Abstract
An effective immune response to antigen challenge is critically dependent on the size of the effector cell population generated from clonal activation of antigen-specific T cells. The transcription network involved in regulating the size of the effector population, particularly for CD4 helper T cells, is poorly understood. Here we investigate the role of Id2, an inhibitor of E protein transcription factors, in the generation of CD4 effectors. Utilizing a T cell-specific conditional Id2 knock-out mouse model, we show that Id2 is essential for the development of experimental autoimmune encephalomyelitis (EAE). Although antigen-specific and IL-17 producing CD4 T cells are produced in these mice, the activated CD4 T cells form a smaller pool of effector cells in the peripheral lymphoid organs, exhibit reduced proliferation and increased cell death, and are largely absent in the central nervous system. In the absence of Id2, E protein targets, including the pro-apoptotic protein Bim and Suppressor of cytokine signaling 3 (SOCS3), are expressed at higher levels among activated CD4 T cells. This study reveals a critical role of Id2 in the control of effector CD4 T cell population size and the development of a Th17-mediated autoimmune disease.
Introduction
The magnitude of a CD4 T cell response is a tightly controlled feature of the adaptive immune system. Upon activation through T cell receptor and co-stimulatory molecule signaling, a few antigen-specific CD4 T cells can proliferate to form a large pool of effector cells capable of performing immune functions. The expansion phase is then followed by contraction of the population to leave a small number of long-lived memory cells. Various intrinsic and extrinsic factors, including transcription factors and cytokines, have been implicated in regulating the T cell population size at each stage of this response (1). For example, expression of the transcription factors T-bet (2) and Blimp-1 (3) has been shown to be associated with reduced T cell survival upon contraction, whereas IL-7 has been shown to promote T cell survival through this phase by promoting the expression of anti-apoptotic protein Bcl-2 (4). However, why each individual T cell expresses different levels of these intrinsic factors, why each individual T cell responds differently to extrinsic factors and how these intrinsic-extrinsic factors cross-regulate each other are still not well understood.
Recently, the Inhibitor of DNA Binding (Id) proteins, a family of helix-loop-helix transcriptional regulators, including Id2 and Id3, have been identified to be important in the control of many aspects of T cell responses, including the T cell population size. In CD8 T cells, Id2 and Id3 have been shown to control the numbers of effector and memory cells, at least partially through promoting T cell survival (5-7). In CD4 T cells, Id3 deficiency is also associated with reduced regulatory T cell differentiation (8). In addition, Id3 has been shown to be important for the enforcement of naïve T cell state (9), and Id3-deficient mice spontaneously develop a T cell-mediated autoimmune disease similar to human Sjögren's syndrome (10). However, relatively little is known about the role of Id2 in CD4 T cell responses. One previous report has shown that Id2-deficient mice have increased Th2 dominance, but this difference was largely caused by the lack of a CD8+ dendritic cell subset and therefore was not necessarily related to Id2 function in CD4 T cells (11). Nevertheless, two other studies with double-positive thymocytes (12) and pro-T cell lines (13) showed that E protein transcription factors, the direct target proteins of Id2, may regulate genes important for CD4 T cell responses, including apoptosis-related genes Bcl-2 and Bim, cell cycle-related genes Rb and Cdk6, and cytokine signal regulators SOCS1 and SOCS3(12, 13). SOCS1 and SOCS3 directly control the CD4 T cell response to multiple cytokines regulating effector and/or memory function and population size, such as IL-7, IL-6, IL-12 and IL-15 (14). Many of those cytokines can also regulate the expression of Id2 (6). Thus, Id2 may be involved in the cross-regulation of intrinsic and extrinsic factors for CD4 T cell population control. Thus far, studies based on Id2 knock-out mice cannot resolve these possibilities because the mice do not have normal development of lymph nodes (15), and the model cannot separate CD4 T cell-intrinsic role of Id2 from extrinsic ones.
To investigate how Id2 is involved in CD4 T cell responses, we studied Id2 conditional knock-out mice (16) with the experimental autoimmune encephalomyelitis (EAE) model, a CD4 T cell-dominant autoimmune disease model. EAE is a rodent model of human multiple sclerosis. By administering exogenous neuroautoantigens, a small number of pre-existing autoreactive CD4 T cells in the mice can be activated and induce central nervous system inflammation, demyelination and paralytic symptoms. The EAE model is an ideal tool to reveal potential roles of Id2 in many aspects of CD4 T cell responses, including T cell activation, differentiation, migration and population maintenance. With an Id2 reporter mouse model, we found that Id2 is dynamically regulated in the process of CD4 T cell activation. More strikingly, mice with T cell-specific Id2 deficiency are resistant to EAE, developing a smaller effector population in their peripheral lymphoid organs that fail to infiltrate the central nervous system (CNS). This defect is at least in part due to reduced percentage of proliferating cells and increased death of effector CD4 T cells, and analysis of genes dysregulated in the absence of Id2 showed higher expression of Bim and SOCS3 in these cells. These results establish the importance of Id2 in effector CD4 T cell population size control.
