Abstract
Background:
The Spine Patient Outcomes Research Trial (SPORT) is a prospective, multicenter study of operative versus nonoperative treatment of lumbar intervertebral disc herniation. It has been suggested that epidural steroid injections may help improve patient outcomes and lower the rate of crossover to surgical treatment.
Methods:
One hundred and fifty-four patients included in the intervertebral disc herniation arm of the SPORT who had received an epidural steroid injection during the first three months of the study and no injection prior to the study (the ESI group) were compared with 453 patients who had not received an injection during the first three months of the study or prior to the study (the No-ESI group).
Results:
There was a significant difference in the preference for surgery between groups (19% in the ESI group compared with 56% in the No-ESI group, p < 0.001). There was no difference in primary or secondary outcome measures at four years between the groups. A higher percentage of patients changed from surgical to nonsurgical treatment in the ESI group (41% versus 12% in the No-ESI, p < 0.001).
Conclusions:
Patients with lumbar disc herniation treated with epidural steroid injection had no improvement in short or long-term outcomes compared with patients who were not treated with epidural steroid injection. There was a higher prevalence of crossover to nonsurgical treatment among surgically assigned ESI-group patients, although this was confounded by the increased baseline desire to avoid surgery among patients in the ESI group. Given these data, we concluded that more studies are necessary to establish the value of epidural steroid injection for symptomatic lumbar intervertebral disc herniation.
Level of Evidence:
Therapeutic Level II. See Instructions for Authors for a complete description of levels of evidence.
Lumbar disc herniation is the most common cause of lumbar radiculopathy1. Nonoperative management of this condition may include physical therapy, anti-inflammatory medications, and epidural steroid injections2-5. Epidural steroid injection may modulate the inflammatory cells, cytokines, or other pain mediators associated with lumbar disc herniation-related pain6-9, although it is not believed that an epidural steroid injection directly causes regression of a herniated nucleus pulposus10.
There is considerable controversy about the clinical efficacy of epidural steroid injections in the management of lumbar disc herniation. Improvements in outcome have been reported at three5, six11, sixteen12, and eighteen13 months after epidural steroid injections. Other studies have demonstrated no difference in outcome after epidural steroid injection1,12,14-18. The largest study of epidural injections is a prospective, randomized trial of 160 patients, and this study showed a benefit of steroid treatment at two weeks but none at three, six, or twelve months15.
To our knowledge, the study with the longest follow-up after epidural steroid injections was reported by Riew et al.19. The study included fifty-five nonsurgically treated patients who received an epidural steroid injection or a placebo injection in a mixed cohort of patients with both intervertebral disc herniation and spinal stenosis. The authors’ main finding was that patients who received an epidural steroid injection were significantly more likely to cross over from surgical to nonoperative treatment. However, the authors reported no difference in North American Spine Society20 outcome scores between the epidural steroid and placebo-injection groups after a minimum of one year21 or five years19 of follow-up to account for this “surgical avoidance.”
In the previous literature regarding epidural steroid injection, the experimental variable was injection of a specific agent compared with a placebo10,13,18,22-24. We are aware of only one other study that compared an epidural steroid injection with non-injection, nonsurgical treatment of lumbar disc herniation without an injection. Buchner et al. randomized thirty-eight patients with a lumbar disc herniation to either receive or not receive an epidural steroid injection22. The authors found a nonsignificant trend toward improvement in the epidural steroid injection group. Despite this uncertainty about therapeutic benefit, the utilization of epidural steroid injections has increased significantly in the United States25. Previous studies have also demonstrated an association between epidural steroid injection and an increased rate of surgery25,26.
The purpose of this study was to determine how the administration of epidural steroid injections affects the outcome of patients with lumbar radiculopathy from lumbar disc herniation in the SPORT study. In particular, our goal was to measure the effect of epidural steroid injections on primary outcome measures of pain and function and disease-specific measures. We hypothesized that patients receiving epidural steroid injections during treatment would have improved outcomes and would be more likely to cross over from operative to nonoperative treatment when compared with patients not receiving an epidural steroid injection.
