Abstract
Purpose
To study the effect of supplementing biotin to sperm preparation medium on the motility of frozen-thawed spermatozoa.
Methods
Semen samples of men attending the University infertility clinic (n = 105) were cryopreserved using glycerol-egg yolk-citrate buffered cryoprotective medium in liquid nitrogen. After a period of two weeks, the semen samples were thawed and the motile spermatozoa were extracted by swim-up technique using Earle’s balanced salt solution (EBSS) medium supplemented with either biotin (10 nM) or pentoxifylline (1 mM). The post-wash motility was observed up to 4 h after incubation.
Results
Both biotin and pentoxifylline supplementation resulted in significant increase in total motility (p < 0.05), progressive motility (p < 0.001) and rapid progressive motility (p < 0.05 v/s biotin and p < 0.01 v/s pentoxifylline) compared to the control at 1 h post-incubation period. Significantly higher percentage of total (p < 0.01, p < 0.05 in biotin and pentoxifylline respectively), progressive (p < 0.001) and rapid progressive motilities (p < 0.01) were observed in these two groups even at 2 h compared to the control. In the control group at 4 h after incubation, ~11% decline in total motility and ~8% decline in progressive motility was observed. However, in both biotin and pentoxifylline group the motility was significantly higher than control (p < 0.001). No significant difference in the motility was observed between biotin and pentoxifylline groups at any of the time intervals studied.
Conclusions
Biotin can enhance the sperm motility and prolong the survival of frozen-thawed semen samples which may have potential benefit in assisted reproductive technology field.
Keywords: Biotin, pentoxifylline, spermatozoa, cryopreservation, post-thaw motility
Introduction
Poor structural and functional characteristics of spermatozoa are common consequences of human semen cryopreservation. Efforts have been made to increase the post-thaw motility and sperm function by using pharmacological agents, vitamins and antioxidants. Among these, pentoxifylline is currently the most widely used pharmacological agent, which has been shown to enhance the motility and longevity of spermatozoa under in vitro conditions [1]. However, the studies suggesting toxic effect of pentoxifylline on embryo development [2–4] have raised concern about its application in human assisted reproductive technology (ART) set up. Therefore, the search for safe alternate agents that can enhance the sperm function without exerting any toxic effect on gametes and embryos is of clinical significance.
Biotin (vitamin B7 or vitamin H), is an essential micronutrient required for the normal growth and development of the body. It acts as a coenzyme in all carboxylation reactions involved in the biosynthesis of fatty acids, gluconeogenesis, metabolism of the branched-chain amino acids and de novo synthesis of purine nucleotides. Studies have demonstrated that deficiency of biotin during the gestation period leads to teratogenicity in mouse [5] and hamster [6] indicating its role in reproduction and development. However, its effect on sperm function was not known until now. In the present investigation, we report for the first time that biotin can act as a promising sperm motility enhancer under in vitro conditions.
Materials and methods
Patients
The semen samples were collected from 105 men (mean age of 33.48 ± 2.1 years), who visited the University infertility clinic for semen analysis between 2008 and 2010. The study was approved by the Institutional Ethical Committee. The subjects were explained about the study, and a written consent was taken from them.
Men with sexual abstinence of 3–5 days were asked to provide their semen samples in a sterile container by masturbation. Out of 105 samples, 70 were normozoospermic subjects and 35 men were with abnormal semen parameters. After liquefaction of the ejaculate, sperm density was determined using Makler’s counting chamber (Sefi Instruments, Israel). Sperm motility and morphology were assessed according to WHO criteria [7].
To determine the optimum concentration of biotin fresh ejaculates from normozoospermic men (n = 10) were divided into aliquots of equal volume and washed with Earl’s Balanced Salt Solution (EBSS) medium supplemented with Human serum albumin (HSA). The pellets were overlaid with EBSS medium supplemented with different concentrations of biotin (0, 1, 10, 50, 100, 500 and 1000 nM). Pentoxifylline (1 mM) was used as the positive control [8]. In 10 nM biotin group, the progressive motility was maintained until 4 h (Table 1), which was higher than the control (p > 0.05) and pentoxifylline (p < 0.05). In the control group, the progressive motility declined by approximately 6% (86.25 ± 4.33 v/s 80.38 ± 3.04) while in the pentoxifylline group, the decline was almost 20% (93.33 ± 2.17 v/s 73.00 ± 4.91) when compared to the motility at 1 h (p < 0.01). Hence, 10 nM of biotin was considered as optimum concentration for the present study.
