Abstract
The clear cell variant of hepatocellular carcinoma is a rare entity, occurring at a frequency of less than 10% of hepatocellular carcinoma, with a female prevalence and usually associated with hepatitis C and cirrhosis. We reported a case of primary clear cell hepatocellular carcinoma occurring in a non-cirrhotic liver without history of hepatitis. Our examination included gross pathology, histopathology, immunohistochemistry, special stains, and electron microscopy evaluation. The tumor was composed of sheets of medium-to-large cells with foamy and reticulated cytoplasm and small-to-medium sized nuclei with variably prominent nucleoli. Oil red O stain showed abundant intracellular lipid. Periodic Acid-Schiff stain confirmed the presence of abundant glycogen deposition. Immunohistochemically the tumor cells were positive for Hep Par1, negative for epithelial membrane antigen, steroidogenic factor-1, HMB45, melan A, CK7 and CK20. Electron microscopy study was performed, which was first done in a clear cell hepatocellular carcinoma occurring in a non-cirrhotic liver without elevation of liver function tests. Ultrastructural evaluation of the clear cells showed scarce cellular organelles, cytoplasmic lipid vacuoles and swollen mitochondria.
Key words: clear cell hepatocellular carcinoma, immunohistochemisty, ultrastructural study.
Introduction
The clear cell variant of hepatocellular carcinoma is a rare entity, with a reported frequency ranging from 0.4–37%.1–6 Focal clear cell changes may be able to be identified in over 50% of the cases,7 however, tumors composed of more than 90% clear cell are rare.6,8,9 Primary clear cell carcinoma of liver usually occurs against a background of hepatitis6 or cirrhosis.9 Primary clear cell carcinoma in non-cirrhotic liver is very rare.1 We reported here a primary clear cell carcinoma in a non-cirrotic liver with immunohistochemical stain and ultrastructural study. The electron microscopy study was not reported previously in such kind of case.
Case Report
Clinical history and methods
A 62-year-old man with a history of hypertension, hyperlipidimia and type 2 diabetes mellitus was found to have elevated alkaline phosphatase of 111 units/L on routine laboratory tests. The remainder of his liver function tests were within normal range (AST 34 units/L, ALT 35 units/L, total bilirubin 1.0 mg/dL). Physical exam revealed a 179.1 cm man weighing 95.3 kg (Body Mass Index: 29.7). Subsequent work-up revealed an 11 cm mass in the right lobe of the liver. No mass lesion was identified in other organs including bilateral kidneys. Other laboratory tests included total cholesterol 208 mg/dL, LDL cholesterol 134 mg/dL, HDL cholesterol 41 mg/dL, triglycerides 167 mg/dL, HgA1c 6.1%, alpha-fetoprotein of 6.4, and negative hepatitis serologies, anti-smooth muscle and anti-mitochondrial antibodies. His medications included Zetia 10 mg daily, Niaspan 2000 mg daily, Gemfibrozil 600 mg twice daily, Metformin 1000 mg twice daily, Lopressor 50 mg daily, Lasix 40 mg daily, and Lisinopril 5 mg daily. There was no known exposure to occupational chemicals such as vinyl chloride.
Light microscopy and immunohis-tochemistry
Non-neoplastic liver tissue and clear cell carcinoma were fixed in 10% formalin, routinely processed, and embedded in paraffin. Sections were routinely stained with hematoxylin-eosin and Periodic Acid-Schiff with or without digestion. Immunohistochemical study was performed by using antibodies against Hep Par1 (Dako; Glostrup, Denmark; #M7158; 1:100), epithelial membrane antigen (Dako; Glostrup, Denmark; #M0613; 1:100), HMB-45 (Lab Vision; Fremont, CA; #MS-364-S; 1:40), Melan A (Dako; Glostrup, Denmark; #M7196; 1:50), steroidogenic factor-1(SF-1) (R&D Systems; Minneapolis, MN; #PP-N1665-00; 1:100).
