Abstract
Background:
Implant-associated infections contribute to patient morbidity and health care costs. We hypothesized that surface modification of titanium fracture hardware with vancomycin would support bone-healing and prevent bacterial colonization of the implant in a large-animal model.
Methods:
A unilateral transverse mid-diaphyseal tibial osteotomy was performed and repaired with a titanium locking compression plate in nine sheep. Four control animals were treated with an unmodified plate and five experimental animals were treated with a vancomycin-modified plate. The osteotomy was inoculated with 2.5 × 106 colony-forming units of Staphylococcus aureus. The animals were killed at three months postoperatively, and implants were retrieved aseptically. Microbiologic and histologic analyses, scanning electron and confocal microscopy, and microcomputed tomography were performed.
Results:
All animals completed the study. Compared with the treatment cohort, control animals exhibited protracted lameness in the operatively treated leg. Gross findings during necropsy were consistent with an infected osteotomy accompanied by a florid and lytic callus. Microcomputed tomography and histologic analysis of the tibiae further supported the presence of septic osteomyelitis in the control cohort. Thick biofilms were also evident, and bacterial cultures were positive for Staphylococcus aureus in three of four control animals. In contrast, animals treated with vancomycin-treated plates exhibited a healed osteotomy site with homogenous remodeling, there was no evidence of biofilm formation on the retrieved plate, and bacterial cultures from only one of five animals were positive for Staphylococcus aureus.
Conclusions:
Vancomycin-derivatized plate surfaces inhibited implant colonization with Staphylococcus aureus and supported bone-healing in an infected large-animal model.
Clinical Relevance:
Binding of vancomycin to the surface of implants holds great promise in helping to reduce protracted and recalcitrant implant-associated infections in orthopaedic patients.
Implant-associated infections are a major cause of fixation failure in orthopaedics and result in increased morbidity, mortality, and cost. Despite improved strategies aimed at eradicating implant-associated infections, approximately 5% to 10% of internal fixation devices become infected, with an even higher prevalence of infection after treatment of open fractures and after revision arthroplasty1-5. In the latter cases, the low vascularity at the implant placement site as well as rapid adhesion of serum proteins to the metal surface create an ideal environment for bacterial adhesion and biofilm formation6-9. Bacteria become enclosed in a complex biofilm matrix that protects them from both the host immune response and systemic antimicrobial agents10.
A number of surgical strategies, including extensive local tissue debridement and prolonged systemic antimicrobial therapy, have been devised to avoid the need for removal of the implant when infection develops. In addition, systems for local antibiotic delivery have been developed for the treatment and prevention of orthopaedic infections; these systems include use of polymethylmethacrylate cement (PMMA), use of biodegradable polymers, and regional limb perfusion11-15. The elution kinetics of these carrier systems are characterized by an initially supratherapeutic antibiotic release that often drops below the minimal inhibitory concentration (MIC) toward the end of the elution period. Unfortunately, antimicrobial concentrations below the MIC favor emergence of resistant bacterial populations, further complicating successful resolution of the infection. Furthermore, local antimicrobial therapies inefficiently penetrate established biofilms, thereby triggering changes in quorum sensing and gene expression that further contribute to antimicrobial resistance16. In the case of PMMA, the porous, nondegradable carrier matrix itself may also serve as a substrate for bacterial adhesion and subsequent biofilm formation17,18.
We previously tethered antimicrobial compounds to titanium implant surfaces to address the need to prevent bacterial adhesion and biofilm formation. We showed that vancomycin tethered to titanium alloys prevented bacterial adhesion, proliferation, and biofilm formation in vitro. This permanent bactericidal surface was stable, maintained its activity in bodily fluids, and inhibited bacterial colonization without leading to the development of antimicrobial resistance19-22. In the present study, we extended our experiments from model materials to clinically relevant orthopaedic implants to examine the utility of these engineered surfaces. Our hypothesis was that vancomycin covalently bonded to orthopaedic trauma locking compression plates made of commercially pure titanium would support bone-healing and prevent bacterial implant colonization in a large-animal model.
Materials and Methods
Surface Derivatization of the Plate
Determination of Optimum Titanium Surface Treatments for Vancomycin Tethering
Prior to beginning the in vivo evaluation, it was necessary to assess which surface treatments would yield an implant surface that was most amenable to tethering vancomycin to the titanium. Four different surface treatments were evaluated with the use of slugs milled from grade-4 commercially pure titanium stock (Synthes, West Chester, Pennsylvania): (1) no finishing treatment, resulting in a control titanium surface; (2) treatment with nitrohydrofluoric acid; (3) treatment with nitrohydrofluoric acid followed by anodization with gold Ti 101, a standard treatment for titanium orthopaedic implants23; and (4) treatment with nitrohydrofluoric acid, anodization with gold Ti 101, and reimmersion in the nitrohydrofluoric acid bath to remove the anodized surface.
Vancomycin was covalently bonded to these four surfaces, and the distribution of surface-bound vancomycin was visualized by immunofluorescence (Invitrogen anti-vancomycin Alexa Fluor 488) and imaged by confocal laser scanning microscopy (FluoView 300; Olympus America, Center Valley, Pennsylvania). The surface was also imaged with a scanning electron microscope (SEM). The antimicrobial activity of the surface was evaluated by challenging it with 104 colony-forming units (CFUs) of Staphylococcus aureus strain ATCC 25923, followed by visualization of the adherent bacteria with use of the LIVE/DEAD BacLight Bacterial Viability Kit (Invitrogen). The number of adherent bacteria was determined by plating bacterial sonicate samples at 1:10, 1:100, and 1:1000 dilutions. Assays, including positive controls (bacterial challenge without coating) and negative controls (no bacterial challenge) for each surface derivatization, were executed in triplicate.
Vancomycin Bonding to Titanium
Each titanium plate (Locking Compression Plate; Synthes) was autoclaved three times for thirty minutes each at 121°C to passivate it by hydrothermal aging24. The passivated plate was washed with dimethylformamide (DMF) and anhydrous toluene, aminopropylated with 5% aminopropyltriethoxysilane (APTS) in anhydrous toluene under argon, and incubated in the dark with agitation for sixteen hours at room temperature. The plate was then washed three times with anhydrous toluene and sonicated three times for five minutes each with DMF to remove any excess, noncovalently bound APTS. Two fluorenylmethyloxycarbonyl chloride-aminoethoxyethoxyacetate (FMOC-AEEA) linkers in DMF were sequentially coupled to the surface after activation by O-(7-azabenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU) and 1% diisopropylethylamine (DIEA). This was followed by deprotection with 20% piperidine in DMF for thirty minutes, yielding an AEEA-AEEA-APTS-Ti surface. A fourfold molar excess of vancomycin was then coupled to this surface in the presence of HATU and DIEA as described above to yield a vancomycin-AEEA-AEEA-APTS-Ti surface. The plate was then incubated for twenty-four hours in phosphate-buffered saline solution (PBS) to elute any noncovalently bound vancomycin, washed multiple times with deionized water, and stored in deionized water until implantation.
