Abstract
Purpose
To verify whether carriers of common single-nucleotide polymorphisms (SNPs) of the FSH receptor (FSHR) show reduced responsiveness of antral follicles to FSH administration as assessed by the FORT.
Methods
We performed a prospective study in a university hospital. Study population consisted of 124 Caucasian IVF-ET candidates. FSHR 307Ala and 680Ser variants were analyzed in haplotypes and as separated genes. Serum FSH, estradiol (E2), and anti-Müllerian hormone (AMH) were measured on cycle-day 3. Antral follicle (3–8 mm) count (AFC) and preovulatory follicle (16–22 mm) count (PFC) were performed, respectively, at the achievement of pituitary suppression (before FSH administration) and on the day of hCG administration. Antral follicle responsiveness to FSH administration assessed by the FORT (PFCx100/AFC).
Results
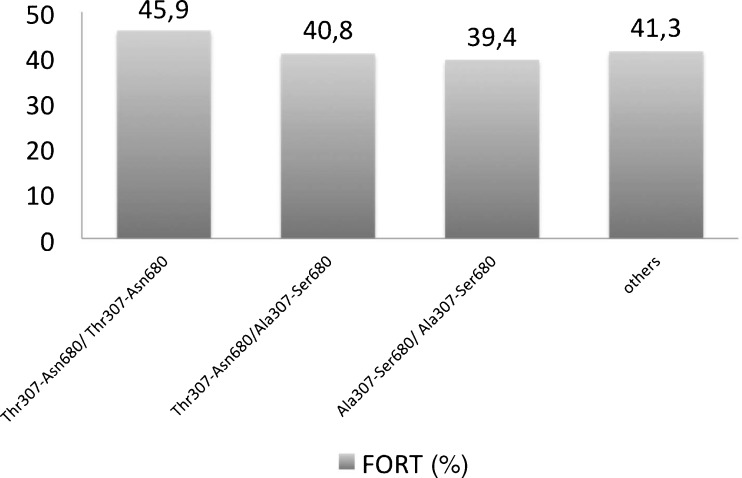
Data concerning baseline and IVF-ET parameters were similar between SNPs carriers and controls. Moreover, FORT was similar for different haplotypes Thr307-Asn680 (45.9%) and Ala307-Ser680 (39.4%) and 307Thr/Ala-Ala/Ala (41.1%; 5.0–91.6%) versus 307Thr/Thr (44.4%; 17.3–83.3%) and in 680Asn/Ser-Ser/Ser (40.0%; 5.0–91.6%) versus 680Asn/Asn (42.2%; 8.3–90.0%) carriers.
Conclusions
Antral follicle responsiveness to FSH, as far as measured by the FORT, is not influenced by the presence of SNPs of FSHR 307Ala and 680Ser.
Keywords: FSHR polymorphisms, SNP, Genetic polymorphisms, IVF-ET outcome, Biomarkers
Introduction
The adequate understanding of factors driving responsiveness to FSH administration of antral follicles is key to custom-tailoring controlled ovarian hyperstimulation (COH) regimens according to individual patient characteristics. Among the multiple factors that have been hitherto proposed, rank some specific single nucleotide polymorphisms (SNPs) of the FSH receptor (FSHR) [1,12]. In particular, the occurrence of two frequent allelic variants localized in the exon 10 of the human FSHR gene, which are observed in strong linkage disequilibrium (Thr307Ala and Asn680Ser), have been shown to influence the ovarian response to endogenous [11] or exogenous gonadotropins [2,4,12]. Yet, the association between these SNPs and ovarian response to COH could not be duplicated in other investigations [3,9].
Since the ovarian response to COH significantly is influenced by the pre-therapeutic amount of small antral follicles [8], the reckoning of the absolute number of growing/preovulatory follicles obtained in response to FSH offers an insufficient reflection of the actual antral follicle responsiveness to FSH. This methodological limitation constitutes a possible explanation for the conflicting conclusions drawn by previous clinical studies on the relationship between FSHR genotype and ovarian response to COH [2–4,12].