Materials and Methods
Study Design
The Spine Patient Outcomes Research Trial (SPORT) was conducted at thirteen multidisciplinary spine practices in eleven states across the United States. The details of the methods have been reported previously4,5,27,28. The prospective data collection in SPORT provides a method to evaluate the effect of epidural steroid injection on the outcome of treatment of patients with lumbar radiculopathy. In the observational cohort of the lumbar intervertebral disc herniation study, 50% (360) of the patients received preenrollment epidural steroid injection and 38% (274) received epidural steroid injection during treatment. In the randomized cohort, 42% (199) received preenrollment epidural steroid injection and 50% (236) received epidural steroid injection during treatment29.
Patients
The human subject committees at each center approved the standardized protocol. Inclusion criteria for the study were an age of over eighteen years, radicular pain for six weeks, and a positive nerve root tension sign and/or neurological deficit. The diagnosis of radiculopathy was confirmed by cross-sectional images (magnetic resonance imaging [MRI], or a computed tomography [CT] myelogram if the patient was unable to undergo MRI) that demonstrated intervertebral disc herniation at the level that corresponded to the symptoms. Exclusion criteria included cauda equina syndrome, progressive neurological deficit, malignant tumor, scoliosis of >15°, disc herniation cephalad to L2, prior back surgery, and other established contraindications to elective surgery. Patients were offered participation in either a randomized or an observational cohort.
Study Interventions
The patients in the surgery group were designated to receive lumbar discectomy. The nonoperative protocol was ‘‘usual recommended care,’’ which included, at least, active physical therapy, education and counseling with instructions regarding home exercise, and nonsteroidal anti-inflammatory drugs if the patient could tolerate them. Nonoperative treatment options also included epidural steroid injections.
Study Measures
Data used in this study were obtained prospectively, and reviewed retrospectively, from patient questionnaires completed at baseline as well as six weeks, three months, six months, one year, two years, three years, and four years following surgery. Primary outcome measures included the bodily pain (BP), physical function (PF), and the physical and mental component summary (PCS and MCS) domains of the Short Form-36 (SF-36)30 as well as the American Academy of Orthopaedic Surgeons version of the Oswestry Disability Index (ODI)20. Secondary measures included patient self-reported improvement, work status, and satisfaction with current symptoms31. Symptom severity was measured by the low back pain bothersomeness (LBP) scale and the sciatica bothersomeness (SBI) and leg pain bothersomeness (LPI) indices32,33. The SF-36 and the ODI range from 0 to 100; the SBI, from 0 to 24; and the LBP scale, from 0 to 6. Higher scores indicate more severe symptoms on the ODI, SBI, and LBP scale, whereas higher scores indicate less severe symptoms on the SF-36.
Comparison
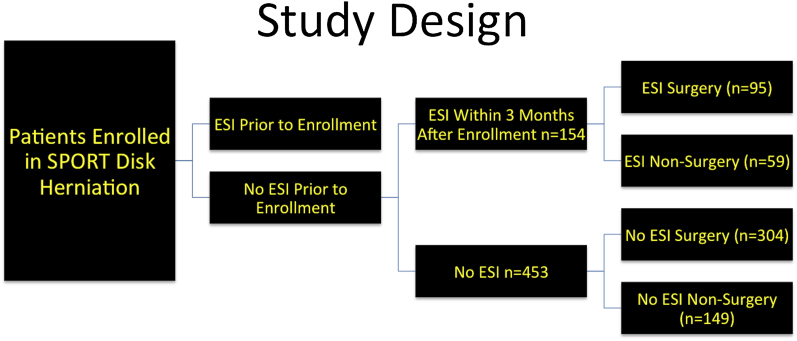
The change in primary outcome measures in patients who received epidural steroid injection (the ESI group) was compared with that in a group of patients who did not receive epidural steroid injection (the No-ESI group) as part of their treatment for intervertebral disc herniation within SPORT. To fairly assess the effect of epidural steroid injection, we excluded patients who underwent such an injection prior to their enrollment in SPORT (Fig. 1). We also included only patients who had received an epidural injection during the first three months of enrollment in the SPORT. The three-month time point was selected to exclude patients who received epidural steroid injection later in the course of the SPORT as a “salvage” intervention after a failed initial attempt at nonsurgical treatment. The majority (77%) of patients in the ESI group received the injection within the first three months of enrollment.