For the in vivo study, derivatization of the plate surface was timed with respect to the surgical procedure to mitigate possible variations due to shelf life. A set of plates underwent surface preparation in parallel, and in vitro verification of the quality of the vancomycin bonding was performed on plates not destined for implantation to ensure that each animal received a vancomycin-modified plate with a validated surface derivatization. The locking head screws (LHS; Synthes), which were made of TAN titanium alloy (Ti-Al6-7Nb), were not coated prior to use in the in vivo study.
Animal Model
We previously developed a sheep infection model and validated it in terms of reproducibility25,26. This model recapitulated the clinical signs of osseous infection, including pain, soft-tissue swelling, osteomyelitis, and delayed osseous union, following a long-bone fracture (see Appendix).
Surgical Procedure
Nine mature (2.5 to 3.5-year-old) Dorset-cross ewes were used for this study, which was approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Perioperative analgesia in all sheep consisted of flunixin meglumine (1.1 mg/kg), buprenorphine hydrochloride (0.01 mg/kg), and transdermal fentanyl patches (2.5 μg/kg). One intramuscular dose of preoperative antimicrobials consisting of 5 mg/kg ceftiofur sodium and 22,000 IU/kg procaine penicillin was given to all animals. General anesthesia was induced and maintained with a mixture of isoflurane in oxygen administered through a standard small-animal, semi-closed circle system.
The animal was placed in left lateral recumbency, and a medial skin incision was made extending from the level of the tibial tuberosity distal to the level of the medial malleolus. The underlying fascia and periosteum were incised at the mid-diaphysis and a 1-cm section was circumferentially elevated. A sample plate was fitted to the medial aspect of the diaphysis, and the most proximal and distal screw holes were predrilled. The plate was removed and a transverse mid-diaphyseal tibial osteotomy was performed. The osteotomy was created under constant copious irrigation with use of a Synthes Large Battery Drive fitted with a standard oscillating saw and blade (50 × 27 × 0.6 mm) to yield an osteotomy gap of 0.6 mm. The osteotomy was reduced and repaired with a titanium plate. The five osteotomies in the treatment cohort were repaired with a vancomycin-modified plate, and the four osteotomies in the control animals were repaired with an uncoated plate. The modified plates had been treated with nitrohydrofluoric acid, anodized with gold Ti 101, and then reimmersed in the nitrohydrofluoric acid bath to remove the anodized surface prior to derivatization with vancomycin. All plate holes were filled with bicortical locking-head screws.
Prior to closure, a temporary 14-gauge silicone catheter was placed parallel to the plate, with its tip at the osteotomy site, and run out the proximal aspect of the incision. After closure of all soft-tissue layers, the plate was inoculated with 2.5 mL of saline solution containing 106 CFU/mL of Staphylococcus aureus via the indwelling catheter. The catheter was removed and the site was sealed with stainless steel staples and tissue glue. An impervious wound dressing was applied and the animal was taken to the recovery area, where a light bivalve fiberglass full-limb splint was placed for recovery from general anesthesia.
Preparation of Bacteria
Before surgery, bacteria were passaged into 5 mL of trypticase soy broth supplemented with 1.5% dextrose and incubated at 37°C for sixteen hours. The bacterial suspensions were pelleted, washed, and resuspended in saline solution at a concentration of 1 × 106 CFU/mL as determined by comparison with a 0.5 McFarland standard. Aliquots containing 2.5 mL of this bacterial dilution were stored on ice prior to inoculation.
Preoperative Clinical Pathologic Evaluation
A venous blood sample was collected for routine preoperative hematologic analysis and measurements of fibrinogen and serum chemistry (performed by the Clinical Diagnostic Laboratory at the University of Pennsylvania’s New Bolton Center). The fibrinogen level was analyzed with use of a HemosIL* PT-Fibrinogen Kit (Beckman Coulter, Brea, California)25-29.
Postoperative Evaluation
Clinical Care
Sheep were monitored during the postoperative period by a veterinary surgeon. Clinical evaluation included daily examinations for general demeanor, appetite, fever, pain, swelling and/or discharge at the incision, and amount of weight-bearing of the operatively treated limb. The fibrinogen level was analyzed at weekly intervals, as described above, until the animal was killed.
In Vivo Digital Radiography
Radiographs (Sound-Eklin Digital X-Ray, Carlsbad, California) of the operatively treated tibia in the standing animal were obtained immediately postoperatively and at thirty-day intervals until the animal was killed. Standard craniocaudal and lateral views of the operatively treated tibia were used to evaluate the degree of periosteal reaction, degree of callus formation, and presence or absence of radiographic signs consistent with infection. Assessment of the radiographs was performed by three blinded observers who graded the images for radiographic signs of infection and evaluation of fracture-healing according to a semiquantitative scoring system (see Appendix)30,31.
Postmortem Evaluation
Ex Vivo Microcomputed Tomography
The osteotomy site was fixed in 10% formalin and analyzed by microcomputed tomography (microCT) (μCT 80; Scanco Medical, Brüttisellen, Switzerland) using an isotropic voxel resolution of 50 μm, x-ray energy of 55 kVp, and current of 145 μA at medium resolution. The scan time ranged from approximately 3.0 to 3.9 hours per sample to acquire 1024 projections per 180° with a 2048-detector CCD (charge coupled device) array. A calibration phantom of hydroxyapatite was used to account for variations in the CT-derived electron density of each voxel over time. Segmented images were obtained with use of a Gaussian filter with sigma = 1.0, support = 0.8, and threshold = 300. The resulting three-dimensional images serve to demonstrate differences in the morphology of the osteotomy site between the two study cohorts.
Explantation Procedure
The sheep were killed at three months postoperatively with use of an intravenous overdose of barbiturate. An aseptic dissection was performed and the soft tissues were inspected for gross evidence of inflammation and infection. The gross pathologic appearance of the surgical site was evaluated with use of a semiquantitative scoring system (see Appendix).
A sterile bacterial culture sample was obtained at the level of the osteotomy after implant removal. Using an aseptic technique, the osteotomy site was swabbed and the inoculum was processed according to standard protocols for diagnostic microbiology at the Clinical Microbiology Laboratory at the University of Pennsylvania’s New Bolton Center. Briefly, preliminary bacterial growth was determined with use of trypticase soy agar32. Columbia CNA agar with blood was used for selective isolation of gram-positive cocci. The specific strain of Staphylococcus aureus was not verified, as contamination of cultures is more typically by Enterococcus faecalis than by staphylococci in our clinical experience in a large animal hospital with a high orthopaedic caseload.
