Abstract
Purpose
To determine the optimal volume or density of embryos for the well-of-the-well (WOW) system in order to track the development of individual embryos and to determine whether the WOW system can reverse the negative impact of culturing embryos singly.
Methods
(1) Mouse embryos (groups of nine at the 2-cell stage) were cultured in 6.25 μl, 12.50 μl, 25.00 μl and 50.00 μl of droplets of culture medium under paraffin oil; (2) Groups of three, six, nine and twelve embryos at the 2-cell stage were cultured in 50 μl of droplet of culture medium under paraffin oil; (3) Groups of nine embryos at the 2-cell stage were cultured in 50 μl of droplet under paraffin oil with or without nine micro-wells made on the bottom of the Petri dish into each of which were placed one of the nine embryos (WOW system). Also single 2-cell stage embryos was cultured individually in 5.5 μl of droplet of culture medium under paraffin oil with or without a single micro-well made on the bottom of the Petri dish (WOW system for single culture). At the end of culture, the percentages of blastocyst development, hatching and hatched blastocysts were compared in each group. The blastocysts were fixed for differential staining.
Results
The blastocyst development was significantly higher (P < 0.05) when nine embryos were cultured in 50 μl of droplet of culture medium compared with other volumes. The blastocyst development was significantly reduced (P < 0.05) in single embryo culture compared to group embryo culture with or without the WOW system. The blastocyst development was not improved when single embryo cultured individually in a micro-well was compared to single embryo cultured individually without micro-well. The total cell numbers of blastocysts were significantly higher in group embryo culture than single embryo culture regardless of whether the WOW system was used. In addition, the total cell numbers of blastocysts were significantly higher (P < 0.05) in single embryo culture with the WOW system than without.
Conclusions
Group embryo culture is superior to single embryo culture for blastocyst development. The WOW system with 50 μl of droplet of culture medium can be used to track the individual development of embryo cultured in groups while preserving good embryonic development. The reduced embryonic development with single embryo culture cannot be ameliorated by the WOW system.
Keywords: Zygote, Embryo, Micro-well culture system, Blastocyst, Development
Introduction
Embryo quality is a key factor to successful in vitro fertilization (IVF). Establishing an optimal culture system in vitro for embryos is one of the main goals in assisted reproductive technologies (ART). The development potential and viability of the embryos cultured in vitro is gradually reduced by the culture process, despite the tremendous advances in culture media and conditions in recent years. Apart from the improvement of culture media, relatively little work has been done to determine the physical requirements of embryos cultured in vitro. Currently the preferred culture system is micro-drop culture on a Petri dish surface covered with mineral oil or paraffin oil.
Although some reports indicated that mammalian embryos cultured in groups exhibit better embryonic development than those cultured singly [1–4], it remains unclear by what mechanism group culture promotes embryonic development in vitro. It has been postulated that embryos secrete certain growth factors as embryonic autocoids to support or promote their development in vitro [5–10]. Group culture with different numbers of embryos in different volumes of culture medium resulted in differential embryonic development in vitro [2, 3, 11]. Therefore, the volume of micro-drop (culture medium) or density of embryos is an important factor for embryonic development in vitro. Increased embryo density improves embryonic development, possibly through the increased concentration of trophic autocrine and paracrine factors [12–14]. On the other hand, when using improved formulations of sequential media, reduced volume of culture medium was not observed to be beneficial to embryonic development when an embryo was cultured alone [15], indicating that the group culture is preferred when using sequential media and the beneficial effects cannot be mimicked by volume reduction in the single embryo culture.
Tracking the developmental progress of the individual embryo has practical importance in research and clinical ART, allowing one to identify the viability of biomarkers to be used to assess oocyte and embryo quality as well as pregnancy success after embryo transfer. For this purpose, the well-of-the-well (WOW) system has been adapted in order to track the development of individual embryo [8, 16–19]. An embryo to culture dish volume ratio of 1:30 μl has been identified to be optimal when each embryo was cultured in a 0.27 μl micro-well, producing developmental rates up to the level of mass embryo production in cattle [18]. However, the optimal volume of medium droplets or density of embryos to be used with the WOW system remains to be determined. In addition, it is also unknown whether the WOW system will enable us to culture embryo singly while obtaining results comparable to group culture.