To overcome this contingency, we elected to use an objective index to assessing antral follicle responsiveness to exogenous FSH, the Follicular Output RaTe (FORT) [6]. The FORT is calculated by the ratio between the number of preovulatory follicles obtained in response to FSH administration and the preexisting pool of small antral follicles, and it is not, by design, influenced by the number of small antral follicles before FSH treatment. The availability and objectivity of the FORT prompted us to investigate whether the two most frequent allelic variants of the human FSHR gene Thr307Ala and Asn680Ser are related to antral follicle responsiveness to FSH administration.
Materials and methods
Subjects
We prospectively studied 124 patients, 24 to 41 years of age, having accomplished COH for IVF-ET (In vitro Fertilization-Embryo Transfer). All of them met the following inclusion criteria: 1) both ovaries present, deprived of morphological abnormalities (such as cysts, endometriomas, etc.), and adequately visualized in transvaginal ultrasound scans; 2) regular menstrual cycles lasting between 25 and 35 days; 3) total number of antral follicles measuring 3–8 mm in diameter before COH <25; 4) body mass index (BMI) ranging from 17–29 kg/m2. Non-inclusion criteria were: 1) current or past diseases affecting ovaries or gonadotropin or sex steroid secretion, clearance, or excretion; 2) clinical and/or biological signs of hyperandrogenism. Exclusion criteria was: 1) patients who were not expected to meet the criteria set for hCG administration, defined as ≥4 preovulatory follicles (16–22 mm in diameter) and estradiol (E2) levels per preovulatory follicle were >200 pg/mL, and who underwent, therefore, COH cancellation in the present cycle. Incidentally, indications for IVF-ET were male factor (35.2%), tubal factor (10.2%), endometriosis without ovarian lesions (7.8%), idiopathic infertility (28.9%), or mixed (19.9%). All patients signed a written informed consent and this investigation received the approval of ethical review boards of both collaborative institutions (Comité de Protection des Personnes, France and Hospital de Clínicas de Porto Alegre, Rio Grande do Sul, Brazil).
Controlled ovarian hyperstimulation protocol
Within the 3 months that preceded IVF-ET, participants had serum anti-Müllerian hormone (AMH), E2, and FSH levels measured on cycle-day 3. Small amounts of total blood were also collected in proper cardboards (QIAcard FTA Four Spots®, QIAGEN, Hilden, Allemagne) for later DNA extraction. Between cycle-days 1–3 of a subsequent menstrual cycle, they received a single-dose, time-release gonadotropin releasing-hormone (GnRH) agonist, triptorelin (3 mg, IM, Decapeptyl®, Beaufour Ipsen Pharma, Paris, France). Three weeks later, complete pituitary desensitization was confirmed by the detection of low serum levels of progesterone (P4), E2, and LH (baseline). The absence of ovarian cysts and an endometrial thickness <5 mm were also verified. Thereafter, recombinant FSH therapy (Gonal-F, Serono Pharmaceuticals, Boulogne, France) was initiated, at a dosage of 300 IU/day for at least 5 days, and continued until the day of hCG (Gonadotrophine Chorionique “Endo”, Organon Pharmaceuticals, Saint-Denis, France, 10,000 IU, IM) administration. From the 6th day of recombinant FSH therapy onwards, daily FSH doses were adjusted according to E2 levels and/or the number of growing follicles. During the last days of COH, patients had daily visits for ultrasonographic and hormonal examinations to define the proper timing for hCG administration. To improve reliability of FORT calculation, any unsuitable extending of COH duration was avoided by administering hCG (day of hCG administration; dhCG) as soon as ≥4 preovulatory follicles (≥16 mm in diameter) were observed and E2 levels per preovulatory follicle were >200 pg/mL. Oocytes were then retrieved 35 hours after hCG administration by transvaginal ultrasound-guided aspiration and ETs were performed 2 days after oocyte retrieval. Luteal phase was supported with micronized P4, 600 mg/day, administered continuously by vaginal route, starting on the evening of ET. Clinical pregnancy was defined as presence of a gestational sac observed at ultrasound scan at around 7 weeks of amenorrhea. Embryo implantation rate was defined as total number of gestational sacs x 100/total number of embryos transferred.