Following bacterial culture sample collection, the plate was sectioned into 15-mm pieces and prepared for biofilm evaluation by SEM. The plate pieces were fixed in 4% paraformaldehyde for two hours, dehydrated in a series of increasingly concentrated ethanol solutions (10% to 100%), and placed in Freon 113 until completely dry. The pieces were then sputter-coated and viewed at ×1800 and ×4000. The distribution of surface-bound vancomycin was determined by immunofluorescence and confocal laser microscopy as described above.
Histology
The osteotomy region was then processed for nondecalcified histology and embedded in methylmethacrylate (MMA). Specimen blocks were cut in the sagittal plane at a section thickness of 5 to 8 μm with use of a rotary microtome (Leica Microsystems, Nussloch, Germany) and mounted (ColorFrost Plus; Thermo Fisher Scientific, Kalamazoo, Michigan). All samples were stained with Harris hematoxylin and eosin and von Kossa stain (Poly Scientific, Bay Shore, New York) to assess the morphology of the soft-tissue envelope and the tissue within the osteotomy region. Toluidine blue 2% at pH 2.5 was used to evaluate the degree of osseous healing at the level of the osteotomy. Brown and Brenn stains were used to determine whether bacteria were present within the bone. The histologic evaluation was performed by a blinded veterinary pathologist at three months postoperatively for the safety studies and at one month postoperatively for the efficacy studies. The evaluation utilized a semiquantitative grading scale that evaluated callus architecture, the degree of the tissue remodeling response, cellular infiltration, and the presence of bacteria (see Appendix).
Statistical Analysis
The raw scores from the subjective and objective measurements obtained by blinded observers for gross pathology, radiography, and histology were converted to a point scale on which the lowest sum equaled the best result. Interobserver agreement was determined, and statistical analysis was performed. Radiographic scores at one and three months postoperatively and histologic scores were analyzed with use of the point scale grading systems (see Appendix) and reported as the mean and the standard deviation. The Wilcoxon-Mann-Whitney signed rank test was used to assess the significance of differences between the vancomycin and control cohorts. A p value of <0.05 was considered significant for all comparisons. A post hoc analysis demonstrated that the sample size yielded 90% power to detect a significant difference between the cohorts.
Source of Funding
This work was supported by the Grayson Jockey Club Research Foundation, the National Institutes of Health (NIH grants DE-13319, DE-10875, AR-051303, DE-019901, and HD-061053), and the Department of Defense (grant DAMD17-03-1-0713).
Results
Surface Derivatization of the Plate
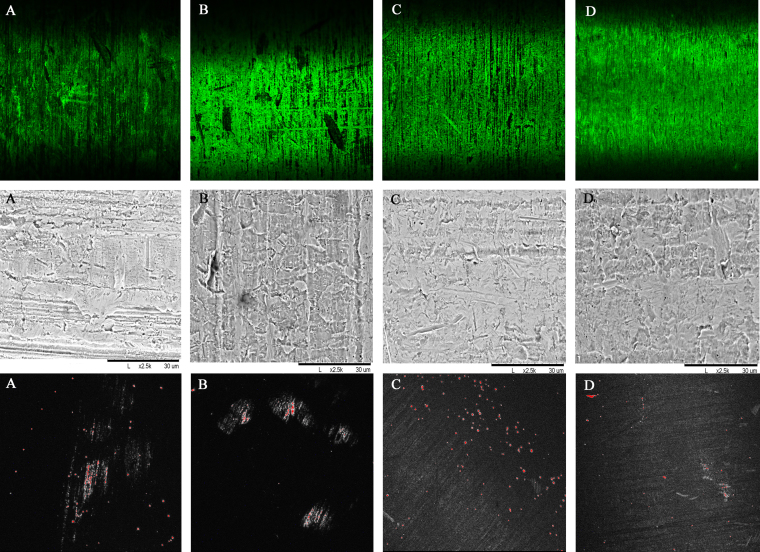
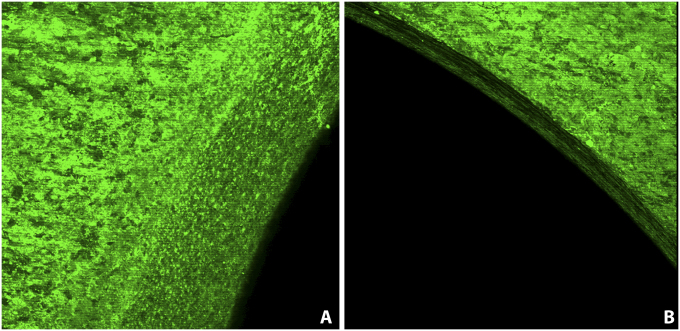
Immunostaining with anti-vancomycin antibody showed abundant and homogenous fluorescence on the surface of the titanium plate treated with nitrohydrofluoric acid, anodized with gold Ti 101 and then reimmersed in the nitrohydrofluoric acid bath, and a smooth surface topography that successfully resisted colonization was apparent in the SEM images after the bacterial challenge assay (Fig. 1, panels labeled “D”). Using this same optimized surface preparation protocol, we successfully derivatized all titanium plate surfaces with vancomycin for the in vivo study. The anti-vancomycin immunofluorescence staining and the LIVE/DEAD BacLight Bacterial Viability Kit confirmed that the surface was homogenously covered with vancomycin, the antimicrobial coating was stable and active, and there was minimal disturbance of the titanium surface (Fig. 2 and Appendix).
Fig. 1.
The top row shows immunofluorescent staining and confocal laser imaging of four different surface modifications of titanium slugs, confirming vancomycin tethering (Invitrogen anti-vancomycin Alexa Fluor 488, ×10). The middle row shows corresponding scanning electron microscope (SEM) images depicting surface topography of the slugs with the four different surface treatments (×2500). The bottom row shows bacteria stained for viability with the LIVE/DEAD BacLight Bacterial Viability Kit (×10). Live bacteria fluoresce green whereas bacteria with damaged cell membranes fluoresce red, indicating lack of viability. Note that surface modification 4 (panels labeled D) consistently achieved the best results, with homogenous vancomycin tethering in the top row, the smoothest surface topography in the middle row, and the least amount of bacterial adhesion in the bottom row.
Fig. 2.
Immunofluorescent staining and confocal laser imaging confirming vancomycin tethering on two regions of the plate (anti-vancomycin Alexa Fluor 488, ×10). Intense green fluorescence indicates uniform tethering of vancomycin. Vancomycin tethering is also demonstrated on the screw holes in the plate shaft.