Table 1.
Effect of different concentration of biotin supplemented to sperm preparation medium on progressive motility in normozoospermic semen samples (n = 10)
| Incubation time (h) | Percentage progressive motility (Mean ± SE) | |||||||
|---|---|---|---|---|---|---|---|---|
| Concentration of biotin (nM) | Pentoxifylline | |||||||
| 0 | 5 | 10 | 50 | 100 | 500 | 1000 | (1 mM) | |
| 1 | 86.25 ± 4.33 | 86.25 ± 6.81 | 91.25 ± 2.77 | 91.63 ± 3.34 | 91.63 ± 2.31 | 90.50 ± 3.21 | 86.75 ± 4.72 | 93.33 ± 2.17* |
| 2 | 83.25 ± 2.74 | 85.88 ± 1.78 | 89.00 ± 4.55 | 88.38 ± 3.77 | 87.38 ± 1.83 | 85.00 ± 4.22 | 84.38 ± 3.59 | 86.67 ± 3.44** |
| 4 | 80.38 ± 3.04 | 82.50 ± 2.20 | 88.50 ± 1.82* | 86.75 ± 2.10** | 83.63 ± 2.35 | 82.88 ± 1.92 | 79.88 ± 2.03 | 73.00 ± 4.91 |
*p < 0.05; **p < 0.01 V/s pentoxifylline at 4 h
During cryopreservation, semen samples were mixed with equal volume of glycerol- egg yolk-citrate buffered freezing medium and stored in liquid nitrogen as described elsewhere [9]. To avoid observer bias, all the cryo-vials were coded by a person who was not aware of the study. After two weeks, the frozen samples were rapidly thawed at 37°C and divided into three equal parts. The cryoprotectants were completely removed by mixing the sperm suspension with 2 ml of EBSS supplemented with HSA and by centrifuging at 1800 rpm for 8 min. The motile spermatozoa were extracted from the pellet by swim-up technique [10]. Briefly, the sperm pellet was carefully overlaid with 200–300 μl of EBSS medium supplemented with HSA, EBSS with 10 nM biotin (Fluka, Cat. No. 14400) or EBSS with 1 mM pentoxifylline (Sigma, Cat. No. P1784).
The influence of biotin supplementation to sperm preparation medium was assessed by motility evaluation at 1, 2 and 4 hours after incubation at 37°C and 5% CO2 (Hera Cell 150i, Germany). About 10 μl of sperm suspension was placed on a clean glass slide, and a cover slip was placed carefully over it. A total of 200 spermatozoa was scored for the motility evaluation using the light microscope (Olympus, India) under 400X magnification, and graded as rapid progressive (grade a), slow or sluggish (grade b), non-progressive (grade c) and immotile (grade d) spermatozoa [7].
The motility data are presented as Mean±SE and analyzed by one-way ANOVA using GraphPadInstat 3.0 software (USA). Multiple comparisons were made using Bonferroni procedure and the values with P < 0.05 were considered as statistically significant.
Results
Out of the 105 study subjects, 70 were normozoospermic and 35 were with abnormal semen parameters (25 asthenozoospermic, 1 oligozoospermic, 6 oligoasthenozoospermic, 2 asthenoteratozoospermic and 1 oligoasthenoteratozoospermic). The study group had a mean sperm density of 49.56 ± 3.28 Millions/ml, 59.51 ± 2.09% total motility, 42.54 ± 2.2% progressive motility, 60.54 ± 2.48% viability and 26.29 ± 1.62% spermatozoa with normal morphology. The post-wash findings in frozen-thawed spermatozoa processed in the presence of biotin or pentoxifylline did not show any significant difference between normozoospermic and semen samples with abnormal parameters. Hence, the results are discussed below taking both normozoospermic and abnormal group together.
