Abstract
Background:
Hospital compliance with the Surgical Care Improvement Project (SCIP) measures has increased recently for patients undergoing hip arthroplasty. However, reductions in postoperative infections were less than expected, and concern remains about complications associated with prophylaxis against venous thromboembolism (VTE). We sought to examine the association between hospital adherence to SCIP measures and postoperative infections.
Methods:
We conducted an observational study of 17,714 patients who underwent hip replacement in 2008 at 128 New York state hospitals. These hospitals were divided into less compliant and highly compliant groups, on the basis of their levels of compliance compared with the median value of compliance with SCIP measures. From the New York State Department of Health annual report, we collected the confirmed postoperative infections at the facility level. From the Healthcare Cost and Utilization Project state inpatient database, we identified incidences of postoperative infections at the patient level, using International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes.
Results:
During 2008, mean hospital compliance increased from 93.5% to 96.0% for the infection prevention measure and from 91.4% to 97.5% for the VTE prevention measure. Higher adherence to infection prevention measures was not associated with a significant reduction in infection (p ≥ 0.09 for all). Hospitals that were at least 97% compliant with the SCIP VTE-2 measure (patients receiving VTE prophylaxis around the time of surgery) reported significantly higher infection rates compared with less compliant hospitals (1.60% versus 0.93%; p < 0.001). Similarly, patients from highly compliant hospitals (for the VTE-2 measure) were at significant risk of postoperative infection (adjusted odds ratio, 1.50; 95% confidence interval, 1.07 to 2.12; p = 0.02).
Conclusions:
Targeting complete compliance with SCIP infection prevention measures was not associated with additional reductions in infection outcomes following hip replacement. Furthermore, significant risk of postoperative infections may result from increased perioperative use of VTE prophylactics.
Level of Evidence:
Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
In the United States, approximately 300,000 patients underwent total hip arthroplasty in 2005, with a projected increase of up to 200% by 20301. Although hospital-associated infections following hip replacement develop in 0.2% to 1.1% of patients2, they are serious complications that increase the rate of morbidity and the burden to the health-care system3 and remain as one of the main reasons for revision arthoplasty4. Additionally, patients having a hip replacement are at high risk for venous thromboembolism (VTE) and, without the use of prophylaxis, 15% to 60% of them would develop deep vein thrombosis and 0.5% to 2% would have a fatal pulmonary embolism5. In 2008, the U.S. Centers for Medicare and Medicaid Services (CMS) added VTE to the list of preventable complications that are referred to as “never events,” thereby reducing the reimbursement amount payable to hospitals if patients experienced these events following hip replacement6.
Concerns over hospital-associated complications led to the development of clinical guidelines and implementation of process measures such as those from the Surgical Care Improvement Project (SCIP)7. There are four SCIP measures against postoperative infections (INF) and two against VTE related to hip arthroplasty. SCIP INF-1 measures the percentage of hospital patients who received prophylactic antibiotics within one hour prior to surgical incision. SCIP INF-2 measures the percentage of hospital patients who received prophylactic antibiotics recommended for their specific surgical procedure. SCIP INF-3 measures the percentage of hospital patients whose prophylactic antibiotics were discontinued within twenty-four hours after surgery end time. SCIP INF-6 measures the percentage of hospital patients with appropriate removal of surgical site hair with clippers or depilatory or those not requiring removal of surgical site hair. SCIP VTE-1 measures the percentage of hospital patients with recommended VTE prophylaxis ordered. SCIP VTE-2 measures the percentage of hospital patients who received appropriate venous thromboembolism prophylaxis (except aspirin) within twenty-four hours before surgery to twenty-four hours after surgery. SCIP measures against infections have been evaluated recently in a multi-institutional setting with minimal reduction in infections identified8-10.
Process measures related to VTE prophylaxis are not without controversy. Anticoagulation has been associated with a higher risk of surgical complications, as shown in meta-analysis11, case-control12-14, retrospective15, and prospective16 cohort studies. These results challenge those of earlier clinical trials in which patients with a higher risk of bleeding and other complications were excluded. Clinicians have long suspected that patients with chemical prophylaxis against VTE have increased wound drainage and hematoma rates, which results in an increased risk of infection14. Thus, there remains disagreement regarding the optimal VTE prophylaxis after total hip arthroplasty17. One committee18 has highly recommended pharmacologic prophylaxis, while another committee report19 has expressed substantial concerns over high rates of bleeding and hematoma among patients with use of prophylaxis.
In this study, we sought to evaluate the effect of the SCIP prevention measures against postoperative infection. Furthermore, we addressed the issue of possible cross-measure association between compliance with SCIP VTE prevention measures and levels of postoperative infections.