Electron microscopy
1 mm cubes of tumor and non-neoplastic liver tissue were cut into 1 mm cubes and immediately fixed in 4% paraformaldehyde and 1% glutaraldehyde in 0.1 M sodium cacodylate buffer and epon-embedded. Ultrathin sections were stained with uranyl acetate and lead citrate in combination, and examined with a Hitachi 7000 electron microscope.
Pathology findings
The initial liver biopsy consisted of tissue cores measuring approximately 1.3 cm in length. They were largely composed of fibrous tissue and necrosis with scant areas of tumor cells with clear cytoplasm. No normal liver parenchyma was identified. Review of immunohistochemical stains performed at an outside institution showed the neoplastic cells were positive in a canalicular pattern for polyclonal carcinoembryonic antigen and negative for CK7 and CK20. An iron stain was focally positive and a reticulin stain showed decreased reticulin fibers.
The patient underwent a right hepatic lobectomy at our institution. Gross examination of the 1610.3 g partial hepatectomy specimen revealed an 11.7 cm unencapsulated mass with a variegated cut surface showing focal greentan discoloration and yellow-tan necrotic areas (Figure 1A). The uninvolved liver parenchyma was non-cirrhotic. Histologic examination of the mass revealed sheets of medium-to-large cells with foamy and reticulated cytoplasm and small to medium sized nuclei with variably prominent nucleoli (Figure 1B). There was no obvious trabecular or pseudoglandular architecture. Mitoses were rare to absent (1 in 25 high-power fields). The clear cells comprised greater than 95% of the tumor and were positive for Hep Par1 (Figure 1D) and negative for epithelial membrane antigen, HMB45, melan A, steroidogenic factor-1 (SF-1) (Table 1)
Figure 1.
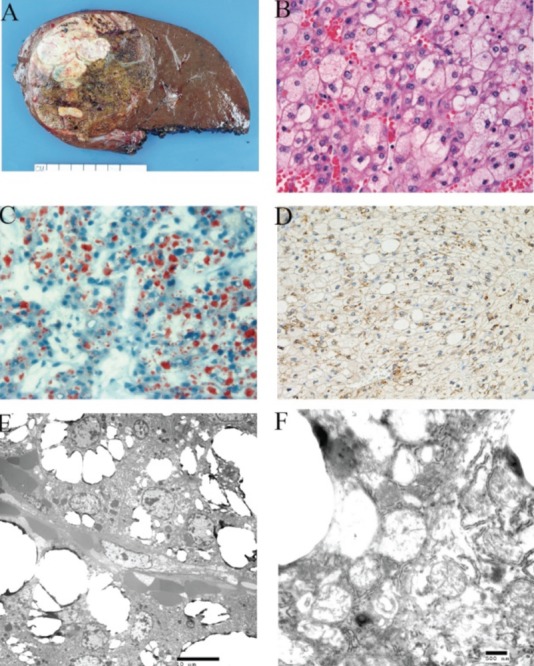
Clear cell variant of hepatocellular carcinoma. A)macroscopic appearance of the tumor; B) Sheet of clear cells in the tumor (×400); C) Oil red O stain showed cytoplasmic lipid in the tumor cells (×400); D) Hep Par1 immunohistochemical stain in the tumor (×400); E) Abundant vacuoles in the tumor cells by electron microscopy (×2,000); F) Mitochondrial swelling in the tumor cells (×20,000).
Table 1. Immunohistochemical stains.
| Markers | Hep Par1 | EMA | HMB-45 | Melan A | SF-1 | CK7 | CK20 |
|---|---|---|---|---|---|---|---|
| Positivity | + | - | - | - | - | - | - |
EMA, epithelial membrane antigen; SF-1 steroidogenic factor-1.
No significant inflammation, steatosis, or fibrosis was identified in the non-neoplastic liver. A diagnosis of moderately differentiated clear cell hepatocellular carcinoma was rendered.