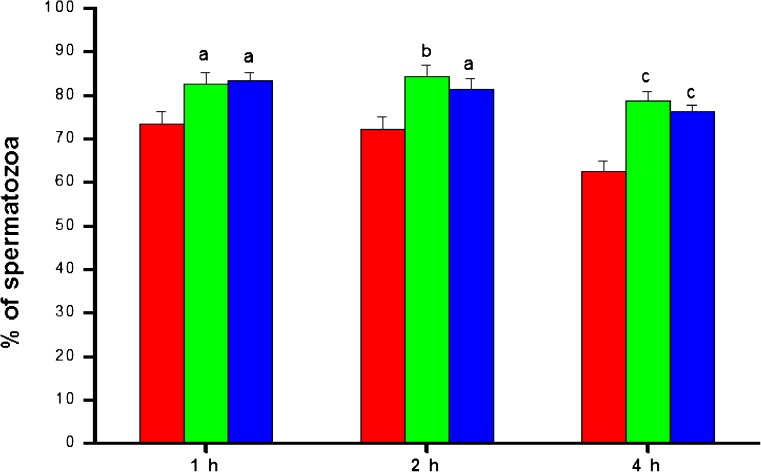
Cryopreservation resulted in approximately 50% reduction in total motility of spermatozoa compared to the fresh ejaculate. At 1 hour after incubation, the total motility (grade a + grade b + grade c) in post-wash samples of the control group was 73.51 ± 2.8% (Fig. 1), which was approximately 10% higher in both biotin (82.67 ± 2.56) and pentoxifylline group (83.24 ± 2.04) (p < 0.05). The difference in total motility between these two groups was also observed even at 2 and 4 hour after the incubation. At 4 h, the total motility decreased by ~11% in the control group (62.57 ± 2.4 v/s 73.51 ± 2.8), whereas, in biotin and penoxifylline group motility decreased by ~4% (78.69 ± 2.17 v/s 82.67 ± 2.56) and ~7% (76.24 ± 1.47 v/s 83.24 ± 2.04) respectively (p < 0.001 compared to the control).
Fig. 1.
The total motility pattern in frozen-thawed semen samples processed in media supplemented with biotin (10 nM) and pentoxifylline (1 mM) (n = 105)at different hours after incubation in vitro.  —Control;
—Control;  —Biotin;
—Biotin;  —Pentoxifylline. a:p < 0.05; b:p < 0.01; c: < 0.001 compared to control at respective intervals
—Pentoxifylline. a:p < 0.05; b:p < 0.01; c: < 0.001 compared to control at respective intervals
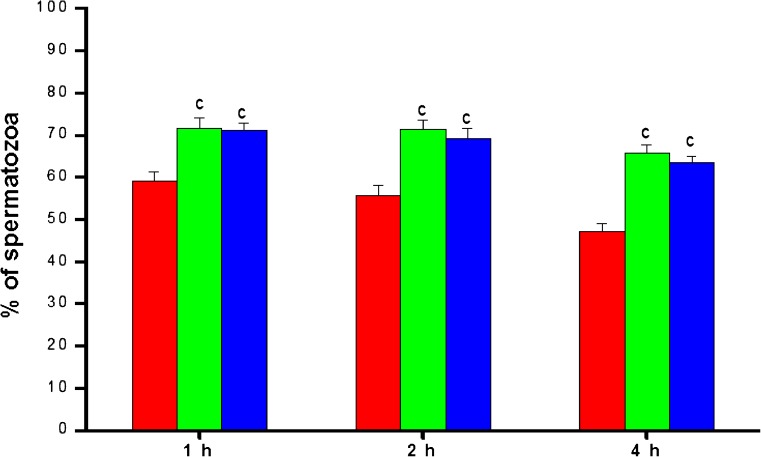
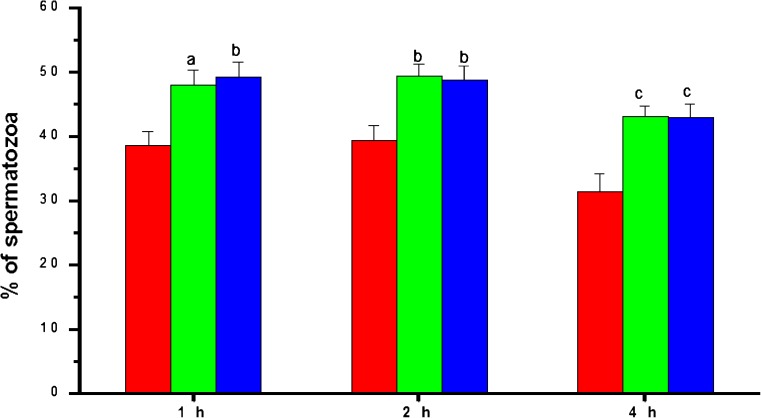
The progressive (grade a + b) motility in post-wash samples decreased gradually with incubation time (Fig. 2). Both biotin and pentoxifylline groups had significantly higher (p < 0.001) progressive motility compared to the control at all the time intervals studied. At 4 h after incubation, the progressive motility in the control group was 47.00 ± 2.13%, while in the biotin and pentoxifylline supplemented groups, it was 65.73 ± 1.98% and 63.43 ± 1.46% respectively. The similar trend was observed in rapid progressive motility. Control group had 38.63 ± 2.09% of sperm with rapid progressive (grade a) motility at 1 h after incubation, which was significantly higher in the biotin (p < 0.05) and pentoxifylline (p < 0.01) group (Fig. 3). At 4 h after incubation, the rapid progressive motility dropped to 31.33 ± 2.9% in the control group. However, in biotin and pentoxifylline supplemented groups, it was significantly higher (p < 0.001) than in the control group (43.05 ± 1.56 and 42.98 ± 2.04% respectively). Even though the biotin group had a marginally higher percentage of total, progressive or rapid progressive motility, statistically, it was not found to be significant (p > 0.05) compared to pentoxifylline group at any of the time intervals studied except for rapid progressive motility at 1 h.
