Abstract
We report the widespread occurrence of structurally diverse oleuropeyl glucose esters, including the new diester eucaglobulin B, localized specifically to the essential oil secretory cavities of myrtaceous species. Clear taxonomic patterns in the composition of cavity extracts within the genus Eucalyptus are shown with species from subgenus Symphyomyrtus dominated by oleuropeyl glucose esters and species from subgenus Eucalyptus dominated instead by the flavanone, pinocembrin. We also examined the intra-species occurrence of oleuropeyl glucose esters by quantifying the abundant constituents cuniloside B and froggattiside A in trees from two populations of Eucalyptus polybractea R.T. Baker. All trees contained both compounds, which were positively correlated with total essential oil concentration. This apparent ubiquity of oleuropeyl glucose esters at both intra- and inter-specific levels in Eucalyptus is indicative of important physiological or ecological functions. The significance of their prevalence and the sequestration of these esters and also pinocembrin to the extracellular domain of secretory cavities is discussed in light of their potential biological activities and our findings that they are spatially segregated to the exterior of cavity lumina. The localization of oleuropeyl glucose esters to a specific and isolatable tissue type has the potential to aid in future elucidation of function and biosynthesis.
Introduction
Myrtaceous plants are rich sources of a range of biologically active compounds. In particular, species within the large genus Eucalyptus are well known for the abundant essential oils found in their leaves. Eucalyptus oil is composed of mono- and sesquiterpenes that possess strong antimicrobial activities [1] and has been implicated in plant defence responses to fungal infection, wounding [2] and folivory [3], [4]. Eucalyptus foliage is also characterised by high levels of phenolic compounds including ellagitannins, proanthocyanidins, flavanones, flavonol glycosides, phenolic acids and phloroglucinol derivatives [5], [6], [7]. Many of these are strong antioxidants [8] that may act to quench reactive oxygen species resulting from wounding, pathogen attack and photoinhibition [9], [10]. Eucalyptus phenolics can also deter folivory by reducing palatability and the nutritional value of ingested leaves [4], [11].
Another group of potentially important biologically active compounds are the monoterpene acid glucose esters. These have been found in a diverse range of plant families but are particularly prevalent in Eucalyptus where they have commonly been isolated from bulk leaf extracts, or less often from extracts of fruit capsules (reviewed by [12]). The esters are predominantly composed of the monoterpenoid oleuropeic acid esterified to glucopyranose, generally at the primary hydroxyl position, and either a second monoterpene acid or a phenolic group esterified or glycosylated at the anomeric position (see Fig. 1). They possess an electrophilic α,β-unsaturated carbonyl group in the monoterpenoid and often in the phenolic moiety, and some also have the ability to act as antioxidants through the reducing potential of phenolic hydroxyl groups [12]. Accordingly, some of the Eucalyptus esters show significant in vitro activity against a range of targets including Epstein-Barr virus [13], Escherichia coli, Candida albicans and tumor cell lines [14]. These chemical properties combined with their biological activity suggest that this group of compounds may have potential pharmaceutical and therapeutic applications in addition to important physiological and ecological functions.
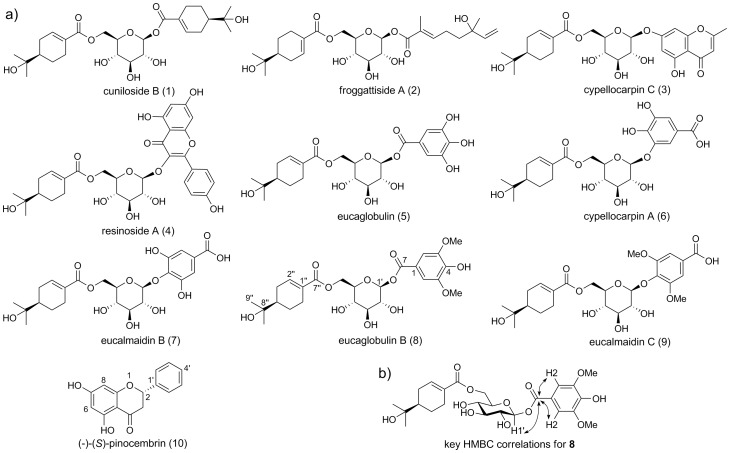
Figure 1. Structures of non-volatile compounds localized to the foliar secretory cavities of Eucalyptus species and Melaleuca armillaris.
(a) Oleuropeyl glucose esters and the flavanone, pinocembrin (10) and (b), key HMBC correlations used in the structural elucidation of eucaglobulin B (8).
Unlike the well characterised terpenes and phenolics from Eucalyptus, no work has been carried out towards establishing a functional role for oleuropeyl glucose esters in planta. Establishing a function is a relatively complex task which is aided by a good understanding of the diversity and ubiquity of the glucose esters in the genus. Some progress has been made in this area. Recently, two of these esters, cuniloside B (1) and cypellocarpin C (3) were shown to occur in bulk leaf extracts from over 20 species of Eucalyptus [15]. In addition, in E. polybractea, 1 and froggattiside A (2) were found to be exclusively localized to the foliar essential oil secretory cavities [12]. It is not known if secretory cavities are the common site of storage of oleuropeyl glucose esters more generally, but if so it may indicate a possible functional role for the esters within the cavities. Indeed, recent work on enzymatically isolated secretory cavities from E. polybractea using multiphoton fluorescence lifetime imaging (MP-FLIM) revealed a unique spatial distribution of the non-volatile component (dominated by 1 and 2) to the exterior of cavity lumina, abutting the secretory cells [16]. This finding raised the prospect of a functional role of these compounds relating to their localization, possibly as a barrier protecting secretory cells from potentially autotoxic volatile terpenes [16], [17].
The work presented here has three aims, each relating to the function of oleuropeyl glucose esters. The first is to document their ubiquity, abundance and diversity in secretory cavities from a range of Eucalyptus species from different sub-genera as well as a species from the sister genus Melaleuca. The second aim is to quantify variation in the abundant oleuropeyl glucose esters 1 and 2 in two natural populations of E. polybractea, and to investigate their relationship with the essential oils co-housed within secretory cavities. The third aim is to explore the consistency of the recently observed spatial arrangement of the non-volatile fraction within cavity lumina [16] by applying MP-FLIM to isolated secretory cavities from three species of Eucalyptus.