Materials and methods
Animals
The Id2hCD5/hCD5 reporter mouse model has been reported previously (16). Briefly, an IRES-driven, truncated human CD5 (hCD5) cDNA, without the sequence encoding the intracellular signaling domain, was knocked-in to the 3′ untranslated region of the Id2 gene. The Id2f/f mouse was used to generate conditional Id2 knock-out mice and has been described (27). The entire protein coding region of the Id2 gene was flanked by loxP sites. These mice were crossed with CD4-Cre transgenic mice (Taconic, Hudson, NY) to generate T cell-specific Id2-deficient mice (Δ/Δ, Id2f/f CD4Cre+). The CD4Cre transgene-negative littermates were used as wild type controls (f/f, Id2f/f CD4Cre-). All animal work was reviewed and approved by the Duke IACUC.
T cell culture and stimulation
Splenic CD44lowCD62L+ naïve CD4 T cells were sorted and cultured in RPMI1640 medium supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin and 55 μM 2-mercaptoethanol. For TCR stimulation, cells were treated with plate-bound anti-CD3ε (1 μg/mL) and anti-CD28 (2.5 μg/mL) (both from Biolegend, San Diego, CA) plus IL-2 (2 ng/mL, PeproTech, Rocky Hill, NJ). For un-stimulated wells, cells were treated with IL-2 only.
Induction of EAE
EAE was induced in 6-week-old male mice by subcutaneous injection with 100 μg myelin oligodendrocyte glycoprotein35-55 (MOG35-55) peptide (MEVGWYRSPFSRVVHLYRNGK, United Peptide Corporation, Rockville, MD) emulsified in 100μL CFA (Sigma-Aldrich, St. Louis, MO) containing 2 mg/ml of heat-killed Mycobacterium tuberculosis H37RA (Difco, Detroit, MI). Mice were also injected intraperitoneally with 200ng of pertussis toxin (List Biological Laboratories, Campbell, CA) on day 0 and day 2. Clinical signs of EAE were recorded daily using a 0 to 4 scoring system: 0, normal; 1, limp tail; 2, unsteady gait; 3, hind-limb paralysis; 4, four-limb paralysis.
Cell preparation and flow cytometry analysis
CD4 T cells were harvested from spleens and draining lymph nodes (inguinal and axillary). Mononuclear cells from the CNS were obtained as following: brain and spinal cord tissue were digested with 2.5 mg/mL collagenase D (Roche Applied Science, Indianapolis, IN) at 37°C for 50 minutes. The tissue was then passed through a 70μm cell strainer and applied to Percoll gradient (30%/70%) centrifugation. Lymphocytes were collected from the interface.
Antibodies against CD4 (RM4-5), CD8α (53-6.7), CD44 (IM7), CD62L (MEL-14), IL-17A (Tc11-18H10.1), IFN-γ (XMG1.2) were from Biolegend. The antibody against human CD5 (hCD5, UCHT2) was from BD Bioscience (San Jose, CA). 7AAD was from Life Technologies (Grand Island, NY). The mouse MOG38-49 I-A (b) tetramer was supplied by the NIH Tetramer Core Facility to identify the MOG-specific CD4 T cells. For surface staining, single-cell suspensions (2×106 cells) were stained for 15 minutes at 4°C. For tetramer staining, cells were stained for 3 hours at 37°C as previously reported (17). For intracellular staining, cells were stimulated with PMA (10 ng/mL) and ionomycin (1 μg/mL) in the presence of monensin (3μM) (all from Sigma-Aldrich) for 5 hours at 37°C, stained for surface markers and 7AAD, then fixed and permeabilized with the Cytofix/Cytoperm kit (BD), immediately followed by intracellular staining and flow cytometry analysis. For BrdU staining, 1 mg of BrdU in D-PBS was injected intraperitoneally 15 hours before sacrificing the animal, and the staining was performed with the BD BrdU Flow Kit according to manufacturer protocols. Cells were analyzed with a FACSCanto flow cytometer (BD) or sorted with a MoFlo cell sorter (Beckman Coulter, Indianapolis, IN).