Materials and Methods
Data Sources
Each year since 2007, the New York State Department of Health has audited 186 hospitals and its annual report has identified infections from 100% of reporting hospitals. The New York State Department of Health joined the National Healthcare Safety Network organized by the Centers for Disease Control and Prevention and became the first state to publicly report hospital-acquired infections for all hospitals that performed selected surgical procedures. According to its 2008 annual report, 171 hospitals performed a total of 23,611 hip arthroplasties and reported 274 surgical site infections following hip replacement20.
To incorporate patient-level data and assess the postoperative infections, we used the 2008 New York State Inpatient Database from the Healthcare Cost and Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality (AHRQ)21. This file was linked to the 2008 state inpatient database revisit supplemental file to track patients longitudinally for initial hospitalization and readmission within a year. In addition, process measures from the New York quality improvement organization (IPRO, Lake Success, New York) were also merged by hospital. We successfully linked those discharge records from 17,882 patients to 153 hospitals from the New York State Department of Health report, and then to the American Hospital Association 2008 survey guide, such that infection outcomes and hospital characteristics from matched hospitals were linked to each discharge record related to hip replacement.
Because data from the New York State Department of Health were publicly available and the database from AHRQ contained only deidentified information, the study was exempted by the institutional review boards at the National Institutes of Health. Linkage of AHRQ data with other data was performed at the hospital level.
Outcome Measures
We obtained a hospital-level rate of surgical site infections following hip replacements from the New York State Department of Health 2008 annual report. Infections were defined as those identified during the patient’s initial hospital discharge or readmission, with the clinical definitions as superficial, deep, and organ space infections on the basis of the National Healthcare Safety Network criteria, although the specific types were not listed in the public release file20. Each case of surgical site infection was reported by the individual hospital. During the pilot year 2007, reports of infection from each hospital were audited for accuracy according to guidelines from the National Healthcare Safety Network22.
We also captured the infection outcomes using patient-level data from the state inpatient database. The postoperative infection was ascertained with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. We identified index hospitalizations for hip replacements using ICD-9-CM procedure codes. The postoperative infections were captured either as a secondary diagnosis during initial hospitalization or a readmission with any related diagnosis within the calendar year, into the same hospital or any other HCUP hospitals in the state of New York. We excluded patients with a diagnosis of infection or VTE on admission. The ICD-9-CM codes for postoperative infection were 998.5, 998.51, or 998.59 for those occurring within thirty days after hip replacement and 996.6, 996.60, 996.66, or 996.67 for those occurring within a year after hip replacement.
Data Analysis
We estimated that, on the basis of the assumption that the baseline rate of surgical site infection was 1%, the sample size that was needed to detect a 50% increase in this rate, with 80% power and a 95% confidence level, was 7963 patients for each group. Since we had 17,714 patients in the cohort, we divided the cohort into two groups on the basis of the levels of compliance with SCIP measures. Hospitals with greater than the median level of compliance were designated as the highly compliant group, leaving the others that were equal to or below the median level of compliance as the less compliant group. With surgical volume treated the same way, we analyzed all SCIP measure and surgical volume covariates as binary variables.
We used locally weighted polynomial regression or scatterplot smoothing (LOESS)23 to visualize the nonlinear relationship between the risk-adjusted infection rates and surgical volume or compliance with VTE prevention measures. To further quantify the association between compliance with SCIP measures and infection outcomes, we used generalized linear regression to study such relationships on facility levels and generalized linear mixed models to analyze the patient-level data.
We included the following covariates: age; sex; admission type (emergency, urgent, or elective); comorbidity index (0, 1, 2, or ≥3); hospital surgical volume (twenty to 102, or 103 to 645 total hip replacements [hospitals with a volume of less than twenty total hip arthroplasties per year were excluded, since data from these hospitals were reported as “not applicable” in the New York State annual report]); hospital teaching status, location, bed size, and ownership; primary payer; and individual SCIP measures (INF-1, INF-2, INF-3, INF-6, VTE-1, and VTE-2). We incorporated all of the SCIP measures in the final model and eliminated other covariates that were not relevant on the basis of univariate analysis (p > 0.2). The comorbidity index was based on comorbidities identified in hospital discharge records with use of the diagnosis coding of ICD-9-CM24. For hospital-level analyses, we used the numbers of infections as outcomes and procedures reported by each hospital as offset to model the infection rates. Because of excessive zero outcomes (forty-five of 128 hospitals), we used the zero-inflated negative binomial regression of the generalized linear model for the hospital-level data. As for patient-level analysis, we used hierarchical logistic regression from the generalized linear mixed model, taking into consideration patient clustering by hospital. For these two models, we respectively used procedures COUNTREG and GLIMMIX of SAS 9.2 software (SAS Institute, Cary, North Carolina). Statistical tests were performed at a two-tailed significance level of <0.05.
Source of Funding
This research was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH). Foster Chen was supported by the Clinical Research Training Program at NIH.