Fig. 2.
The progressive motility pattern in frozen-thawed semen samples processed in media supplemented with biotin (10 nM) and pentoxifylline (1 mM) (n = 105) at different hours after incubation in vitro.  —Control;
—Control;  —Biotin;
—Biotin;  —Pentoxifylline. c:< 0.001 compared to control at respective intervals
—Pentoxifylline. c:< 0.001 compared to control at respective intervals
Fig. 3.
The rapid progressive motility pattern in frozen-thawed semen samples processed in media supplemented with biotin (10 nM) and pentoxifylline (1 mM) (n = 105) at different hours after incubation in vitro.  —Control;
—Control;  —Biotin;
—Biotin;  —Pentoxifylline. a:p < 0.05; b:p < 0.01; c < 0.001 compared to control at respective intervals
—Pentoxifylline. a:p < 0.05; b:p < 0.01; c < 0.001 compared to control at respective intervals
Discussion
Decrease in motility and poor survival of frozen-thawed spermatozoa has been consistently observed in earlier studies [11, 12]. Improving the motility in frozen-thawed spermatozoa and maintaining their survival till fertilization is a real challenge in assisted reproduction. In the present investigation, we observed a time-dependent decline in rapid progressive motility as well as total motility in frozen-thawed spermatozoa under in vitro conditions.
Pentoxifylline has been used extensively as a sperm motility enhancer in fresh ejaculates, post-thaw semen samples and testicular sperm [10, 13, 14]. The clinical experience with pentoxifylline has contradictory reports in the literature. Even though a majority of the studies found a significant improvement in the motility after pentoxifylline treatment [10, 13–15], few have observed either no improvement [16] or adverse effect [17]. However, our observations confirm that pentoxifylline can significantly enhance the motility of spermatozoa subjected to freeze-thaw process, both in normozoospermic and abnormal semen samples. The enhanced motility induced by pentoxifylline is due to its inhibitory effect on phosphodiesterase, which in turn helps in maintaining a high intracellular cyclic adenosine monophosphate (cAMP) level in the spermatozoa [18]. A marginal decline in motility with the increase in the incubation time observed in pentoxifylline group may be due to the failure of maintaining an elevated level of cAMP in the cytoplasm of the frozen-thawed spermatozoa.
In the present study we observed that supplementation of biotin to sperm preparation medium not only resulted in significant enhancement in the sperm motility but also maintained the motility until 4 hours of incubation. In addition, the biotin supplemented group maintained significantly higher percentage of sperm with rapid progressive motility even at 4 h after incubation. The motility was marginally higher in the biotin group compared to pentoxifylline group at all the time intervals studied, which indicates its potential application as sperm motility enhancer.
From this preliminary data, it is difficult to elucidate the exact mechanism of action of biotin. Nonetheless, using an indirect approach, we made an attempt to know whether the effect is mediated through inhibition of phosphodiesterase enzyme activity. The sperm motility was inhibited by addition of different concentration of phosphodiesterase IV to the sperm suspension extracted by swim-up technique. The motility did not improve even after adding different concentrations of biotin to phosphodiesterase-treated sperm suspension (unpublished data). This suggests that biotin-induced motility enhancement may not be mediated through the inhibition of phosphodiesterase enzyme. However, further studies are required to elucidate the exact molecular mechanism behind biotin-induced sperm motility enhancement.