A representative section of the mass was taken for frozen section and stained with Oil Red O. Oil Red O staining revealed abundant intracellular lipid in the neoplastic cells (Figure 1C) with minimal to absent lipid in the non-neoplastic liver.
Fresh tissue from the tumor was taken for electron microscopy examination. Ultrastructural evaluation revealed features of hepatocellular carcinoma including the presence of bile canaliculi bounded by short tight junctions, and presence of microvilli lacking filamentous core rootlets.
The neoplastic cells were filled with empty vacuoles corresponding to cytoplasmic lipid (Figure 1E). There were scarce numbers of organelles in the cytoplasm. The majority of the mitochondria in the tumor cells were swollen. The hydropic mitochondria showed varying degrees of swelling with disorientation, displacement and disintegration cristae (Figures 1F).
Discussion
Clear cell hepatocellular carcinoma is a rare variant of hepatocellular carcinoma that is morphologically similar to other clear cell tumors. We reported a case of primary clear cell hepatocellular carcinoma characterized by >95% of clear cells in a background of non-cir-rhotic liver, which was challenging for diagnosis just by morphology. The differential diagnosis included metastatic clear cell renal cell carcinoma,10 primary clear cell cholangiocarcinoma,11,12 epithelioid angiomyolipoma (pecoma),13–15 metastatic adrenal cortical carcinoma.16 Clear cell renal cell carcinoma can be morphologically indistinguishable from the primary clear cell hepatocellar carcinoma. Immunohistochemical stain plays an important role in reaching an accurate diagnosis. Murakata et al. performed a battery of immunohistochemical markers on a group of primary clear cell hepatocellular carcinoma (HCC) and metastatic clear cell carcinoma and found that Hep Par1 had a high sensitivity and specificity to differentiate primary clear cell HCC from metastatic renal cell carcinoma.10 Epithelioid angiomyolipoma is a rare variant of classical angiomyolipoma and is composed of polygonal cells with clear or eosinophilic cytoplasm and has a carcinoma-like growth.14,17 Melanocytic markers are characteristic for this type of tumor. Melan A and HMB-45 may account for all of this type of tumor. Our case was negaitive for HMB45, therefore excluded such a possibility. Primary clear cell cholangiocarcinoma is a very rare variant of cholangiocarcinoma. Histologically, the tumor is composed of copious clear cytoplasm and forms glandular structures or solid nests, which mimics the primary clear cell hepatocellular carcinoma. Immunohistochemically, this tumor is usually positive for CK7 and CK19,11,12 negative for CK20. In our case, the tumor cells were negative for CK7 and CK20, excluding such possibility. Adrenal cortical carcinoma is rare and follows an aggressive course. This tumor can sometimes metastasize to liver; therefore, it may be included in the differential diagnosis when a clear cell tumor is encountered in the liver. SF-1 is a recently described marker and usually positive in tumor arising from adrenal glands.18 In our case, the tumor was negative for this marker, consistent with report.19 In summary, immunohistochemical study in our cases showed tumor cells were positive for Hep Par1, negative for EMA, HMB45, CK7, CK20 and SF-1, confirmed the diagnosis.
Only fourteen cases of clear cell hepatocellular carcinoma has undergone ultrastructural study,20,21 none was clearly indicated to be performed on the clear cell carcinoma in a non-cirrhotic liver with negative hepatitis infection. We found that the tumor had abundant cytoplasmic lipid and swollen mitochondria. The cytoplasmic lipid was confirmed by oil red O stain. Cytoplasmic clearing of hepatocytes can be observed in a variety of benign and pathologic conditions. In steatosis and other nonspecific forms of hepatocyte injury, hepatocytes undergo ballooning degeneration characterized by enlarged cells with reticulated cytoplasm. Ultrastructurally, ballooning degeneration has been attributed to cytoskeletal rearrangements.22 It was unlikely that neoplastic clear cells of hepatocellular carcinoma represented invasion of steatotic liver parenchyma, as the non-neoplastic liver in this case demonstrated no significant steatosis.
