Abstract
Hepatic epithelioid hemangioendothelioma (HEH) is a rare disease of unknown etiology for which a standard systemic treatment has not been established. The common expression of vascular endothelial growth factor (VEGF) and its receptor in HEH provide a rationale for the reported use of antiangiogenic drugs, including bevacizumab, lenalidomide and thalidomide. We report a case of a young male patient with HEH who was treated with sorafenib for almost 2 years. Sorafenib was used instead of other VEGF inhibitors due to its convenient oral route, its dual antiangiogenic and antiproliferative activity, and its favorable safety profile. Sorafenib therapy resulted in durable stabilization with progressive calcification of liver tumors and minor but stable response of lung lesions.
Key words: hemangioendothelioma, sorafenib, liver.
Introduction
Epithelioid hemangioendothelioma (EH) is a rare malignant vascular soft tissue sarcoma of unknown etiology. Liver is the organ which is most frequently involved by primary EH, although this tumor can develop from any visceral or soft tissue site. For hepatic epithelioid hemangioendothelioma (HEH), a comprehensive review of literature from 1984 to 2005 includes 402 published cases and shows a light female predominance (3/2) as well as a median age of 42 years with a very wide range (3–86).1 Also, this review showed that the most frequent first-symptom was right upper quadrant pain (49%); the tumor frequently involved both hepatic lobes (81%) and than, although 63% of the patients were non-metastatic when extrahepatic lesions were present, lung was the most frequent site of metastases (13% of the patients with metastases).
EH is characterized by an often unpredictable clinical course. Whereas some patients present a rapidly progressive disease, others may remain stable for several years. Liver primary location carries a poor prognosis among the primary sites of EH origin. However, 5-year overall survival of HEH patients after standard primary radical treatment is 55%, and thereby, it is one of the liver malignancies that is associated with a better prognosis among all liver malignancies.2 Moreover, HEH patients undergoing liver transplantation have a 10-year survival rate above 70%.3 Standard treatment options for liver-limited HEH include liver resection, liver transplantation or transcatheter arterial chemoembolization.1,4 Radioembolization is a promising but yet investigational option for these patients.5 A standard systemic treatment has not been established for advanced HEH. Previous literature highlights single case reports of patients treated with several anti-cancer agents, but case series or phase II clinical trials are lacking. Some of these case reports showed successful treatment with interferon, an agent with immunomodulatory and antiangiogenic activity.6–8 Besides, the expression of both vascular endothelial growth factor (VEGF) or its receptor (VEGFR) VEGF in HEH provide a rationale for the use of antiangiogenic drugs.9 Among the more than 200 studies published in English over the last 5 years about EH, those reporting the results of new systemic agents only consider antiangiogenic agents such as bevacizumab, lenalidomide and thalidomide. Therefore, antiangiogenic therapy could be an option to treat EH and HEH. We are reporting herein a case of HEH successfully treated with the antiangiogenic agent sorafenib.
Case Report
A 22 years old male patient, with a previous history of type 1 diabetes, presented with pain in the left side of the chest for about 2 months and a slightly altered performance status (Eastern Cooperative Oncology Group score 1). General examination disclosed nothing but a small hepatomegaly and the results from blood tests including liver function tests within normal limits. A chest and abdominal computed tomography (CT) scan showed multiple bilateral pulmonary nodules and at least 6 hypodense liver lesions suggestive of metastases, one of them in close contact with the right branch of the portal vein. No bone metastases were detected by bone scintigraphy. Serum tumor markers including carcinoembryonic antigen and alpha-fetoprotein were normal. An ultrasound-guided percutaneous liver biopsy showed that the liver parenchyma was massively replaced by a fibrotic tissue containing poligonal tumor cells with round nuclei and a relatively scarce cytoplasm containing vacuolae. In the boundaries of the tumor areas tumor cells showed a sinusoidal growth pattern. Immunohistochemically, tumor cells had a positive staining by CD-34, (cytoplasmatic diffuse 100%), CD-31 (cytoplasmatic diffuse 100%), keratine AE3/AE1 (cytoplasmatic focal), VEGF (intense immunoreactivity, in both membrane and cytoplasm in almost 100% of cells) and were negative by c-erb-B2, EGFR and c-kit. The final pathologic diagnosis was liver epithelioid hemangioendothelioma.