Surgical Procedure
All surgical procedures and recovery from general anesthesia were uneventful, and no animals were lost to infection or perioperative complications. The decision to administer one systemic dose of perioperative antimicrobial agents via the indwelling intravenous catheter is supported by our previous animal model development studies, in which we observed that a single perioperative dose of the antimicrobial agent attenuated the clinical side effects (high fever and lethargy) caused by the bacterial inoculum administered during surgery. However, the dose regimen would not be expected to impact the infection status at the surgical site.
Postoperative Evaluation
Clinical Care
Animals in the control cohort had a clear tendency to exhibit postoperative lameness in the operatively treated leg compared with animals in the treatment cohort. The lameness was categorized as non-weight-bearing during the first few postoperative days and then decreased to toe-touch lameness (with the animal setting the toes on the ground but reluctant to place the heel). Although these findings corroborated the radiographic, gross pathologic, and histologic data, they represent subjective clinical scores obtained during daily physical examinations. Control sheep also displayed an overall mild to moderate hyperfibrinogenemia compared with sheep in the vancomycin cohort. No signs of systemic infection were evident in any of the sheep in either cohort, and all appeared bright and alert with good appetites. The surgical wounds healed without visible signs of infection or the formation of draining tracts.
Radiography
Radiographic follow-up examinations in the control cohort showed progressive signs of cortical thinning and disruption, widening of the medullary canal, periosteal reaction, and osteolysis consistent with bacterial osteomyelitis. Overall, the animals in the treatment cohort showed signs of normal bone-healing with smooth, bridging remodeling of the osteotomy (Figs. 3-A and 3-C).
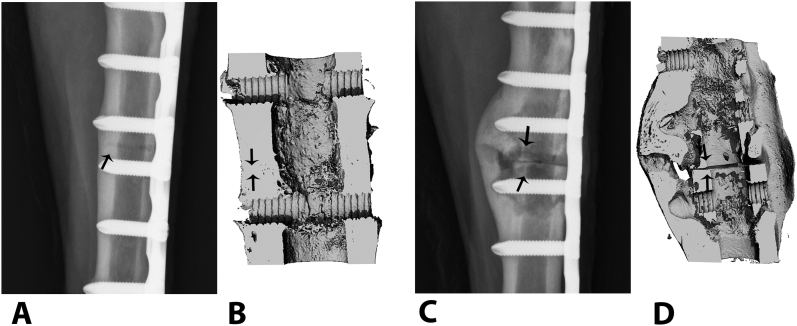
Fig. 3.
Osteotomies repaired with vancomycin-treated (Figs. 3-A and 3-B) and untreated, control plates (Figs. 3-C and 3-D). The representative craniocaudal digital radiograph of the vancomycin-treated animal made ninety days after osteotomy (Fig. 3-A) is consistent with normal bone-healing and callus formation at the osteotomy site (arrow), but the control (Fig. 3-C) shows progressive signs of cortical thinning, periosteal disruption, and osteolysis consistent with septic osteomyelitis at the osteotomy site (arrows). Three-dimensional reconstructed microcomputed tomography views of the same osteotomy sites in the vancomycin-treated (Fig. 3-B) and control animals (Fig. 3-D) show persistence of the osteotomy gap (arrows) in the control, with a poorly organized lytic callus, enlarged medullary canal, and cortical thinning.
Postmortem Evaluation
Microcomputed Tomography
The three-dimensional reconstructed microCT images from both cohorts supported the clinical and gross observations. Compared with the treatment cohort, there was a tendency for less advanced healing of the osteotomy sites in the control cohort as evidenced by a poorly organized, lytic callus with an expanded medullary canal and persistence of the osteotomy (Figs. 3-B and 3-D).
Explantation and Gross Pathology
No implant failure occurred. Aseptic dissection and gross evaluation of the osteotomy region and associated soft tissues suggested a preponderance of infection in all of the control animals. One control animal had a completely unstable osteotomy with frank pus, whereas the remaining control animals presented with a grossly stable osteotomy encased in a large, irregular, and florid callus containing necrotic debris with microabscesses (Fig. 4-D). The presence of infection was supported by the bacterial cultures from the callus region, which were positive for Staphylococcus aureus in three of four control animals (see Appendix). A pure growth of Staphylococcus aureus was achieved in all of these animals. In the treatment cohort, all explanted tibiae were healed and had a callus that was more uniformly smooth and remodeled, and four of five animals yielded no bacterial growth from culture swabs obtained from the osteotomy site. The adjacent soft-tissue envelope appeared grossly healthy (Fig. 4-A).
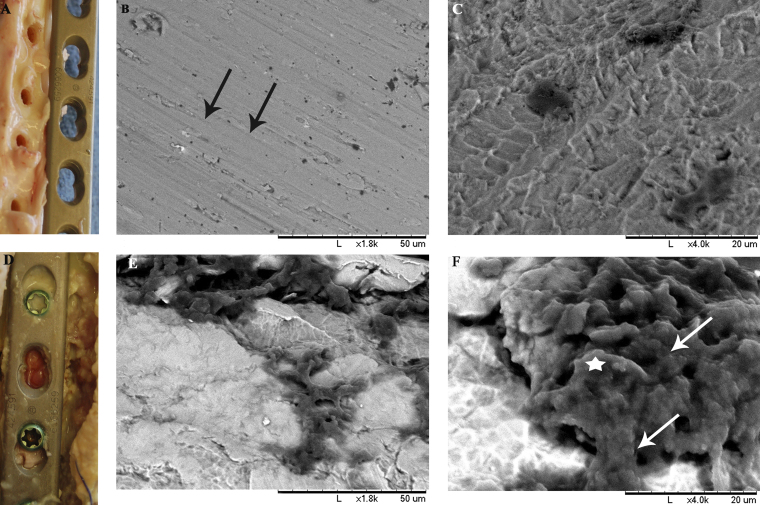
Fig. 4.
Representative gross pathologic photograph of the surgical site in an animal in the vancomycin-treated cohort at ninety days postoperatively, during explantation and tissue harvesting (Fig. 4-A), demonstrates a normal soft-tissue envelope with little to no necrotic debris. In contrast, a photograph of the surgical site in an animal in the control cohort (Fig. 4-D) demonstrated evidence of septic osteomyelitis accompanied by abscess formation about the osteotomy site and microabscesses along the plate. Representative scanning electron microscope (SEM) images of plates at ×1800 (Figs. 4-B and 4-E) and ×4000 (Figs. 4-C and 4-F) demonstrate the degree of biofilm formation in vivo. No biofilm formation or bacterial colonies are visible on the plates from the treatment cohort (Figs. 4-B and 4-C), and normal machining lines can be noted on the plate surface (Fig. 4-B, black arrows). In contrast, the plates from the control cohort are covered with colonies of coccoid bacteria (Fig. 4-F, white arrows) that are encased in an extracellular matrix (Fig. 4-F, white star).