The objective of this study was two-fold. First to determine the optimal volume or density of embryos for the WOW system in order to track the development of individual embryo and second to examine the effect of the WOW system on early embryonic development in single embryo culture.
Materials and methods
Animals
Mice (CD1, female: 8-week old; male: 10-weeks old) were employed in this study. The mice were housed in a temperature- and light-controlled room with free access to food and water under a photoperiod of 12 h-light and 12 h-dark.
Ovarian stimulation and zygote collection
Female mice were injected intraperitoneally with 10 IU of pregnant mare serum gonadotropin (PMSG; Sigma Chemical Co., St. Louis, MO, USA). 48 h after PMSG injection, the female mice were further injected intraperitoneally with 10 IU of human chorionic gonadotropin (HCG; Sigma). Immediately post HCG injection, each female mouse was caged with a male mouse. 14–16 h post HCG injection, the female mice were sacrificed and the oviducts were dissected and placed into a Petri-dish containing modified human tubal fluid buffered HEPES medium (mHTF-HEPES) [20] supplemented with 1.0 mg/ml bovine serum albumin (BSA; Sigma). Zygotes for embryonic development culture were released by tearing the ampullae of the oviducts.
Embryo culture
The collected zygotes were cultured in 1 ml of Embryo Maintenance Medium (EMM; Coopersurgical/SAGE, USA) in Petri-dish (Falcon, 35 × 10 mm, USA) at 37°C with high humidity and 5 % CO2 incubator for 24 h. Only cleaved 2-cell stage embryos were used for further experiments. The embryos were cultured continuously until day 5 (96 h post-experimental culture) without changing the medium. At the end of culture, the percentages of blastocyst formation, the hatching and hatched blastocysts were assessed.
Differential staining of blastocysts
At the end of culture (96 h), the blastocysts were fixed and underwent differential staining to distinguish inner cell mass (ICM) and trophectoderm (TE) cells. Differential staining was performed using the method described by Wang et al. [21]. Briefly, zona-intact blastocysts were first incubated in solution-1 containing 1 % Triton-100 and 100 μg/ml propidium iodide (PI; Sigma) in BSA-free mHTF-HEPES for 10 s until the trophectoderms have visibly changed color to red and have shrunk slightly, and then immediately transferred to solution-2 containing 25 μg/ml bisbenzimide (Hoechst 33342; Sigma) in 100 % ethanol and stored at 4°C overnight in the dark. Stained blastocysts were mounted onto a slide in a drop of glycerol without excessive amounts of solution 2, and then gently flattened with a cover slip. The slide was observed immediately under fluorescence microscope equipped with a UV filter (with excitation set at 355–530 nm and emission at 465–615 nm with a long pass filter). The nuclei of the ICM were labeled with Hoechst 33342 (blue) and the nuclei of the TE cells were stained with both PI and Hoechst 33342 (fluoresced pink).
Experimental design
Experiment 1. Effect of culture medium volume on embryonic development: Groups of nine 2-cell stage embryos were cultured in 6.25 μl, 12.50 μl, 25.00 μl and 50.00 μl medium droplets under paraffin oil in Petri dish (Falcon, 35.x10 mm, UAS). At the end of culture, the percentages of blastocyst development, the hatching and hatched blastocysts were compared.
Experiment 2. Effect of embryo culture density on embryonic development: Groups of three, six, nine and twelve 2-cell stage embryos were cultured in 50 μl medium droplets under paraffin oil respectively in Petri dish (Falcon, 35.x10 mm, UAS). At the end of culture, the percentages of blastocyst development, the hatching and hatched blastocysts were compared.