FORT calculation
At baseline and dhCG, ovarian ultrasound scans were performed using a 5.0-9.0 MHz multi-frequency transvaginal probe (Voluson 730 Expert, General Electric Medical Systems, Paris, France) to evaluate the number and sizes of antral follicles. We carefully determined, at baseline, the number of all follicles measuring 3–8 mm in diameter (antral follicle count, AFC) and, on dhCG, the number of all follicles measuring 16–22 mm in diameter (preovulatory follicle count, PFC), in both ovaries. The FORT was calculated by the ratio between PFC on dhCG x 100/AFC at baseline. The same two equally trained professionals performed all ultrasounds. The choice of considering only 16–22 mm follicles for the calculation of FORT was based in previous investigation of our group [6] and represented a methodological attempt for discriminating, among the cohort of small antral follicles, those that were the most FSH-responsive.
Hormonal measurements
Serum P4, E2, LH, and FSH levels were determined by an automated multi-analysis system using a chemiluminescence technique (Advia-Centaur, Bayer Diagnostics, Puteaux, France). For P4, lower detection limit was 0.10 ng/mL, linearity up to 60 ng/mL, and intra- and interassay coefficients of variation (CV) were 8% and 9%, respectively. For E2, lower detection limit was 30 pg/mL, linearity up to 1,000 pg/mL, and intra- and interassay CV were 8% and 9%, respectively. For LH and FSH, lower detection limit was 0.1 mIU/mL and intra- and interassay CV were 3% and 5%, respectively. Serum AMH levels were determined using a “second generation” enzyme-linked immunosorbent assay (reference A16507; Immunotech Beckman Coulter Laboratories, Villepinte, France). Intra- and interassay coefficients of variation were <6% and <10%, respectively, lower detection limit at 0.13 ng/mL, and linearity up to 21 ng/mL for AMH.
DNA isolation and detection of the polymorphisms Thr307Ala and Asn680Ser
Genomic DNA was extracted from the cardboards according to the manufacturer’s instructions (QIAcard FTA Four Spots®, QIAGEN, Hilden, Allemagne). Overall, methodology for screening both polymorphisms respected STREGA rules [10] and was described elsewhere [15]. All reagents were purchased from Invitrogen (Invitrogen, Carlsbad, USA). Polymerase chain reaction (PCR) was performed in an Eppendorf Personal Cycler Thermocycler (Eppendorf, Hamburg, Germany).
The Thr307Ala variant, in exon 10 of the FSHR gene, was detected by the nested PCR–Restriction Fragment Length Polymorphism (RFLP) method. First, a 657 bp fragment of the FSHR gene was amplified by PCR, using a set of primers (5′-TCTGAGCTTCATCCAATTTGCA-3′ and 5′-GGGAAAGAGGGCA GCTGCAA-3′) at 58°C. This PCR product (1 μL) was further amplified by a second PCR, using another set of primers (5′-CAAATCTATTTTAAGGCAAGAAGTTGATTATATGCCTCAG-3′ and 5′-GTAGATTCCAATGCAGAGATCA-3′), also at 58°C. The amplified fragment of 364 bp was digested with Bsu36I (New England Biolabs, USA) restriction enzyme. A mismatch nucleotide was introduced in the second PCR. This mismatch and the A to G transition created a Bsu36I restriction site, so that the second PCR fragment following Bsu36I digestion and 2.5% agarose gel electrophoresis with ethidium bromide revealed different RFLP patterns. According to them, genotype of FSHR of our patients was labeled as follows: 307Thr/Thr (307TT; n = 29), 307Thr/Ala (307TA; n = 48), and 307Ala/Ala (307AA; n = 32)—results from 15 patients regarding the 307 locus remained inconclusive despite PCR was repeated several times
For the RFLP analysis of the Asn680Ser SNP the methodology was as follows. The FSHR gene was amplified by PCR using genomic DNA as the template and a set of primers (5′-TTTGTGGTCATCTGTGGCTGC-3′ and 5′-CAAAGGCAAGGACTGAATTATCATT-3′), which amplified a DNA fragment of 520 bp at an annealing temperature of 60°C. Since the A to G transition created an endonuclease BsrI recognition site, the PCR fragment following BsrI digestion and 2.5% agarose gel electrophoresis with ethidium bromide revealed different RFLP patterns. According to them, genotype of FSHR of our patients was labeled as follows: 680Asn/Asn (680NN; n = 49), 680Asn/Ser (680NS; n = 62), and 680Ser/Ser (680SS; n = 13).