Real-time PCR
Splenocytes were harvested from mice 9 days after EAE induction. CD4 T cells were enriched with the EasySep mouse CD4+ T cell enrichment kit (Stemcell Technologies, Vancouver, Canada). MOG I-A(b)+CD4+ T cells were sorted, and total RNA from the cells were extracted followed by DNase I treatment using the RNAqueous micro kit (Life Technologies). Reverse transcription was performed with M-MLV reverse transcriptase (Life Technologies). The cDNA was used for real-time PCR with a Mastercycler ep realplex (Eppendorf, Hamburg, Germany). 18s rRNA was used as an internal control. The primer sequences are: Bcl2F, GGACTTGAAGTGCCATTGGTA; Bcl2R, GTTATCATACCCTGTTCTCCCG; Bcl2probe, /56-FAM/TGCGCCATC/ZEN/CTTCCCCGAAA/3IABkFQ/; BimF, GAGATACGGATTGCACAGGAG; BimR, CGGAAGATAAAGCGTAACAGTTG; Bimprobe, /56-FAM/TTCAGCCTC/ZEN/GCGGTAATCATTTGC/3IABkFQ/; SOCS1F, CTGCAGGAGCTGTGTCG; SOCS1R, CCCCACTTAATGCTGCGG; SOCS1probe, /56-FAM/CGCATCCCT/ZEN/CTTAACCCGGTACTC/3IABkFQ/; SOCS3F, CCTATGAGAAAGTGACCCAGC; SOCS3R, TTTGTGCTTGTGCCATGTG; SOCS3probe, /56-FAM/CCCCTCTGA/ZEN/CCCTTTTGCTCCTT/3IABkFQ/; 18s rRNAF, GTT CCT TTG GTC GCT CGC TCC TC; 18S rRNAR, GGC ACG GCG ACT ACC ATC GA
Results
Activated CD4 T cells express higher levels of Id2 than naïve CD4 T cells
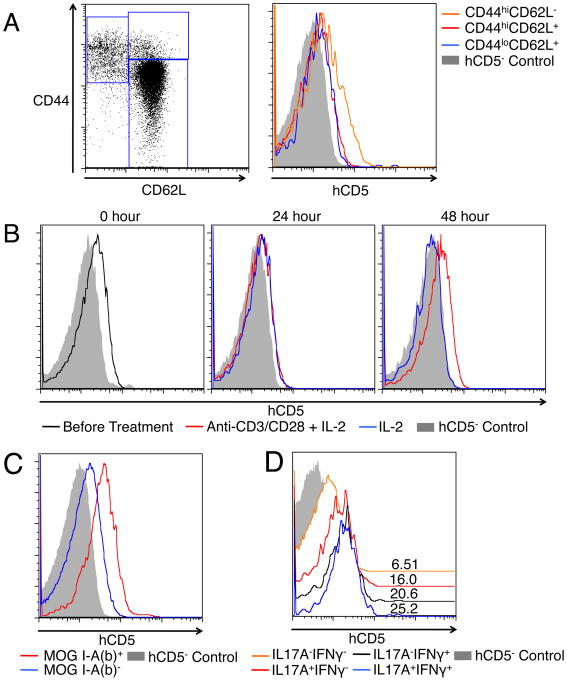
We used an Id2hCD5/hCD5 reporter mouse model, in which surface hCD5 expression can be used as a marker for cellular Id2 expression (16), to first investigate the expression of Id2 in different subsets of CD4 T cells. In the spleen of naïve mice, we found that CD44hiCD62L- effector memory-like CD4 T cells expressed higher levels of hCD5 than the CD44hiCD62L+ central memory-like or the CD44lowCD62L+ naïve CD4 T cells (Figure 1A), suggesting that Id2 expression is correlated with previous T cell activation. Because the exact activation history of the pre-existing effector memory-like CD4 T cells is not defined in these mice, we next sorted CD44lowCD62L+ naïve CD4 T cells and cultured them with anti-CD3/anti-CD28 stimulation plus IL-2 or with IL-2 only. After 48 hours of culture, the TCR-stimulated cells expressed higher levels of hCD5 than the un-stimulated cells (Figure 1B), showing that Id2 is dynamically regulated in the process of T cell activation. These differences of Id2 expression have been verified by quantitative PCR (Supplementary Figure 1). In order to confirm this finding in vivo, we induced EAE, a CD4 T cell-mediated autoimmune disease, in the Id2hCD5/hCD5 reporter mice. Nine days after subcutaneous immunization with MOG peptide, we analyzed the MOG-specific CD4 T cells, as well as IL-17A and/or IFNγ-producing CD4 T cells from the draining lymph nodes. We again found that these cells expressed higher levels of hCD5 than the non-MOG-specific or non-cytokine-producing CD4 T cells (Figure 1C-D). The in vitro and in vivo findings of Id2 up-regulation after CD4 T cell activation suggest that Id2 may play a role during the activation process and/or in the maintenance of the activated T cells.
Figure 1. Activated CD4 T cells express higher levels of Id2 than naïve CD4 T cells.
A: The expression of hCD5 by subsets of splenic CD4 T cells from Id2hCD5/hCD5 reporter mice was analyzed. Left: gating of the subsets. Right: histograms showing the hCD5 expression of CD44hiCD62L- (orange), CD44hiCD62L+ (red) and CD44lowCD62L+ (blue) CD4 T cells. B: Splenic CD44lowCD62L+ naïve CD4 T cells from Id2hCD5/hCD5 reporter mice were sorted and cultured with anti-CD3/anti-CD28 antibodies plus IL-2 (red) or with IL-2 only (blue). Histograms show their hCD5 expression before, 24 hours and 48 hours after culture. C: Expression of hCD5 by MOG I-A (b)+ (red) or MOG I-A (b)- (blue) CD4 T cells isolated from draining lymph nodes of Id2hCD5/hCD5 reporter mice 9 days after EAE induction. D: Expression of hCD5 by IL-17A+IFNγ+ (blue), IL-17A-IFNγ+ (black), IL-17A+IFNγ- (red) and IL-17A-IFNγ- (orange) CD4 T cells from the same lymph nodes as C. Numbers above histograms indicate mean fluorescence intensity. Shaded histograms in each plot showed hCD5 background of comparable populations of wild type CD4 T cells. All plots are representative of three independent experiments.