Histology, SEM, and Anti-Vancomycin Staining
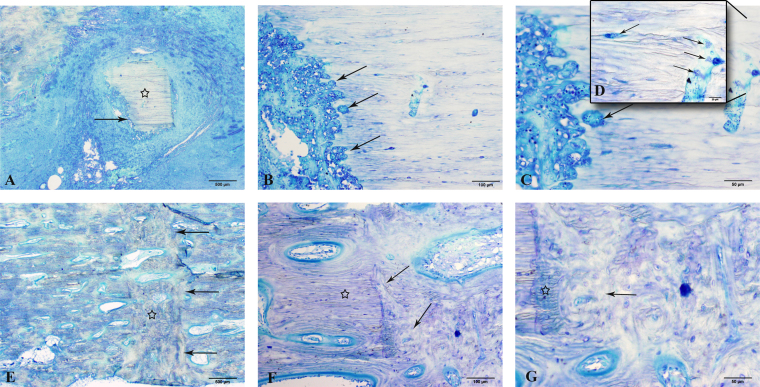
The gross pathologic and radiographic findings during tissue harvesting were further supported by examination of the histology of the callus at the osteotomy region with use of the semiquantitative scoring systems (see Appendix). There was a tendency for less advanced healing at the osteotomy site in control animals, with increased size and overall disorganization of the callus, less remodeling, increased cortical and trabecular osteolysis, and endosteal and medullary inflammation. The main observations in the control cohort were inflammatory aggregates of necrotic neutrophils both within the medullary space and concentrated at the plated cortex extending through fibrous tissue in the osteotomy gap; consequently, the animals in the treatment cohort achieved a lower overall score (p = 0.015) compared with the control cohort (Appendix and Fig. 5). In addition, endosteal callus was not present in the control animals; this was a hallmark difference between the treatment and control cohorts and was also associated with the presence of osteomyelitis. Moreover, the osteotomy site in the control animals was lytic, containing multiple fragments of necrotic bone (Figs. 5-A, 5-B, and 5-C). Large clusters of bacteria were evident within the canaliculi and haversian canals of dead bone that opened to the osteotomy site (Figs. 5-C and 5-D). In contrast, tibiae from animals in the vancomycin cohort presented with bridging callus and a remodeling osteotomy (Figs. 5-E, 5-F, and 5-G). The key characteristic morphologic findings in the treatment cohort included various degrees of bridging callus consisting of both lamellar and woven bone admixed with islands of hyaline cartilage and fibrovascular tissue (Fig. 5-E). The inflammatory cell infiltrate at the osteotomy site in the treatment cohort was predominantly lymphocytes and plasma cells, with only occasional neutrophils (Figs. 5-F and 5-G). In summary, radiographic, gross pathologic, and histologic scores of animals in the treatment cohort were significantly lower compared with the control cohort (Table I), supporting our clinical observation of normal fracture-healing (Fig. 3).
Fig. 5.
Representative photomicrographs of calcified histologic sections, comparing fracture-healing at three months after osteotomy in the control and vancomycin cohorts at ×2 (Figs. 5-A and 5-E), ×10 (Figs. 5-B and 5-F), ×20 (Figs. 5-C and 5-G), and ×40 (Fig. 5-D) magnification. The controls (Figs. 5-A through 5-D) had evidence of bone sequestrum formation (Fig. 5-A, star) with inflammatory aggregates of necrotic neutrophils resulting in cortical lysis (Figs. 5-A and 5-B, arrows) at the level of the osteotomy and multiple colonies of bacterial clusters (Figs. 5-C and 5-D, arrows), consistent with a delayed union. In comparison, vancomycin-treated plates had uniform woven bone formation (Fig. 5-E, star) across the osteotomy gap (Fig. 5-E, arrows), with formation of bridging callus consisting of both lamellar bone (Figs. 5-F and 5-G, star) and woven bone (Figs. 5-F and 5-G, arrows). Further evidence of normal bone resorption and haversian remodeling is present at the interface between the cortex and the osteotomy gap, consistent with normal fracture-healing (Fig. 5-G, arrow and star).
TABLE I.
Outcome Scores
| Score* | Treatment Cohort† | Control Cohort† | P Value |
| Radiographic, 1 month postop. | 1.46 ± 0.299 | 3.1 ± 0.32 | 0.016 |
| Radiographic, 3 months postop. | 0.33 ± 0.23 | 1.24 ± 2.06 | 0.90 |
| Gross pathologic | 0 ± 0 | 1.25 ± 0.57 | 0.014 |
| Histologic | 0.52 ± 0.16 | 2.05 ± 0.48 | 0.015 |
According to the semiquantitative scoring system in the Appendix. A low score was more desirable and consistent with normal fracture-healing, whereas a high score was reflective of osteomyelitis.
The values are given as the mean and the standard deviation.
SEM visualization of plates from the control cohort revealed large areas of bacterial aggregates covering 60% to 80% of the plate surface. These characteristic grapelike clusters were associated with an abundant biofilm, which was characterized by thick carpets of sessile bacteria and covered by extracellular matrix that appeared similar to the exopolysaccharide matrix described in the literature (Figs. 4-E and 4-F)33,34. Plates from the treatment cohort showed clean surfaces with grooves resulting from machining (Figs. 4-B and 4-C). Sporadic small clusters of planktonic cocci were visible, especially adjacent to screw holes.
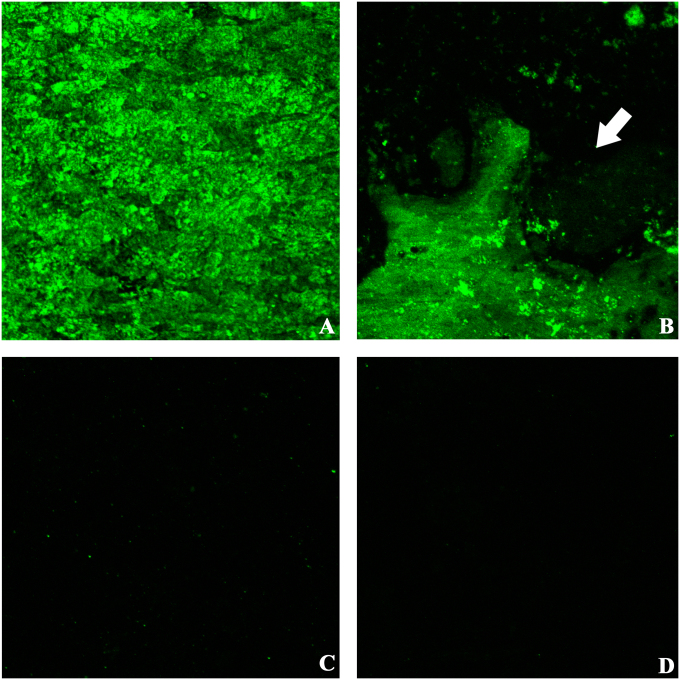
Anti-vancomycin immunostaining of explanted plates confirmed the presence of vancomycin on the surface of the derivatized plates, whereas the control plates did not have any uptake of stain (Figs. 6-C and 6-D). The derivatized plates exhibited patchy, uneven immunofluorescence on the undersurface of the plate in contact with bone, with some areas exhibiting little or no evidence of anti-vancomycin staining (Figs. 6-A and 6-B).
