epidermal surface as intracellular chytrid thalli (white arrow) in the stratum corneum of L. caerulea are observed; haematoxylin and eosin stain; scale bar = 10 µm.
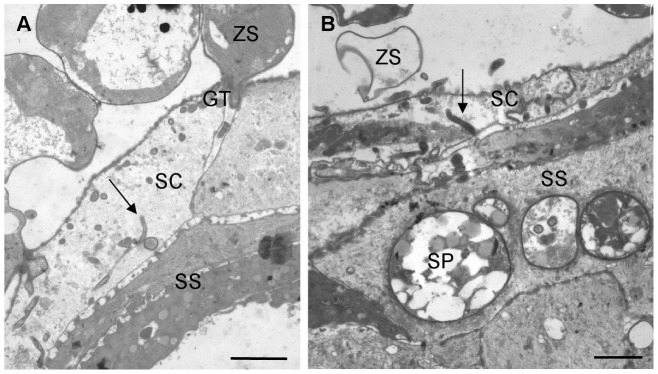
Figure 4. TEM overview of the development of Bd in skin explants of Alytes muletesis and Litoria caerulea.
(A) infected epidermis of A. mulentensis at 1 dpi, with loss of the host cell cytoplasma and the presence of germ tube fragments inside the infected cell in cross and longitudinal section (arrow); scale bar = 2 µm; (B) infected epidermis of L. caerulea at 2 dpi showing colonization of the stratum corneum, loss of the host cell cytoplasm and the presence of germ tube fragments (arrow); intracellular chytrid sporangia are observed in the stratum spinosum; scale bar = 2 µm; GT; germ tube, SC: stratum corneum, SP: sporangium, SS: stratum corneum, ZS: encysted zoospore.
To support the validity of the observations made in vitro, A. muletensis, L. caerulea and X. laevis frogs were infected in vivo. During the course of the infection trial no clinical signs were observed in A. muletensis (n = 3). In 1 out of 3 L. caerulea excessive shedding of skin and erythema of the hind limbs occurred. In all X. laevis frogs (n = 3) only excessive shedding of skin was observed. At 12 days post infection, all A. muletensis and L. caerulea frogs were infected, with mean genomic equivalents of Bd ± standard error detected by qPCR of 517 ± 636 for A. muletensis (n = 3) and 350 ± 589 for L. caerulea (n = 3). All X. laevis frogs tested negative (n = 3).
In skin samples taken at 14 days after exposure, the epidermis of experimentally infected X. laevis frogs was still intact. No adhering zoospores nor sporangia could be observed. In contrast, in all infected A. muletensis (n = 3) ( Fig. 5A , LM) and L. caerulea frogs (n = 3) the stratum corneum was colonized with intracellular sporangia. Germlings or developing sporangia adhering to the epidermis were not observed. Colonization was more abundant in L. caerulea. One out of 3 infected L. caerulea frogs carried a high infection load (1020 GE), was colonized to broad extent ( Fig. 5B , LM) but did not show any clinical signs. The other L. caerulea individuals were infected to the same and lesser extent (9 and 11 GE), with only one individual presenting clinical signs.
Figure 5. Skin sections of Alytes muletensis and Litoria caerulea experimentally infected with Bd at 14 days post infection.
Exclusively intracellular chytrid thalli (arrows) are observed by light microscopy in the stratum corneum of A. muletensis (A) and L.caerulea (B); haematoxylin and eosin stain; scale bar = 10 µm.