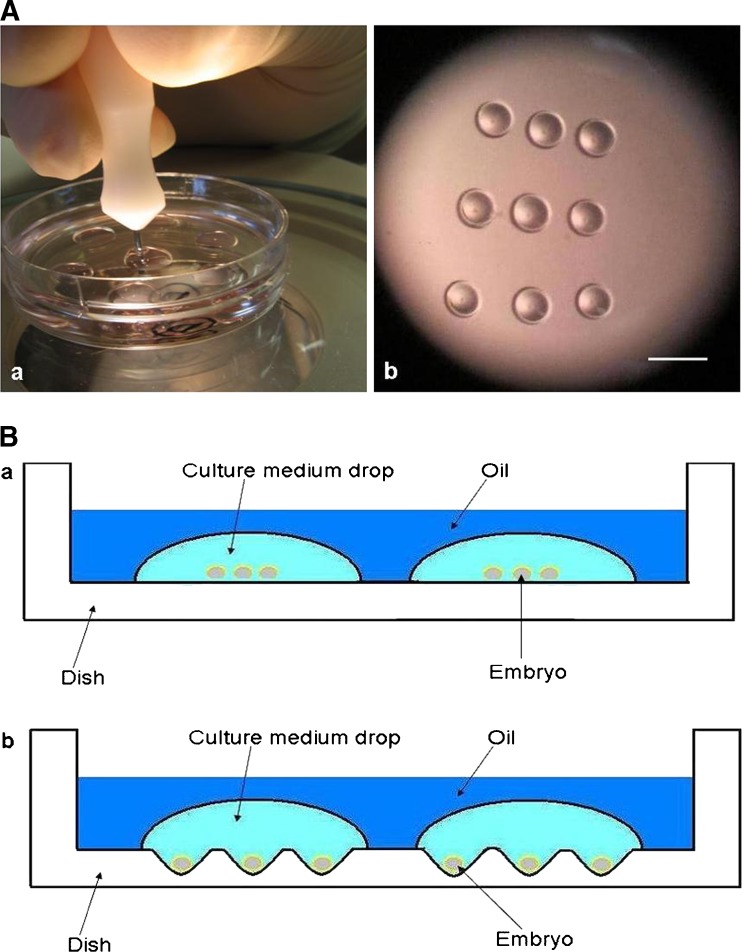
Experiment 3. Effect of culture systems on embryonic development: Groups of nine 2-cell stage embryos were cultured in 50 μl medium droplets under paraffin either with or without nine micro-wells made on the bottom of the Petri dish (Falcon, 35 × 10 mm, USA) (Fig. 1A). The dimension of each microwell was approximately1.2 × 1.2 × 0.5 mm (Fig. 1B), and each of the nine embryos was placed into one micro-well in the WOW group. In addition single 2-cell stage embryo was cultured in 5.5 μl medium droplets under paraffin oil either with or without a single micro-well made on the bottom of the Petri dish (Falcon, 35 × 10 mm, USA) to contain the embryo. At the end of culture, the percentages of blastocyst development, the hatching and hatched blastocysts were assessed in each of the three groups and compared. At the same time, the blastocysts were fixed for the differential staining.
Fig. 1.
The well-of-the-well (WOW) system for group embryo culture. (A) Preparation of the WOW system. The wells were made manually with a strong mechanical force using a BLS aggregation needle under paraffin oil (a). The 9 wells were made in 50 μl medium droplets under paraffin oil (b). Scale bar indicates 2 mm. (B) Schematic drawing of cross-sectional profiles for two culture systems. The medium droplets (50 μl each) covered by paraffin oil on flat surface of Petri dish (a). The medium droplets (50 μl each) with the WOW system on the bottom of the Petri dish, covered by paraffin oil, each well containing an embryo (b)
Statistical analysis
The difference in percentages of blastocyst development, hatching/hatched blastocysts, the cell number of blastocyst and the ration of ICM/TE (%) in each group were analyzed using the SPSS 13.0 / PC Statistics package (SPSS Inc., Chicago, IL, USA). Data expressed as mean ± SD were analyzed by Independent-Samples T Test. Data expressed as cardinal numbers and percentages were analyzed by the exact χ2 or Fisher’s exact test. A P value of less than 0.05 was considered to be statistically significant.