Fig. 6.
Immunofluorescent staining and confocal laser imaging of explanted plates to assess vancomycin stability and biofilm formation (anti-vancomycin Alexa Fluor 488, ×10). Fig. 6-A Intact top surface of a vancomycin-treated plate. Fig. 6-B Patchy fluorescence on the undersurface of the plate (arrow). Figs. 6-C and 6-D Absence of anti-vancomycin staining on the top and bottom, respectively, of a control plate.
Discussion
The goal of this investigation was to link vancomycin to titanium plates, using our previously published technology, and to assess the utility of this system in combating infection in a large-animal osteotomy model. Although the number of animals was small, our results lend considerable strength to the notion that the metal-antimicrobial hybrid provides a new and exciting approach to preventing or limiting implant-associated infection in orthopaedic surgery.
In many preclinical studies, a major challenge in translating novel treatment strategies from concept to clinical practice is the lack of suitably refined large-animal models. Although most animal models for the study of orthopaedic infections have been well established in dogs, rabbits, guinea pigs, and rats30,31, these models are not without limitations. In order for the results to be translatable to humans, the animal needs to be large enough to accommodate the use of human orthopaedic implants, and it should recapitulate the clinical scenario—in this case, orthopaedic trauma, perioperative bacterial contamination of the surgical site, implant colonization, and infection resulting in an altered healing response that eventually leads to delayed union or nonunion.
The use of a sheep model in studies of orthopaedic infection was first reported in 1973, and since then they have been developed as models in studies of chronic osteomyelitis35,36. Orthopaedic models utilizing sheep and goats37,38 are well accepted because their larger bone and medullary canal sizes allow evaluation of fixation devices (e.g., external fixators and intramedullary nails) that otherwise would need to be modified for use in smaller animals such as rabbits or rats. Establishment of infection has been achieved by either direct inoculation37 or hematogenous spread36. In our model, we consistently achieved infection and a resulting nonunion of the osteotomized tibia in 100% of the control animals.
We used a high bacterial load of Staphylococcus aureus ATCC 25923, a clinical isolate and a standard strain that is reported to form biofilms39-44. Staphylococcus aureus is the most common causative agent in periprosthetic infection42-46, and approximately 56% of clinical isolates of Staphylococcus aureus formed biofilms in one study44. This particular strain was used in the present study because it has been used in many other studies of antibiotic sensitivity and it produced aggressive infection in our preliminary studies using a rodent model47. There are many other biofilm-forming organisms, including coagulase-negative staphylococci and Pseudomonas aeruginosa. Although there are undoubtedly differences in biofilm characteristics among strains of the same species, and more importantly among different species of pathogenic bacteria, the gross characteristics of the biofilm—i.e., reduced immune surveillance, antimicrobial resistance and asking, and strong adhesion—are felt to be the qualities that determine initiation of infection and that are shared among biofilms. The presence of the bacteria adjacent to the implant was observed to produce a biofilm in the present study, subverting natural clearance of the bacteria by the immune system. The fact that bacterial adhesion and biofilm formation were prevented on the vancomycin-treated titanium hardware allowed the robust immune system of the sheep to clear the contaminating pathogens, ultimately resulting in the observed difference in bone stock between the control and vancomycin-treated animals (Fig. 3).
The high bacterial load of Staphylococcus aureus ATCC 25923 (2.5 mL of 106 CFU/mL of microorganisms) was not representative of surgical practice, but it was necessitated by the sheep’s strong immune response. Nevertheless, this large-animal model presented all of the hallmark signs and ensuing complications of local infection associated with a tibial osteotomy.
We chose readily available long-bone fracture repair implants made from titanium, and we adapted and optimized our previously published protocols for bonding the antimicrobial agent to the metal surface. The full presentation of data from the surface optimization portion of the study is beyond the scope of this report. Nevertheless, there was clear evidence of a copious biofilm on the explanted hardware of the control animals, whereas the treated hardware exhibited only a few clusters of bacterial colonies and remained devoid of biofilm (Figs. 4-B, 4-C, 4-E, and 4-F). This finding is consistent with previous studies in which the absence of bacterial clustering lent strength to the concept that vancomycin-modified titanium surfaces are protective against biofilm formation21. It is also important to note that the vancomycin-derivatized titanium surface does not have bactericidal activity beyond the plate surfaces. Thus, conferring bactericidal protection to indwelling implants would indeed integrate with the host’s immune surveillance by preventing establishment of a biofilm on the device surface and would thereby support the overall physiological tissue response by an immunocompetent host in the presence of infection.
We observed an absence of vancomycin staining on portions of the undersurface of all retrieved vancomycin-derivatized titanium plates. The plate surfaces had been evaluated following derivatization and had generated an intense and homogenous anti-vancomycin staining reaction at that time. Thus, we are confident that each of the surface-derivatized plates was indeed coated with vancomycin at the time of surgery. The lack of tethered vancomycin on portions of the plates retrieved three months postoperatively is intriguing. Although we could not rule out the explant processing technique, the loss of staining may be linked to several factors capable of contributing to instability of the titanium-vancomycin surface. Localized bacterial infection has been shown to be capable of generating a low pH48, which could result in cleavage of the vancomycin-titanium bond. A second factor, which may be due to vancomycin-induced complement activation resulting in upregulated osteoclastogenesis, is the high population of osteoclasts visible under the plate49,50. Osteoclastic cleavage of the vancomycin bond could diminish anti-vancomycin reactivity and generate a low level of staining. Finally and most importantly, micromotion between the bone and the plate could serve to disrupt the titanium-vancomycin layer.
We argue that the vancomycin release would be unlikely to impact acquisition of vancomycin resistance. Since the amount of surface-bound vancomycin is low, the amount of active drug released would be insufficient to provide a selective growth advantage42,47. It is important to note, however, that the current study was not designed to address issues of emerging resistance. Our primary goal in this study was to study initial fracture-healing after bacterial challenge in the presence of coated and noncoated implants; a longer-term study will be needed to corroborate our ninety-day findings. If device-associated infections encased by biofilm are the source of recalcitrant infections, then protecting hardware with a permanent coating could at least mitigate that risk. How long-lasting or permanent this coating will be in vivo is a subject for future investigations.