The nutritional value of biotin has been well established. Earlier studies have shown that biotin deficiency can cause teratogenesis in lower species of animals [6] and in humans [19, 20]. Reduced activity of the biotin-dependent enzymes alters the lipid metabolism and can lead to biotin-responsive inborn errors of lipid metabolism [21, 22]. Now it is clearly evident that biotin has a significant role to play in regulation of chromatin structure [23], heme synthesis [24] and prevention of DNA damage [25]. Zempleni and Mock [26] suggest that teratogenic effect of biotin deficiency in mice and humans is due to the genomic instability caused by decrease in biotinylated histones. Incidence of the birth defects was found to reduce when the women were given multivitamin supplements (including biotin) prior to and during pregnancy [27]. These evidences clearly emphasize the nutritional importance of biotin during pregnancy or fetal development. However, its effect on sperm function enhancement was not known until now.
In conclusion, our novel finding has demonstrated that supplementing biotin to sperm preparation medium can significantly enhance the post-thaw sperm motility and their longevity in vitro. In our view, since biotin is an essential micronutrient it may not exert any toxic effect on sperm or the developing embryo at the tested concentration (10 nM). Therefore, biotin may be a promising and safe alternative agent to enhance the post-thaw sperm quality in an ART set up.
Acknowledgments
Authors kindly acknowledge the technical support provided by Mrs. Jalyalaxmi Pai, Mrs. Kirthi Patil, and Ms. Anita Poojary. Authors thank Dr. Prashanth Naik and Mr. Naveenchandra Kumar, Mangalore University for editing the language of the manuscript.
Footnotes
Capsule
Biotin supplementation to sperm wash medium can enhance the sperm motility and prolong the sperm survival.
References
- 1.Stanic P, Sonicki Z, Suchanek E. Effect of pentoxifylline on motility and membrane integrity of human cryopreserved spermatozoa. Int J Androl. 2002;25:186–90. doi: 10.1046/j.1365-2605.2002.00348.x. [DOI] [PubMed] [Google Scholar]
- 2.Tournaye H, Linden M, Abbeel E, Devroey P, Steirteghem A. Effects of pentoxifylline on in-vitro development of preimplantation mouse embryos. Hum Reprod. 1993;8:1475–80. doi: 10.1093/oxfordjournals.humrep.a138282. [DOI] [PubMed] [Google Scholar]
- 3.Tournaye H, Linden M, Abbeel E, Devroey P, Steirteghem A. Mouse in vitro fertilization using sperm treated with pentoxifylline and 2-deoxyadenosine. Fertil Steril. 1994;62:644–7. doi: 10.1016/s0015-0282(16)56960-x. [DOI] [PubMed] [Google Scholar]
- 4.Scott L, Smith S. Human sperm motility enhancing agents have detrimental effect on mouse oocytes and embryos. Fertil Steril. 1995;63:166–75. [PubMed] [Google Scholar]
- 5.Mock DM. Marginal biotin deficiency is common in normal human pregnancy and is highly teratogenic in mice. J Nutr. 2009;139:154–7. doi: 10.3945/jn.108.095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe T. Dietary biotin deficiency affects reproductive function and prenatal development in hamsters. J Nutr. 1993;123:2101–8. doi: 10.1093/jn/123.12.2101. [DOI] [PubMed] [Google Scholar]
- 7.Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 8.Kovacic B, Vlaisavljevic V, Reljic M. Clinical use of pentoxifylline for activation of immotile testicular sperm before ICSI in patients with non-obstructive azoospermia. J Androl. 2006;27:45–52. doi: 10.2164/jandrol.05079. [DOI] [PubMed] [Google Scholar]
- 9.Kalthur G, Adiga SK, Upadhya D, Rao S, Kumar P. Effect of cryopreservation on sperm DNA integrity in patients with teratospermia. Fertil Steril. 2008;89:1723–7. doi: 10.1016/j.fertnstert.2007.06.087. [DOI] [PubMed] [Google Scholar]
- 10.Mahadevan M, Baker G. Assessment and preparation of semen for in vitro fertilization. In: Wood C, Trounson A, editors. Clinical in vitro fertilization. Berlin: Springer; 83. pp. 97–1984. [Google Scholar]