Results
Demographic Data
Table I shows the demographic characteristics of 17,714 patients discharged from the 128 hospitals after twenty-five hospitals that performed less than twenty hip arthroplasties per year had been excluded from the total of 153 hospitals (see Materials and Methods). The patient cohort was dichotomized on the basis of the median value of the SCIP VTE-2 prevention measure. Hospitals with higher compliance were more likely to be teaching hospitals, smaller hospitals, and private hospitals in rural areas.
TABLE I.
Patient Characteristics
| Total (N = 17,714) |
Less Compliance* (N = 9568) |
High Compliance† (N = 8146) |
||||
| Patient characteristics | No. | % | No. | % | No. | % |
| Age (y) | ||||||
| 18-24 | 32 | 0.2 | 20 | 0.2 | 12 | 0.1 |
| 25-44 | 665 | 3.8 | 392 | 4.1 | 273 | 3.4 |
| 45-64 | 5790 | 32.7 | 3206 | 33.5 | 2584 | 31.7 |
| 65-74 | 3991 | 22.5 | 2114 | 22.1 | 1877 | 23.0 |
| 75-84 | 4607 | 26.0 | 2393 | 25.0 | 2214 | 27.2 |
| ≥85 | 2629 | 14.8 | 1443 | 15.1 | 1186 | 14.6 |
| Race | ||||||
| White | 15,213 | 85.9 | 8048 | 84.1 | 7165 | 88.0 |
| Black | 1134 | 6.4 | 689 | 7.2 | 445 | 5.5 |
| Other‡ | 1367 | 7.7 | 831 | 8.7 | 536 | 6.6 |
| Sex | ||||||
| Male | 7182 | 40.5 | 3871 | 40.5 | 3311 | 40.6 |
| Female | 10,532 | 59.5 | 5697 | 59.5 | 4835 | 59.4 |
| Comorbidity | ||||||
| 0 | 2739 | 15.5 | 1480 | 15.5 | 1259 | 15.5 |
| 1 | 4760 | 26.9 | 2562 | 26.8 | 2198 | 27.0 |
| 2 | 4540 | 25.6 | 2452 | 25.6 | 2088 | 25.6 |
| ≥3 | 5675 | 32.0 | 3074 | 32.1 | 2601 | 31.9 |
| Admission type§ (n = 17,770) | ||||||
| Emergency | 5093 | 28.8 | 2718 | 28.4 | 2375 | 29.2 |
| Urgent | 1101 | 6.2 | 590 | 6.2 | 511 | 6.3 |
| Elective | 11,506 | 65.0 | 6251 | 65.3 | 5255 | 64.5 |
| Primary payer | ||||||
| Medicare | 10,760 | 60.7 | 5734 | 59.9 | 5026 | 61.7 |
| Medicaid | 615 | 3.5 | 370 | 3.9 | 245 | 3.0 |
| Private | 5788 | 32.7 | 3213 | 33.6 | 2575 | 31.6 |
| Uninsured | 140 | 0.8 | 46 | 0.5 | 94 | 1.2 |
| Other | 411 | 2.3 | 205 | 2.1 | 206 | 2.5 |
| Teaching hospital§ (n = 17,342) | ||||||
| No | 11,649 | 67.2 | 7291 | 76.4 | 4358 | 55.9 |
| Yes | 5693 | 32.8 | 2249 | 23.6 | 3444 | 44.1 |
| Hospital bed size§ (n = 17,686) | ||||||
| Small | 711 | 4.0 | 17 | 0.2 | 694 | 8.5 |
| Medium | 2183 | 12.3 | 1097 | 11.5 | 1086 | 13.3 |
| Large | 14,792 | 83.6 | 8426 | 88.1 | 6366 | 78.1 |
| Hospital ownership§ (n = 17,686) | ||||||
| Government | 2602 | 14.7 | 1721 | 18.0 | 881 | 10.8 |
| Private | 15,084 | 85.2 | 7819 | 81.9 | 7265 | 89.2 |
| Hospital location§ (n = 17,686) | ||||||
| Rural | 1310 | 7.4 | 470 | 4.9 | 840 | 10.3 |
| Urban | 16,376 | 92.6 | 9070 | 95.0 | 7306 | 89.7 |
| Hospital volume (no. of total hip replacements per year) | ||||||
| 20-48 | 1412 | 8.0 | 928 | 9.7 | 484 | 5.9 |
| 50-102 | 2397 | 13.5 | 934 | 9.8 | 1463 | 18.0 |
| 103-169 | 4185 | 23.6 | 2326 | 24.3 | 1859 | 22.8 |
| 187-645 | 9720 | 54.9 | 5380 | 56.2 | 4340 | 53.3 |
Based on hospital compliance that was less than the median value of the SCIP VTE-2 measure.
Based on hospital compliance that was higher than the median value of the SCIP VTE-2 measure.
All individuals with data on race as missing or those identified as other than white or black.
Data were unavailable for some patients.