In summary, clinical observations, digital radiography, and multiple additional ex vivo analytical methods indicated that tethering of vancomycin to a modified surface on a titanium plate was able to decrease clinical signs of infection in a large-animal infection model. Moreover, this technology prevented biofilm formation and promoted bone-healing in the presence of a substantial bacterial load. Our observations show that this system has promise when compared with current systems for local antimicrobial delivery, which are characterized by unpredictable elution kinetics, lack of long-term efficacy, and the inability to eradicate implant-associated infection. We believe that these encouraging results should drive the translation of this technology into clinical practice.
Appendix
A description of the animal model as well as tables showing the outcome scoring system, the optimization of the surface modification, and histologic scores and culture results in the study animals are available with the online version of this article as a data supplement at jbjs.org.
Acknowledgments
Note: The authors thank Drs. Rolf Modesto and Bambi Jasmin, Ms. Allison Volpe, Ms. Karie Durynski, Ms. Annie Ireland, Ms. Jan Ramberger, Mr. Jeremy Maurer (Penn Vet CORL), James Gilbert (Thomas Jefferson University), and Mr. Chris Scholl and Ms. Payel Patel (Synthes, West Chester, Pennsylvania) for their technical assistance with this study.
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Cierny G, 3rd, DiPasquale D. Periprosthetic total joint infections: staging, treatment, and outcomes. Clin Orthop Relat Res. 2002. Oct;(403):23-8 [PubMed] [Google Scholar]
- 2.Geipel U, Herrmann M. [The infected implant. Part 1: bacteriology]. Orthopade. 2004. Dec;33(12):1411-26; 1427-8. German [DOI] [PubMed] [Google Scholar]
- 3.McPherson EJ, Woodson C, Holtom P, Roidis N, Shufelt C, Patzakis M. Periprosthetic total hip infection: outcomes using a staging system. Clin Orthop Relat Res. 2002. Oct;(403):8-15 [PubMed] [Google Scholar]
- 4.Puolakka TJ, Pajamäki KJ, Halonen PJ, Pulkkinen PO, Paavolainen P, Nevalainen JK. The Finnish Arthroplasty Register: report of the hip register. Acta Orthop Scand. 2001. Oct;72(5):433-41 [DOI] [PubMed] [Google Scholar]
- 5.Ahern BJ, Richardson DW, Boston RC, Schaer TP. Orthopedic infections in equine long bone fractures and arthrodeses treated by internal fixation: 192 cases (1990-2006). Vet Surg. 2010. Jul;39(5):588-93 Epub 2010 Apr 29 [DOI] [PubMed] [Google Scholar]
- 6.Costerton JW, Montanaro L, Arciola CR. Biofilm in implant infections: its production and regulation. Int J Artif Organs. 2005. Nov;28(11):1062-8 [DOI] [PubMed] [Google Scholar]
- 7.Sheehan E, McKenna J, Mulhall KJ, Marks P, McCormack D. Adhesion of Staphylococcus to orthopaedic metals, an in vivo study. J Orthop Res. 2004. Jan;22(1):39-43 [DOI] [PubMed] [Google Scholar]
- 8.Mack D, Rohde H, Harris LG, Davies AP, Horstkotte MA, Knobloch JK. Biofilm formation in medical device-related infection. Int J Artif Organs. 2006. Apr;29(4):343-59 [DOI] [PubMed] [Google Scholar]
- 9.Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008;322:207-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002. Jul;292(2):107-13 [DOI] [PubMed] [Google Scholar]
- 11.Garvin K, Feschuk C. Polylactide-polyglycolide antibiotic implants. Clin Orthop Relat Res. 2005. Aug;(437):105-10 [DOI] [PubMed] [Google Scholar]
- 12.Kanellakopoulou K, Giamarellos-Bourboulis EJ. Carrier systems for the local delivery of antibiotics in bone infections. Drugs. 2000. Jun;59(6):1223-32 [DOI] [PubMed] [Google Scholar]
- 13.Rutledge B, Huyette D, Day D, Anglen J. Treatment of osteomyelitis with local antibiotics delivered via bioabsorbable polymer. Clin Orthop Relat Res. 2003. Jun;(411):280-7 [DOI] [PubMed] [Google Scholar]
- 14.Zalavras CG, Patzakis MJ, Holtom P. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin Orthop Relat Res. 2004. Oct;(427):86-93 [DOI] [PubMed] [Google Scholar]
- 15.Wininger DA, Fass RJ. Antibiotic-impregnated cement and beads for orthopedic infections. Antimicrob Agents Chemother. 1996. Dec;40(12):2675-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reading NC, Sperandio V. Quorum sensing: the many languages of bacteria. FEMS Microbiol Lett. 2006. Jan;254(1):1-11 [DOI] [PubMed] [Google Scholar]
- 17.Burny F, Donkerwolcke M, Moulart F, Bourgois R, Puers R, Van Schuylenbergh K, Barbosa M, Paiva O, Rodes F, Bégueret JB, Lawes P. Concept, design and fabrication of smart orthopedic implants. Med Eng Phys. 2000. Sep;22(7):469-79 [DOI] [PubMed] [Google Scholar]
- 18.Fairman R, Akerfeldt KS. Peptides as novel smart materials. Curr Opin Struct Biol. 2005. Aug;15(4):453-63 [DOI] [PubMed] [Google Scholar]
- 19.Antoci V, Jr, Adams CS, Parvizi J, Ducheyne P, Shapiro IM, Hickok NJ. Covalently attached vancomycin provides a nanoscale antibacterial surface. Clin Orthop Relat Res. 2007. Aug;461:81-7 [DOI] [PubMed] [Google Scholar]
- 20.Antoci V, Jr, King SB, Jose B, Parvizi J, Zeiger AR, Wickstrom E, Freeman TA, Composto RJ, Ducheyne P, Shapiro IM, Hickok NJ, Adams CS. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J Orthop Res. 2007. Jul;25(7):858-66 [DOI] [PubMed] [Google Scholar]
- 21.Antoci V, Jr, Adams CS, Parvizi J, Davidson HM, Composto RJ, Freeman TA, Wickstrom E, Ducheyne P, Jungkind D, Shapiro IM, Hickok NJ. The inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials. 2008. Dec;29(35):4684-90 Epub 2008 Sep 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antoci V, Jr, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Vancomycin bound to Ti rods reduces periprosthetic infection: preliminary study. Clin Orthop Relat Res. 2007. Aug;461:88-95 [DOI] [PubMed] [Google Scholar]
- 23. Scholl C, Synthes, West Chester, PA. Personal communication; 2009 Feb.