Results and Discussion
Localization of Oleuropeyl Glucose Esters to the Secretory Cavities of Eucalyptus
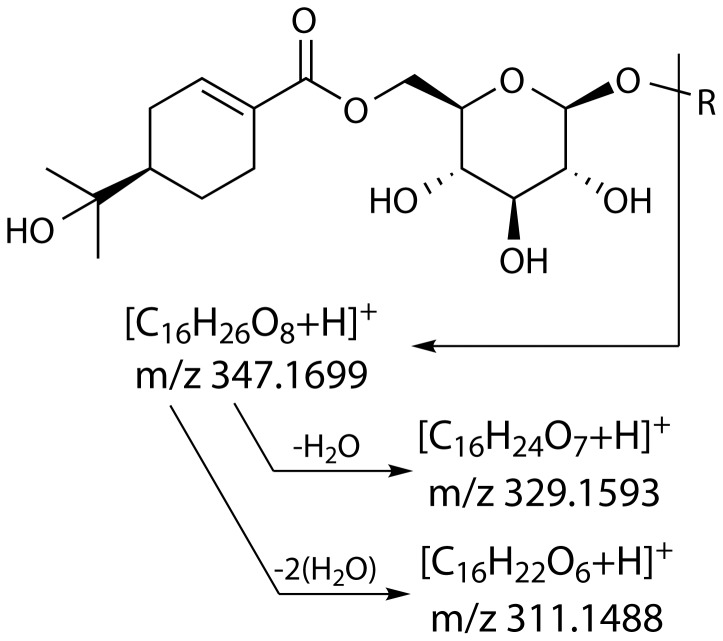
A selective analysis of the genus Eucalyptus was undertaken to examine the occurrence and composition of a non-volatile fraction localised to foliar essential oil secretory cavities. Cavity extracts from 19 species of Eucalyptus in three subgenera and a single species of Melaleuca were analysed using LC-ESI-FTMS (Table 1). Comparison of MS spectra, UV absorbance and retention times with natural standards showed the secretory cavities of all 19 species of Eucalyptus contained 1, whereas 2 was detected in 15 species and also in Melaleuca armillaris (Table 2). In addition, 3 was identified in 15 Eucalyptus species based on comparison with a synthetic standard (Table 2). Both 1 and 2 have a distinctive MS2 fragmentation pattern with the production of two highly abundant C16 fragments of m/z 329.1593 and 311.1488, corresponding to an oleuropeic acid esterified to glucose, with the loss of one or two water molecules, respectively ([18]; Fig. 2). A lower abundance C16 fragment with m/z 347.1699 was also observed, corresponding to an oleuropeyl glucose ester with no loss of water. The fragmentation of 3 resulted in the production of fragment m/z 311.1492, also consistent with its structure containing an oleuropeyl glucose ester (Fig. 1a).
Table 1. Eucalyptus and Melaleuca species surveyed for the presence of non-volatile compounds in the lumen of foliar secretory cavities‡.
| Species # | Species | Subgenus | Section | Series | Authority |
| 1 | Eucalyptus muelleriana | Eucalyptus | Capillulus | Pachyphloius | A.W.Howitt |
| 2 | E. gregsoniana | Eucalyptus | Cineraceae | Pauciflorae | L.A.S.Johnson & Blaxell |
| 3 | E. pauciflora | Eucalyptus | Cineraceae | Pauciflorae | Sieber ex Spreng. |
| 4 | E. olsenii | Eucalyptus | Nebulosa | L.A.S.Johnson & Blaxell | |
| 5 | E. erythrocorys | Eudesmia | Limbatae | Heteropterae | F.Muell. |
| 6 | E. froggattii | Symphyomyrtus | Adnataria | Buxeales | Blakely |
| 7 | E. dielsii | Symphyomyrtus | Bisectae | Elongatae | C.A.Gardner |
| 8 | E. platypus | Symphyomyrtus | Bisectae | Erectae | Hook |
| 9 | E. spathulata | Symphyomyrtus | Bisectae | Erectae | Hook |
| 10 | E. halophila | Symphyomyrtus | Bisectae | Halophilae | D.J.Carr & S.G.M.Carr |
| 11 | E. loxophleba ssp. lissophloia | Symphyomyrtus | Bisectae | Loxophlebae | L.A.S.Johnson & K.D.Hill |
| 12 | E. leptophylla | Symphyomyrtus | Bisectae | Porantherae | F.Muell. |
| 13 | E. myriadena | Symphyomyrtus | Dumaria | Ovulares | Brooker |
| 14 | E. torquata | Symphyomyrtus | Dumaria | Torquatae | Luehm. |
| 15 | E. resinifera | Symphyomyrtus | Latoangulatae | Annulares | Sm. |
| 16 | E. cypellocarpa | Symphyomyrtus | Maidenaria | Globulares | L.A.S.Johnson |
| 17 | E. globulus | Symphyomyrtus | Maidenaria | Globulares | Labill. |
| 18 | E. pulverulenta | Symphyomyrtus | Maidenaria | Orbiculares | Sims |
| 19 | E. dalrympleana | Symphyomyrtus | Maidenaria | Viminales | Maiden |
| 20 | Melaleuca armillaris | (Sol. ex Gaertn.) Sm. |
Eucalyptus taxonomic classification is according to [45].
Table 2. Oleuropeyl glucose esters and a flavanone found in secretory cavity extracts of Eucalyptus species and Melaleuca armillaris.
| Rt (min) | Observed ESI-LC-FTMS parent ions | Formula | Observed MS2 ions of parent ion (bold type) | Compound name | Species # (see Table 1) |
| 5.25 | 516.2067 [M+NH4]+; 521.1624 [M+Na]+ | C23H30O12 | 329.1594 (100); 311.1488 (80); 481.1703 (24);293.1383 (13); 463.1597 (12) | eucaglobulin (5),cypellocarpin A (6),or eucalmaidin B (7)1 | 7,16–19 |
| 5.61 | 546.2899 [M+NH4]+; 551.2456 [M+Na]+; 529.2637 [M+H]+ | C26H40O11 | 511.2536 (100); 493.2430 (45); 329.1594 (35);347.1700 (21); 311.1490 (19); 475.2321 (14) | unknown 1 | 6,11,20 |
| 6.75 | 544.2378 [M+NH4]+; 549.1935 [M+Na]+ | C25H34O12 | 329.1593 (100); 311.1488 (98); 509.2018 (29) | eucalmaidin C (9) | 7–9,14 |
| 6.77 | 546.2899 [M+NH4]+; 551.2457 [M+Na]+; 529.2637 [M+H]+ | C26H40O11 | 511.2535 (100); 329.1594 (59); 493.2430 (29);311.1488 (14); 347.1699 (13) | unknown 2 | 6,7,11,19,20 |
| 7.20 | 530.2951 [M+NH4]+; 535.2505 [M+Na]+; 513.2687 [M+H]+ | C26H40O10 | 329.1593 (100); 311.1488 (81); 495.2583 (36);477.2479 (14); 347.1699 (13) | cuniloside B (1)2 | 1–20 |
| 7.77 | 544.2378 [M+NH4]+; 549.1935 [M+Na]+; 527.2114 [M+H]+ | C25H34O12 | 311.1488 (100); 509.2015 (53);491.1909 (39); 329.1595 (21);347.1490 (7); 275.1278 (6);293.1383 (6); 167.1066 (6) | eucaglobulin B (8)2 | 7–9 |
| 8.00 | 615.2061 [M+H]+; 637.1880 [M+Na]+ | C31H34O13 | 287.0550 (100); 311.1490 (92) | resinoside A (4) | 11,16,17 |
| 8.06 | 521.2009 [M+H]+ | C26H32O11 | 503.1904 (100); 311.1492 (1) | cypellocarpin C (3)2 | 2,5–13,15–19 |
| 8.23 | 505.2635 [M+H]+; 522.2900 [M+NH4]+; 527.2453 [M+Na]+; 487.2529 [M-H2O+H]+ | C24H40O11 | 193.0495 (100); 311.1488 (86) | unknown 3 | 5,7–9,13–15 |
| 8.39 | 530.2950 [M+NH4]+; 535.2505 [M+Na]+; 513.2685 [M+H]+ | C26H40O10 | 329.1593 (100); 311.1488 (71);495.2583 (42); 347.1699 (41);477.2479 (31); 459.2378 (7) | froggattiside A (2)2 | 2,3,6–14,16–20 |
| 9.27 | 602.3884 [M+NH4]+607.3439 [M+Na]+; 585.3621 [M+H]+ | C31H52O10 | 329.1594 (100); 549.3417 (82);311.1487 (32); 347.1699 (21);567.3525 (19);531.3312 (11) | unknown 4 | 7–9,13,16 |
| 9.37 | 521.2009 [M+H]+ | C26H32O11 | 503.1904 (100); 485.1809 (2);467.1702 (1); 311.1492 (1) | unknown 5 | 2–4,7,9,10,12,13,17–19 |
| 9.56 | 556.2744 [M+NH4]+; 561.2296 [M+Na]+; 539.2480 [M+H]+ | C27H38O11 | 521.2378 (100); 311.1489 (23);539.2488 (14); 503.2272 (11);329.1595 (5) | unknown 6 | 9,11,17,19 |
| 9.66 | 522.2900 [M+NH4]+; 527.2453 [M+Na]+; 505.2635 [M+H]+; 487.2530 [M−H2O+H]+ | C24H40O11 | 487.2535 (100); 469.2431 (43);311.1489 (31); 451.2325 (16);321.1542 (4) | unknown 7 | 7–9,13 |
| 10.79 | 602.3885 [M+NH4]+; 607.3441 [M+Na]+; 585.3625 [M+H]+ | C31H52O10 | 549.3419 (100); 531.3314 (95);329.1594 (77); 567.3527 (58);311.1489 (35); 347.1698 (9) | unknown 8 | 7–9 |
| 13.27 | 553.2635 [M+H]+ | C28H40O11 | 535.2537 (100); 517.2427 (28);311.1487 (24) | unknown 9 | 7–9,11,16,17,19 |
| 17.06 | 255.0661 [M−H]−; 511.1386 [2 M−H]- | C15H12O4 | 213.0551 (100); 151.0032 (97);187.0761 (48); 211.0760 (35);169.0655 (30); 183.0813 (21) | pinocembrin (10)2 | 1–4 |
Spectral data consistent with all three compounds.