Results
Experiment 1. Effect of culture medium volume on embryonic development: As shown in Table 1, although there were no significant differences in terms of blastocyst development (70.8 %, 69.4 % and 75.0 %) and hatching and hatched blastocyst rates (58.9 %, 64.0 % and 70.4 %) among the three medium volumes of 6.25, 12.50, 25.00 μl, the blastocyst development (84.7 %) and hatching and hatched blastocyst rate (86.9 %) were significantly higher (P < 0.05) in the 50 μl medium droplet group compared with the other volumes.
Experiment 2. Effect of embryo culture density on embryonic development: As shown in Table 2, blastocyst development (84.7 %) was significantly higher (P < 0.05) when groups of nine embryos were cultured in 50 μl medium droplets compared to the other embryos densities (65.3 %, 68.1 % and 66.7 %). Although there were no significant differences in the hatching and hatched blastocyst rates (56.3 %, 67.3 % and 70.8 %) among three, six and twelve embryos per 50 μl group, the percentage of hatching and hatched blastocysts (75.4 %) was significantly higher (P < 0.05) when nine embryos rather than three embryos were cultured in 50 μl of medium droplet (56.3 %).
Experiment 3. Effect of culture systems on embryonic development: As shown in Table 3, the blastocyst development as well as the hatching and hatched blastocyst rates were significantly reduced (P < 0.05) in single embryo culture regardless of whether the WOW system was used (64.3 % and 56.7 %) or not used (70.3 % and 57.8 %), compared with group embryo culture with (88.2 % and 81.9 %) or without (84.2 % and 77.8 %) the WOW system. However, there was no benefit observed with the WOW system in terms of either blastocyst development (88.2 % vs. 84.2 %) or the rate of hatching and hatched blastocysts (81.9 % vs. 77.8 %) in the group embryo culture. Similarly, neither blastocyst development nor the hatching and hatched blastocyst rates were improved in single embryo culture with the WOW system (70.3 % and 57.8 %) compared to without the WOW system (64.3 % and 56.7 %).
Table 1.
The effect of volume of culture medium on mouse embryonic development (6 replicates)*
| Volume of culture medium drop (μl) | No. of embryos cultured in each drop | Total No. of embryos cultured | No. of blastocysts developed (%) | No. of blastocysts hatching and hatched (%) |
|---|---|---|---|---|
| 6.25 | 9 | 72 | 51 (70.8)a | 30 (58.9)a |
| 12.50 | 9 | 72 | 50 (69.4)a | 32 (64.0)a |
| 25.00 | 9 | 72 | 54 (75.0)ab | 38 (70.4)a |
| 50.00 | 9 | 72 | 61 (84.7)b | 53 (86.9)b |
*Different superscripts in each column indicate significant differences (P < 0.05)
Table 2.
The effect of embryo density on mouse embryonic development (6 replicates)*
| No. of embryos cultured in each drop (50 μl) | Total No. of embryos cultured | No. of blastocysts developed (%) | No. of blastocysts hatching and hatched (%) |
|---|---|---|---|
| 3 | 72 | 47 (65.3)a | 27 (56.3)a |
| 6 | 72 | 49 (68.1)a | 33 (67.3)ab |
| 9 | 72 | 61 (84.7)b | 46 (75.4)b |
| 12 | 72 | 48 (66.7)a | 34 (70.8)ab |
*Different superscripts in each column indicate significant differences (P < 0.05)
Table 3.