- 24.Ketonis C, Parvizi J, Adams CS, Shapiro IM, Hickok NJ. Topographic features retained after antibiotic modification of Ti alloy surfaces: retention of topography with attachment of antibiotics. Clin Orthop Relat Res. 2009. Jul;467(7):1678-87 Epub 2009 Apr 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radtke CL, Armbruster D, DePaula A, Harten R, Schaer TP. Validation of infected ovine tibial osteotomy model for fracture repair with antimicrobial loaded polymer sleeve. Vet Surg. 2009;38:E42 [Google Scholar]
- 26.Schaer TP, Stewart S, Hsu BB, Klibanov AM. Hydrophobic polycationic coatings that inhibit biofilms and support bone healing during infection. Biomaterials. 2012. Feb;33(5):1245-54 Epub 2011 Nov 13 [DOI] [PubMed] [Google Scholar]
- 27.Pfeffer A, Rogers KM, O’Keeffe L, Osborn PJ. Acute phase protein response, food intake, liveweight change and lesions following intrathoracic injection of yeast in sheep. Res Vet Sci. 1993. Nov;55(3):360-6 [DOI] [PubMed] [Google Scholar]
- 28.Hirvonen J, Pyörälä S. Acute-phase response in dairy cows with surgically-treated abdominal disorders. Vet J. 1998. Jan;155(1):53-61 [DOI] [PubMed] [Google Scholar]
- 29.Cheryk LA, Hooper-McGrevy KE, Gentry PA. Alterations in bovine platelet function and acute phase proteins induced by Pasteurella haemolytica A1. Can J Vet Res. 1998. Jan;62(1):1-8 [PMC free article] [PubMed] [Google Scholar]
- 30.An YH, Friedman JR. Animal models of prosthetic infection. : An YH, editor Animal models in orthopaedic research. Boca Raton, FL: CRC Press; 1999. p 443-57 [Google Scholar]
- 31.Voos K, Rosenberg B, Fagrhi M, Seligson D. Use of a tobramycin-impregnated polymethylmethacrylate pin sleeve for the prevention of pin-tract infection in goats. J Orthop Trauma. 1999. Feb;13(2):98-101 [DOI] [PubMed] [Google Scholar]
- 32.Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000. Apr;40(2):175-9 [DOI] [PubMed] [Google Scholar]
- 33.Hannig C, Follo M, Hellwig E, Al-Ahmad A. Visualization of adherent micro-organisms using different techniques. J Med Microbiol. 2010. Jan;59(Pt 1):1-7 [DOI] [PubMed] [Google Scholar]
- 34.Marrie TJ, Noble MA, Costerton JW. Examination of the morphology of bacteria adhering to peritoneal dialysis catheters by scanning and transmission electron microscopy. J Clin Microbiol. 1983. Dec;18(6):1388-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rittmann WW, Brennwald J, Matter P, Allgöwer M. A model of studying bone repair after a standard osteotomy, rigid internal fixation and infection with Staph aureus. Eur Surg Res. 1973; 43 [Google Scholar]
- 36.Kaarsemaker S, Walenkamp GH, vd Bogaard AE. New model for chronic osteomyelitis with Staphylococcus aureus in sheep. Clin Orthop Relat Res. 1997. Jun;(339):246-52 [DOI] [PubMed] [Google Scholar]
- 37.Clasper JC, Stapley SA, Bowley DM, Kenward CE, Taylor V, Watkins PE. Spread of infection, in an animal model, after intramedullary nailing of an infected external fixator pin track. J Orthop Res. 2001. Jan;19(1):155-9 [DOI] [PubMed] [Google Scholar]
- 38.Warme WJ, Brooks D, Carpenter L, McManus AT. External fixator pin tract infection model in the caprine (goat) tibia: a randomized, prospective, blinded study. Am J Orthop (Belle Mead NJ). 2004. Sep;33(9):447-51 [PubMed] [Google Scholar]
- 39.Zmantar T, Kouidhi B, Miladi H, Mahdouani K, Bakhrouf A. A microtiter plate assay for Staphylococcus aureus biofilm quantification at various pH levels and hydrogen peroxide supplementation. New Microbiol. 2010. Apr;33(2):137-45 [PubMed] [Google Scholar]
- 40.Domenico P, Baldassarri L, Schoch PE, Kaehler K, Sasatsu M, Cunha BA. Activities of bismuth thiols against staphylococci and staphylococcal biofilms. Antimicrob Agents Chemother. 2001. May;45(5):1417-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saising J, Ongsakul M, Voravuthikunchai SP. Rhodomyrtus tomentosa (Aiton) Hassk. ethanol extract and rhodomyrtone: a potential strategy for the treatment of biofilm-forming staphylococci. J Med Microbiol. 2011. Dec;60(Pt 12):1793-800 Epub 2011 Aug 4 [DOI] [PubMed] [Google Scholar]
- 42.Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996. Apr;78(4):512-23 [DOI] [PubMed] [Google Scholar]
- 43.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001. Nov;(392):15-23 [PubMed] [Google Scholar]
- 44.Sanderson PJ. Infection in orthopaedic implants. J Hosp Infect. 1991. Jun;18 Suppl A:367-75 [DOI] [PubMed] [Google Scholar]
- 45.Darouiche RO. Antimicrobial approaches for preventing infections associated with surgical implants. Clin Infect Dis. 2003. May 15;36(10):1284-9 Epub 2003 May 9 [DOI] [PubMed] [Google Scholar]
- 46.Harris LG, Richards RG. Staphylococci and implant surfaces: a review. Injury. 2006. May;37 Suppl 2:S3-14 [DOI] [PubMed] [Google Scholar]
- 47.Antoci V, Jr, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Vancomycin bound to Ti rods reduces periprosthetic infection: preliminary study. Clin Orthop Relat Res. 2007. Aug;461:88-95 [DOI] [PubMed] [Google Scholar]
- 48.Epari DR, Lienau J, Schell H, Witt F, Duda GN. Pressure, oxygen tension and temperature in the periosteal callus during bone healing—an in vivo study in sheep. Bone. 2008. Oct;43(4):734-9 Epub 2008 Jun 25 [DOI] [PubMed] [Google Scholar]
- 49.Tu Z, Bu H, Dennis JE, Lin F. Efficient osteoclast differentiation requires local complement activation. Blood. 2010. Nov 25;116(22):4456-63 Epub 2010 Aug 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Von Drygalski A, Curtis BR, Bougie DW, McFarland JG, Ahl S, Limbu I, Baker KR, Aster RH. Vancomycin-induced immune thrombocytopenia. N Engl J Med. 2007. Mar 1;356(9):904-10 [DOI] [PubMed] [Google Scholar]