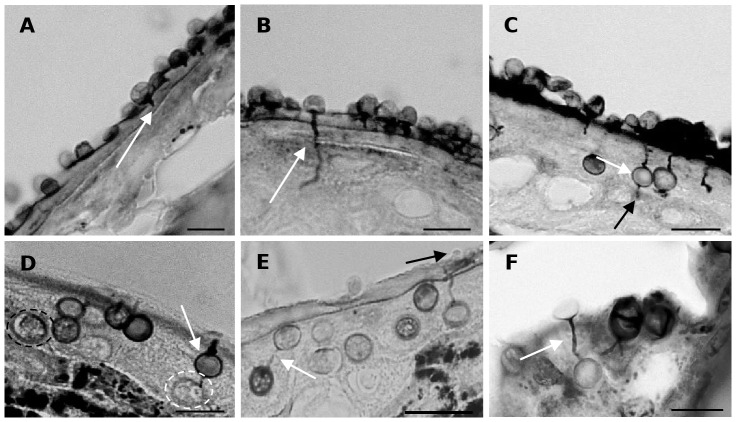
A more detailed study of the invasion process in L. caerulea showed that at earliest, frog skin was invaded by germ tubes 2 hours after exposure to Bd ( Fig. 6A,B ). Eight, 16 and 24 hours of exposure to Bd were defined as most critical time-points to study intracellular colonization and were repeated in triplicate during additional in vitro assays. Chytrid thalli developing intracellularly were observed at 16 to 24 hours after exposure. In one out of the 3 repeats, intracellular colonization occurred 8 hours after exposure. In this experiment the stratum corneum had already detached from the stratum spinosum, probably rendering the epidermis more accessible.
Figure 6. Intracellular colonization of Litoria caerulea skin by Bd.
(A–E): in vitro, (F): in vivo. (A) invasion of the stratum corneum by a germ tube (white arrow) at 2 hour post infection (hpi); (B) strong elongation of the germ tube (white arrow) into the stratum spinosum at 8 hpi; (C) development of intracellular chytrid thalli (white arrow) at the end of a germ tube at 24 hpi; rhizoid-like structures (black arrow) arise from newly developed chytrid thalli; (D) development of a new chytrid thallus at 24 hpi; a swelling is formed at the end of a rhizoid-like structure, a thin cell wall is formed and the cell content of the mother thallus (white arrow) is transferred into the new daughter thallus (white circle); a new thallus in a later developmental stage (black circle); (E) thalli connected by a rhizoid-like structure (white arrow); remnants of a germling, after having injected its cell content into a new intracellular thallus (black arrow); (F) mother thallus connected to a newly formed daughter thallus by a rhizoid-like structure (white arrow) at 14 days post infection. Gomori methenamine silver stain, scale bar = 10 µm.
GMS staining showed that both superficially and deeper localized intracellular chytrid thalli were often connected to a tubular rhizoid-like structure, stretching out to the deeper layers of the epidermis or either to the epidermal surface ( Figs. 6C,D ). In rare cases, remnants of empty zoospore cysts were found at the skin surface ( Fig. 6E ). On several occasions, both in vitro ( Fig. 6E ) and in vivo ( Fig. 6F ) intracellular chytrid thalli apparently connected by a rhizoid-like structure were noticed. As such, older thalli were connected to newly formed thalli. Figure 6D shows a clearly stained older thallus giving rise to a new thallus, outlined by a faintly stained thin cell wall.
Discussion
The present results provide a missing link in the infection process of Bd. Until now established Bd infections had been described with zoospore development occurring in a zoosporangium inside the host cell and the intracellular zoosporangium forming discharge papillae through which zoospores exit [8]. Our results provide a novel insight into the early interaction of Bd zoospores with amphibian skin.
The early pathogenesis consists in the first place of an epibiotic development, upon the host skin and was observed in the 3 species studied. Zoospores matured into thick-walled cysts on the host epidermis and were clustered in foci of infection. Subsequently, invasion of amphibian skin was established by germ tube development. A tubular extension or germ tube arised from the zoospore cysts and penetrated into the epidermal host cells. GMS stained sections showed most clearly that in heavily infected cells germ tubes grew into an irregularly branched mesh work of rhizoids. Histological sections and TEM images strongly suggest an extracellular digestion of the host cytoplasm, followed by an uptake by the germ tubes. A similar effect has been described by Berger et al. [8], who observed dissolution of cellular cytoplasm in infected epidermis of L. gracilenta. However, this was not associated with the presence of germ tubes.
In vitro, X. laevis skin does become infected but the development of Bd in X. laevis skin is apparently limited to an ‘epibiotic’ stage, with epidermal cells solely being used as nutrient source for the growing sporangium upon the epidermis. The typical histological picture of chytridiomycosis with chytrid sporangia developing intracellularly was not observed. Upon examination of stained skin sections from in vivo infected X. laevis frogs, 14 days after exposure to Bd, the skin was still intact and no colonization was observed.