Definition of groups according to FSHR gene polymorphisms
Since these two polymorphisms have been described to be in complete or strong linkage in the literature we have combined genes in haplotypes no analyze our data. We have found that these two polymorphisms are not in complete linkage in our population and Table 1 presents the frequencies of all haplotypes found. Therefore, to clarify the possible relationship between Thr307Ala and Asn680Ser polymorphisms of FSHR and FORT, we have also analyzed the two genes separately. To improve the statistical power of sampling, we decided to combine homozygous and heterozygous carriers for the SNPs, a methodology previously employed by other investigators for similar reasons [2,12]. Therefore, 307TA and 307AA patterns at position 307 (307TA/AA; n = 80) and 680NS and 680SS patterns at position 680 (680NS/SS; n = 75) were analyzed altogether. This led us to set two different study groups for position 307 (307TT; n = 29 and 307TA/AA; n = 80) and two other study groups for position 680 (680NN; n = 49 and 680NS/SS; n = 75).
Table 1.
Haplotype frequencies in total population
| n (%) | |
|---|---|
| Thr307-Asn680/ Thr307-Asn680 | 29 (20.3) |
| Thr307-Asn680/Ala307-Ser680 | 40 (28) |
| Ala307-Ser680/ Ala307-Ser680 | 12 (8.4) |
| Ala307-Asn680/ Ala307-Ser680 | 19 (13.3) |
| Thr307-Ser680/ Ala307-Ser680 | 1 (0.7) |
| Thr307-Asn680/Ala307-Asn680 | 7 (4.9) |
| Ala307-Asn680/ Ala307-Asn80 | 1 (0.7) |
Statistics
The statistical analysis was carried out using the SPSS 12.0 software. The measure of central tendency used was the mean and the measure of variability was the standard deviation. Medians and minimum-maximum values were used when normality of data distribution could not be ascertained. Categorical variables between 307TT and 307TA/AA groups and between 680NN and 680NS/SS groups were compared using the 2-sided Pearson Chi square-test. Continuous variables were compared using the Student’s t-test. Sample size was calculated based on previous study [12] aiming 80% power for a P < 0.05 (110 patients). Genotype distribution was tested for Hardy–Weinberg equilibrium, and the difference in genotype frequencies between the samples was tested using a Chi square-test for independence [10]. A P < 0.05 was considered statistically significant.
Results
Prevalence of allelic variants of the FSHR gene
Genotype analysis was possible in all 124 patients for the FSHR 680 polymorphism and in 109 patients for the FSHR 307 polymorphism, since results from 15 patients regarding the 307 locus remained inconclusive despite PCR was repeated several times. Hence, haplotype combination was only possible in 109 patients. To make data interpretation easier in Tables 2 and 3 we have chosen to demonstrate data regarding classical haplotype combinations, as shown by others [14], and combined all other combinations in the fourth column. Both allelic distributions respected the Hardy-Weinberg Equilibrium [10]. Noteworthy, in the present series, Ser680/Ala307 were not in complete linkage disequilibrium.
Table 2.