Fig. 1.
Study design of this subgroup analysis. Comparison of change in outcome measures between the patients treated with epidural steroid injection (ESI) and those not treated with epidural steroid injection (No ESI) in the surgical and nonsurgical groups was performed in an “as-treated” analysis.
Patients who received epidural steroid injection with any technique (including interlaminar, transforaminal, and caudal) during the first three months of enrollment were included in the ESI group. The prospective data collected in this study did not include injection type or whether fluoroscopic guidance had been used.
Statistical Analysis
Baseline characteristics were compared between the groups by using a chi-square test for categorical variables and a t test for continuous variables. Outcomes were analyzed by using longitudinal mixed-effect models with random individual effect to account for repeated individual observations over time. Covariate adjustment predicted missing data, treatment received, baseline differences, and outcomes included in the model. In addition, outcome, center, age, and sex were included in all longitudinal outcome models. All analyses were as-treated, and treatment was considered to be a time-varying covariate. Therefore, patients categorized at each time point either had received or had not received surgical treatment. Follow-up times were measured from the beginning of treatment, and baseline covariates were updated at the time of surgery. All observations prior to surgery were considered in the nonoperative estimate with follow-up time measured from enrollment. All observations following surgery contributed to the surgical estimate with follow-up time measured from the time of surgery. Secondary and binary outcomes were analyzed by using generalized estimation equations that assumed a compound symmetry working correlation structure. Outcome comparisons between the ESI and No-ESI groups were made at each time point with multiple degrees of freedom by using Wald tests. Across the four-year follow-up interval, overall comparisons of the area under the curve were made by using a Wald test. Significance was defined as p < 0.05 on the basis of a two-sided hypothesis with no adjustment made for multiple comparisons.
A post hoc power analysis34 was performed by using a two-sample t test. On the basis of the standard deviations and sample sizes from our data, there was 80% power to detect an effect size of 0.1 in the SF-36 BP and PF domains and a 0.46-point difference in the ODI between the surgically treated patient groups. The study had an 80% power to detect a 0.7-point difference in the SF-36 BP and PF domains and a 0.5-point difference in the ODI between the nonsurgically treated patient groups.
Because of extensive crossover in the randomized cohort and similar baseline characteristics and outcomes between the patients in the randomized and observational groups when they were analyzed according to treatment, the two groups were combined in this “as-treated” analysis. Patients were considered “surgically assigned” if they had been randomized to receive surgery or had chosen surgical treatment in the observational cohort. A change from the assigned treatment (in the case of the randomized cohort) or the chosen treatment (in the case of the observational cohort) was defined as “crossover.” During the course of the SPORT, patients who initially elected to have, or were assigned to receive, nonsurgical treatment may have changed their minds and ultimately undergone surgical intervention. Conversely, patients who initially elected to undergo surgical intervention or were randomized to have surgery may have changed their minds in the interval between enrollment in the SPORT and their surgery date and thus crossed over to the nonsurgical treatment arm.
Source of Funding
Funding was received from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U01-AR45444) and the Office of Research on Women’s Health, the National Institutes of Health, and the National Institute of Occupational Safety and Health, the Centers for Disease Control and Prevention.
Results
Demographics
The eligible patient population included 625 patients who did not receive epidural steroid injections prior to enrollment in SPORT (Fig. 1). Six hundred and seven patients had complete information about utilization of epidural steroid injections at the six-week or three-month follow-up interval, and eighteen were excluded because of incomplete information. Of these 607 patients, 154 received an epidural steroid injection during the first three months of treatment (the ESI group) and 453 did not receive an epidural steroid injection (the No-ESI group). Seventy-seven percent of the patients who received an epidural steroid injection did so within the first three months.