X. laevis is considered tolerant to clinical chytridiomycosis as defined by Schneider & Ayers [27], i.e. this species can be colonized by Bd but is able to limit the impact of Bd on its health and to maintain a low-level infection [21], [28]. Unfortunately, reports of chytrid infections in X. laevis rely merely on PCR-detection [21], [29], [30] and there is no conclusive histological evidence of how chytrid infections manifest in this species under natural conditions.
Recently, Ramsey et al. [31] found that the level of infection in X. laevis is likely to be determined by both innate and adaptive components of the immune system. As such antimicrobial peptides [32], [33] and antifungal metabolites [34] provide a non-specific protection to potential pathogens, while antibodies in skin secretions of previously exposed frogs provide specific anti-Bd protection. A combined action of these defenses is likely to limit colonization of X. laevis by Bd to mild and non-lethal infections. To which extent epibiosis occurs in other chytrid tolerant species remains to be determined. For example, though the American Bullfrog (Lithobathes catesbeianus) is considered a notorious carrier of Bd, there is solid evidence of Bd developing intracellularly in the skin of this species [35], [36].
In A. muletensis and L. caerulea epibiotic development was followed by extensive intracellular colonization of the stratum corneum. Intracellular growth of chytrid thalli was established within 24 hours in L. caerulea and in the later stages of infection in A. muletensis. Additional infection assays in L. caerulea confirm that colonization propagates to the deeper skin layers within 24 hours, at earliest at 16 hours after exposure to Bd. Both in the wild [22]–[24] as in experimental infection trials [25], [26], [37] A. muletensis and L. caerulea can be severely colonized by Bd.
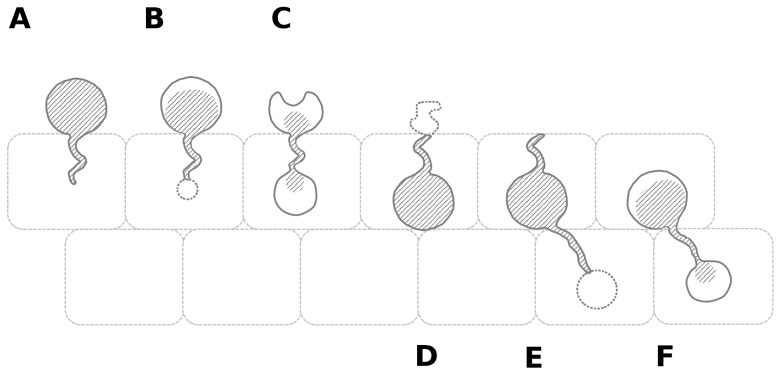
Especially GMS staining proved its usefulness in visualising fungal cell walls and revealing structures that were overlooked using HE staining. In A. muletensis and L. caerulea intracellular chytrid thalli with rhizoid-like structures stretching out either to the epidermal surface or to the deeper layers of the epidermis were observed. On several occasions, both in vitro and in vivo, intracellular chytrid thalli apparently connected by a rhizoid-like structure were noticed. These observations provide consistent evidence of how intracellular colonization is established, as summarized in Figure 7 . Together with the presence of germ tubes these data confirm the hypothesis of an endobiotic development of Bd as formulated by Longcore [14]. During endobiotic development zoospores encyst upon the host cell and inject their nucleus and cytoplasm into the host cell via a germ tube. The germ tube forms a swelling inside the cell and enlarges. Finally, the contents undergo mitosis, zoospores are formed and are released into the environment through discharge papillae [14]. Analogous invasion mechanisms are seen in chytrids parasitizing plants and algae e.g. Entophlyctis spp. [38], [39].
Figure 7. Schematic summary of the intracellular colonization process by Bd in amphibian skin.
(A) Germination of a zoospore cyst or germling is followed by the development of a germ tube that invades an epidermal cell; (B) at the end of the germ tube a swelling is formed, that gives rise to a new thallus; (C) cell contents of the germling migrate into the newly formed thallus; (D) the emptied germling evanesces; (E) the new intracellular thallus forms a rhizoid-like structure that extends to a deeper epidermal layer and develops a swelling at its end; (F) a new intracellular thallus is formed.
In addition, our observations indicate how colonization by Bd proceeds to deeper skin layers. It seems that older mother thalli develop rhizoid-like structures that spread to the deeper skin layers and form a swelling inside the cell. This swelling enlarges and gives rise to a new daughter thallus.