Improvements in Compliance with SCIP Measures
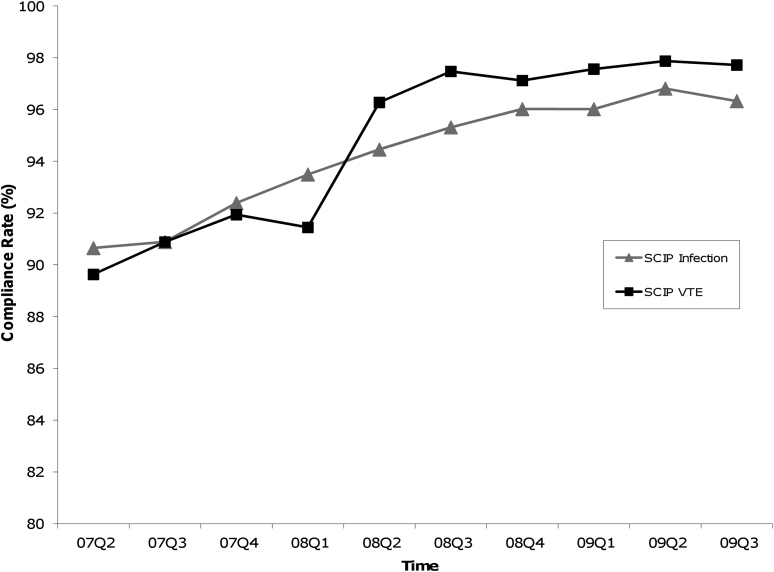
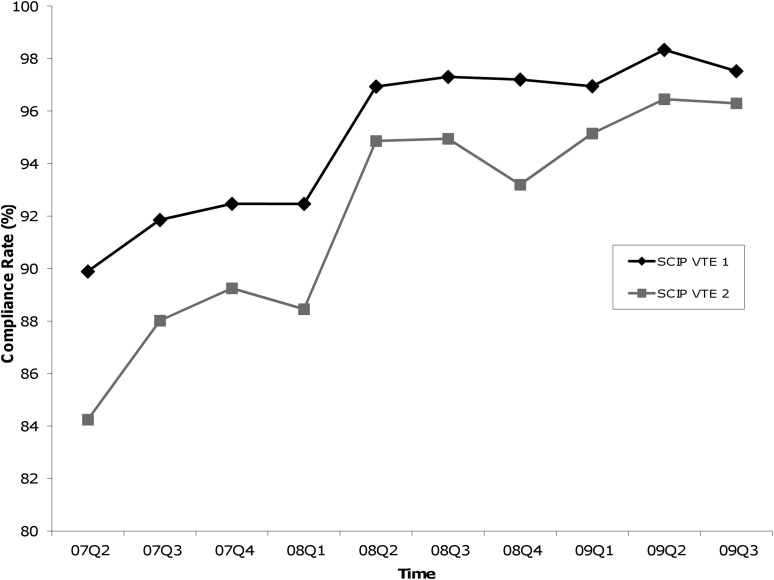
Figures 1-A and 1-B show the quarterly means of hospital compliance from April 1, 2007, through September 30, 2009. While there was a gradual improvement of SCIP infection prevention measures during this period, a marked increase in VTE prevention measures was seen early in 2008, particularly in the first two quarters. Additionally, the mean compliance on the overall infection prevention measure increased from 93.5% to 96.0% during 2008, whereas the overall VTE prevention measure increased from 91.4% to 97.5%. Specifically, the mean VTE prevention measures increased from 92.5% to 97.2% for VTE-1 and from 88.4% to 93.2% for VTE-2.
Graphs showing quarterly hospital compliance with SCIP (Surgical Care Improvement Project) process measures from April 1, 2007, through September 30, 2009. The values are given as the mean of the overall infection or VTE (venous thromboembolism) prevention measures from all reported hospitals. The overall infection or VTE prevention measure was the average of individual infection or VTE prevention measures, respectively.
Fig. 1-A.
A gradual improvement was seen in the overall SCIP infection prevention measures, compared with a step increase in the overall SCIP VTE prevention measure right before the third quarter of 2008.
Fig. 1-B.
Substantial increases were also seen in individual SCIP VTE-1 and VTE-2 measures.
Associations Between Hospital-Associated Infections and Case Volume, or SCIP Compliance
The Appendix shows the unadjusted incidence rate of postoperative infections at the hospital level. Hospitals with higher adherence to SCIP infection prevention measures (INF-1, INF-2, INF-3, and INF-6) did not manifest a significantly lower infection rate. For example, for hospitals that were highly compliant (95.2% to 100% for SCIP INF-1), the mean infection rate was 1.31%, which was not significantly different from the rate of 1.17% for less compliant hospitals (50.0% to 95.1% for SCIP INF-1; p = 0.39). However, higher levels of adherence to SCIP VTE-1 or VTE-2 measures were associated with significantly higher rates of infection (p < 0.001 for all; see Appendix). Specifically, postoperative infection rates for hospitals with higher than median values of compliance with VTE prevention measures tended to be higher than the state average of 1.4% (1.52% [95% confidence interval (CI), 1.29% to 1.80%] for VTE-1 and 1.60% [95% CI, 1.35% to 1.91%] for VTE-2). On the other hand, postoperative infection rates for hospitals with lower than median values of compliance with VTE prevention measures were significantly lower than the state average (0.95% [95% CI, 0.77% to 1.17%] for VTE-1 and 0.93% [95% CI, 0.76% to 1.14%] for VTE-2).