The baseline characteristics and demographics of the ESI and No-ESI cohorts are presented in the Appendix. There were baseline differences between groups with regard to the percentages of patients of white race (ESI: 79% versus No-ESI: 87%, p = 0.041), with full or part-time work status (ESI: 65% versus No-ESI: 60%, p = 0.031), with depression (ESI: 16% versus No-ESI: 9%, p = 0.034), with other comorbidity (ESI: 53% versus No-ESI: 43%, p = 0.039), whose treatment preference at baseline was surgery (ESI: 19% versus No-ESI: 56%, p < 0.001), with any neurological deficit (ESI: 70% versus No-ESI: 79%, p = 0.027), with an asymmetric sensory decrease (ESI: 43% versus No-ESI: 53%, p = 0.03), and who received surgery (ESI: 48% versus No-ESI: 69%, p < 0.001). The groups also differed with regard to the mean baseline SF-36 MCS scores, favoring the No-ESI group (ESI: 43.9 versus No-ESI: 46.5, p = 0.012).
Operative details are presented in the Appendix. There was no significant difference in the details of the surgery, including operative time, blood loss, dural tear rate, or complications of surgery, between the ESI and No-ESI groups. There was also no difference in the rate of revision surgery at one to four years.
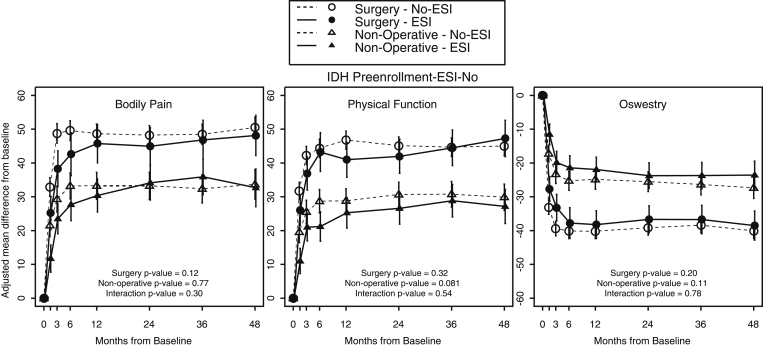
The adjusted change in the outcome measures over four years in the ESI group compared with the No-ESI group is illustrated in Figure 2 and the Appendix. The change in outcome measures was adjusted for age, sex, marital status, smoking status, race, compensation (Workers’ Compensation, Social Security compensation, or other compensation), herniation level and type, work status, depression, self-rated health trend, treatment preference at baseline, baseline SF-36 score, ODI, SBI, and symptom duration. There were no significant differences in primary or secondary outcome measures between the surgically treated ESI and No-ESI groups or between the nonsurgically treated ESI and No-ESI groups at the four year time point or in the area-under-the-curve average measurements. There were no significant differences in the primary outcome measures between the ESI and Non-ESI groups at one, two, or three years regardless of whether they were treated surgically or nonsurgically. Outcome measures were further measured at each individual time point (see Appendix); there was significantly less satisfaction in the surgically treated ESI group (64.5%) than in the surgically treated non-ESI group (80.6%) at one year (p = 0.02). There was a corresponding increase in the treatment effect of surgery on patient satisfaction in the No-ESI group (ESI: 15.8 versus No-ESI: 40, p = 0.012). At one year, there was also an increase in the percentage of working patients in the nonsurgically treated ESI group (ESI: 89.9% versus No-ESI: 78.2%, p = 0.049). The treatment effect of surgery on work status was increased at one year in the No-ESI group (10.7). The treatment effect on work status was decreased in the ESI group (−11.6, p = 0.005). There were no significant differences at two or three years between the ESI and No-ESI groups. There were also no significant differences in the treatment effect of surgery between the ESI and No-ESI groups at four years or averaged over the study period.