Genetic material of Bd was probably injected into epidermal cells in order to establish intracellular sporangia. As we did not observe any mitochondria or nuclei in the germ tubes additional observations are desirable. However, migration of lipid globules through these germ tubes into the host cell was seen on several occasions. Most probably the lipids function as a source of concentrated energy for the zoospores and as an energy source for the young thallus while it grows into an epidermal cell (pers. comm. J. Longcore).
Host induced morphological variation is peculiar in many chytrids [40], i.e. they exhibit morphological differences between their parasitic and saprophytic state and are able to change from an endobiotic growth to an epibiotic growth depending on nutrients and the substrate [38], [40]. In this perspective, our data suggest that the colonization strategy of Bd is host dependent. The extent of Bd invasion clearly differed between the 3 host species used: from near absence in X. laevis, to moderate in A. muletensis and high in L. caerulea. Moreover, the ability of Bd to enter amphibian skin and to spread in the skin, or its invasiveness, appears to coincide with the susceptibility of the studied species to chytridiomycosis, e.g. low in X. laevis [21], [28], [41], moderate in A. muletensis [23], [37] and high in L. caerulea [24]–[26]).
Why colonization is limited to epibiosis in X. laevis and what makes A. muletensis and L. caerulea more ‘receptive’ to Bd infection remains speculative. However, the influence of certain factors on the outcome of the experiments was minimized. In vitro colonization experiments were carried out under the same conditions. Prior to the isolation of skin explants A. mulentensis, L. caerulea and X. laevis frogs were washed to facilitate the handling of skin tissue and to reduce the risk of bacterial contamination. By washing, skin mucus and skin secretions were also partially removed. Especially X. laevis skin is covered with a prominent mucus layer and skin mucus in itself can be considered as a mechanical barrier and an obstacle for colonization. In addition, skin secretions containing e.g. antimicrobial peptides, antifungal metabolites, are thought to provide protection against chytridiomycosis. However, since the activity of residual skin secretions in skin explants is not yet studied, one must be cautious in assuming a reduced defensive action.
Consequently, we hypothesize that the degree of invasiveness of Bd and amphibian susceptibility to chytridiomycosis is determined by inherent characteristics of the host skin. However, more observations are required to draw definite conclusions about species susceptibility and pathogenesis patterns. The challenge ahead will be to identify which factors mediate these variations in the pathogenesis of Bd infections.
Materials and Methods
Experimental animals
Postmetamorphic wild type X. laevis were purchased from the European Xenopus Resources centre (Portsmouth, UK) and adult outbred X. laevis from Xenopus Express (Le Bourg, France). Subadult A. mulentesis and L. caerulea were captive bred. Upon arrival and before the start-up of all experiments skin swabs from all animals were examined for the presence of Bd by the quantitative PCR (qPCR) of Boyle et al. [42].
Bd strains and culture conditions
Inoculations were carried out with the virulent Bd strain IA042, a representative of the Bd global panzootic lineage [43], isolated from a dead Alytes obstetricans [44]. Cultures were maintained on tryptone/gelatine hydrolysate/lactose (TGhL) broth in 25 cm2 cell culture flasks at 20°C for 5 days. Two ml of a 5 days old broth culture were inoculated on TGhL agar and incubated for 5 to 7 days at 20°C. Zoospores were harvested by flooding the agar plates with 2 ml of distilled water and were immediately counted in lugol with a haemocytometer.
Isolation in vitro culture and infection of anuran skin
For a detailed study of the early interaction between zoospores and host epidermis, full- thickness epidermal (FTE) explants of A. mulentensis, L. caerulea and X. laevis were experimentally infected in an Ussing chamber based model. Isolation and treatment of FTE explants, in vitro culture and infection procedures have been described in detail by Van Rooij et al. [45].