Treatment with sorafenib (Nexavar™ 200 mg tablets), 2 tablets/12 hours (total 800 mg/day) was started in October 2009. Two weeks later, the patient required temporary discontinuation of the treatment due to grade 3 mucocutaneous toxicity (a combination of mucositis, multiform erythema and hand-foot syndrome). After toxicity was resolved, treatment was reinitiated at a reduced dose of 200 mg bid which resulted in partial improvement of side effects. Three months later, in January 2010, chest pain (the primary symptom) had subsided and toxicity consisted of grade 1 multiform erythema, grade 2 hand-foot-syndrome, grade 1 alopecia and grade 1 mucositis. Sorafenib dose was further reduced and since January 2011 a final dose of 200 mg every 36 hours resulted in no mucositis or multiform erythema, and grade 1 hand-foot syndrome.
As shown in Figures 1 and 2, evaluation of tumor response by means of serial CT scans showed a favorable response with a decrease in the size of the lung metastases, disappearance of the left pleural thickening, and a progressive calcification of the liver metastases that nevertheless experienced no major changes in size. Altogether, using Response Evaluation Criteria In Solid Tumors criteria v. 1.0,10 tumor response consisted in a prolonged stabilization. As it can also be observed, after a distinct lung infiltrate appeared in June 2011, a bronchoalveolar lavage disclosed Mycobacterium tuberculosis and the patient is currently receiving tuberculostatic agents.
Figure 1.
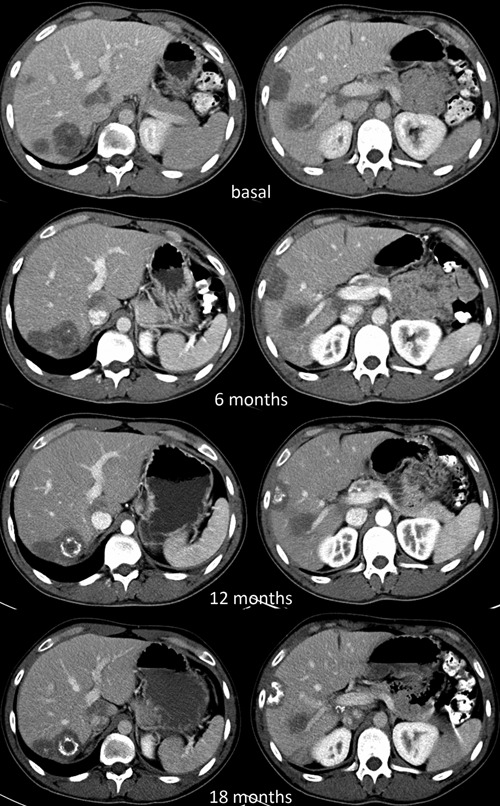
Computed tomography scan showing the evolution of liver metastases at different times.
Figure 2.
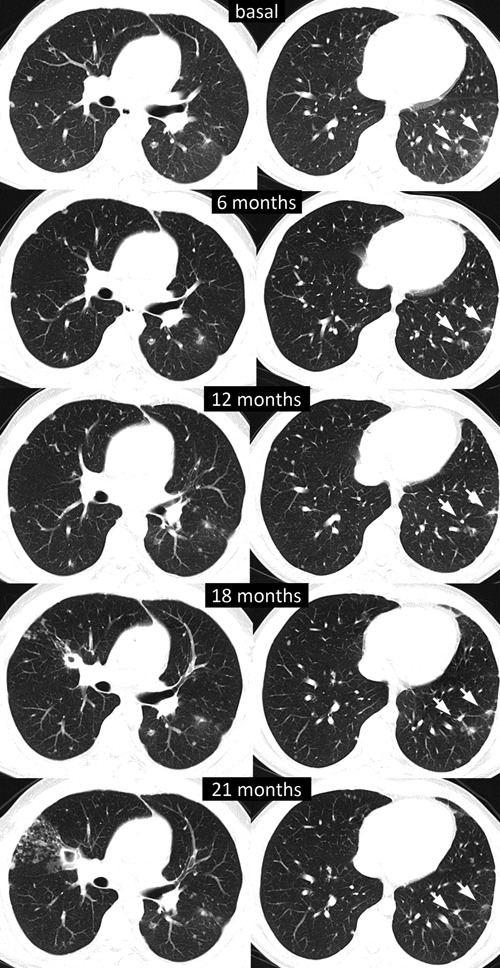
Computed tomography scan showing the evolution of lung metastases at different times.