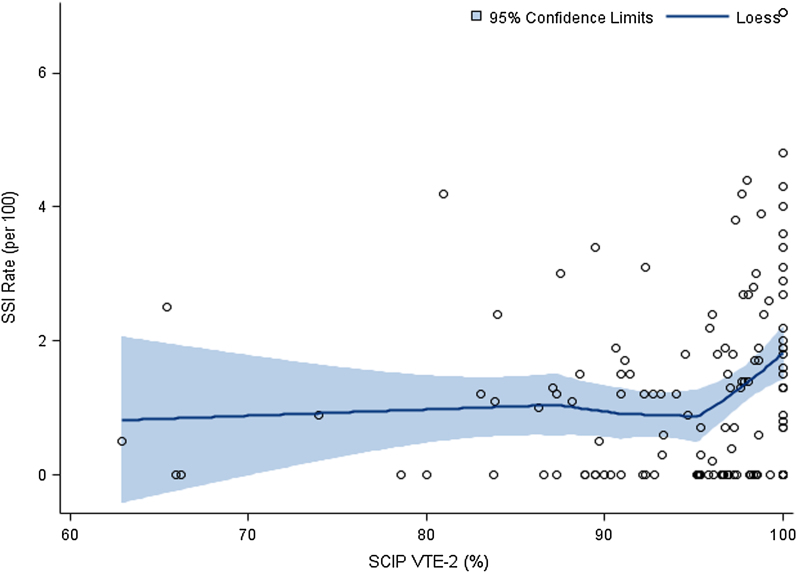
There was a significant and negative association between hospital case volumes and risk-adjusted infection rates for the hospitals with ≤150 hip replacements per year (p < 0.0001) (see Appendix). For hospitals that had >150 hip replacements per year, the association was not significant. In contrast, for hospitals with SCIP VTE-2 compliance higher than approximately 95%, a positive association was found between this compliance and risk-adjusted postoperative infection rates (Fig. 2). For hospitals with SCIP VTE-2 compliance of ≤95%, the association was absent. The apparent 54% reduction in postoperative infections seen among high-volume hospitals was mirrored by a 60% increase in infection rates associated with higher adherence to the SCIP VTE-2 measure.
Fig. 2.
Locally weighted scatterplot smoothing (LOESS) estimates of hospital-reported risk-adjusted rates of surgical site infections (SSI) following total hip replacement by compliance with the SCIP VTE-2 measure. The fitted lines indicate the mean; shaded areas indicate the 95% CI.
Risk of Postoperative Infections Associated with SCIP VTE Compliance
In the final model for hospital-level analysis that included all of the SCIP measures, hospital case volume, and teaching status, the last two factors were both associated with significantly lower risks of postoperative infections (Table II). Among all of the SCIP measures, only that with VTE-2 was associated with a significantly higher rate of infections (adjusted odds ratio [OR]: 1.91; 95% CI, 1.31 to 2.79). Similarly, on the patient-level analysis, the adjusted risks of infection were significantly higher for hospitals with higher compliance with VTE-2 (adjusted OR: 1.50; 95% CI, 1.07 to 2.12; p = 0.02), but not with VTE-1 (p = 0.63).
TABLE II.
Risk Ratio of Postoperative Infections by Adherence to SCIP Prevention Measures and Volume
| Measure* | Hospital Data†‡ | Patient Data†§ |
| SCIP INF-1 adherence | 0.98 (0.70-1.38) | 0.83 (0.61-1.15) |
| SCIP INF-2 adherence | 0.93 (0.66-1.32) | 1.02 (0.74-1.42) |
| SCIP INF-3 adherence | 0.74 (0.52-1.04) | 1.21 (0.88-1.67) |
| SCIP INF-6 adherence | 1.35 (0.94-1.92) | 0.93 (0.67-1.28) |
| SCIP VTE-1 adherence | 0.98 (0.68-1.42) | 0.92 (0.66-1.29) |
| SCIP VTE-2 adherence | 1.91 (1.31-2.79) | 1.50 (1.07-2.12) |
| Hospital case volume | 0.64 (0.44-0.93) | 1.05 (0.75-1.47) |
Adherence was based on whether hospital compliance was lower or higher than the median value of respective SCIP measure compliance or case volume. INF-1 indicates that the prophylactic antibiotic was received within one hour prior to surgical incision; INF-2, that prophylactic antibiotics were recommended for the specific surgical procedure; INF-3, that prophylactic antibiotics were discontinued within twenty-four hours after surgery end time; INF-6, that appropriate surgical site hair removal was done or surgical site hair removal was not required; VTE-1, that patients having surgery had recommended VTE prophylaxis ordered; and VTE-2, that patients having surgery received appropriate VTE prophylaxis within twenty-four hours before surgery to twenty-four hours after surgery.