Early interactions of Bd zoospores with amphibian epidermis and the in situ development of zoospores to sporangium were observed during 5 consecutive days in X. laevis and 3 days in A. muletensis and L. caerulea. Immediately after euthanasia with intracoelomically-injected T 61® (Intervet, Mechelen, Belgium) frogs are washed according to the protocol of Nishikawa et al. [46] to facilitate the handling of the tissue and to reduce the risk of contamination. Briefly, frogs were washed in plastic containers containing respectively 70% ethanol, Leibovitz L-15 medium 70% (3 times; Gibco, Life technologies Europe, Gent, Belgium), Ca2+/Mg2+-free Barth's solution (CMFB), 1.25 mM ethylenediaminetetraacetic acid (EDTA; Sigma, St. Louis, MO, USA) in CMFB for 5 min and 70% L15 medium (twice) at 4°C. FTE explants (10×25 mm for A. muletensis and 20×25 mm for L. caerulea and X. laevis) were excised and were mounted in an Ussing chamber (exposed surface area of 0.28 cm2 for A. muletensis and 1.07 cm2 for L. caerulea and X. laevis). From each donor animal a skin sample was tested for the presence of Bd by qPCR [42].
Explants were apically exposed to 7 ml inoculum (2.8×107 zoospores/ml distilled water). In the course of the subsequent 3 to 5-days incubation period at 20°C, skin samples were removed at 1 to 3–5 days post infection (dpi) and the exposed skin surface area was excised and processed for histology and transmission electron microscopy (TEM).
Additional experiments in skin of L. caerulea were performed under the same conditions as described above. In short, FTE skin explants were mounted in an Ussing chamber with an exposed surface area of 1.07 cm2. Explants were exposed to an inoculum of 2.8×107 zoospores/ml distilled water. In the course of a 24 hours incubation period at 20°C, skin samples were removed after 1, 2, 4, 8, 16 and 24 hours of exposure. The experiment was then repeated in triplicate, with sampling after 8, 16 and 24 hours of exposure.
For light microscopic studies, skin samples were fixed in 10% neutral buffered formalin and embedded in paraffin. Five µm sections were stained with haematoxylin and eosin (HE) and Gomori methenamine silver (GMS). For TEM, skin samples were fixed in 4% formaldehyde containing 1% CaCl2 (w/v) in 0.121 M Na-cacodylate adjusted to pH 7. The samples were washed and postfixed in 1% OsO4 (w/v). Subsequently the skin samples were dehydrated through a graded series of alcohol and embedded in LX-112 resin (Ladd Research Industries, Burlington, Vermont, USA). Semi-thin sections (2 µm) were cut and stained with toluidin blue to select regions for ultrathin sectioning (90 nm) with an ultratome (Ultracut E; Reichert-Jung, Nussloch, Germany). The ultrathin sections (90 nm) were stained with uranyl acetate and lead citrate solutions and examined under a JEOL EX II transmission electron microscope (JEOL Ltd, Zaventem, Belgium) at 80 kV. Measurements of all structures are given as (Min.)-Av.-(Max.), with Min. = minimum value for the measured collection of structures (n), Av. = average value and Max. = maximum value.
In vivo infection of A. muletensis, L. caerulea and X. laevis
To assess the validity of the in vitro results, A. mulentensis, L. caerulea and X. laevis frogs were experimentally infected. All animal experiments were approved by the Ethical Committee of the Faculty of Veterinary Medicine, Ghent University, Belgium (EC2008/120, EC2010/98). Experiments were performed following all necessary ethical and biosecurity standards.
For inoculation, respectively three subadult A. mulentensis, L. caerulea and X. laevis frogs were individually housed for 24 hours in plastic containers (18×13×4 cm) containing moistened paper tissue, terracotta flower-pots as shelter and a petri-dish filled with dechlorinated tap water for bathing. Frogs were inoculated by topical application of 2×106 zoospores/100 µl distilled water (Bd strain IA042). Twenty-four hours after exposure all frogs were transferred to fresh containers. X. laevis frogs were housed in plastic containers (32×17×21 cm), containing dechlorinated tap water (water depth 10 cm) at 20°C. Terracotta flower-pots were provided as shelter. Frogs were fed twice weekly with trout pellets (Skretting, Cheshire, UK). Water was changed three times weekly. A. muletensis and L. caerulea frogs were housed in plastic containers (18×13×4 cm), with moistened paper tissue, terracotta flower-pots as shelter and a petri-dish filled with dechlorinated tap water for bathing. Containers were regularly sprayed with dechlorinated tap water to maintain humidity. Tissue and bathing water was changed three times weekly. Ambient temperature varied between 19 and 20°C. A. muletensis and L. cearulea frogs were fed calcium powdered crickets, fruit flies (Drosophila melanogaster) or buffalo worms (Alphitobius laevigatus) ad libitum. The photoperiod of the experimental animal facilities followed natural ambient conditions (12–15 h light). At day 12 post inoculation all animals were sampled by passing a sterile synthetic swab (160 C, Copan Italia S.p.A., Brescia, Italy) along the pelvic region 10 times, fore- and hind limbs 5 times. Gloves were changed between handling of each animal. Swabs were examined for the presence of B. dendrobatidis by qPCR [42]. At day 14 post inoculation all animals were sacrificed by intracoelomically-injected T 61® (Intervet, Mechelen, Belgium). Skin samples were collected from the pelvic region, fixed in10% neutral buffered formalin, processed for histology and stained with HE and GMS.