Patient’s characteristics and COH data in the all FSHR haplotype groups
| Thr307-Asn680/Thr307-Asn680 | Thr307-Asn680/Ala307-Ser680 | Ala307-Ser680/Ala307-Ser680 | Othersb | P | |
|---|---|---|---|---|---|
| n | 29 | 40 | 12 | 28 | |
| Ages (years) | 35.2 ± 3.7 | 34.6 ± 3.8 | 35.1 ± 3.7 | 34.9 ± 4.1 | 0.913 |
| BMI (kg/m2) | 21.4 ± 5.1 | 22.1 ± 6.7 | 21.7 ± 3.0 | 21.5 ± 5.2 | 0.932 |
| Serum Day-3 FSH levels (mUI/mL) | 6.2 ± 1.9 | 6.7 ± 2.3 | 7.0 ± 1.8 | 6.3 ± 1.6 | 0.609 |
| Serum Day-3 E2 levels (pg/mL) | 37.6 ± 20.2 | 37.6 ± 18.4 | 49.1 ± 38.8 | 40.2 ± 17.6 | 0.484 |
| Serum AMH levels (ng/mL) | 2.9 ± 1.1 | 2.9 ± 1.9 | 3.6 ± 1.5 | 3.1 ± 1.6 | 0.654 |
| AFC at baselinea | 16.5 ± 5.2 | 17.3 ± 6.4 | 20.6 ± 7.7 | 17.9 ± 5.4 | 0.367 |
| Total dose of recombinant FSH (IU) | 2,790 ± 550 | 2,769 ± 622 | 2,598 ± 580 | 2,559 ± 608 | 0.435 |
| Duration of recFSH therapy (days) | 10.56 ± 1.41 | 10.42 ± 1.77 | 10.38 ± 1.38 | 10.41 ± 1.58 | 0.940 |
| Serum E2 levels on dhCG (pg/mL) | 2,340 ± 960 | 2,246 ± 961 | 1,918 ± 1124 | 2,174 ± 980 | 0.435 |
| Serum P4 levels on dhCG (ng/mL) | 0.81 ± 0.35 | 0.74 ± 0.41 | 0.94 ± 0.73 | 0.84 ± 0.49 | 0.269 |
aAntral follicle count (follicles measuring 3–10 mm)
b Ala307-Asn680/Ala307-Ser680; Thr307-Ser680/Ala307-Ser680; Thr307-Asn680/Ala307-Asn680; Ala307-Asn680/Ala307-Asn680
Table 3.
Embryology and IVF-ET outcome data in the all FSHR haplotype groups
| Thr307-Asn680/Thr307-Asn680 | Thr307-Asn680/Ala307-Ser680 | Ala307-Ser680/Ala307-Ser680 | Othersa | P | |
|---|---|---|---|---|---|
| n | 29 | 40 | 12 | 28 | |
| No. of oocytes retrieved | 12.6 ± 6.7 | 9.6 ± 4.9 | 12.0 ± 5.6 | 10.6 ± 4.7 | 0.233 |
| No. of metaphase II oocytes | 10.5 ± 6.4 | 8.5 ± 4.30 | 10.9 ± 5.6 | 10.1 ± 4.2 | 0.388 |
| No. of embryos | 7.4 ± 4.9 | 6.3 ± 2.8 | 6.7 ± 3.4 | 5.5 ± 2.6 | 0.358 |
| Top-morphology embryos (%) | 2.0 ± 2.3 | 1.3 ± 1.4 | 2.1 ± 2.0 | 1.5 ± 1.3 | 0.497 |
| Clinical Pregnancy Rate/OR (%) | 37.9 | 35.9 | 46.4 | 38.0 | 0.620 |
| Embryo Implantation Rate (%) | 24,1% | 23.4% | 27,0% | 21,3% | 0.420 |
aAla307-Asn680/Ala307-Ser680; Thr307-Ser680/Ala307-Ser680; Thr307-Asn680/Ala307-Asn680; Ala307-Asn680/Ala307-Asn680
Overall population and COH characteristics and IVF-ET results
At the time of inclusion, women were aged 34.8 ± 3.8 years and presented BMI values at 22.0 ± 3.9 kg/m2. On cycle day 3, serum E2 and FSH levels were 39 ± 22 pg/mL and 6.4 ± 1.8 mIU/mL, respectively. At baseline, AFC was at 16.5 ± 6.2 follicles. On dhCG, PFC was at 6.6 ± 2.9 follicles and serum P4 and E2 levels were 0.85 ± 0.48 ng/mL, 2,211 ± 1001 pg/mL, respectively. COH lasted 10.5 ± 1.8 days and required a total dose of 2,730 ± 621 IU of recombinant FSH. Overall, FORT was 42.2% (range, 5.0–91.7%). An average of 10.9 ±5.4 oocytes were retrieved, 6.5 ± 3.7 embryos were obtained, and 1.64 ± 0.65 embryos were transferred into the uterus.