The values are given as the adjusted OR with the 95% CI in parentheses.
Another significant factor was teaching hospitals (p = 0.004).
Another significant factor was admission type (p < 0.0001), adjusted additionally for sex (p = 0.1) and comorbidity (p = 0.1).
Subgroup Analysis: Patients with Elective Hip Arthroplasty
Because admission type was a strong confounder for our study (Table II), we conducted separate analyses for elective and nonelective hip replacements. Table III showed that the risks of postoperative infections were significantly elevated for the patients undergoing elective hip arthroplasty in hospitals that were highly compliant with SCIP VTE-2 measures compared with similar patients from less compliant hospitals (adjusted OR: 2.30; 95% CI, 1.20 to 4.40).
TABLE III.
Risk Ratio of Postoperative Infection After Elective and Nonelective Hip Arthroplasty
| Measure* | Elective Surgery†‡ | Nonelective Surgery†§ |
| SCIP INF-1 adherence | 0.59 (0.32-1.08) | 0.99 (0.69-1.41) |
| SCIP INF-2 adherence | 0.88 (0.48-1.61) | 0.97 (0.68-1.40) |
| SCIP INF-3 adherence | 1.54 (0.84-2.84) | 1.17 (0.82-1.67) |
| SCIP INF-6 adherence | 0.74 (0.41-1.33) | 1.04 (0.72-1.49) |
| SCIP VTE-1 adherence | 0.84 (0.44-1.57) | 0.88 (0.61-1.28) |
| SCIP VTE-2 adherence | 2.30 (1.20-4.40) | 1.32 (0.90-1.91) |
| Hospital case volume | 0.57 (0.30-1.07) | 1.10 (0.74-1.63) |
Adherence was based on whether hospital compliance was lower or higher than the median value of respective SCIP measure compliance or case volume. INF-1 indicates that the prophylactic antibiotic was received within one hour prior to surgical incision; INF-2, that prophylactic antibiotics were recommended for the specific surgical procedure; INF-3, that prophylactic antibiotics were discontinued within twenty-four hours after surgery end time; INF-6, that appropriate surgical site hair removal was done or surgical site hair removal was not required; VTE-1, that surgery patients had recommended VTE prophylaxis ordered; and VTE-2, that surgery patients received appropriate VTE prophylaxis within twenty-four hours before surgery to twenty-four hours after surgery.
The values are given as the adjusted OR with the 95% CI in parentheses.
Other significant factors were age (p = 0.001) and comorbidity (p = 0.03).
Another significant factor was primary payer (p = 0.002).
Sensitivity Analysis: Patients with Elective Hip Arthroplasty
Considering the arbitrary nature of setting cutoff at the median value for comparison, we conducted sensitivity analyses to vary the cutoff point for the dichotomization from the default at 50% down to 25% or up to 75%. The results are presented in the Appendix. The cross-measure associations of SCIP VTE-2 compliance with infection outcomes were robust from approximately 42% to about 58% (within 15% of the median [50%]).
Discussion
Implementation and expansion of the SCIP has greatly improved hospital compliance with its process measures, while mandatory reporting of hospital-associated infections has made the evaluation of these process measures possible. In this report, we linked the publicly available report with hospital discharge records to estimate the impact of hospital compliance with SCIP measures on clinical outcomes of surgical care across a large state in the U.S.
Consistent with recent studies8-10, we did not find any significant effect on postoperative infections due to hospital compliance with SCIP infection prevention measures. Instead, we found an association between hospital compliance with VTE prevention measures and higher levels or risks of postoperative infections. This implies that mandating a complete compliance with SCIP VTE prevention measures may have unintended consequences related to the infection outcomes following hip replacement. To our knowledge, this study is the first to address the potential issue of a cross-measure effect.
Furthermore, we showed that such an association was specific to the VTE-2 measure. For most of the hip replacements, compliance with the VTE-2 measure modality is defined as the timely application of prophylactic agents, including warfarin, low-molecular-weight heparin, or factor Xa inhibitor. Our results are consistent with an etiological relationship between the administration of low-molecular-weight heparin and the ensuing risk related to surgical site infections16. A recent meta-analysis study indicated that low-molecular-weight heparin reduced nonfatal VTE at the expense of hematoma formation25. Postoperative hematoma formation, wound drainage, and a mean international normalized ratio of >1.5 were risk factors for periprosthetic infection14. Therefore, the cross-measure association we observed is not unexpected because of the potential risks of bleeding and other complications following VTE pharmacologic prophylaxis. Additionally, given that the infection rate following hip replacement was low, the absolute increase in the rate of postoperative infections associated with higher compliance with SCIP VTE measures remained small.