Identification by comparison with authentic standards or NMR analyses.
Figure 2. MS2 fragmentation of oleuropeyl glucose esters resulting in diagnostic C16 fragments.
We used the characteristic fragmentation pattern of the oleuropeyl glucose ester moiety as a means to search for other structurally related compounds in the non-volatile extracts of secretory cavities. The resultant mass spectra were consistent with eight oleuropeyl glucose esters previously reported from bulk leaf extracts of Eucalyptus (Table 2, Fig. 1a). For example, a pseudomolecular ion peak [M+H]+ at m/z 615.2061 and [M+Na]+ at m/z 637.1880, corresponding to the molecular formula C31H34O13, matched published data for resinoside A (4; [19]). Furthermore, fragmentation of the [M+H]+ ion resulted in the fragments m/z 287.0550 and 311.1490, consistent with the presence of a kaempferol moiety and an oleuropeyl glucose ester, respectively. Interestingly, no mass spectral data matching 4 was detected in the extract from E. resinifera secretory cavities, the species from which this compound was first isolated from whole leaf extracts; however, a spectral match was detected in the extract of E. cypellocarpa cavities, a species from which 4 has been previously reported in leaves [20]. The mass spectrum of a compound eluting at 5.25 min in five of the species matched three structurally related compounds previously reported from Eucalyptus leaf extracts: eucaglobulin (5) [21], cypellocarpin A (6) [13] and eucalmaidin B (7) [22]. Further analyses are required to differentiate between isomers that have gallic acid attached in different ways to the anomeric position of glucose (Fig. 1a).
The cavity extracts of the majority of species also contained up to nine unidentified oleuropeyl glucose ester containing compounds (Table 2). The subgenus Symphyomyrtus was found to be particularly rich in these putative esters with E. dielsii, and E. spathulata containing eight and E. platypus containing six of these compounds. Many of the unknown esters were found in extracts of multiple species. One such compound was present in three species and was particularly abundant in the cavity extract from E. platypus. The compound was purified from that species, subjected to MS and NMR analyses and identified as a new compound, named here as eucaglobulin B (8), owing to its close structural relationship to eucaglobulin (5).
The molecular formula of 8 was established by HR-ESI-FTMS (m/z 544.2378 [M+NH4]+; 549.1935 [M+Na]+; 527.2114 [M+H]+) as C25H34O12. Fragmentation of the [M+NH4]+ ion resulted in the characteristic 311, 329 and 347 daughter ions, consistent with the presence of an oleuropeyl glucose ester. Fragmentation of the [M−H]− ion gave a daughter ion of m/z 197.0448 consistent with a dimethylgallic acid group. The presence of a dimethylgallyl group and an oleuropeyl group suggested structural similarity or identity with the known compound eucalmaidin C (9). The 1H NMR spectrum appeared almost identical to that reported for 9, supporting the presence of oleuropeyl and 3,5-dimethylgallyl esters, with the exception of the anomeric proton being present at δ 5.67 ppm (rather than δ 4.88 as reported for 9). In particular, the downfield shifts of both H1′ and H6′a and H6′b are consistent with ester linkages at these positions. HMBC spectroscopy revealed correlations between H2 and C7 of the 3,5-dimethylgallyl fragment, and between C7 of the 3,5-dimethylgallyl fragment and the glucopyranose H1′, confirming that the 3,5-dimethylgallic acid group was attached to the anomeric position of the glucose (C1′), and the oleuropeic acid group at C6′ (Fig. 1b). Based on these analyses, the structure of 8 was proposed.
It appears the occurrence of oleuropeyl glucose esters is widespread in eucalypts. Along with the evidence presented here, compounds 1 and 3 were recently found in bulk leaf extracts of a further 10 species from three Eucalyptus subgenera and the sister genus Corymbia [15]. Moreover, it appears that most, if not all of these compounds are localised to the lumen of foliar secretory cavities. Furthermore, the positive identification of 1 and 2 as well as the identification of two other putative esters in a species from the genus Melaleuca suggests that non-volatiles localized to secretory cavities may be more widespread in the family Myrtaceae. Interestingly, all species from other families in which oleuropeyl glucose esters have been isolated from bulk leaf extracts produce essential oils and, with the exception of Olea europea, all produce and store essential oils in specialised storage structures [23], [24], [25]. It is tempting to speculate that the esters may also be housed alongside essential oils in specialised secretory structures in the non-myrtaceous species.
The diversity, ubiquity and abundance of oleuropeyl glucose esters across many Eucalyptus species are suggestive of important ecological or physiological roles. A common structural theme shared among these esters is the presence of at least one α,β-unsaturated carbonyl group in the monoterpene acid moieties and often another in the phenolic moiety, if present. This functional group has been shown to be an important determinant of biological activity in a range of molecules including those specifically involved in plant defence [26], [27]. The reactivity of the α,β-unsaturated carbonyls in combination with the reducing potential of any phenolic hydroxyls suggests sequestration to extracellular domains may be a mechanism to avoid potential auto-toxicity within leaves, similar to that proposed for the sequestration of mono- and sesquiterpenes to specialized secretory structures [28].