The effect of culture systems on mouse embryonic development (8 replicates)*
| Culture system | No. of embryos cultured in each drop | Total No. of embryos cultured | No. of blastocysts developed (%) | No. of blastocysts hatching and hatched (%) |
|---|---|---|---|---|
| Non-WOW | ||||
| (50.0 μl) | 9 | 139 | 117 (84.2)a | 91 (77.8)a |
| Non-WOW | ||||
| (5.5 μl) | 1 | 145 | 102 (70.3)b | 59 (57.8)b |
| WOW | ||||
| (50.0 μl) | 9 | 144 | 127 (88.2)a | 104 (81.9)a |
| WOW | ||||
| (5.50 μl) | 1 | 140 | 90 (64.3)b | 51 (56.7)b |
*Different superscripts in each column indicate significant differences (P < 0.05)
The total cell numbers of blastocysts were significantly higher in group embryo culture (66.7 ± 10.7 and 61.8 ± 12.3 ) compared to single embryo culture (54.9 ± 12.4 and 48.4 ± 12.0 ) regardless of whether the WOW system was used (Table 4). Interestingly, although the total cell numbers of blastocysts were not different in group embryo culture with (66.7 ± 10.7) compared to without (61.8 ± 12.3) the WOW system, the ICM cell number was significantly higher (P < 0.05) in the WOW system group (18.4 ± 2.7 versus13.2 ± 1.7). In addition, in single embryo culture, the total cell numbers of blastocysts were significantly higher (P < 0.05) with the WOW system (54.9 ± 12.4) than without (48.4 ± 12.0). Similarly, the ICM cell numbers in single embryo culture were significantly higher in the WOW group (12.9 ± 2.1) compared to the control group (11.5 ± 1.4). Lastly, in group embryo culture, the ratio of ICM/TE (%) was significantly higher with (40.6 ± 12.3) than without (28.8 ± 8.5) the WOW system.
Table 4.
Comparison of mean cell number and inner cell mass -to- trophectoderm ratio (ICM/TE) in blastocysts produced by different culture systems*
| Culture system | No. of blastocysts examined | Cell number (mean ± SD) | |||
|---|---|---|---|---|---|
| Total | TE | ICM | ICM / TE (%) | ||
| Non-WOW | |||||
| with group culture | 30 | 61.8 ± 12.3a | 48.6 ± 11.9a | 13.2 ± 1.7a | 28.8 ± 8.5a |
| Non-WOW | |||||
| with single culture | 30 | 48.4 ± 12.0b | 36.9 ± 11.3b | 11.5 ± 1.4b | 33.9 ± 10.1ab |
| WOW | |||||
| with group culture | 30 | 66.7 ± 10.7a | 48.3 ± 10.9a | 18.4 ± 2.7c | 40.6 ± 12.3b |
| WOW | |||||
| with single culture | 30 | 54.9 ± 12.4c | 42.0 ± 11.2b | 12.9 ± 2.1a | 32.5 ± 8.1a |
*Different superscripts in each column indicate significant differences (P < 0.05)
Discussion
The results of the present study demonstrate that group embryo culture is superior to single embryo culture in promoting in vitro embryonic development, that 50 μl medium droplets (embryo density being 1:5.5 μl) with the WOW system seems to be preferable for mouse embryonic development in vitro, and that the decrease in embryonic development due to single embryo culture cannot be overcome by the WOW system.
Compared with single embryo culture, embryos cultured in groups exhibit higher rates of blastocyst development and increased blastocyst cell numbers [1–4, 22, 23]. It has been proposed that embryos mutually benefit from the production of growth factors or interferon-tau via cooperative autocrine or paracrine interactions, which are not present in single embryo culture [1, 24, 25]. In fact, studies of the embryonic secretome indicate that more than one factor is involved in promoting early embryonic development during culture in vitro [26]. Accordingly, it has been reported that embryo quality was greatly improved by culturing the embryos in reduced volumes and/or by increasing embryo density [27–29], implying that a detrimental developmental effect on cultured embryos was observed when the size of the droplet was increased. However, the results of our present study suggest that the development of mouse embryos is not improved by increasing the embryo density of culture (Tables 1 and 2).