Fig. 2.
Change in primary outcome measures between patients with an intradiscal disc herniation (IDH) who received epidural steroid injection (ESI) or did not receive epidural steroid injection (No-ESI) within the first three months after enrollment.
Change from the assigned or chosen treatment (defined as “crossover”) by the patients in the surgical and nonsurgical groups is reported in the Appendix. The percentage of patients assigned to receive surgical treatment who crossed over to undergo nonsurgical treatment was significantly increased in the ESI group (ESI: 41% versus No-ESI: 12%, p < 0.001). There was also a trend toward an increased percentage of patients assigned to nonoperative treatment crossing over to undergo surgical treatment in the ESI group (ESI: 42% versus No-ESI: 30%, p = 0.057). The crossover was further subdivided according to baseline preference for surgery. Analysis of the patients who had a baseline preference for surgery (see Appendix) showed a significant increase in crossover to nonsurgical treatment in the ESI group (13%) compared with the non-ESI group (1%, p = 0.044). There was no significant difference in crossover from nonsurgical to surgical treatment between the ESI group (60%) and the No-ESI group (79%) (p = 0.276) who had a baseline preference for surgery. Analysis of the patients who had a baseline preference for nonsurgical treatment (see Appendix) showed no significant difference in crossover from surgery to nonsurgical treatment between the ESI (48%) and No-ESI (56%) groups (p = 0.623). Conversely, there was no significant difference in crossover from nonsurgical to surgical treatment between the ESI (33%) and No-ESI (20%) groups who had a baseline preference for nonsurgical treatment (p = 0.084).
Discussion
These results indicate no significant difference in outcome at one, two, three, or four years between patients who received an epidural steroid injection and those who did not receive an epidural injection for the treatment of lumbar disc herniation in the SPORT study. However, an increased rate of surgical avoidance was noted in the group treated with an epidural steroid injection.
These results are similar to those of Buchner et al.22, who also found no difference in the outcomes of injection and no-injection treatment. At the one year follow-up evaluation in our study, there was less satisfaction among the surgically treated patients who had had an epidural steroid injection, suggesting that patients who ultimately had surgery were less satisfied as a result of having had the additional procedure. However, the early outcome differences were not significant over the course of the remainder of the study period.
Similar to previous studies13,21, our results suggest that epidural steroid injections are associated with surgical avoidance (a 41% rate of crossover by surgically assigned patients who received epidural steroid injection compared with a 12% rate of crossover by surgically assigned patients who did not receive an epidural steroid injection), although this is heavily influenced by the baseline preference for surgery. Epidural steroid injection was not associated with surgical avoidance or increased crossover to surgery by patients with a baseline preference for nonsurgical treatment. Among patients who had a baseline preference for surgery, epidural steroid injection was associated with a higher rate of crossover from surgical to nonsurgical treatment. One plausible explanation for the differences is possible selection bias—i.e., patients who received an epidural steroid injection may have been less ideal surgical candidates. This assessment of baseline preference has not been reported in other studies on “surgical avoidance,” including that of Riew et al.19. Furthermore, surgical avoidance may not be an ideal goal in the absence of a long-term improvement in outcome with nonsurgical treatment or even equivalence in outcome between surgical and nonsurgical treatment. Our results suggest that patients who “avoided surgery” may have had less improvement than they would have otherwise had.