Based on the LC-PDA and MS-TIC data, the non-volatile extracted from secretory cavities of four species belonging to the subgenus Eucalyptus (E. meulleriana, E. pauciflora, E. gregsoniana and E. olsenii) were dominated by a compound that did not show the characteristic MS2 fragmentation of an oleuropeyl glucose ester, despite such esters being present at lower levels. Instead, the dominant compound had a pseudomolecular ion peak [M-H]− at m/z 255.0661, corresponding to the molecular formula C15H12O4. 1H and 13C NMR analysis and optical rotation identified this compound as (−)-(S)-pinocembrin (10), a flavanone found in a diverse array of species. Of special relevance is its identification in the resinous exudates of leaf glands (including glandular trichomes) of several species including Populus deltoides (Salicaceae) [29], Lychnophora ericoides (Asteraceae) [30], Adenostoma sparsifolium (Rosaceae) [31] and Acacia neovernicosa (Fabaceae) [32]. There is only a single report of its isolation from the leaves of a eucalypt (E. sieberi, also from subgenus Eucalyptus [33]), although it has been reported as a constituent of unifloral honey derived from several eucalypt species [34]. Its localization to the surface secretions of plants is suggestive of a role in defence and indeed it has been shown to be an effective antibacterial, antifungal and antifeedant agent [29], [35], [36]. These activities may relate to the presence of the phenolic groups in its structure (Fig. 2a). Once again, it is possible that sequestration of 10 to secretory cavities is related to its potential for auto-toxicity to the plant.
Intraspecific Variation of 1, 2 and Total Essential Oils
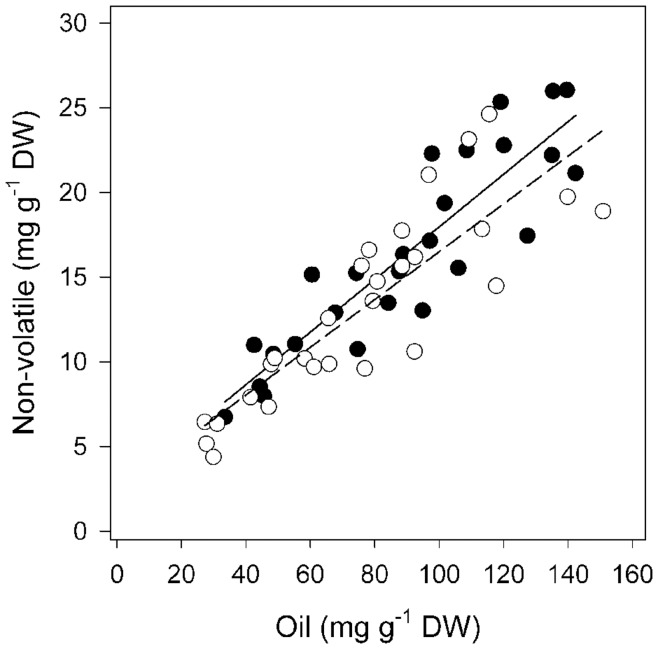
Leaf samples from two E. polybractea populations were analysed for foliar non-volatiles (based on HPLC quantification of 1 and 2 only) and total essential oil content (GC-FID). Mean (± SE) total oil in population A was 89.7±6.5 mg g−1 dry weight (DW) and in population B was 76.7±6.2 mg g−1 DW. The GC profile of all trees was dominated by 1,8-cineole with respective mean percentage abundances (±SE) of 78.5±0.8 and 71.9±1.4. All trees tested contained both 1 and 2 with mean values (± SE) of 14.8±1.0 and 1.5±0.1 mg g−1 DW, respectively for population A and 11.6±0.9 and 1.6±0.2 mg g−1 DW, respectively for population B. Strong positive correlations were found between the concentration of 1 and 2 and the concentration of oil on a dry leaf basis in both populations (r 2 = 0.80 and 0.73 in populations A and B, respectively; Fig. 3).
Figure 3. Relationship between total oil (mg g−1 DW) and non-volatiles (combined cuniloside B (1) and froggattiside A (2); mg g−1 DW) for two populations of E. polybractea (closed circles population A; open circles population B).
Linear regressions were significant with equations of non-volatiles = 2.44+0.15×total oil for Population A (solid line; r 2 = 0.80; F = 96.23, P = 0.00) and non-volatile = 2.40+0.14×total oil for Population B (dashed line; r 2 = 0.73; F = 70.99, P = 0.00).
The strong positive correlations could arise from various factors. First, the components that make up the non-volatile and oil may be biosynthetically linked. Secondly, the non-volatiles and oil may be responding to related selection pressures. Thirdly, the non-volatiles may play a functional role in oil storage. A similarly positive correlation was found in multiple Eucalyptus species between the monoterpene 1,8-cineole and sideroxylonal, a non-volatile formylated phloroglucinol compound (FPC) [37]. FPCs are not known to be stored in secretory cavities and are thought not to be biosynthetically related to monoterpenes, but they were found to map to the same quantitative trait loci in E. nitens [38]. It was suggested that a common regulatory region controlling gene transcription may be responsible for the observed correlations [38]. Nonetheless, a link between sideroxylonal and terpene concentration and possum feeding choice can also explain the positive correlations in evolutionary terms due to the demonstrated role of volatile terpenes in creating a conditioned flavour aversion in marsupial folivores [39]. A similar interaction may be occurring between the non-volatile oleuropeyl esters and terpenes in Eucalyptus, although in this case, the strong likelihood of a shared biosynthetic pathway must be taken into consideration due to the presence of the monoterpene-derived terpenoid moieties.
MP-FLIM of in situ Secretory Cavity Components
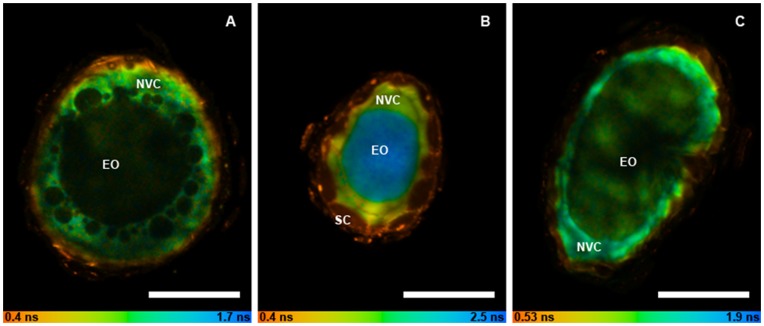
We examined the localization of autofluorescent constituents of the non-volatile component within isolated secretory cavities from E. spathulata, and E. froggattii to see if the non-volatile and oil were spatially segregated as recently reported for E. polybractea [16]. The non-volatile component in cavities from E. spathulata and E. froggattii was found to be highly autofluorescent and images of the cavity lumina clearly show it abutting the secretory cells in a comparable manner to E. polybractea, albeit with markedly different fluorescence lifetimes (as indicated by the pseudo-colour mapping; Fig. 4). Purified compound 8, identified in E. spathulata, was analysed for its fluorescent properties. It was found to be autofluorescent, with respective excitation and emission maxima of 275 nm and 343 nm (consistent with the presence of a gallic acid moiety), and capable of multiphoton excitation. Hence it is likely 8 is contributing to the fluorescence of the non-volatile component in the isolated cavities from this species. In addition, the gallic acid glycoside 9 was detected in E. spathulata and may also be contributing to the observed fluorescence. The major non-volatile constituents from E. polybractea and E. froggattii cavities (compounds 1 and 2) are non-fluorescent and no evidence was found for the presence of esters with gallic acid moieties in the extract of E. froggattii; however, there is evidence for other phenolic esters which are likely responsible for the observed fluorescence (Table 2).