Discussion
The present case report describes a typical case of HEH. The symptoms (abdominal pain, hepatomegaly), the site of metastases (lung) and the bilobar liver involvement are all characteristic of the initial presentation of this tumor. A small number of single case reports of HEH patients treated with cytotoxic chemotherapy have been published. The most frequently used agent is doxorubicin,11,12 or its liposomal formulation.13,14 However, the results have been far from ideal and HEH is considered a poorly chemosensitive tumor. In the last years antiangiogenic agents that block the VEGF or its receptor, VEGFR, have emerged as anticancer agents. The Raf/MEK/ERK signaling pathway is a key step in both proliferation and angiogenesis. Sorafenib is an inhibitor of the tyrosine-kinase (TK) associated to this signaling pathway. In addition, sorafenib strongly inhibits several TK associated with molecules involved in the angiogenesis process, such as VEGFR-2 and 3 and platelet derived growth factor-β.15 Sorafenib is currently a standard treatment for the treatment of both advanced hepatocellular carcinoma and advanced renal-cell carcinoma.16,17 A phase I clinical trial of sorafenib in patients with solid tumors showed promising efficacy in patients with advanced sarcomas.18 Furthermore, a phase II clinical trial, enrolled 145 patients and showed a response rate of 14% in angiosarcoma patients but minimal activity against other sarcomas.19 A difference in clinical benefit between vascular and nonvascular sarcomas (liposarcoma and leyomiosarcoma) was also observed in another clinical trial which enrolled 51 patients.20 Vascular-sarcoma patients in this trial achieved a higher rate of disease stabilization and a longer progression-free survival when compared with the group of nonvascular-sarcoma patients.
Vascular sarcomas are inherently a possible target for antiangiogenic therapy. Emamaulee et al. investigated the expression of VEGF by means of immunohistochemical assessment in a small study. They found positivity in 6 out of 6 samples of HEH.21 This result, together with the efficacy shown by interferon and other antiangiogenic agents,6–8 such as thalidomide and lenalidomide,22–24 in patients with advanced HEH supports the role of angiogenic pathways as possible targets in patients with HEH. Thus, the initiation of the treatment with the antiangiogenic agent sorafenib was supported by this background. The reason for choosing sorafenib instead of other VEGF inhibitors was mainly the convenience of the oral route, its dual antitumor activity (antiangiogenic and antiproliferative), and the different safety profile. The lower incidence of fatigue and hypertension of sorafenib compared to sunitinib (37% vs. 74% and 17% vs. 28%, respectively, as reported in the package inserts) were judged important due to the age of the patient and the coexistence of diabetes. Its better tolerance and stronger antiangiogenic activity moved us to prefer sorafenib to interferon.
The response achieved in our case was a prolonged stabilization associated with significant intratumoral changes. The response was dissociated, with minor tumor size reduction being observed in the lungs as compared with liver, where progressive calcification, instead of tumor shrinkage, was the main sign of response. Calcification and other changes in tumor density without clear tumor shrinkage are considered as highly suggestive of tumor response, especially after the introduction of the newest antineoplastic molecular-targeted therapies.25 Sorafenib may have the advantage, over other previous antiangiogenic agents, of its dual antitumor activity (antiangiogenic and antiproliferative). HEH is a rare tumor for which clinical trials involving a relevant number of patients are not feasible. In fact, a major objective response was not observed in our case. Nevertheless, we believe that this case provides encouraging evidence of the potential benefit of continued sorafenib treatment in patients with HEH.