- 11.Critser JK, Huse-Benda AR, Aaker DV, Arneson BW, Ball GD. Cryopreservation of human spermatozoa. The effect of cryoprotectants on motility. Fertil Steril. 1988;50:314–20. [PubMed] [Google Scholar]
- 12.Kalthur G, Raj S, Thiyagarajan A, Kumar S, Kumar P, Adiga SK. Vitamin E supplementation in semen cryopreservation medium improves the motility and protects sperm from freeze-thaw-induced DNA damage. Fertil Steril. 2011;95:1149–51. doi: 10.1016/j.fertnstert.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Yovich JM, Edirisinghe WR, Cummins JM, Yovich JL. Influence of pentoxifylline in severe male factor infertility. Fertil Steril. 1990;53:715–22. doi: 10.1016/s0015-0282(16)53470-0. [DOI] [PubMed] [Google Scholar]
- 14.Yovich JL. Pentoxifylline: actions and applications in assisted reproduction. Hum Reprod. 1993;8:1786–91. doi: 10.1093/oxfordjournals.humrep.a137935. [DOI] [PubMed] [Google Scholar]
- 15.Yunes R, Fernadez P, Doncel GF, Acosta AA. Cyclin nucleotide phosphodiesterase inhibition increases tyrosine phosphorylation and hyper motility in normal and pathological human spermatozoa. Biocell. 2005;29:287–93. [PubMed] [Google Scholar]
- 16.Gil MA, Hernandez M, Roca J, et al. Pentoxifylline added to freezing or post-thaw extenders does not improve the survival or in vitro fertilizing ability of boar spermatozoa. Reproduction. 2010;139:557–64. doi: 10.1530/REP-09-0274. [DOI] [PubMed] [Google Scholar]
- 17.Centola GM, Cartie RJ, Cox C. Differential responses of human sperm to varying concentrations of pentoxifylline with demonstration of toxicity. J Androl. 1995;16:136–42. [PubMed] [Google Scholar]
- 18.Tash JS, Means AR. Cyclic adenosine 3′,5′ monophosphate, calcium and protein phosphorylation in flagellar motility. Biol Reprod. 1983;28:75–104. doi: 10.1095/biolreprod28.1.75. [DOI] [PubMed] [Google Scholar]
- 19.Karl PI, Fisher SE. Biotin transport in microvillous membrane vesicles, cultured trophoblasts and the isolated perfused cotyledon of the human placenta. Am J Physiol. 1992;262:C302–8. doi: 10.1152/ajpcell.1992.262.2.C302. [DOI] [PubMed] [Google Scholar]
- 20.Schenker S, Hu Z, Johnson RF, Yang Y, Frosto T, Elliott BD, Henderson GI, Mock DM. Human placental biotin transport: normal characteristics and effect of ethanol. Alcohol Clin Exp Res. 1993;17:566–75. doi: 10.1111/j.1530-0277.1993.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 21.Feldman G, Wolf B. Deficient acetyl CoA carboxylase activity in multiple carboxylase deficiency. Clin Chim Acta. 1981;111:147–51. doi: 10.1016/0009-8981(81)90181-9. [DOI] [PubMed] [Google Scholar]
- 22.Packman S, Caswell N, Gonzales-Rios M, Kadlecek T, Cann H, Rassin D, McKay C. Acetyl CoA carboxylase in cultured fibroblasts: differential biotin dependence in the two types of biotin-responsive multiple carboxylase deficiency. Am J Hum Genet. 1984;36:80–92. [PMC free article] [PubMed] [Google Scholar]
- 23.Kuroishi T, Rios-Avila L, Pestinger V, Wijeratne SS, Zempleni J. Biotinylation is a natural, albeit rare, modification of human histones. Mol Genet Metab. 2011;104:537–45. doi: 10.1016/j.ymgme.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atamna H, Newberry J, Erlitzki R, Schultz CS, Ames BN. Biotin deficiency inhibits heme synthesis and impairs mitochondria in human lung fibroblasts. J Nutr. 2007;137:25–30. doi: 10.1093/jn/137.1.25. [DOI] [PubMed] [Google Scholar]
- 25.Zempleni J, Teixeira DC, Kuroishi T, Cordonier EL, Baier S. Biotin requirements for DNA damage prevention. Mutat Res 2011;doi:10.1016j/mrfmmm2011.08.001. [DOI] [PMC free article] [PubMed]
- 26.Zempleni J, Mock DM. Marginal biotin deficiency is teratogenic. Proc Soc Exp Biol Med. 2000;223:14–21. doi: 10.1046/j.1525-1373.2000.22303.x. [DOI] [PubMed] [Google Scholar]
- 27.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–5. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]