Since October 1, 2008, CMS has ceased payment for “reasonably preventable” events that included VTE following hip replacement. Around that date, we saw marked increases in hospital compliance with VTE prevention measures. It is likely that these increases were the result of changes in the types of prophylactics and/or timing of prophylaxis that surgeons prescribed for patients. For example, by switching from warfarin to low-molecular-weight heparin, compliance with VTE-2 measures could be more easily implemented and tracked. For surgeons who predominantly prescribed low-molecular-weight heparin, perioperative application may be the determining factor for measure compliance. Both changes have been shown to be associated with increased bleeding episodes26,27, which might lead to more infections.
Our epidemiological investigation provides a unique opportunity to study rare postoperative events such as surgical site infection and VTE. However, there are several important limitations in our study. First, our patient-level data were derived from administrative data, which have questionable sensitivity and specificity, and may introduce biases. Second, both compliance and hospital-reported data were at the facility level, making the association we described ecological in nature. However, the high compliance rates among hospitals mean that most, if not all, patients were compliant, making the ecological fallacy less problematic. Third, although we adjusted for age, sex, insurance status, hospital volume, and comorbidities in our patient-level analysis, we did not have information about other risk factors such as duration of surgery, American Society of Anesthesiologists (ASA) classification score (a measure of the severity of illness), surgeon volume, posttraumatic osteoarthritis, and preoperative stay2. Additionally, we did not single out risk factors such as obesity, diabetes, and coagulation deficiency. However, in our hospital-level analyses, we visualized the cross-measure association by plotting infection rates that had been risk-adjusted by wound class, ASA score, and duration of surgery. Fourth, we did not have data separately for superficial and deep prosthetic infections, which may represent distinctly different clinical outcomes. It should be mentioned that the New York State annual report only summarized the overall proportions of superficial and deep incision infections at 33% and 45%, respectively (with the rest being organ space infections).
Finally, our results from one state may not be generalized to other states or to the U.S. as a whole. Our findings may be specific to the year 2008, a year when the CMS ruling took effect. Overall, in the current state of high compliances for most hospitals, a lack of utility in the SCIP measures with limited cross-measure effect size is not unexpected and may be inherent within the design of this study. Further studies are needed to replicate these findings and address the utility of SCIP VTE measures for the prevention of their intended outcomes of VTE.
Despite these limitations, our study suggests that hospitals participating in the SCIP might need to consider not only bleeding risk but surgical site infections as well when implementing their VTE prophylaxis guidelines for patients undergoing hip replacement.
In conclusion, for hospitals that provide surgical care for patients undergoing total hip arthroplasty, incremental compliances with SCIP infection prevention measures were not associated with significant reduction in postoperative surgical site infections. Yet, the relationship between VTE prophylaxis compliance and surgical site infections is alarming, given that both VTE and infections are targeted by SCIP performance measures. The nature and clinical importance of this cross-measure association are not known and warrant further investigation.
Appendix
Figures and a table showing the association between compliances and infection rates and a sensitivity analysis are available with the electronic version of this article on our web site at jbjs.org.
Acknowledgments
Note: IPRO, Inc., provided the SCIP compliance data. The authors thank the state of New York for participating in the Healthcare Cost and Utilization Project state inpatient database in 2008. The authors also thank Dr. Remington Nevin for initial review of this manuscript.