Mice with T cell-specific Id2 deficiency do not develop EAE
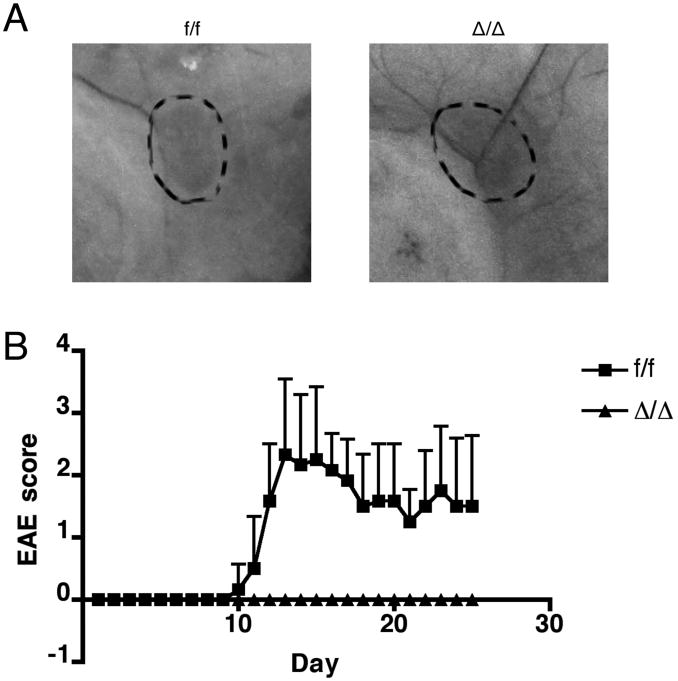
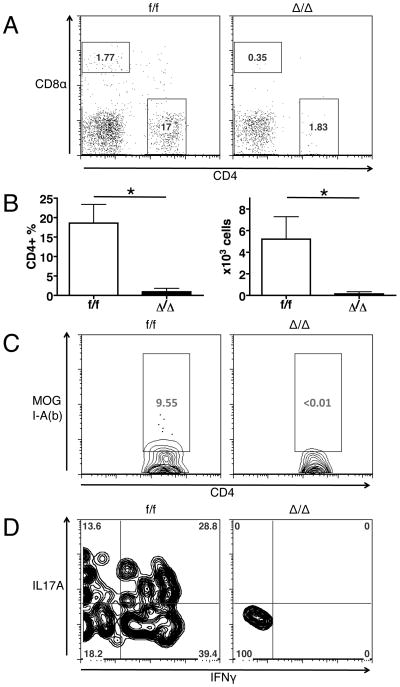
To test the functional significance of the up-regulation of Id2 expression in CD4 T cells during immune responses in vivo, we utilized the EAE model to compare the control (f/f, Id2f/fCD4Cre-) mice versus T cell-specific Id2-deficient (Δ/Δ, Id2f/f CD4Cre+) mice. Both mice have grossly normal inguinal (Figure 2A) and axillary (data not shown) lymph node development. In the naïve mice, despite a small reduction in total thymocyte numbers, the T cell-specific Id2-deficient mice have similar thymocyte development and splenic CD4 T cell composition compared to the control mice (Supplementary Figure 2A to H). Similarly, no difference was observed with inguinal and axillary lymph node cell analysis (data not shown). The Id2-deficient CD4 T cells are also able to differentiate into IL-17-producing Th17 cells in vitro, with ability to survive and proliferate comparable to the control cells (Supplementary Figure 2I). After immunization with MOG peptide, all control mice developed paralytic symptoms characteristic of EAE; however, none of the T cell-specific Id2-deficient mice developed the disease (Figure 2B). Because the development of disease symptoms in the EAE model is critically dependent on CD4 T cell infiltration into the CNS, we analyzed the CNS tissue from the mice 15 days after EAE induction, when the disease severity was at its peak in control mice. While we found a significant number of CD4 T cells in the brain and spinal cord of control mice, nearly no CD4 T cell infiltration was observed in the CNS of T cell-specific Id2-deficient mice (Figure 3A-B). The CD4 T cells found in the CNS of control mice consist of a significant proportion of MOG-specific cells, as well as IL-17 and/or IFNγ producing cells, while the few CD4 T cells found in the CNS of T cell-specific Id2-deficient mice do not contain these populations (Figure 3C-D). Together, the complete resistance to the disease and the absence of T cell infiltration in the CNS showed that Id2 expression is indeed functionally relevant to CD4 T cell responses in vivo, and Id2 deficiency may cause a significant defect in CD4 T cell response to MOG immunization, possibly early in the initiation phase of the disease.
Figure 2. Mice with T cell-specific Id2 deficiency are resistant to EAE.