Supporting Information
TEM image of negative control skin explant of Xenopus laevis . Negative control sample, incubated with distilled water during 5 days under the same conditions as skin explants exposed to Bd. zoospores; all cell layers are still intact; scale bar = 5 µm.
(TIF)
Acknowledgments
We gratefully acknowledge Christian Puttevils, Delphine Ameye and Dominique Jacobus for the preparation of histological and TEM slides with meticulous care. Strain IA042 was kindly provided by Trent Garner (Institute of Zoology, Zoological Society of London, London, UK) and Matthew Fisher (Imperial college, London, UK). We thank Joyce Longcore (Department of Biological Sciences, University of Maine, USA) and two anonymous referees for providing helpful comments that improved the manuscript considerably.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Funding for this work was provided to PVR by research grant BOF08/24J/004 from Ghent University. No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 2.Wake DB, Vredenburg VT. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA. 2008;105:11466–11473. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lötters S, Kielgast J, Bielby J, Schmidtlein S, Bosch J, et al. The link between rapid enigmatic amphibian decline and the globally emerging chytrid fungus. Ecohealth. 2009;6:358–372. doi: 10.1007/s10393-010-0281-6. [DOI] [PubMed] [Google Scholar]
- 4.Crawford AJ, Lips KR, Bermingham E. Epidemic disease decimates amphibian abundance, species diversity, and evolutionary history in the highlands of central Panama. Proc Natl Acad Sci USA. 2010;107:13777–13782. doi: 10.1073/pnas.0914115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger L, Speare R, Daszak P, Green DE, Cunningham AA, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pessier AP, Nichols DK, Longcore JE, Fuller MS. Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White's tree frogs (Litoria caerulea). J Vet Diagn Invest. 1999;11:194–199. doi: 10.1177/104063879901100219. [DOI] [PubMed] [Google Scholar]
- 7.Marantelli G, Berger L, Speare R, Keegan L. Distribution of the amphibian chytrid Batrachochytrium dendrobatidis and keratin during tadpole development. Pac Conservat Biol. 2004;10:173–179. [Google Scholar]
- 8.Berger L, Hyatt AD, Speare R, Longcore JE. Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Dis Aquat Organ. 2005;68:51–63. doi: 10.3354/dao068051. [DOI] [PubMed] [Google Scholar]
- 9.Voyles J, Berger L, Young S, Speare R, Webb R, et al. Electrolyte depletion and osmotic imbalance in amphibians with chytridiomycosis. Dis Aquat Organ. 2007;77:113–118. doi: 10.3354/dao01838. [DOI] [PubMed] [Google Scholar]
- 10.Voyles J, Young S, Berger L, Campbell C, Voyles WF, et al. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science. 2009;326:582–585. doi: 10.1126/science.1176765. [DOI] [PubMed] [Google Scholar]
- 11.Carver S, Bell BD, Waldman B. Does chytridiomycosis disrupt amphibian skin function? Copeia. 2010;3:487–495. [Google Scholar]
- 12.Marcum RD, St-Hilaire S, Murphy PJ, Rodnick KJ. Effects of Batrachochytrium dendrobatidis infection on ion concentrations in the boreal toad Anaxyrus (Bufo) boreas boreas. Dis Aquat Organ. 2010;91:17–21. doi: 10.3354/dao02235. [DOI] [PubMed] [Google Scholar]
- 13.Campbell CR, Voyles J, Cook DI, Dinudom A. Frog skin epithelium: Electrolyte transport and chytridiomycosis. Int J Biochem Cell Biol. 2012;44:431–434. doi: 10.1016/j.biocel.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longcore JE, Pessier AP, Nichols DK. Batrachochytrium dendrobatidis gen et sp nov, a chytrid pathogenic to amphibians. Mycologia. 1999;91:219–227. [Google Scholar]