Figure 4. Fluorescence lifetime images of enzymatically isolated eucalypt secretory cavities showing localization of the lumen non-volatile component.
Eucalypt species: E. polybractea (A), E. froggattii (B) and E. spathulata (C). Pseudocolour mapping represents mean fluorescence lifetime. Secretory cells (SC); non-volatile component (NVC); essential oil (EO). Scale bar represents 50 µm.
The occurrence of non-volatile components in essential oil secretory structures has been noted in other species and a protective function was proposed for secretions observed in the subcuticular space of mint trichomes [40]. The common localization of these components to the exterior of cavity lumina supports their speculated function as a region of low diffusivity between the secretory cells and essential oil that may act to protect the cells from the potential autotoxic effects of the oil [12], [16]. Nonetheless, as noted above, sequestration of such potentially biologically active metabolites to extracellular secretory cavities may simply be a means of avoiding auto-toxicity from the non-volatiles themselves. If so, their spatial arrangement within cavity lumina may merely be a result of their relative hydrophilicity compared to terpenes, resulting in secretion into and accretion around the periphery of the lumina, rather than any specific functional role related to this arrangement. More research is required to probe the relationship between the non-volatile component and essential oils co-housed within secretory cavities.
Conclusion
Oleuropeyl glucose esters were identified as common components of secretory cavity extracts in all Eucalyptus species examined. Particular species within the subgenus Symphyomyrtus proved to be rich in these esters and would make ideal targets for future biosynthetic studies or could act as sources of these interesting natural products. The common distribution of these compounds within the genus Eucalyptus and potentially more broadly within the Myrtaceae, as well as their commonality at an intra-specific level and tight correlation with essential oils, is indicative of important functional roles perhaps in plant defence. Moreover, the localisation of these biologically active compounds to secretory cavity lumina provides a strong indication that they may be sequestered there to avoid possible autotoxic effects. The localization of non-volatile constituents to secretory cavities is of particular interest given the recent development of a protocol to isolate live Eucalyptus secretory cavities from leaves [17], which may be used to help elucidate their biosynthetic pathways and function in planta.
Materials and Methods
Ethics Statement
All necessary permits were obtained for the field studies as described below.
Plant Material
Species were selected based on published descriptions of large foliar secretory cavities and essential oil yield and also to represent different subgenera of the genus Eucalyptus. Bulk samples of fully expanded leaves were collected in April 2009 from Eucalyptus trees growing in the Peter Francis Points Arboretum (Colerain, Australia, 37°36.57′S, 141°41.05′E; permission for sample collection was gained from the arboretum curator Ray Clay) and stored at −80°C until analysis. Leaves of Melaleuca armillaris were collected and analysed immediately from trees growing at the University of Melbourne, Parkville, Victoria.
Eucalyptus Polybractea Population Survey
Bulk samples of fully expanded leaves were collected in February 2011 from two natural populations of E. polybractea from north-western Victoria, Australia (population A: 36°31.87′S, 143°44.68′E, population B: 36°32.78′S, 143°44.93′E) located on public land (Parks Victoria collection permit: 10004785) subjected to short-rotation coppicing approximately 2 km apart (see [41]). At the time of harvesting the coppice was 18 months of age. Samples were collected along a 30 m north-south transect with only fully expanded leaves from the current seasons growth harvested. Leaves were kept on ice for no longer than 5 h before being transferred to a −80°C freezer. Duplicate leaves for each tree were ground to a fine powder under liquid nitrogen in a mortar and pestle, extracted in 3 mL hexane containing 100 µg mL−1 tridecane as an internal standard and incubated at 50°C for 4 days. Samples were analysed by GC-FID following the protocol of [42]. Tissue extracted for oil was allowed to dry and then re-extracted with 3 mL 70% acetone at 25°C for 24 h. 180 µL aliquots of acetone extract were made up to 1.20 mL with deionized H2O and loaded onto 30 mg Strata-X reverse phase cartridges for solid-phase extraction (Phenomenex, Torrance, USA,) preconditioned with 100% methanol followed by H2O. Samples were washed with 1 mL 30% methanol in water and then 1 and 2 were eluted with 3 mL 80% methanol. This fraction was further fractionated by RP-HPLC using the Shimadzu HPLC system under the following conditions: Gemini C18 analytical column (Phenomenex, 5 µm, 150×4.6 mm); flow rate, 1 mL min−1; column temperature, 23°C; gradient (acetonitrile/water), 30–45% over 2 min followed by 45–65% over 8 min. Both 1 and 2 were quantified based on PDA responses at 220 nm compared to a standard series of synthetic 1 [15].
Collection of Non-volatile Fraction from Secretory Cavities
With the aid of a stereomicroscope, individual leaves from each species were cut into ∼2 mm wide strips, held under water with forceps and the non-volatile contents of secretory cavities physically removed from the cut edges of the leaf strips using a microprobe with a 1 µm tip (World Precision Instruments, Sarasota, USA). The microprobe tip was then rinsed in a vial of acetonitrile (100%) to dissolve the collected non-volatile material. This process was continued until sufficient non-volatile was collected for analysis. Each acetonitrile collection was dried under a constant stream of N2, re-dissolved in acetonitrile (70%), passed through a 0.45 µm filter and analysed using the LC-MS system.
LC-MS Analyses
The LC-MS system used for fractionation and accurate mass measurements was comprised of a Finnigan Surveyor LC Pump, Surveyor AutoSampler and a linear ion trap coupled to a FT-ICR mass spectrometer LTQ FTMS (Finnigan MAT, Bremen, Germany). The instrument was calibrated weekly with Agilent G2421A solution (Agilent Technologies, Santa Clara, USA) for positive and negative ion mode. The following chromatographic conditions were used for the separation of secretory cavity extracts: Gemini C18 analytical column (Phenomenex, 5 µm, 150×4.6 mm); flow rate, 0.5 mL min−1; column temperature, 23°C; gradient, 20–35% acetonitrile/water acidified with acetic acid (0.1%) over 2 min, followed by 35–65% over 14 min, then 65–100% over 3 min. The following MS source conditions were used in positive ion mode: sheath gas, 60 arbitrary units; spray voltage, 5.3 kV; capillary temperature, 250°C; capillary voltage, 33 V; and tube lens voltage, 80 V. In negative ion mode: sheath gas, 60 arbitrary units; spray voltage, 2.7 kV; capillary temperature, 250°C; capillary voltage, −12 V; and tube lens voltage, −140 V. In both positive and negative mode a scan range of 200–1000 m/z was used and CID carried out at 35% normalized collision energy. Mass spectra were analysed with Xcalibur software (Thermo Electron, San Jose, USA). Identification of 1 and 2 was based on spectral similarity, UV absorbance and retention time of natural standards purified from E. froggattii [18]. Identification of 3 was based on spectral similarity and retention time of a synthetic standard [15].