Relationships between Asn680Ser and Thr307Ala genotypes and patient’s characteristics, COH data, IVF-ET results, and FORT
Patient’s characteristics and COH data in the all FSHR haplotypes groups are summarized in Table 2. As shown, ages, BMIs, serum E2 and AMH levels and AFC were comparable in all groups. Similarly, indications for IVF-ET were also not different between groups (data not shown). Data regarding polymorphisms analysis separately (680NN vs. 680NS/SS and 307TT vs. 307TA/AA) are not shown however results are not different.
Embryology and IVF-ET outcome data in all FSHR genotype groups are shown in Table 3. The number of oocytes, metaphase II oocytes, and total embryos, the prevalence of top-morphology embryos, as well as fertilization rates were also comparable in all haplotype groups as well as in 307TA/AA versus 307TT patients and in 680NN versus 680NS/SS groups. Despite the present investigation was underpowered to detect possible differences in IVF-ET outcome in FSHR SNP carriers as compared to controls, incidentally, clinical and ongoing pregnancy rates per oocyte retrieval as well as embryo implantation rates remained comparable in all groups. Finally, as illustrated in Fig. 1, antral follicle responsiveness to exogenous FSH, as assessed by the FORT, was comparable in Thr307Ala and Asn680Ser carriers or non-carriers.
Fig. 1.
Bar-chart showing FORT (as calculated by PFC × 100/AFC) in different haplotypes. Differences were not statistically significant
Discussion
The present investigation aimed at testing the hypothesis that FSHR genotype influences antral follicle responsiveness to exogenous FSH in adult women. Contrasting with previous publications that addressed this issue [2–4,9,11,12], to quantify follicle responsiveness to FSH, instead of using the number of follicles undergoing preovulatory maturation or the number of oocytes retrieved, we used the FORT, an innovative measure that has the advantage of being independent from the pretreatment mass of FSH-sensitive follicles [6]. The present results indicated that, after administration of substantial FSH doses, FORT was not significantly influenced by the presence of FSHR SNPs (Thr307Ala and AsnSer680).
As discussed elsewhere [6], FORT is a promising clinical strategy to assess the ability of antral follicles to respond to exogenous FSH. Yet, this index presents a number of limitations. In its present design, FORT was not assessable in patients having discontinued FSH treatment before hCG administration (often due to a markedly insufficient number of recruited follicles by COH), since their 16–22 mm follicle count could not be established. If FORT still is instrumental when the number of average-sized follicles at mid-follicular phase of COH, instead of that of 16–22 mm follicles on dhCG, is used remains to be established. Undoubtedly, this development could reincorporate patients vowed to cancellation into FORT calculation, thereby allowing to elucidating whether some FSHR genotypes can be more frequently observed among this group of patients. In addition, FORT implies that 3–8 mm follicles before COH respond coordinately to FSH, which is not always the case [5]. To overcome this limitation, the individual tracking of the development of each follicle in response to FSH would be required, which is practically unrealistic. Instead, two alternative methodological measures were taken. First, to reduce follicle size discrepancies and coordinate as far as possible follicle growth during COH, endogenous FSH was beforehand profoundly suppressed by a GnRH agonist. Second, to recruit as many follicles as possible despite their pretreatment sizes, relatively high initial FSH doses were used. Finally, to prevent the possible influence of any unsuitable extending of COH duration on PFC and, therefore, FORT calculation, strict follicle monitoring and ovulation triggering policies were respected. Indeed, all participants received hCG as early as ≥4 follicles reached preovulatory maturation (and E2 levels per preovulatory follicle were >200 pg/mL) and only those who definitely would not satisfy this criteria were cancelled. Furthermore, FORT assumed that, on dhCG, only 16–22 mm follicles effectively responded to FSH, while it is conceivable that smaller follicles also presented some degree of FSH responsiveness. However, as very small follicles, which were not countable by ultrasound at baseline, may have also initiated their FSH-driven maturation after the start of COH and reached intermediate sizes on dhCG, the inclusion of average-sized follicles on dhCG into the calculation of FORT could puzzle its interpretation. Moreover, additional studies focusing on other follicle sizes will be helpful to fine-tune alternative relevant cutoffs for the calculation of this new parameter. Together, these FORT characteristics bring out its great flexibility and spur us to further develop this new concept. Future evaluation of alternative ways of calculating the FORT, in particular using different numerators and including patients treated with weaker and possibly more discriminating exogenous FSH signals will undoubtedly contributive to broaden its clinical applications.