A: Representative photographs of inguinal lymph nodes (circled by dashed lines) from control (f/f, Id2f/fCD4Cre-) and T cell-specific Id2-deficient (Δ/Δ, Id2f/fCD4Cre+) mice. n=5 for each group. B: EAE was induced in six-week-old mice, and their disease scores were recorded daily. Score 0: normal, 1: limp tail, 2: unsteady gait, 3: hind limb paralysis, 4: four limb paralysis. f/f, n=6; Δ/Δ, n=7. Error bars indicate S.D.
Figure 3. Mice with T cell-specific Id2 deficiency do not recruit CD4 T cells into the CNS 15 days after EAE induction.
A: Representative plots of CD4 and CD8α staining of CNS infiltrating cells. B: Percentage and number of CD4 T cells in the CNS. C: Representative plots of MOG I-A(b) staining of CNS infiltrating CD4+ cells. D: Representative plots of IL-17A and IFNγ staining of CNS infiltrating CD4+ cells. n=3 for each group. *p<0.05. Error bars indicate S.D.
EAE induction generates a smaller pool of effector CD4 T cells in mice with T cell-specific Id2 deficiency
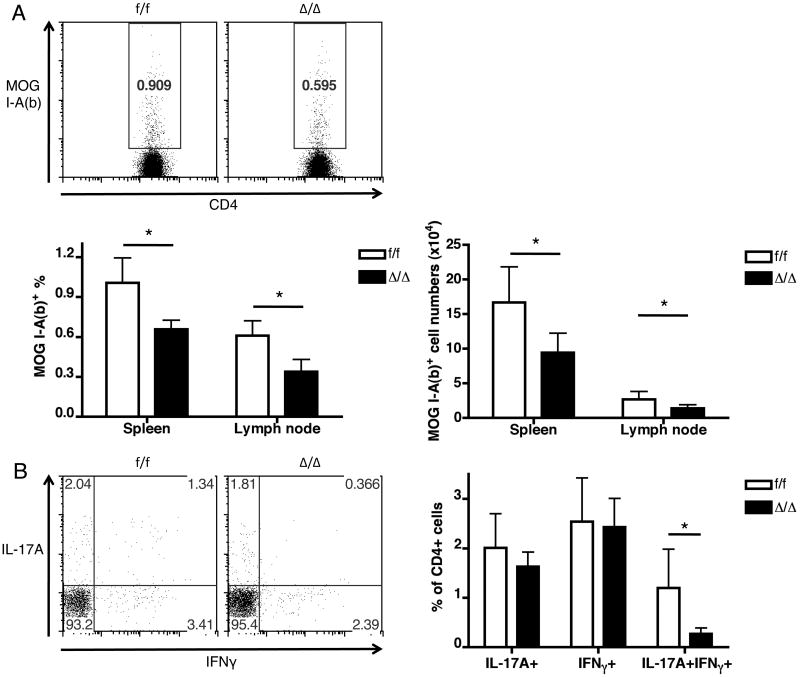
Two possibilities may lead to the absence of CD4 T cell infiltration to the CNS in mice with Id2 deficiency: the effector CD4 T cells may develop in the periphery normally, but fail to migrate to the CNS, or the development of the effector T cells may be defective. To distinguish these possibilities, we next examined the CD4 T cell response in peripheral lymphoid organs of the mice 9 days after immunization, when control mice started to show paralytic symptoms. Compared to control mice, the percentage and number of MOG-specific CD4 T cells was lower in the T cell-specific Id2-deficient mice both in the spleen and draining lymph nodes (Figure 4A). Intracellular cytokine staining also revealed a decrease of splenic cytokine-producing CD4 T cell populations in the T cell-specific Id2-deficient mice (Figure 4B), especially the IL-17A+IFN-γ+ cells, which have been reported to be especially encephalitogenic (18). These results correlate with the higher expression of Id2 seen in these populations in the Id2hCD5/hCD5 model (Figure 1C-D). It is noteworthy that residual MOG-specific and cytokine-producing CD4 T cells can be detected in the T cell-specific Id2-deficient mice, suggesting that these T cells have not totally lost their capability of activation and differentiation; presence of these cells in the spleen but not in the CNS also suggests that they have entered circulation but possibly failed to enter CNS. Several adhesion molecules and chemokine receptors have been shown to be important for the migration of T cells in EAE, including α4β1 integrin (19) and CCR6 (20). We examined the expression levels of the two molecules by MOG-specific T cells from mice 9 days after EAE induction but found no difference between Id2-deficient and control cells (Supplementary Figure 3). Therefore, the absence of Id2-deficient CD4 T cells in CNS is unlikely due to change in the expression of α4β1 integrin or CCR6.
Figure 4. Mice with T cell-specific Id2 deficiency have a smaller pool of effector CD4 T cells in the secondary lymphoid organs 9 days after EAE induction.