IR and NMR Spectroscopy
NMR spectra were recorded on Varian 500, Bruker AV 600 (600, 150 MHz), or Bruker Biospin-Avance 800. Chemical shifts (δ) for 1H NMR spectra are reported in parts per million and are followed by multiplicity, coupling constant(s) (J, Hz), integration and assignments. The following abbreviations are used in reporting multiplicities: s, singlet; d, doublet; ABq, AB quartet; br, broad. Residual solvent signals were used for reference: δ 7.26 for 1H NMR in CHCl3, δ 2.05 for 1H NMR and δ 29.84 for 13C NMR in d 6-acetone. IR spectra were obtained on a Perkin–Elmer Spectrum One FTIR spectrometer with a zinc selenide/diamond Universal ATR sampling accessory as a thin film.
Structural Elucidation of Eucaglobulin B
Eucaglobulin B (8) was purified for structure elucidation from bulk leaf extracts of E. platypus. Leaf samples were ground under liquid N2 to a fine powder and extracted in 70% acetone for 24 h at 25°C. The extract was loaded onto a 200 mg Strata-X reverse phase cartridge for solid-phase extraction (Phenomenex) and eluted successively with 10, 20 and 30% acetonitrile. The 20% fraction was dried under a stream of N2 and then redissolved in 50% acetonitrile acidified with 0.1% formic acid. Purification of 8 from this fraction was carried out on a HPLC system comprised of a Shimadzu LC-20AT pump, SIL-20A HT autosampler, SPD-M20A detector and FRC-10A fraction collector (Shimadzu Corporation, Kyoto, Japan) under the following conditions: Gemini C18 analytical column (Phenomenex, 5 µm, 150×4.6 mm); flow rate, 1 mL min−1; column temperature, 23°C; gradient, 20–36% acidified acetonitrile (0.1% formic acid) over 15 min.
[α]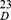 +8.9 (c 0.04, CHCl3); IR ν 3380, 2937, 1703, 1599, 1462, 1426, 1337, 1257, 1216, 1185, 1070, 1035, 762 cm−1; 1H NMR (600 MHz, d
4-MeOH) δ 7.41 (s, 2 H, ArH), 7.03-7.02 (m, 1H, H2′′), 5.68-5.67 (m, 1H, H1′), 4.45 (dd, J = 12.0, 1.8 Hz, 1H, H6′), 4.27 (dd, J = 12.0, 5.4 Hz, 1H, H6′), 3.90 (s, 6H, OMe), 3.70-3.67 (m, 1H, H5′), 3.54-3.49 (m, 2H, H2′,3′), 3.46-3.42 (m, 1H, H4′), 3.33 (m, 1H, H2′), 2.51-2.47 (m, 1H, H6′′), 2.39-2.31 (m, 1H, H3′′), 2.17-2.10 (m, 1H, H6′′), 2.04-1.98 (m, 2H, H5′′/H3′′), 1.56-1.51 (m, 1H, H4′′), 1.20 (dd, J = 12.6, 4.8 Hz, 1H, H5′′), 1.17 (2 s, 2×3H, H9′′,H10′′); 13C NMR (200 MHz, d4-methanol) δ 167.33 (C7′′), 165.13 (C7), 147.37 (C3), 141.18 (C4), 129.59 (C1′′), 118.87 (C1), 107.18 (C2), 104.75 (C2′′), 94.55 (C1′), 44.13 (C6′′), 27.13 (C5′′), 25.34 (C4′′) (Note: Owing to the small amount of material available, the reported 13C data was obtained from HMBC spectrum. Non-ambiguous data could not be obtained for the following carbons: C3′′, C8′′, C9′′, C2′, C3′, C4′, C5′, C6′.). HRMS (ESI+) m/z: 544.2378 [M+NH4]+ (calcd. 544.2399 for C25H38O12NH4) and m/z 549.1935 [M+Na]+ (calcd. 549.1953 for C25H34O12Na). MS2 fragmentation of [M+NH4]+: 311.1488 (100); 509.2015 (53); 491.1909 (39); 329.1595 (21); 347.1490 (7); 275.1278 (6); 293.1383 (6); 167.1066 (6).
+8.9 (c 0.04, CHCl3); IR ν 3380, 2937, 1703, 1599, 1462, 1426, 1337, 1257, 1216, 1185, 1070, 1035, 762 cm−1; 1H NMR (600 MHz, d
4-MeOH) δ 7.41 (s, 2 H, ArH), 7.03-7.02 (m, 1H, H2′′), 5.68-5.67 (m, 1H, H1′), 4.45 (dd, J = 12.0, 1.8 Hz, 1H, H6′), 4.27 (dd, J = 12.0, 5.4 Hz, 1H, H6′), 3.90 (s, 6H, OMe), 3.70-3.67 (m, 1H, H5′), 3.54-3.49 (m, 2H, H2′,3′), 3.46-3.42 (m, 1H, H4′), 3.33 (m, 1H, H2′), 2.51-2.47 (m, 1H, H6′′), 2.39-2.31 (m, 1H, H3′′), 2.17-2.10 (m, 1H, H6′′), 2.04-1.98 (m, 2H, H5′′/H3′′), 1.56-1.51 (m, 1H, H4′′), 1.20 (dd, J = 12.6, 4.8 Hz, 1H, H5′′), 1.17 (2 s, 2×3H, H9′′,H10′′); 13C NMR (200 MHz, d4-methanol) δ 167.33 (C7′′), 165.13 (C7), 147.37 (C3), 141.18 (C4), 129.59 (C1′′), 118.87 (C1), 107.18 (C2), 104.75 (C2′′), 94.55 (C1′), 44.13 (C6′′), 27.13 (C5′′), 25.34 (C4′′) (Note: Owing to the small amount of material available, the reported 13C data was obtained from HMBC spectrum. Non-ambiguous data could not be obtained for the following carbons: C3′′, C8′′, C9′′, C2′, C3′, C4′, C5′, C6′.). HRMS (ESI+) m/z: 544.2378 [M+NH4]+ (calcd. 544.2399 for C25H38O12NH4) and m/z 549.1935 [M+Na]+ (calcd. 549.1953 for C25H34O12Na). MS2 fragmentation of [M+NH4]+: 311.1488 (100); 509.2015 (53); 491.1909 (39); 329.1595 (21); 347.1490 (7); 275.1278 (6); 293.1383 (6); 167.1066 (6).
Structural Elucidation of (–)-(S)-pinocembrin
Pinocembrin (10) was purified for structure elucidation from secretory cavity extracts of E. olsenii. Purification of 10 was carried out on the Shimadzu LC system under the following conditions: Gemini C18 analytical column (Phenomenex, 5 µm, 150×4.6 mm); flow rate, 1 mL min−1; column temperature, 23°C; gradient, 65–100% acetonitrile/water over 5 min.