The central finding of the present investigation, i.e. the lack of relationship between the presence of the FSHR SNPs in homozyogous or heterozygous state (307TA/AA or 680NS/SS) and FORT, may be at least in part attributable to the relatively high initial FSH doses administered to our patients [7]. As stated previously, this measure, which was implemented to recruit as many follicles as possible despite their heterogeneous pretreatment sizes, may have overcome the possible “resistance” to FSH of antral follicles in patients carrying Asn680Ser and/or Thr307Ala SNPs. Indeed, it has been shown that the reported reduction of follicular sensitivity to exogenous FSH, resulting of FSHR polymorphisms, may be surmounted by increasing gonadotropin doses [2,7,12,15]. Whether this constitutes an explanation to the fact that, despite serum day-3 FSH levels were discretely yet significantly increased in 680NS/SS carriers, these patients displayed a comparable FORT as controls remains to be elucidated in further studies.
Incidentally, we observed that roughly 4% of infertile French population studied showed a lack of linkage between Thr307Ala and Asn680Ser SNPs. This contrasts with previous observations made in other Caucasian populations indicating that both variants of FSHR were in complete linkage, which led some authors to suggest that only one SNP needs to be tested (as a TAG SNP) [12]. However, homogeneity of the studied populations has raised questions about the actual relationship between these polymorphisms [4,12,15]. The present data evidencing an incomplete association of these two alleles corroborates previous studies made in a group of fertile Brazilian women [13]. Furthermore, both of the studied alleles are in the Hardy-Weinberg equilibrium.
In conclusion, the present findings indicate that FSHR genotype does not influence antral follicle responsiveness to strong FSH doses, as far as it is measurable by the FORT. Further studies using lower, yet more discriminating, FSH doses are needed to verify whether this lack of difference is due to the intensity of the FSH signal or to a lack of functional relationship between these SNPs of FSHR and follicle reactivity to FSH. These next research steps, possibly conducted using the FORT as the outcome measure, will be undoubtedly helpful to define the actual role of FSHR genomics in the individualization of COH treatments.
Acknowledgments
Financial support
This study was supported by a scholarship from the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and a grant from the Fundo de Incentivo a Pesquisa (FIPE) of Hospital de Clínicas de Porto Alegre—RS—Brazil.
Footnotes
Capsule
FSH receptor genotype does not influence antral follicle responsiveness to exogenous gonadotropin as far as measured by FORT.
References
- 1.Aittomaki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82(6):959–968. doi: 10.1016/0092-8674(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 2.Behre HM, Greb RR, Mempel A, Sonntag B, Kiesel L, Kaltwasser P, et al. Significance of a common single nucleotide polymorphism in exon 10 of the follicle-stimulating hormone (FSH) receptor gene for the ovarian response to FSH: a pharmacogenetic approach to controlled ovarian hyperstimulation. Pharmacogenet Genomics. 2005;15(7):451–456. doi: 10.1097/01.fpc.0000167330.92786.5e. [DOI] [PubMed] [Google Scholar]
- 3.Daelemans C, Smits G, Maertelaer V, Costagliola S, Englert Y, Vassart G, et al. Prediction of severity of symptoms in iatrogenic ovarian hyperstimulation syndrome by follicle-stimulating hormone receptor Ser680Asn polymorphism. J Clin Endocrinol Metab. 2004;89(12):6310–6315. doi: 10.1210/jc.2004-1044. [DOI] [PubMed] [Google Scholar]