A: Representative plots and bar graphs showing percentages and numbers of MOG-specific cells among CD4 T cells from spleens of control (f/f) and Id2 conditional knock-out (Δ/Δ) mice. Similar changes were observed in the lymph nodes as shown in the bar graphs. B: Representative plots and bar graphs of cytokine-producing CD4 T cells from spleens of control (f/f) and Id2 conditional knock-out (Δ/Δ) mice. n≥3 for each group. *p < 0.05. Error bars indicate S.D.
Id2-deficient CD4 T cells show reduced percentage of proliferating cells and increased cell death
A smaller population of effector T cells may be the result of reduced cell proliferation or increased cell death. We examined the proliferation of MOG-specific CD4 T cells in the expansion phase of the immune response (6 days after EAE induction) by in vivo BrdU labeling. We found a significant decrease of percentage of BrdU+ cells from the draining lymph nodes of T cell-specific Id2-deficient mice compared to the control mice (Figure 5A. f/f: 18.4±2.7%, Δ/Δ: 11.3±3.1%, p=0.002). We next compared cell death between control and Id2-deficient CD4 T cells with 7AAD staining. Corresponding to their reduced population size, we found increased cell death in MOG-specific CD4 T cells as well as IL-17A+IFN-γ+ CD4 T cells from T cell-specific Id2-deficient mice 9 days after EAE induction (Figure 5B-C.). The percentage of 7AAD+ cells among MOG I-A(b)+ cells in the spleen were: f/f: 43.7±11.2%, Δ/Δ: 61.0±9.9%, p=0.018. The percentage of 7AAD+ cells among MOG I-A(b)+ cells in the lymph nodes were: f/f: 49.9±2.7%, Δ/Δ: 60.1±4.1%, p=0.023. The percentage of 7AAD+ cells among IL-17A+IFN-γ+ cells in the spleen were: f/f: 20.6±13.4%, Δ/Δ: 60.5±23.6%, p=0.001. Repeating the experiments with a fixable Live/Dead stain (Life Technologies) generated similar results (data not shown). These findings indicate that Id2-deficient CD4 T cells, after activation, suffer from reduced proliferation, increased cell death, form a smaller effector cell population, and are unable to induce EAE.
Figure 5. Id2-deficient CD4 T cells show reduced percentage of proliferating cells and undergo increased cell death.
A: Proliferating cells were labeled in vivo with BrdU 6 days after EAE induction. Cells from draining lymph nodes were analyzed 15 hours after BrdU injection. Line histogram: MOG I-A (b)+ CD4+ cells; shaded histogram: MOG I-A (b)+ CD4+ cells from mice not injected with BrdU. (B and C) Cell death of the Id2-deficient MOG-specific CD4 T cell and IL-17A+IFNγ+ CD4 T cell populations 9 days after EAE induction were shown by 7AAD staining. B: Line histogram: splenic MOG I-A (b)+ cells; shaded histogram: MOG I-A (b)- cells. C: Line histogram: splenic IL-17A+IFNγ+ CD4 T cells. Bar graph shown is analysis of splenic CD4 T cells. n≥3 for each group. *p < 0.05. Error bars indicate S.D.
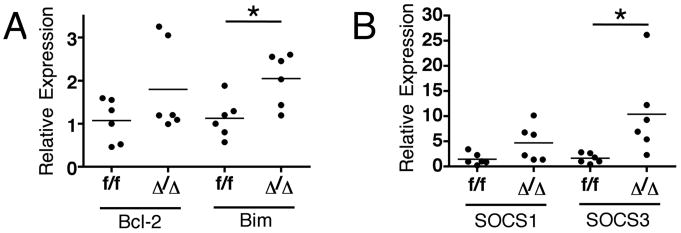
Id2-deficient CD4 T cells express higher levels of Bim and SOCS3
Two possible mechanisms may explain how Id2 affects the CD4 T cell population size: direct control of apoptosis or inhibition of cytokine signaling. Altered expression of both anti-apoptotic and pro-apoptotic genes, such as Bcl-2 and Bim, has been observed in Id2-deficient CD8 T cells (7). We used real-time PCR to examine their expressions in splenic MOG-specific CD4 T cells from animals 9 days after EAE induction, and we found that the expression of the pro-apoptotic Bim was increased in Id2-deficient CD4 T cells but not Bcl-2 (Figure 6A). This result correlates with the increased cell death of this population and is compatible with a previous report that E proteins can up-regulate Bim expression (13).
Figure 6. Id2-deficient MOG-specific CD4 T cells express higher levels of Bim and Socs3 mRNA.