[α]D 22 −64.6° (c 0.025 in acetone). Lit. [33] −45.3 (c 0.9 in acetone, 15°C); lit. [43] −45.6° (c 0.5 in MeOH); 1H NMR (CDCl3, 500 MHz) δ 12.04 (s, 1H, 5-OH), 7.47-7.38 (m, 5H, Ar), 6.00 (ABq, J = 2.5 Hz, 2H, H6 and H8), 5.43 (dd, J = 12.5, 3.0 Hz, 1H, H2), 3.09 (dd, J = 17.0, 13.0 Hz, 1H, H3b), 2.83 (dd, J = 17.0, 3.0 Hz, 1H, H3b). This data is consistent with that reported [43]; 1H NMR (d 6-acetone, 500 MHz) δ 12.19 (br s, 1H, 5-OH), 7.57-7.55 (m, 3H, Ar), 7.46-7.38 (m, 2H, Ar), 5.96 (d, J = 2.0 Hz, 1H, H8), 5.92 (d, J = 2.0 Hz, 1H, H6), 5.54 (dd, J = 12.5, 3.2 Hz, 1H, H2), 3.12 (dd, J = 17.0, 12.5 Hz, 1H, H3a), 2.77 (dd, J = 17.0, 3.2 Hz, 1H, H3b); 13C NMR (d 6-acetone, 150 MHz) δ 140.0 (C1’), 129.4 (C3’), 129.2 (C4’), 127.2 (C2’), 97.4 (C6), 96.5 (C8), 79.7 (C2), 43.6 (C3) (Note: No signals were observed for the quaternary carbons in the 13C NMR spectrum due to the low concentration of the sample; however, the non-quaternary signals in the 13C NMR spectrum and all the signals in the 1H NMR spectrum were consistent with literature values [44]). HR-FTMS (ESI−) m/z: 255.0661 [M-H]− (calcd. 255.0663 for C15H11O4). MS2 fragmentation of [M-H]−: 213.0551 (100); 151.0032 (97); 187.0761 (48); 211.0760 (35); 169.0655 (30); 183.0813 (21).
MP-FLIM Imaging and Fluorescence Spectrometry
Secretory cavities were isolated from leaf tissue using a pectinase leaf digestion protocol as described in [17]. However, the digestion protocol was altered for E. spathulata from 12 h to 2 h. Isolated cavities were imaged and analysed as reported in [16]. Purified 8 was dissolved in 100% acetonitrile and excitation and emission spectra collected using a Cary Eclipse fluorescence spectrophotometer (Varian Inc, Palo Alto, CA, USA) with a spectral bandwidth of 5 nm. To test for the ability of 8 to undergo multiphoton excitation the compound was dried onto a coverslip and imaged as per the cavities.
Acknowledgments
We thank Ray Clay (Peter Francis Points Arboretum) for access to trees, Jan Heskes for assistance in the field, Mark Richardson and Hamish Blake (University of Melbourne) for NMR assistance and Adrian Lam (University of Melbourne) and Metabolomics Australia for LC-MS support. We also acknowledge Trevor Smith and the Australian Research Council (ARC) Centre of Excellence for Coherent X-Ray Science for MP-FLIM use and assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Allison M. Heskes was supported by the Holsworth Wildlife Research Endowment (managed by ANZ Trustees). Jason Q. D. Goodger and Ian E. Woodrow were supported by the Australian Research Council (Project DP1094530 and LP110100138), and Spencer J. Williams, Sammi Tsegay and Tim Quach (Project DP1093356). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gilles M, Zhao J, An M, Agboola S. Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem. 2010;119:731–737. [Google Scholar]
- 2.Eyles A, Davies NW, Mohammed CM. Traumatic oil glands induced by pruning in the wound-associated phloem of Eucalyptus globulus: chemistry and histology. Trees- Struct Funct. 2004;18:204–210. [Google Scholar]
- 3.Edwards PB, Wanjura WJ, Brown WV. Selective herbivory by Christmas beetles in response to intraspecific variation in Eucalyptus terpenoids. Oecologia. 1993;95:551–557. doi: 10.1007/BF00317440. [DOI] [PubMed] [Google Scholar]
- 4.Matsuki M, Foley WJ, Floyd R. Role of volatile and non-volatile plant secondary metabolites in host tree selection by Christmas beetles. J Chem Ecol. 2011;37:286–300. doi: 10.1007/s10886-011-9916-5. [DOI] [PubMed] [Google Scholar]
- 5.Boulekbache-Makhlouf L, Meudec E, Chibane M, Mazauric J, Slimani S, et al. Analysis by high-performance liquid chromatography diode array detection mass spectrometry of phenolic compounds in fruit of Eucalyptus globulus cultivated in Algeria. J Agric Food Chem. 2010;58:12615–12624. doi: 10.1021/jf1029509. [DOI] [PubMed] [Google Scholar]
- 6.Cadahía E, Conde E, García-Vallejo MC, Fernández de Simón B. High pressure liquid chromatographic analysis of polyphenols in leaves of Eucalyptus camaldulensis, E. globulus and E. rudis: Proanthocyanidins, ellagitannins and flavonol glycosides. Phytochem Anal. 1997;8:78–83. [Google Scholar]
- 7.Ghisalberti EL. Bioactive acylphloroglucinol derivatives from Eucalyptus species. Phytochemistry. 1996;41:7–22. doi: 10.1016/0031-9422(95)00484-x. [DOI] [PubMed] [Google Scholar]
- 8.Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- 9.Close DC, Davies NW, Beadle CL. Temporal variation of tannins (galloylglucoses), flavonols and anthocyanins in leaves of Eucalyptus nitens seedlings: implications for light attenuation and antioxidant activities. Funct Plant Biol. 2001;28:269–278. [Google Scholar]
- 10.Eyles A, Davies NW, Yuan ZQ, Mohammed CL. Host responses to natural infection by Cytonaema sp. in the aerial bark of Eucalyptus globulus. For Pathol. 2003;33:317–331. [Google Scholar]
- 11.DeGabriel JL, Moore BD, Foley WJ, Johnson CN. The effects of plant defensive chemistry on nutrient availability predict reproductive success in a mammal. Ecology. 2009;90:711–719. doi: 10.1890/08-0940.1. [DOI] [PubMed] [Google Scholar]
- 12.Goodger JQD, Woodrow IE. α,β-Unsaturated monoterpene acid glucose esters: structural diversity, bioactivities and functional roles. Phytochemistry. 2011;72:2259–2266. doi: 10.1016/j.phytochem.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Ito H, Koreishi M, Tokuda H, Nishino H, Yoshida T. Cypellocarpins A-C, phenol glycosides esterified with oleuropeic acid, from Eucalyptus cypellocarpa. J Nat Prod. 2000;63:1253–1257. doi: 10.1021/np0001981. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa T, Takano F, Takata T, Niiyama M, Ohta T. Bioactive monoterpene glycosides conjugated with gallic acid from the leaves of Eucalyptus globulus. Phytochemistry. 2008;69:747–753. doi: 10.1016/j.phytochem.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Hakki Z, Cao B, Heskes AM, Goodger JQD, Woodrow IE, et al. Synthesis of the monoterpenoid esters cypellocarpin C and cuniloside B and evidence for their widespread occurrence in Eucalyptus. Carbohydr Res. 2010;345:2079–2084. doi: 10.1016/j.carres.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 16.Heskes AM, Lincoln CN, Goodger JQD, Woodrow IE, Smith TA. Multiphoton fluorescence lifetime imaging shows spatial segregation of secondary metabolites in Eucalyptus secretory cavities. J Microsc. 2012;247:33–42. doi: 10.1111/j.1365-2818.2011.03593.x. [DOI] [PubMed] [Google Scholar]