- 4.Castro F, Ruiz R, Montoro L, Perez-Hernandez D, Sanchez-Casas Padilla E, Real LM, et al. Role of follicle-stimulating hormone receptor Ser680Asn polymorphism in the efficacy of follicle-stimulating hormone. Fertil Steril. 2003;80(3):571–576. doi: 10.1016/S0015-0282(03)00795-7. [DOI] [PubMed] [Google Scholar]
- 5.Fanchin R, Schonauer LM, Cunha-Filho JS, Mendez Lozano DH, Frydman R. Coordination of antral follicle growth: basis for innovative concepts of controlled ovarian hyperstimulation. Semin Reprod Med. 2005;23(4):354–362. doi: 10.1055/s-2005-923393. [DOI] [PubMed] [Google Scholar]
- 6.Genro VK, Grynberg M, Scheffer JB, Roux I, Frydman R, Fanchin R. Serum anti-Mullerian hormone levels are negatively related to Follicular Output RaTe (FORT) in normo-cycling women undergoing controlled ovarian hyperstimulation. Hum Reprod. 2011;26(3):671–677. doi: 10.1093/humrep/deq361. [DOI] [PubMed] [Google Scholar]
- 7.Greb RR, Grieshaber K, Gromoll J, Sonntag B, Nieschlag E, Kiesel L, et al. A common single nucleotide polymorphism in exon 10 of the human follicle stimulating hormone receptor is a major determinant of length and hormonal dynamics of the menstrual cycle. J Clin Endocrinol Metab. 2005;90(8):4866–4872. doi: 10.1210/jc.2004-2268. [DOI] [PubMed] [Google Scholar]
- 8.Hendriks DJ, Mol BW, Bancsi LF, Velde ER, Broekmans FJ. Antral follicle count in the prediction of poor ovarian response and pregnancy after in vitro fertilization: a meta-analysis and comparison with basal follicle-stimulating hormone level. Fertil Steril. 2005;83(2):291–301. doi: 10.1016/j.fertnstert.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Klinkert ER, Velde ER, Weima S, Zandvoort PM, Hanssen RG, Nilsson PR, et al. FSH receptor genotype is associated with pregnancy but not with ovarian response in IVF. Reprod Biomed Online. 2006;13(5):687–695. doi: 10.1016/S1472-6483(10)60660-8. [DOI] [PubMed] [Google Scholar]
- 10.Little J, Higgins JP, Ioannidis JP, Moher D, Gagnon F, Elm E, et al. Strengthening the reporting of genetic association studies (STREGA): an extension of the strengthening the reporting of observational studies in epidemiology (STROBE) statement. J Clin Epidemiol. 2009;62(6):597–608. doi: 10.1016/j.jclinepi.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Overbeek A, Kuijper EA, Hendriks ML, Blankenstein MA, Ketel IJ, Twisk JW, et al. Clomiphene citrate resistance in relation to follicle-stimulating hormone receptor Ser680Ser-polymorphism in polycystic ovary syndrome. Hum Reprod. 2009;24(8):2007–2013. doi: 10.1093/humrep/dep114. [DOI] [PubMed] [Google Scholar]
- 12.Perez Mayorga M, Gromoll J, Behre HM, Gassner C, Nieschlag E, Simoni M. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptor genotype. J Clin Endocrinol Metab. 2000;85(9):3365–3369. doi: 10.1210/jc.85.9.3365. [DOI] [PubMed] [Google Scholar]
- 13.Rodini GP, Genro VK, Matte U, Pereira FS, Bilibio JP, Greggianin C, et al. There is no complete linkage between the polymorphisms N680S and T307A of the follicular stimulating hormone receptor gene in fertile women. J Assist Reprod Genet. 2011;28(3):221–224. doi: 10.1007/s10815-010-9503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simoni M, Gromoll J, Hoppner W, Kamischke A, Krafft T, Stahle D, et al. Mutational analysis of the follicle-stimulating hormone (FSH) receptor in normal and infertile men: identification and characterization of two discrete FSH receptor isoforms. J Clin Endocrinol Metab. 1999;84(2):751–755. doi: 10.1210/jc.84.2.751. [DOI] [PubMed] [Google Scholar]
- 15.Sudo S, Kudo M, Wada S, Sato O, Hsueh AJ, Fujimoto S. Genetic and functional analyses of polymorphisms in the human FSH receptor gene. Mol Hum Reprod. 2002;8(10):893–899. doi: 10.1093/molehr/8.10.893. [DOI] [PubMed] [Google Scholar]