This study had several unique strengths compared with the previous studies. Patient enrollment in our study (n = 607) far exceeded that of the next largest published study (n = 160)27. This reduced the possibility of type-II error confounding our results. Since this was a retrospective analysis of prospectively collected data, an a priori power analysis was not performed. We performed a post hoc power analysis to determine the effect size that we were powered to detect in the SF-36 and ODI outcome instruments34. Based on the numbers available, there was adequate power to detect the mean differences between groups in the four-year area-under-curve results with the exception of the SF-36 BP domain among the nonsurgically treated patients. The study had an 80% power to detect a difference of 0.7 in the SF-36 BP domain among the nonsurgically treated patients. However, the observed effect size was 0.6. Furthermore, the post hoc analysis showed adequate statistical power to detect a minimum clinically important difference in primary outcome measures. The strict SPORT inclusion criteria limit our results to patients with intervertebral disc herniation only, as opposed to those with either intervertebral disc herniation or spinal stenosis19,21. Furthermore, the duration of follow-up (four years) in this study is significantly longer than that in many previous studies1-3,9,10,13,14,17,18,22,23,29. The current study also includes outcome assessments of surgically treated patients with and without epidural steroid injection, which enables an estimation of the treatment effect of surgery and crossover in both directions. We did not find any significant increase in the incidence of dural tear, spinal fluid leak, postoperative infection, or neurological injury when comparing surgical patients who had had a preoperative epidural steroid injection with those who had not. Therefore, we do not believe that there is a substantial disadvantage of epidural steroid injection in patients who ultimately undergo surgical intervention.
Weaknesses of this study include the possibility of an unknown baseline confounder or selection bias between the ESI and No-ESI groups that would limit the generalizability of the results. Although the known confounders of lumbar disc herniation treatment outcomes (obesity, duration of symptoms, and compensation status) did not differ significantly among the patient cohorts, there is the possibility that an unknown confounder influenced the results. At baseline, there were differences between the ESI and No-ESI groups in SF-36 MCS score and prevalence of depression, suggesting worse overall baseline mental health in the ESI group. There were also clinical differences, including a decreased prevalence of physical findings including neurological deficits and asymmetric sensory decreases in the ESI group. Despite these baseline differences in demographic parameters and clinical factors, there were no significant differences in the primary outcome measures: SF-36 PCS or ODI.
We conclude that further study is necessary to determine the long-term value of epidural steroid injections, given the substantial growing national volume of use and cost of epidural steroid injection.
Appendix
Tables showing baseline demographics, comorbidities, clinical findings, and health status measures; operative treatments, complications, and events; change scores and treatment effects for primary and secondary outcomes according to treatment received; average area-under-the-curve results over four years; crossover of assigned/chosen treatment groups; percentage of patients who underwent surgery at each time point; and crossover of assigned/chosen treatment groups for patients who had baseline preference for surgery and those with a baseline preference for nonoperative treatment are available with the online version of this article as a data supplement at jbjs.org.