Likewise, little to no steatosis has been observed in the non-neoplastic liver of explants at our institution that contain hepatocellular carcinomas with focal clear cell morphology. Glycogen storage diseases can have morphologically similar hepatic manifestations due to enzyme deficiencies resulting in defective glycogen metabolism. However, ultrastructural evaluation has localized the glycogen accumulation to the cytoplasm in Von Gierke's disease and lysosomes in Pompe's disease.23 Our ultrastructural evaluation of clear cell hepatocellular carcinoma demonstrated lipid accumulation in the cytoplasm as well as swollen mitochondria, which differed from the aforementioned conditions with respect to the nature of accumulated material as well as affected organelle.
Conclusions
While conventional hepatocellular carcinomas generally have a poor prognosis, the prognosis of clear cell hepatocellular carcinoma is controversial. Some studies report no significant difference in prognosis as compared to conventional hepatocellular carcinoma.9 Others report later recurrence in patients with the clear cell variant,24 as well as improved survival, with survival correlating to the proportion of clear cells.6 In our case, the clear cell hepatocellular carcinoma arose in a male without a history of hepatitis C or cirrhosis, a demographic in which the prognosis is less clear.
References
- 1.Adamek HE, Spiethoff A, Kaufmann V, et al. Primary clear cell carcinoma of noncirrhotic liver: immunohistochemical discrimination of hepatocellular and cholangiocellular origin. Dig Dis Sci. 1998;43:33–8. doi: 10.1023/a:1018859617522. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan TF, Jr, Huvos AG. Clear-cell carcinoma of the liver. A clinicopathologic study of 13 patients, Am J Clin Pathol. 1974;61:529–39. doi: 10.1093/ajcp/61.4.529. [DOI] [PubMed] [Google Scholar]
- 3.Emile JF, Lemoine A, Azoulay D, et al. Histological, genomic and clinical heterogeneity of clear cell hepatocellular carcinoma. Histopathology. 2001;38:225–31. doi: 10.1046/j.1365-2559.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- 4.Kashala LO, Conne B, Kalengayi MM, et al. Histopathologic features of hepatocellular carcinoma in Zaire. Cancer. 1990;65:130–4. doi: 10.1002/1097-0142(19900101)65:1<130::aid-cncr2820650126>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Lai CL, Wu PC, Lam KC, Todd D. Histologic prognostic indicators in hepatocellular carcinoma. Cancer. 1979;44:1677–83. doi: 10.1002/1097-0142(197911)44:5<1677::aid-cncr2820440522>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Ma W, Li H, Li Q. Clinicopathological and prognostic features of primary clear cell carcinoma of the liver. Hepatol Res. 2008;38:291–9. doi: 10.1111/j.1872-034X.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- 7.Ng IO, Lai EC, Fan ST, et al. Prognostic significance of pathologic features of hepatocellular carcinoma. A multivariate analysis of 278 patients. Cancer. 1995;76:2443–8. doi: 10.1002/1097-0142(19951215)76:12<2443::aid-cncr2820761207>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Pecorella I, Ciardi A, Aiello E, et al. Clear cell hepatocellular carcinoma treated with liver transplantation. Pathologica. 1994;86:307–10. [PubMed] [Google Scholar]
- 9.Yang SH, Watanabe J, Nakashima O, Kojiro M. Clinicopathologic study on clear cell hepatocellular carcinoma. Pathol Int. 1996;46:503–9. doi: 10.1111/j.1440-1827.1996.tb03645.x. [DOI] [PubMed] [Google Scholar]