Footnotes
Disclosure: None of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of any aspect of this work. One or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, one or more of the authors has had another relationship, or has engaged in another activity, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007. Apr;89(4):780-5 [DOI] [PubMed] [Google Scholar]
- 2.Urquhart DM, Hanna FS, Brennan SL, Wluka AE, Leder K, Cameron PA, Graves SE, Cicuttini FM. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010. Dec;25(8):1216-22e1-3. Epub 2009 Oct 30 [DOI] [PubMed] [Google Scholar]
- 3.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008. Oct;23(7):984-91 Epub 2008 Apr 10 [DOI] [PubMed] [Google Scholar]
- 4.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009. Jan;91(1):128-33 [DOI] [PubMed] [Google Scholar]
- 5.Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004. Sep;126(3 Suppl):338S-400S [DOI] [PubMed] [Google Scholar]
- 6.Deep vein thrombosis/pulmonary embolism federal registrar 2008;73(161):48480-48482 http://edocket.access.gpo.gov/2008/pdf/E8-17914.pdf. Accessed 2011 Jan 11 [Google Scholar]
- 7.Bratzler DW, Hunt DR. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis. 2006. Aug 1;43(3):322-30 Epub 2006 Jun 16 [DOI] [PubMed] [Google Scholar]
- 8.Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA. 2010. Jun 23;303(24):2479-85 [DOI] [PubMed] [Google Scholar]
- 9.Hawn MT, Itani KM, Gray SH, Vick CC, Henderson W, Houston TK. Association of timely administration of prophylactic antibiotics for major surgical procedures and surgical site infection. J Am Coll Surg. 2008. May;206(5):814-9; discussion 819-21. Epub 2008 Mar 4 [DOI] [PubMed] [Google Scholar]
- 10.Ingraham AM, Cohen ME, Bilimoria KY, Dimick JB, Richards KE, Raval MV, Fleisher LA, Hall BL, Ko CY. Association of surgical care improvement project infection-related process measure compliance with risk-adjusted outcomes: implications for quality measurement. J Am Coll Surg. 2010. Dec;211(6):705-14 [DOI] [PubMed] [Google Scholar]
- 11.Freedman KB, Brookenthal KR, Fitzgerald RH, Jr, Williams S, Lonner JH. A meta-analysis of thromboembolic prophylaxis following elective total hip arthroplasty. J Bone Joint Surg Am. 2000. Jul;82-A(7):929-38 [DOI] [PubMed] [Google Scholar]
- 12.Asensio A, Ramos A, Múñez E, Vilanova JL, Torrijos P, García FJ. Preoperative low molecular weight heparin as venous thromboembolism prophylaxis in patients at risk for prosthetic infection after knee arthroplasty. Infect Control Hosp Epidemiol. 2005. Dec;26(12):903-9 [DOI] [PubMed] [Google Scholar]
- 13.Minnema B, Vearncombe M, Augustin A, Gollish J, Simor AE. Risk factors for surgical-site infection following primary total knee arthroplasty. Infect Control Hosp Epidemiol. 2004. Jun;25(6):477-80 [DOI] [PubMed] [Google Scholar]
- 14.Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007. Sep;22(6 Suppl 2):24-8 Epub 2007 Jul 26 [DOI] [PubMed] [Google Scholar]
- 15.Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007. Jan;89(1):33-8 [DOI] [PubMed] [Google Scholar]
- 16.Burnett RS, Clohisy JC, Wright RW, McDonald DJ, Shively RA, Givens SA, Barrack RL. Failure of the American College of Chest Physicians-1A protocol for lovenox in clinical outcomes for thromboembolic prophylaxis. J Arthroplasty. 2007. Apr;22(3):317-24 [DOI] [PubMed] [Google Scholar]
- 17.Streiff MB, Haut ER. The CMS ruling on venous thromboembolism after total knee or hip arthroplasty: weighing risks and benefits. JAMA. 2009. Mar 11;301(10):1063-5 [DOI] [PubMed] [Google Scholar]
- 18.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008. Jun;133(6 Suppl):381S-453S [DOI] [PubMed] [Google Scholar]
- 19.Haas SB, Barrack RL, Westrich G, Lachiewicz PF. Venous thromboembolic disease after total hip and knee arthroplasty. J Bone Joint Surg Am. 2008. Dec;90(12):2764-80 [PubMed] [Google Scholar]
- 20.New York State Department of Health. Hospital-acquired infections - 2008. 2009. http://www.health.ny.gov/statistics/facilities/hospital/hospital_acquired_infections/2008/docs/hospital-acquired_infection.pdf. Accessed 2011 Jan 11.
- 21.State inpatient databases of healthcare cost and utilization project (HCUP) Agency for Healthcare Research and Quality, Rockville, MD: www.hcup-us.ahrq.gov/sidoverview.jsp. Accessed 2011 Jan 11 [PubMed] [Google Scholar]
- 22. New York State Department of Health. Hospital-acquired infection reporting system – 2007. 2008. http://www.health.ny.gov/statistics/facilities/hospital/hospital_acquired_infections/2007/docs/hospital-acquired_infection-full_report.pdf. Accessed 2011 Jan 11.
- 23.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assn. 1979. Dec;74(368):829-836 [Google Scholar]
- 24.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998. Jan;36(1):8-27 [DOI] [PubMed] [Google Scholar]
- 25.Tasker A, Harbord R, Bannister GC. Meta-analysis of low molecular weight heparin versus placebo in patients undergoing total hip replacement and post-operative morbidity and mortality since their introduction. Hip Int. 2010. Jan-Mar;20(1):64-74 [DOI] [PubMed] [Google Scholar]
- 26.Hull RD, Pineo GF, Francis C, Bergqvist D, Fellenius C, Soderberg K, et al. Low-molecular-weight heparin prophylaxis using dalteparin in close proximity to surgery vs warfarin in hip arthroplasty patients: A double-blind, randomized comparison. Arch Intern Med. 2000. July 24;160(14):2199-207 [DOI] [PubMed] [Google Scholar]
- 27.Strebel N, Prins M, Agnelli G, Büller HR. Preoperative or postoperative start of prophylaxis for venous thromboembolism with low-molecular-weight heparin in elective hip surgery? Arch Intern Med. 2002. Jul 8;162(13):1451-6 [DOI] [PubMed] [Google Scholar]