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, one or more of the authors has had another relationship, or has engaged in another activity, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Atlas SJ, Keller RB, Wu YA, Deyo RA, Singer DE. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the maine lumbar spine study. Spine (Phila Pa 1976). 2005. Apr 15;30(8):927-35 [DOI] [PubMed] [Google Scholar]
- 2.Buttermann GR. Treatment of lumbar disc herniation: epidural steroid injection compared with discectomy. A prospective, randomized study. J Bone Joint Surg Am. 2004. Apr;86-A(4):670-9 [PubMed] [Google Scholar]
- 3.Rasmussen S, Krum-Møller DS, Lauridsen LR, Jensen SE, Mandøe H, Gerlif C, Kehlet H. Epidural steroid following discectomy for herniated lumbar disc reduces neurological impairment and enhances recovery: a randomized study with two-year follow-up. Spine (Phila Pa 1976). 2008. Sep 1;33(19):2028-33 [DOI] [PubMed] [Google Scholar]
- 4.Lurie JD, Faucett SC, Hanscom B, Tosteson TD, Ball PA, Abdu WA, Frymoyer JW, Weinstein JN. Lumbar discectomy outcomes vary by herniation level in the Spine Patient Outcomes Research Trial. J Bone Joint Surg Am. 2008. Sep;90(9):1811-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinstein JN, Lurie JD, Tosteson TD, Tosteson AN, Blood EA, Abdu WA, Herkowitz H, Hilibrand A, Albert T, Fischgrund J. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976). 2008. Dec 1;33(25):2789-800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goupille P, Jayson MI, Valat JP, Freemont AJ. The role of inflammation in disk herniation-associated radiculopathy. Semin Arthritis Rheum. 1998. Aug;28(1):60-71 [DOI] [PubMed] [Google Scholar]
- 7.Grönblad M, Virri J, Seitsalo S, Habtemariam A, Karaharju E. Inflammatory cells, motor weakness, and straight leg raising in transligamentous disc herniations. Spine (Phila Pa 1976). 2000. Nov 1;25(21):2803-7 [DOI] [PubMed] [Google Scholar]
- 8.Lee HM, Weinstein JN, Meller ST, Hayashi N, Spratt KF, Gebhart GF. The role of steroids and their effects on phospholipase A2. An animal model of radiculopathy. Spine (Phila Pa 1976). 1998. Jun 1;23(11):1191-6 [DOI] [PubMed] [Google Scholar]
- 9.Olmarker K, Byröd G, Cornefjord M, Nordborg C, Rydevik B. Effects of methylprednisolone on nucleus pulposus-induced nerve root injury. Spine (Phila Pa 1976). 1994. Aug 15;19(16):1803-8 [DOI] [PubMed] [Google Scholar]
- 10.Buttermann GR. Lumbar disc herniation regression after successful epidural steroid injection. J Spinal Disord Tech. 2002. Dec;15(6):469-76 [DOI] [PubMed] [Google Scholar]
- 11.Tosteson AN, Skinner JS, Tosteson TD, Lurie JD, Andersson GB, Berven S, Grove MR, Hanscom B, Blood EA, Weinstein JN. The cost effectiveness of surgical versus nonoperative treatment for lumbar disc herniation over two years: evidence from the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976). 2008. Sep 1;33(19):2108-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vad VB, Bhat AL, Lutz GE, Cammisa F. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine (Phila Pa 1976). 2002. Jan 1;27(1):11-6 [DOI] [PubMed] [Google Scholar]
- 13.Wang JC, Lin E, Brodke DS, Youssef JA. Epidural injections for the treatment of symptomatic lumbar herniated discs. J Spinal Disord Tech. 2002. Aug;15(4):269-72 [DOI] [PubMed] [Google Scholar]
- 14.Carette S, Leclaire R, Marcoux S, Morin F, Blaise GA, St-Pierre A, Truchon R, Parent F, Levésque J, Bergeron V, Montminy P, Blanchette C. Epidural corticosteroid injections for sciatica due to herniated nucleus pulposus. N Engl J Med. 1997. Jun 5;336(23):1634-40 [DOI] [PubMed] [Google Scholar]
- 15.Karppinen J, Malmivaara A, Kurunlahti M, Kyllönen E, Pienimäki T, Nieminen P, Ohinmaa A, Tervonen O, Vanharanta H. Periradicular infiltration for sciatica: a randomized controlled trial. Spine (Phila Pa 1976). 2001. May 1;26(9):1059-67 [DOI] [PubMed] [Google Scholar]
- 16.Manchikanti L, Singh V, Cash KA, Pampati V, Damron KS, Boswell MV. Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 2–Disc herniation and radiculitis. Pain Physician. 2008. Nov-Dec;11(6):801-15 [PubMed] [Google Scholar]