- 10.Murakata LA, Ishak KG, Nzeako UC. Clear cell carcinoma of the liver: a comparative immunohistochemical study with renal clear cell carcinoma. Mod Pathol. 2000;13:874–81. doi: 10.1038/modpathol.3880156. [DOI] [PubMed] [Google Scholar]
- 11.Haas S, Gutgemann I, Wolff M, Fischer HP. Intrahepatic clear cell cholangiocarcinoma: immunohistochemical aspects in a very rare type of cholangiocarcinoma. Am J Surg Pathol. 2007;31:902–6. doi: 10.1097/PAS.0b013e31802c0c8a. [DOI] [PubMed] [Google Scholar]
- 12.Toriyama E, Nanashima A, Hayashi H, et al. A case of intrahepatic clear cell cholangiocarcinoma. World J Gastroenterol. 2010;16:2571–6. doi: 10.3748/wjg.v16.i20.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen P, Yuan T, Liu H. Hepatic angiomyolipoma mimicking hepatic clear cell carcinoma. J Int Med Res. 2009;37:257–63. doi: 10.1177/147323000903700132. [DOI] [PubMed] [Google Scholar]
- 14.Mete O, van der Kwast TH. Epithelioid angiomyolipoma: a morphologically distinct variant that mimics a variety of intra-abdominal neoplasms. Arch Pathol Lab Med. 2011;135:665–70. doi: 10.5858/2009-0637-RSR.1. [DOI] [PubMed] [Google Scholar]
- 15.Tsui WM, Yuen AK, Ma KF, Tse CC. Hepatic angiomyolipomas with a deceptive trabecular pattern and HMB-45 reactivity. Histopathology. 1992;21:569–73. doi: 10.1111/j.1365-2559.1992.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 16.Hermsen IG, Gelderblom H, Kievit J, et al. Extremely long survival in six patients despite recurrent and metastatic adrenal carcinoma. Eur J Endocrinol. 2008;158:911–9. doi: 10.1530/EJE-07-0723. [DOI] [PubMed] [Google Scholar]
- 17.Nese N, Martignoni G, Fletcher CD, et al. Pure epithelioid PEComas (so-called epithelioid angiomyolipoma) of the kidney: a clinicopathologic study of 41 cases: detailed assessment of morphology and risk stratification. Am J Surg Pathol. 2011;35:161–76. doi: 10.1097/PAS.0b013e318206f2a9. [DOI] [PubMed] [Google Scholar]
- 18.Sangoi AR, Fujiwara M, West RB, et al. Immunohistochemical distinction of primary adrenal cortical lesions from metastatic clear cell renal cell carcinoma: a study of 248 cases. Am J Surg Pathol. 2011;35:678–86. doi: 10.1097/PAS.0b013e3182152629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasano H, Shizawa S, Suzuki T, et al. Transcription factor adrenal 4 binding protein as a marker of adrenocortical malignancy. Hum Pathol. 1995;26:1154–6. doi: 10.1016/0046-8177(95)90280-5. [DOI] [PubMed] [Google Scholar]
- 20.Singh HK, Silverman JF, Geisinger KR. Fine-needle aspiration cytomorphology of clearcell hepatocellular carcinoma. Diagn Cytopathol. 1997;17:306–10. doi: 10.1002/(sici)1097-0339(199710)17:4<306::aid-dc13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Wu PC, Lai CL, Lam KC, et al. Clear cell carcinoma of liver. An ultrastructural study. Cancer. 1983;52:504–7. doi: 10.1002/1097-0142(19830801)52:3<504::aid-cncr2820520321>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 22.Hubscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006;49:450–65. doi: 10.1111/j.1365-2559.2006.02416.x. [DOI] [PubMed] [Google Scholar]
- 23.Hug G. Glycogen storage diseases. Birth Defects Orig Artic Ser. 1976;12:145–75. [PubMed] [Google Scholar]
- 24.Li T, Fan J, Qin LX, et al. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol. 2011;18:1955–63. doi: 10.1245/s10434-010-1540-z. [DOI] [PubMed] [Google Scholar]