An accumulation of toxic metabolites in the smaller volume of culture medium may help explain these effects. In higher embryo density conditions, more products of metabolism and oxygen-derived radicals might be discharged and accumulated in the culture medium droplets [30, 31]. The higher concentrations of these toxic products may be detrimental to embryo development [30–33]. Therefore, it is important to confirm the optimal volume of the culture medium droplet as well as embryo density in order to maximize early embryonic development in vitro. The optimal size of the culture medium droplet should minimize on the one hand the accumulation of toxic metabolites, such as ammonium and oxygen derived free radicals, at the same time, while retaining, on the other hand, the positive acting autocrine and/or paracrine factors. Our results indicate that 9 mouse embryos cultured in 50 μl of culture medium droplet can reach the optimal early embryonic development (Tables 1 and 2). Of course, this culture condition may be different based on different culture media and culture systems.
The WOW systen provides a suitable microenvironment for nutritional support. It has been postulated to dilute metabolized toxic products while accumulating autocrine and/or paracrine factors released by the embryos, resulting in improved embryonic development [8, 16, 18, 19]. Although the WOW system was developed initially for short-term co-culture of zona-free mouse embryos to produce chimerae [34], the system has been shown to be useful in tracking individual zona-intact embryo cultured in groups [8, 16, 18, 19], which presents an alternative to single embryo culture. Our future research will be aimed at developing a culture system for tracking individual embryo development following in vitro oocyte maturation (IVM) without imparirment of embryo development to the blastocyst stage.
We report in the present study that the proportion of embryos developed to blastocyst stage were same in both culture systems when the embryos were cultured in groups (Table 3), but the ICM cell numbers of blastocysts were significantly higher in the WOW system (Table 4), indicating a beneficial effect of the WOW system for early embryonic development. Interestingly, in single embryo culture, the total cell numbers of blastocysts developed with the WOW system were significantly higher compared those cultured without the WOW sytem. However, use of the WOW system in single embryo culture did not improve the rate of blastocyst development. This observation suggests that the problem with single embryo culture for embryonic development cannot be overcome by the WOW system. On the other hand, the results from the present study also provide a piece of information that the WOW system may have clinical application to allow clinical embryologist tracking individual embryo development without reducing the embryo developmental potential.
In conclusion, this study indicates that the volume of medium droplet and embryo density have a significant effect on mouse embryonic development in vitro. There is an optimal density for the group embryo culture. In addition, the WOW system can be used to improve the quality of embryos and for tracking the development of individual embryo in group culture. The WOW system, however, does not overcome the problem of decreased embryo development encountered in single embryo culture.
Acknowledgments
Declaration of Interest
There is no conflict of interest that could affect the impartiality of the research reported.
Footnotes
Capsule
Group embryo culture is superior to single embryo culture in terms of early embryonic development. The well-of-the-well (WOW) system with 50 μl culture medium droplets can be used to track the individual development of group cultured embryo with better embryonic development. However, the impairment of embryonic development with single embryo culture is not overcome using the WOW system.
Contributor Information
Ying-Pu Sun, Email: syp2008@vip.sina.com.
Ri-Cheng Chian, FAX: +1-514-8431662, Email: ri-cheng.chian@muhc.mcgill.ca.