- 15.Moss AS, Reddy NS, Dortaj IM, Francisco MJS. Chemotaxis of the amphibian pathogen Batrachochytrium dendrobatidis and its response to a variety of attractants. Mycologia. 2008;100:1–5. doi: 10.3852/mycologia.100.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Voyles J, Rosenblum EB, Berger L. Interactions between Batrachochytrium dendrobatidis and its amphibian hosts: a review of pathogenesis and immunity. Microbes Infect. 2011;13:25–32. doi: 10.1016/j.micinf.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Naglik JK, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Mol Biol Rev. 2003;67:400–428. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermout S, Tabart J, Baldo A, Mathy A, Losson B, et al. Pathogenesis of dermatophytosis. Mycopathologia. 2008;166:267–275. doi: 10.1007/s11046-008-9104-5. [DOI] [PubMed] [Google Scholar]
- 19.Rosenblum EB, Stajich JE, Maddox N, Eisen MB. Global gene expression profiles for life stages of the deadly amphibian pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci USA. 2008;105:17034–17039. doi: 10.1073/pnas.0804173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moss AS, Carty N, Francisco MJS. Identification and partial characterization of an elastolytic protease in the amphibian pathogen Batrachochytrium dendrobatidis. Dis Aquat Organ. 2010;92:149–158. doi: 10.3354/dao02223. [DOI] [PubMed] [Google Scholar]
- 21.Weldon C, du Preez LH, Hyatt AD, Muller R, Speare R. Origin of the amphibian chytrid fungus. Emerg Infect Dis. 2004;10:2100–2105. doi: 10.3201/eid1012.030804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.IUCN. 2011.1 IUCN Red List of Threatened Species. 2011. Available: http://www.iucnredlist.org. Accessed 2011 Jun 30.
- 23.Walker SF, Bosch J, James TY, Litvintseva AP, Oliver Valls JA, et al. Invasive pathogens threaten species recovery programs. Curr Biol. 2008;18:R853–R854. doi: 10.1016/j.cub.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 24.Berger L, Speare R, Skerratt LF. Distribution of Batrachochytrium dendrobatidis and pathology in the skin of green tree frogs Litoria caerulea with severe chytridiomycosis. Dis Aquat Organ. 2005;68:65–70. doi: 10.3354/dao068065. [DOI] [PubMed] [Google Scholar]
- 25.Woodhams DC, Ardipradja K, Alford RA, Marantelli G, Reinert LK, et al. Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses. Anim Conserv. 2007;10:409–417. [Google Scholar]
- 26.Berger L, Speare R, Marantelli G, Skerratt LF. A zoospore inhibition technique to evaluate the activity of antifungal compounds against Batrachochytrium dendrobatidis and unsuccessful treatment of experimentally infected green tree frogs (Litoria caerulea) by fluconazole and benzalkonium chloride. Res Vet Sci. 2009;87:106–10. doi: 10.1016/j.rvsc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher MC. Endemic and introduced haplotypes of Batrachochytrium dendrobatidis in Japanese amphibians: sink or source? Mol Ecol. 2009;18:4731–4733. doi: 10.1111/j.1365-294X.2009.04385.x. [DOI] [PubMed] [Google Scholar]
- 29.Hill WA, Newman SJ, Craig L, Carter C, Czarra J, et al. Diagnosis of Aeromonas hydrophila, Mycobacterium species, and Batrachochytrium dendrobatidis in an African Clawed Frog (Xenopus laevis). J Am Assoc Lab Anim. 2010;49:215–220. [PMC free article] [PubMed] [Google Scholar]
- 30.Solis R, Lobos G, Walker SF, Fisher M, Bosch J. Presence of Batrachochytrium dendrobatidis in feral populations of Xenopus laevis in Chile. Biol Invasions. 2010;12:1641–1646. [Google Scholar]