Real-time PCR analysis of Bcl-2, Bim (A), Socs1 and Socs3(B) mRNA expression by sorted Id2-deficient MOG I-A (b)+ CD4 T cells from spleens of mice 9 days after EAE induction, normalized to their control counter parts. Each dot represents an individual animal. *p < 0.05
In addition to direct regulation of apoptosis, Id2 may also regulate cell survival and other aspects of T cell responses through regulation of cytokine signaling. Two important suppressors of cytokine signaling in T cells, SOCS1 and SOCS3, have been reported to be positively regulated by E proteins (12, 13). In particular, increased SOCS3 can inhibit cytokine signaling through STAT3, an important signal transduction mediator of pro-inflammatory cytokines such as IL-6 (21). T cell-specific STAT3 knock-out mice are also resistant to EAE and also demonstrate a reduced size of IL-17-producing effector CD4 T cell population (22). We examined the expression of Socs1 and Socs3 in Id2-deficient MOG-specific CD4 T cells by real-time PCR and found that Socs3 expression was profoundly up-regulated compared to control CD4 T cells; there was also a trend toward increased Socs1 expression in these cells (Figure 6B). These results indicate that Id2 is required for the maintenance of the effector CD4 T cell population size through regulating the expression of genes related to apoptosis control and cytokine signaling.
Discussion
Once CD4 T cells get activated in an immune response, both intrinsic and extrinsic factors contribute to the maintenance and regulation of the effector T cells, controlling the size of the population and the function of individual cells. Here we demonstrate that transcriptional regulator Id2, previously best known for its role in the development of various lineages of hematopoietic cells (23), is also an important regulator of CD4 T cell responses, especially in the Th17-mediated EAE disease model.
In the absence of Id2, the population size of MOG-specific CD4 T cells is reduced. The cytokine-producing CD4 T cells are also reduced; specifically, we found that IL-17A+IFNγ+ CD4 T cells almost completely disappeared in the T cell-specific Id2-deficient mice. The findings implied that this population is particularly dependent on Id2 for its formation and/or survival. Previous studies reported that this population is more encephalitogenic and has a stronger propensity to migrate into the central nervous system (18). In a tumor model, the IFNγ production capacity of Th17 cells has also been shown to be crucial for their anti-tumor activity (24). These reports suggest that the IL-17A+IFNγ+ CD4 T cells may be important effector cells of a Th17-mediated immune response. They also help explain that, despite relatively comparable development of IL-17A+ or IFNγ+ CD4 T cells in the peripheral lymphoid organs of the T cell-specific Id2-deficient mice, and a reduced but still significant population of MOG-specific CD4 T cell, no CNS infiltration or disease symptoms developed in these mice. Why this population is particularly dependent on Id2, and whether the population can be specifically targeted for controlling Th17-mediated autoimmune diseases, remain important questions to be addressed.
One possible explanation lies in Id2-mediated regulation of SOCS3. By removing the inhibitor Id2, SOCS3 expression increases. SOCS3 can suppress signaling of many Th17-related cytokines, including IL-21 and IL-23, through its association with the cytokine receptor and inhibition of JAK kinases, reducing the phosphorylation and activation of the downstream signal transduction molecule STAT3 (21). IL-21 production is up-regulated in Th17 cells upon IL-6 stimulation, and it can up-regulate the expression of IL-23 receptor by Th17 cells, increasing the cellular responsiveness to IL-23 and thus “stabilize” Th17 cells (25). IL-23 signaling is critical for the development of IL-17+IFNγ+ as well as IL-17-IFNγ+ cells from IL-17+ cells during chronic inflammation, such as in EAE (26). In the absence of Id2, these signaling processes may be impaired by SOCS3, thus impairing Th17 maintenance and evolution. In fact, T cell-specific STAT3 deficient mice are also resistant to EAE and experimental autoimmune uveitis (EAU) (22). These mice also have significantly reduced IL-17A+IFNγ+ CD4 T cells in the periphery. The phenotype similarity between STAT3-deficient CD4 T cells and Id2-deficeint CD4 T cells, and the up-regulation of SOCS3 in the latter, strongly suggests that Id2 influence CD4 T cells through their regulation of cellular responses to cytokines. However, because E proteins and Id proteins regulate many genes, it is possible that other important pathways are also affected in the Id2-deficient CD4 T cells.
The current study focuses on the role of Id2 in the effector stage of the CD4 T cell immune response. Whether Id2 play a role in the formation of CD4 T cell memory is also an important question. Yang et al previously showed that Id2 is important for the formation of short-lived effector memory CD8 T cells in a Listeria infection model (6). The possibility that Id2 contributes to the survival of effector memory CD4 T cells can be tested in the future with a variety of prime-boost immunization models or infection-rechallenge models. In addition, we have not completely ruled out a potential role of Id2 in regulating T cell migration and effector functions; a comprehensive examination in these aspects may reveal additional mechanisms leading to the total resistance to EAE in T cell-specific Id2-deficient mice.
We have shown in this study that mice with T cell-specific Id2 deficiency do not develop EAE. However, whether removing Id2 after the disease already develops, such as using chemical inhibitors of Id2 or inducible Id2 deletion models, can alter the disease course, is another interesting question. We predict that removal of Id2 after T cell activation should also be effective in inhibiting the T cell response, possibly by shrinking the effector T cell population through increased cell death. If confirmed, modulation of E protein-Id protein activity may become a possible direction of the development of future immune suppressive treatments.
Supplementary Material
Acknowledgments
We thank the NIH tetramer facility for providing us with the MOG I-A (b) tetramer and the Duke Flow Cytometry Shared Resource for technical support.