References
- 1.Paria BC, Dey SK. Preimplantation embryo development in vitro: cooperative interactions among embryos and role of growth factors. Proc Natl Acad Sci U S A. 1990;87:4756–60. doi: 10.1073/pnas.87.12.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canseco RS, Sparks AE, Pearson RE, Gwazdauskas FC. Embryo density and medium volume effects on early murine embryo development. J Assist Reprod Genet. 1992;9:454–7. doi: 10.1007/BF01204051. [DOI] [PubMed] [Google Scholar]
- 3.Lane M, Gardner DK. Effect of incubation volume and embryo density on the development and viability of mouse embryos in vitro. Hum Reprod. 1992;7:558–62. doi: 10.1093/oxfordjournals.humrep.a137690. [DOI] [PubMed] [Google Scholar]
- 4.Kato Y, Tsunoda Y. Effects of the culture density of mouse zygotes on the development in vitro and in vivo. Theriogenology. 1994;41:1315–22. doi: 10.1016/0093-691X(94)90490-A. [DOI] [PubMed] [Google Scholar]
- 5.Keefer CL, Stice SL, Paprocki AM, Golueke P. In vitro culture of bovine IVM-IVF embryos: Cooperative interaction among embryos and the role of growth factors. Theriogenology. 1994;41:1323–31. doi: 10.1016/0093-691X(94)90491-Z. [DOI] [PubMed] [Google Scholar]
- 6.Stoddart NR, Wild AE, Fleming TP. Stimulation of development in vitro by platelet-activating factor receptor ligands released by mouse preimplantation embryos. J Reprod Fertil. 1996;108:47–53. doi: 10.1530/jrf.0.1080047. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill C. Evidence for the requirement of autocrine growth factors for development of mouse preimplantation embryos in vitro. Biol Reprod. 1997;56:229–37. doi: 10.1095/biolreprod56.1.229. [DOI] [PubMed] [Google Scholar]
- 8.Vajta G, Peura TT, Holm P, et al. New method for culture of zona-included or zona-free embryos: the Well of the Well (WOW) system. Mol Reprod Dev. 2000;55:256–64. doi: 10.1002/(SICI)1098-2795(200003)55:3<256::AID-MRD3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Hardy K, Spanos S. Growth factor expression and function in the human and mouse preimplantation embryo. J Endocrinol. 2002;172:221–36. doi: 10.1677/joe.0.1720221. [DOI] [PubMed] [Google Scholar]
- 10.Fujita T, Umeki H, Shimura H, et al. Effect of group culture and embryo-culture conditioned medium on development of bovine embryos. J Reprod Dev. 2006;52:137–42. doi: 10.1262/jrd.16084. [DOI] [PubMed] [Google Scholar]
- 11.Salahuddin S, Ookutsu S, Goto K, et al. Effects of embryo density and co-culture of unfertilized oocytes on embryonic development of in-vitro fertilized mouse embryos. Hum Reprod. 1995;10:2382–5. doi: 10.1093/oxfordjournals.humrep.a136303. [DOI] [PubMed] [Google Scholar]
- 12.Bormann JM, Totir LR, Kachman SD, et al. Pregnancy rate and first-service conception rate in Angus heifers. J Anim Sci. 2006;84:2022–5. doi: 10.2527/jas.2005-615. [DOI] [PubMed] [Google Scholar]
- 13.Katz-Jaffe MG, Schoolcraft WB, Gardner DK. Analysis of protein expression (secretome) by human and mouse preimplantation embryos. Fertil Steril. 2006;86:678–85. doi: 10.1016/j.fertnstert.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Richter KS. The importance of growth factors for preimplantation embryo development and in-vitro culture. Curr Opin Obstet Gynecol. 2008;20:292–304. doi: 10.1097/GCO.0b013e3282fe743b. [DOI] [PubMed] [Google Scholar]
- 15.Vutyavanich T, Saeng-Anan U, Sirisukkasem S, Piromlertamorn W. Effect of embryo density and microdrop volume on the blastocyst development of mouse two-cell embryos. Fertil Steril. 2011;95:1435–9. doi: 10.1016/j.fertnstert.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Vajta G, Korosi T, Du Y, et al. The Well-of-the-Well system: an efficient approach to improve embryo development. Reprod Biomed Online. 2008;17:73–81. doi: 10.1016/S1472-6483(10)60296-9. [DOI] [PubMed] [Google Scholar]
- 17.Tagawa M, Matoba S, Narita M, et al. Production of monozygotic twin calves using the blastomere separation technique and Well of the Well culture system. Theriogenology. 2008;69:574–82. doi: 10.1016/j.theriogenology.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Hoelker M, Rings F, Lund Q, et al. Effect of embryo density on in vitro developmental characteristics of bovine preimplantative embryos with respect to micro and macroenvironments. Reprod Domest Anim. 2010;45:e138–45. doi: 10.1111/j.1439-0531.2009.01535.x. [DOI] [PubMed] [Google Scholar]