- 17.Goodger JQD, Heskes AM, Mitchell MC, King DJ, Neilson EH, et al. Isolation of intact sub-dermal secretory cavities from Eucalyptus. Plant Meth. 2010;6:20. doi: 10.1186/1746-4811-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodger JQD, Cao B, Jayadi I, Williams SJ, Woodrow IE. Non-volatile components of the essential oil secretory cavities of Eucalyptus leaves: discovery of two glucose monoterpene esters, cuniloside B and froggattiside A. Phytochemistry. 2009;70:1187–1194. doi: 10.1016/j.phytochem.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Hyodo S, Etoh H, Yamashita N, Sakata K, Ina K. Structure of resinosides from Eucalyptus resinifera as repellents against the blue mussel Mytilus edulis. Biosci Biotechnol Biochem. 1992;56:138. [Google Scholar]
- 20.Kasajima N, Ito H, Hatano T, Yoshida T, Kaneda M. Cypellogins A, B and C, acylated flavonol glycosides from Eucalyptus cypellocarpa. Chem Pharm Bull. 2005;53:1345–1347. doi: 10.1248/cpb.53.1345. [DOI] [PubMed] [Google Scholar]
- 21.Hou AJ, Liu YZ, Yang H, Lin ZW, Sun HD. Hydrolyzable tannins and related polyphenols from Eucalyptus globulus. J Asian Nat Prod Res. 2000;2:205–212. doi: 10.1080/10286020008039912. [DOI] [PubMed] [Google Scholar]
- 22.Tian LW, Zhang YJ, Wang YF, Lai CC, Yang CR. Eucalmaidins A-E, (+)-oleuropeic acid derivatives from the fresh leaves of Eucalyptus maidenii. J Nat Prod. 2009;72:1608–1611. doi: 10.1021/np900290s. [DOI] [PubMed] [Google Scholar]
- 23.Scarpati ML, Trogolo C. 6-O-Oleuropeoylsucrose from Olea europaea. Tetrahedron Lett. 1966;7:5673–5674. [Google Scholar]
- 24.Manns D, Hartmann R. Monoterpene glucosides from Cunila spicata. Planta Med. 1994;60:467–469. doi: 10.1055/s-2006-959534. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi T, Iida N, Inatomi Y, Murata H, Inada A, et al. A monoterpene glucoside and three megastigmane glycosides from Juniperus communis var. depressa. Chem Pharm Bull (Tokyo) 2005;53:783–787. doi: 10.1248/cpb.53.783. [DOI] [PubMed] [Google Scholar]
- 26.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alméras E, Stolz S, Vollenweider S, Reymond P, Mène-Saffrané L, et al. Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. 2003;34:205–216. doi: 10.1046/j.1365-313x.2003.01718.x. [DOI] [PubMed] [Google Scholar]
- 28.Fahn A. Secretory tissues in vascular plants. New Phytol. 1988;108:229–257. doi: 10.1111/j.1469-8137.1988.tb04159.x. [DOI] [PubMed] [Google Scholar]
- 29.Shain L, Miller JB. Pinocembrin: an antifungal compound secreted by leaf glands of eastern cottonwood. Phytopathology. 1982;72:877–880. [Google Scholar]
- 30.Gobbo-Neto L, Gates PJ, Lopes NP. Negative ion ‘chip-based’ nanospray tandem mass spectrometry for the analysis of flavonoids in glandular trichomes of Lychnophora ericoides Mart. (Asteraceae). Rapid Commun Mass Spectrom. 2008;22:3802–3808. doi: 10.1002/rcm.3802. [DOI] [PubMed] [Google Scholar]
- 31.Proksch M, Weissenböck G, Rodriguez E. Flavonoids and phenolic acids in Adenostoma, a dominant genus of the californian chaparral. Phytochemistry. 1985;24:2889–2891. [Google Scholar]
- 32.Wollenweber E, Seigler DS. Flavonoids from the exudate of Acacia neovernicosa. Phytochemistry. 1982;21:1063–1066. [Google Scholar]
- 33.Bick IRC, Brown RB, Hillis WE. Three flavanones from leaves of Eucalyptus sieberi. Aust J Chem. 1972;25:449–451. [Google Scholar]
- 34.Yao L, Jiang Y, D’Arcy B, Singanusong R, Datta N, et al. Quantitative high-performance liquid chromatography analyses of flavonoids in Australian Eucalyptus honeys. J Agric Food Chem. 2004;52:210–214. doi: 10.1021/jf034990u. [DOI] [PubMed] [Google Scholar]
- 35.Hanawa F, Yamada T, Nakashima T. Phytoalexins from Pinus strobus bark infected with pinewood nematode, Bursaphelenchus xylophilus. Phytochemistry. 2001;57:223–228. doi: 10.1016/s0031-9422(00)00514-8. [DOI] [PubMed] [Google Scholar]
- 36.Diaz Napal GN, Carpinella MC, Palacios SM. Antifeedant activity of ethanolic extract from Flourensia oolepis and isolation of pinocembrin as its active principle compound. Bioresour Technol. 2009;100:3669–3673. doi: 10.1016/j.biortech.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 37.Moore BD, Wallis IR, Palá-Paúl J, Brophy JJ, Willis RH, et al. Antiherbivore chemistry of Eucalyptus- cues and deterrents for marsupial folivores. J Chem Ecol. 2004;30:1743–1769. doi: 10.1023/b:joec.0000042399.06553.c6. [DOI] [PubMed] [Google Scholar]
- 38.Henery ML, Moran GF, Wallis IR, Foley WJ. Identification of quantitative trait loci influencing foliar concentrations of terpenes and formylated phloroglucinol compounds in Eucalyptus nitens. New Phytol. 2007;176:82–95. doi: 10.1111/j.1469-8137.2007.02159.x. [DOI] [PubMed] [Google Scholar]
- 39.Lawler IR, Stapley J, Foley WJ, Eschler BM. Ecological example of conditioned flavor aversion in plant–herbivore interactions: effect of terpenes of Eucalyptus leaves on feeding by common ringtail and brushtail possums. J Chem Ecol. 1999;25:401–415. [Google Scholar]
- 40.Turner GW, Gershenzon J, Croteau RB. Development of peltate glandular trichomes of peppermint. Plant Physiol. 2000;124:665–680. doi: 10.1104/pp.124.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodger JQD, Connelly CA, Woodrow IE. Examination of the consistency of plant traits driving oil yield and quality in short-rotation coppice cultivation of Eucalyptus polybractea. For Ecol Manage. 2007;250:196–205. [Google Scholar]
- 42.King DJ, Gleadow RM, Woodrow IE. Terpene deployment in Eucalyptus polybractea; relationships with leaf structure, environmental stresses, and growth. Funct Plant Biol. 2004;31:451–460. doi: 10.1071/FP03217. [DOI] [PubMed] [Google Scholar]
- 43.Yuan Y, Yang QY, Tong YF, Chen F, Qi Y, et al. Synthesis and enantiomeric resolution of (±)-pinocembrin. J Asian Nat Prod Res. 2008;10:999–1002. doi: 10.1080/10286020802240418. [DOI] [PubMed] [Google Scholar]
- 44.Jung JH, Pummangura S, Chaichantipyuth C, Patarapanich C, McLaughlin JL. Bioactive constituents of Melodorum fruticosum. Phytochemistry. 1990;29:1667–1670. [Google Scholar]
- 45.Brooker MIH. A new classification of the genus Eucalyptus L’Hér.(Myrtaceae). Aust Syst Bot. 2000;13:79–148. [Google Scholar]