- 17.Ng L, Chaudhary N, Sell P. The efficacy of corticosteroids in periradicular infiltration for chronic radicular pain: a randomized, double-blind, controlled trial. Spine (Phila Pa 1976). 2005. Apr 15;30(8):857-62 [DOI] [PubMed] [Google Scholar]
- 18.Wilson-MacDonald J, Burt G, Griffin D, Glynn C. Epidural steroid injection for nerve root compression. A randomised, controlled trial. J Bone Joint Surg Br. 2005. Mar;87(3):352-5 [DOI] [PubMed] [Google Scholar]
- 19.Riew KD, Park JB, Cho YS, Gilula L, Patel A, Lenke LG, Bridwell KH. Nerve root blocks in the treatment of lumbar radicular pain. A minimum five-year follow-up. J Bone Joint Surg Am. 2006. Aug;88(8):1722-5 [DOI] [PubMed] [Google Scholar]
- 20.Daltroy LH, Cats-Baril WL, Katz JN, Fossel AH, Liang MH. The North American spine society lumbar spine outcome assessment Instrument: reliability and validity tests. Spine (Phila Pa 1976). 1996. Mar 15;21(6):741-9 [DOI] [PubMed] [Google Scholar]
- 21.Riew KD, Yin Y, Gilula L, Bridwell KH, Lenke LG, Lauryssen C, Goette K. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am. 2000. Nov;82-A(11):1589-93 [DOI] [PubMed] [Google Scholar]
- 22.Buchner M, Zeifang F, Brocai DR, Schiltenwolf M. Epidural corticosteroid injection in the conservative management of sciatica. Clin Orthop Relat Res. 2000. Jun;(375):149-56 [DOI] [PubMed] [Google Scholar]
- 23.Kolsi I, Delecrin J, Berthelot JM, Thomas L, Prost A, Maugars Y. Efficacy of nerve root versus interspinous injections of glucocorticoids in the treatment of disk-related sciatica. A pilot, prospective, randomized, double-blind study. Joint Bone Spine. 2000;67(2):113-8 [PubMed] [Google Scholar]
- 24.Thomas E, Cyteval C, Abiad L, Picot MC, Taourel P, Blotman F. Efficacy of transforaminal versus interspinous corticosteroid injectionin discal radiculalgia - a prospective, randomised, double-blind study. Clin Rheumatol. 2003. Oct;22(4-5):299-304 [DOI] [PubMed] [Google Scholar]
- 25.Friedly J, Chan L, Deyo R. Geographic variation in epidural steroid injection use in medicare patients. J Bone Joint Surg Am. 2008. Aug;90(8):1730-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedly J, Nishio I, Bishop MJ, Maynard C. The relationship between repeated epidural steroid injections and subsequent opioid use and lumbar surgery. Arch Phys Med Rehabil. 2008. Jun;89(6):1011-5 [DOI] [PubMed] [Google Scholar]
- 27.Birkmeyer NJ, Weinstein JN, Tosteson AN, Tosteson TD, Skinner JS, Lurie JD, Deyo R, Wennberg JE. Design of the Spine Patient outcomes Research Trial (SPORT). Spine (Phila Pa 1976). 2002. Jun 15;27(12):1361-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinstein JN, Lurie JD, Tosteson TD, Zhao W, Blood EA, Tosteson AN, Birkmeyer N, Herkowitz H, Longley M, Lenke L, Emery S, Hu SS. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am. 2009. Jun;91(6):1295-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstein JN, Lurie JD, Tosteson TD, Skinner JS, Hanscom B, Tosteson AN, Herkowitz H, Fischgrund J, Cammisa FP, Albert T, Deyo RA. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006. Nov 22;296(20):2451-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992. Jun;30(6):473-83 [PubMed] [Google Scholar]
- 31.Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine (Phila Pa 1976). 1987. Apr;12(3):264-8 [DOI] [PubMed] [Google Scholar]
- 32.Atlas SJ, Deyo RA, Keller RB, Chapin AM, Patrick DL, Long JM, Singer DE. The Maine Lumbar Spine Study, Part II. 1-year outcomes of surgical and nonsurgical management of sciatica. Spine (Phila Pa 1976). 1996. Aug 1;21(15):1777-86 [DOI] [PubMed] [Google Scholar]
- 33.Patrick DL, Deyo RA, Atlas SJ, Singer DE, Chapin A, Keller RB. Assessing health-related quality of life in patients with sciatica. Spine (Phila Pa 1976). 1995. Sep 1;20(17):1899-908; discussion 1909 [DOI] [PubMed] [Google Scholar]
- 34.Lenth RV. Java Applets for Power and Sample Size [Computer software]. (2006-9). http://www.stat.uiowa.edu/~rlenth/Power. Accessed 18 December 2011 [Google Scholar]