- 19.Sugimura S, Akai T, Somfai T, et al. Time-lapse cinematography-compatible polystyrene-based microwell culture system: a novel tool for tracking the development of individual bovine embryos. Biol Reprod. 2010;83:970–8. doi: 10.1095/biolreprod.110.085522. [DOI] [PubMed] [Google Scholar]
- 20.Quinn P. Enhanced results in mouse and human embryo culture using a modified human tubal fluid medium lacking glucose and phosphate. J Assist Reprod Genet. 1995;12:97–105. doi: 10.1007/BF02211377. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Ock SA, Chian RC. Effect of gonadotrophin stimulation on mouse oocyte quality and subsequent embryonic development in vitro. Reprod Biomed Online. 2006;12:304–14. doi: 10.1016/S1472-6483(10)61002-4. [DOI] [PubMed] [Google Scholar]
- 22.Wiley LM, Yamami S, Muyden D. Effect of potassium concentration, type of protein supplement, and embryo density on mouse preimplantation development in vitro. Fertil Steril. 1986;45:111–9. doi: 10.1016/s0015-0282(16)49107-7. [DOI] [PubMed] [Google Scholar]
- 23.O’Doherty EM, Wade MG, Hill JL, Boland MP. Effects of culturing bovine oocytes either singly or in groups on development to blastocysts. Theriogenology. 1997;48:161–9. doi: 10.1016/S0093-691X(97)00199-4. [DOI] [PubMed] [Google Scholar]
- 24.Larson MA, Kubisch HM. The effects of group size on development and interferon-tau secretion by in-vitro fertilized and cultured bovine blastocysts. Hum Reprod. 1999;14:2075–9. doi: 10.1093/humrep/14.8.2075. [DOI] [PubMed] [Google Scholar]
- 25.Sananmuang T, Tharasanit T, Nguyen C, et al. Culture medium and embryo density influence on developmental competence and gene expression of cat embryos. Theriogenology. 2011;75:1708–19. doi: 10.1016/j.theriogenology.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Katz-Jaffe MG, McCallie BR, Preis KA, et al. Transcriptome analysis of in vivo and in vitro matured bovine MII oocytes. Theriogenology. 2009;71:939–46. doi: 10.1016/j.theriogenology.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Donnay I, Langendonckt A, Auquier P, et al. Effects of co-culture and embryo number on the in vitro development of bovine embryos. Theriogenology. 1997;47:1549–61. doi: 10.1016/S0093-691X(97)00160-X. [DOI] [PubMed] [Google Scholar]
- 28.Gil MA, Abeydeera LR, Day BN, et al. Effect of the volume of medium and number of oocytes during in vitro fertilization on embryo development in pigs. Theriogenology. 2003;60:767–76. doi: 10.1016/S0093-691X(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira AT, Lopes RF, Rodrigues JL. Gene expression and developmental competence of bovine embryos produced in vitro under varying embryo density conditions. Theriogenology. 2005;64:1559–72. doi: 10.1016/j.theriogenology.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Gardner DK, Lane M. Amino acids and ammonium regulate mouse embryo development in culture. Biol Reprod. 1993;48:377–85. doi: 10.1095/biolreprod48.2.377. [DOI] [PubMed] [Google Scholar]
- 31.Malekshah AK, Moghaddam AE. Follicular fluid and cumulus cells synergistically improve mouse early embryo development in vitro. J Reprod Dev. 2005;51:195–9. doi: 10.1262/jrd.16051. [DOI] [PubMed] [Google Scholar]
- 32.Sinclair KD, Dunne LD, Maxfield EK, et al. Fetal growth and development following temporary exposure of day 3 ovine embryos to an advanced uterine environment. Reprod Fertil Dev. 1998;10:263–9. doi: 10.1071/R98021. [DOI] [PubMed] [Google Scholar]
- 33.Johnson MH, Nasr-Esfahani MH. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays. 1994;16:31–8. doi: 10.1002/bies.950160105. [DOI] [PubMed] [Google Scholar]
- 34.Wood SA, Pascoe WS, Schmidt C, et al. Simple and efficient production of embryonic stem cell-embryo chimeras by coculture. Proc Natl Acad Sci U S A. 1993;90:4582–5. doi: 10.1073/pnas.90.10.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]