- 31.Ramsey JP, Reinert LK, Harper LK, Woodhams DC Rollins-Smith. Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African Clawed Frog, Xenopus laevis. Infect Immun. 2010;78:3981–3992. doi: 10.1128/IAI.00402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rollins-Smith LA, Conlon JM. Antimicrobial peptide defenses against chytridiomycosis, an emerging infectious disease of amphibian populations. Dev Comp Immunol. 2005;29:589–598. doi: 10.1016/j.dci.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Rollins-Smith LA. The role of amphibian antimicrobial peptides in protection of amphibians from pathogens linked to global amphibian declines. BBA. 2009;1788:1593–1599. doi: 10.1016/j.bbamem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Becker MH, Brucker RM, Schwantes CR, Harris RN, Minbiole KPC. The bacterially produced metabolite violacein is associated with survival of amphibians infected with a lethal fungus. Appl Environ Microb. 2009;75:6635–6638. doi: 10.1128/AEM.01294-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garner TWJ, Perkins MW, Govindarajulu P, Seglie D, Walker S, et al. The emerging amphibian pathogen Batrachochytrium dendrobatidis globally infects introduced populations of the North American bullfrog, Rana catesbeiana. Biol Lett. 2006;2:455–459. doi: 10.1098/rsbl.2006.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green DE, Dodd KC. Presence of amphibian chytrid fungus Batrachochytrium dendrobatidis and other amphibian pathogens at warm water fish hatcheries in Southeastern North America. Herpetol Conserv Biol. 2007;2:43–47. [Google Scholar]
- 37.Martel A, Van Rooij P, Vercauteren G, Baert K, Van Waeyenberghe L, et al. Developing a safe antifungal treatment protocol to eliminate Batrachochytrium dendrobatidis from amphibians. Med Mycol. 2011;49:143–149. doi: 10.3109/13693786.2010.508185. [DOI] [PubMed] [Google Scholar]
- 38.Longcore JE. Morphology and zoospore ultrastructure of Entophlyctis luteolus sp-nov (Chytridiales) - Implications for chytrid taxonomy. Mycologia. 1995;87:25–33. [Google Scholar]
- 39.Shin W, Boo SM, Longcore JE. Entophlyctis apiculata, a chytrid parasite of Chlamydomonas sp (Chlorophyceae). Can J Bot. 2001;79:1083–1089. [Google Scholar]
- 40.Barr DJS. McLaughlin DJ, McLaughlin EG, Lemke PA, editors. Chytridiomycota. 2001. pp. 93–112. The Mycota VII, Part A, Systematics and evolution. Heidelberg: Springer.
- 41.Kielgast J, Rödder D, Veith M, Lötters S. Widespread occurrence of the amphibian chytrid fungus in Kenya. Anim Conserv. 13, issue. 2010. pp. 36–43.
- 42.Boyle DG, Boyle DB, Olsen V, Morgan JA, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ. 2004;60:141–148. doi: 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- 43.Farrer RA, Weinert LA, Bielby J, Garner TWJ, Balloux F, et al. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc Natl Acad Sci USA. 2011;108:18732–18736. doi: 10.1073/pnas.1111915108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher MC, Bosch J, Yin Z, Stead DA, Walker J, et al. Proteomic and phenotypic profiling of the amphibian pathogen Batrachochytrium dendrobatidis shows that genotype is linked to virulence. Mol Ecol. 2009;18:415–429. doi: 10.1111/j.1365-294X.2008.04041.x. [DOI] [PubMed] [Google Scholar]
- 45.Van Rooij P, Martel A, Brutyn M, Maes S, Chiers K, et al. Development of in vitro models for a better understanding of the early pathogenesis of Batrachochytrium dendrobatidis infections in amphibians. Altern Lab Anim. 2010;38:519–528. doi: 10.1177/026119291003800614. [DOI] [PubMed] [Google Scholar]
- 46.Nishikawa A, Shimizu-Nishikawa K, Miller L. Isolation, characterization, and in vitro culture of larval and adult epidermal cells of the frog Xenopus laevis. In Vitro Cell Dev Biol. 1990;26:1128–1134. doi: 10.1007/BF02623689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TEM image of negative control skin explant of Xenopus laevis . Negative control sample, incubated with distilled water during 5 days under the same conditions as skin explants exposed to Bd. zoospores; all cell layers are still intact; scale bar = 5 µm.
(TIF)