This research is supported by grants from NIH R01GM-059638, R21RR-032742 and Duke Cancer Center Stewart Trust Fund.
References
- 1.Taylor JJ, Jenkins MK. CD4+ memory T cell survival. Curr Opin Immunol. 2011;23:319–323. doi: 10.1016/j.coi.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 5.Ji Y, Pos Z, Rao M, Klebanoff CA, Yu Z, Sukumar M, Reger RN, Palmer DC, Borman ZA, Muranski P, Wang E, Schrump DS, Marincola FM, Restifo NP, Gattinoni L. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat Immunol. 12:1230–1237. doi: 10.1038/ni.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang CY, Best JA, Knell J, Yang E, Sheridan AD, Jesionek AK, Li HS, Rivera RR, Lind KC, D'Cruz LM, Watowich SS, Murre C, Goldrath AW. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat Immunol. 2011;12:1221–1229. doi: 10.1038/ni.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannarile MA, Lind NA, Rivera R, Sheridan AD, Camfield KA, Wu BB, Cheung KP, Ding Z, Goldrath AW. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat Immunol. 2006;7:1317–1325. doi: 10.1038/ni1403. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama T, Li J, Vaque JP, Konkel JE, Wang W, Zhang B, Zhang P, Zamarron BF, Yu D, Wu Y, Zhuang Y, Gutkind JS, Chen W. Control of the differentiation of regulatory T cells and T(H)17 cells by the DNA-binding inhibitor Id3. Nat Immunol. 2011;12:86–95. doi: 10.1038/ni.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyazaki M, Rivera RR, Miyazaki K, Lin YC, Agata Y, Murre C. The opposing roles of the transcription factor E2A and its antagonist Id3 that orchestrate and enforce the naive fate of T cells. Nat Immunol. 2011 doi: 10.1038/ni.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Dai M, Zhuang Y. A T cell intrinsic role of Id3 in a mouse model for primary Sjogren's syndrome. Immunity. 2004;21:551–560. doi: 10.1016/j.immuni.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Kusunoki T, Sugai M, Katakai T, Omatsu Y, Iyoda T, Inaba K, Nakahata T, Shimizu A, Yokota Y. TH2 dominance and defective development of a CD8+ dendritic cell subset in Id2-deficient mice. J Allergy Clin Immunol. 2003;111:136–142. doi: 10.1067/mai.2003.29. [DOI] [PubMed] [Google Scholar]
- 12.Jones ME, Zhuang Y. Acquisition of a functional T cell receptor during T lymphocyte development is enforced by HEB and E2A transcription factors. Immunity. 2007;27:860–870. doi: 10.1016/j.immuni.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz R, Engel I, Fallahi-Sichani M, Petrie HT, Murre C. Gene expression patterns define novel roles for E47 in cell cycle progression, cytokine-mediated signaling, and T lineage development. Proc Natl Acad Sci U S A. 2006;103:9976–9981. doi: 10.1073/pnas.0603728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen XP, Losman JA, Rothman P. SOCS proteins, regulators of intracellular signaling. Immunity. 2000;13:287–290. doi: 10.1016/s1074-7613(00)00028-5. [DOI] [PubMed] [Google Scholar]
- 15.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 16.Jones-Mason ME, Zhao X, Kappes D, Lasorella A, Iavarone A, Zhuang Y. E Protein Transcription Factors Are Required for the Development of CD4(+) Lineage T Cells. Immunity. 2012;36:348–361. doi: 10.1016/j.immuni.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, Duquette P, Prat A. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann Neurol. 2009;66:390–402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- 19.Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64:1336–1342. doi: 10.1212/01.WNL.0000158329.30470.D0. [DOI] [PubMed] [Google Scholar]
- 20.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 21.Baker BJ, Akhtar LN, Benveniste EN. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009;30:392–400. doi: 10.1016/j.it.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Lee YS, Yu CR, Egwuagu CE. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J Immunol. 2008;180:6070–6076. doi: 10.4049/jimmunol.180.9.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kee BL. E and ID proteins branch out. Nat Rev Immunol. 2009;9:175–184. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- 24.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, Roychoudhuri R, Ferreyra GA, Shen W, Durum SK, Feigenbaum L, Palmer DC, Antony PA, Chan CC, Laurence A, Danner RL, Gattinoni L, Restifo NP. Th17 Cells Are Long Lived and Retain a Stem Cell-like Molecular Signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spolski R, Leonard WJ. The Yin and Yang of interleukin-21 in allergy, autoimmunity and cancer. Curr Opin Immunol. 2008;20:295–301. doi: 10.1016/j.coi.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niola F, Zhao X, Singh D, Castano A, Sullivan R, Lauria M, Nam HS, Zhuang Y, Benezra R, Di Bernardo D, Iavarone A, Lasorella A. ID proteins synchronize stemness and anchorage to the niche of neural stem cells. Nat Cell Biol. 2012 Apr 22;14(5):477–87. doi: 10.1038/ncb2490. doi: 10.1038/ncb2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.