References
- 1.Mehrabi A, Kashfi A, Fonouni H, et al. Primary malignant hepatic hemangioendothelioma. A comprehensive review of the literature with emphasis on the surgical therapy. Cancer. 2006;107:2108–21. doi: 10.1002/cncr.22225. [DOI] [PubMed] [Google Scholar]
- 2.Läuffer JM, Zimmermann A, Krähenbühl L, et al. Epithelioid hemangioendothelioma of the liver. A rare hepatic tumor. Cancer. 1996;78:2318–27. doi: 10.1002/(sici)1097-0142(19961201)78:11<2318::aid-cncr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Lewrut JP, Orlando G, Adam R, et al. The place of liver transplantation in the treatment of hepatic epithelioid hemangioendothelioma: report of the European liver transplant registry. Ann Surg. 2007;246:949–57. doi: 10.1097/SLA.0b013e31815c2a70. [DOI] [PubMed] [Google Scholar]
- 4.Cardinal J, de Vera ME, Marsh JW, et al. Treatment of hepatic epithelioid hemangioendothelioma. A single-institution experience with 25 cases. Arch Surg. 2009;144:1035–9. doi: 10.1001/archsurg.2009.121. [DOI] [PubMed] [Google Scholar]
- 5.Laçin S, Küçük O, Oz L, et al. Selective intra-arterial Y-90 microsphere in hemangioendothelioma. Turk J Gastroenterol. 2011;22:89–92. [PubMed] [Google Scholar]
- 6.Calabrò L, Di Giacomo AM, Altomonte M, et al. Primary hepatic hemangioendothelioma progressively responsive to interpheron-alpha: is there room for novel antiangiogenetic treatments? J Exp Clin Cancer Res. 2007;26:145–50. [PubMed] [Google Scholar]
- 7.Galvão FH, Bakonyi-Neto A, Machado MA, et al. Interferon alpha 2B and liver resection to treat multifocal hepatic epithelioid hemangioendothelioma: a relevant approach to avoid liver transplantation. Transplant Proc. 2005;37:4354–8. doi: 10.1016/j.transproceed.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Roudier-Pujol C, Enjolras O, Lacronique J, et al. Multifocal epithelioid hemangioendothelioma with partial remission after interferon alfa-2a treatment. Ann Dermatol Venereol. 1994;121:898–904. [PubMed] [Google Scholar]
- 9.Salech F, Valderrama S, Nervi B, et al. Thalidomide for the treatment of metastatic epithelioid hemangioendothelioma: a case report with long-term follow-up. Ann Hepatol. 2011;10:99–102. [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors (RECIST Guidelines) J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 11.Idilman R, Dokmeci A, Beyler AR, et al. Successful treatment of an epithelioid hemangioendothelioma of liver. Oncology. 1997;54:171–5. doi: 10.1159/000227683. [DOI] [PubMed] [Google Scholar]
- 12.Bellmunt J, Allende E, Navarro M, et al. Epithelioid hemangioendothelioma of the liver with myocardial metastases. Jpn J Clin Oncol. 1989;19:153–8. [PubMed] [Google Scholar]
- 13.Kelly H, O'Neil BH. Response of epithelioid haemangioendothelioma to liposomal doxorubicin. Lancet Oncol. 2005;6:813–5. doi: 10.1016/S1470-2045(05)70392-2. [DOI] [PubMed] [Google Scholar]
- 14.Grenader T, Vernea F, Reinus C, et al. Malignant epithelioid hemangioendothelioma of the liver successfully treated with pegylated liposomal doxorubicin. J Clin Oncol. 2011;29:e722–4. doi: 10.1200/JCO.2011.35.5891. [DOI] [PubMed] [Google Scholar]
- 15.Smith RA, Dumas J, Adnane L, et al. Sorafenib (BAY 43-9006, Nexavar™), a dual-action inhibitor that targets RAF/MEK/ ERK pathway in tumor cells and tyrosine kinases VEGF/PDGFR in tumor vasculature. Methods Enzymol. 2005;407:597–612. doi: 10.1016/S0076-6879(05)07047-3. [DOI] [PubMed] [Google Scholar]
- 16.Llovet J, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 17.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 18.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–72. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 19.Maki RG, D'Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–40. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Mehren M, Rankin C, Goldblum JR, et al. Phase 2 Southwest Oncology Group-directed intergroup trial (S0505) of sorafenib in advanced soft tissue sarcomas. Cancer. 2012;118:770–6. doi: 10.1002/cncr.26334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emamaullee JA, Edgar R, Toso C, et al. Vascular endothelial growth factor expression in hepatic epithelioid hemangioendothelioma: implications for treatment and surgical management. Liver Transpl. 2010;16:191–7. doi: 10.1002/lt.21964. [DOI] [PubMed] [Google Scholar]
- 22.Raphael C, Hudson E, Williams L, et al. successful treatment of metastatic hepatic epithelioid hemangioendothelioma with thalidomide: a case report. J Med Case Reports. 2010;4:413–413. doi: 10.1186/1752-1947-4-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascarenhas RC, Sanghvi AN, Friedlander L, et al. Thalidomide inhibits the growth and progression of hepatic epithelioid hemangioendothelioma. Oncology. 2004;67:471–5. doi: 10.1159/000082932. [DOI] [PubMed] [Google Scholar]
- 24.Sumrall A, Fredericks R, Berthold A, et al. Lenalidomide stops progression of multifocal epithelioid hemangioendothelioma including intracranial disease. J Neurooncol. 2010;97:275–7. doi: 10.1007/s11060-009-0017-z. [DOI] [PubMed] [Google Scholar]
- 25.Stacchiotti S, Collini P, Messina A, et al. High-grade soft tissue sarcomas: tumor response assessment-pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology. 2009;251:447–56. doi: 10.1148/radiol.2512081403. [DOI] [PubMed] [Google Scholar]


