Abstract
Purpose
To investigate the influence of the peroxisome proliferator activated receptor gamma (PPAR-γ) Pro12Ala polymorphism on the susceptibility of polycystic ovary syndrome (PCOS) and body mass index (BMI), fast insulin levels, homeostasis model assessment of insulin resistance (HOMA-IR) in PCOS patients.
Methods
PubMed, EMBASE, MEDLINE and CENTRAL databases were searched to identify eligible studies. We then conducted a meta-analysis to examine the association between Pro12Ala polymorphism and PCOS.
Results
Seventeen eligible studies, including 2,149 patients and 2,124 controls were enrolled in this meta-analysis. Pro12Ala polymorphism was significantly associated with the susceptibility of PCOS (odds ratio [OR] 0.74, 95 % confidence interval [CI] [0.61, 0.90] for allele; OR 0.70, 95 % CI [0.57, 0.86] for genotype). In the European subgroup of PCOS, the X/Ala genotype was associated with lower BMI (mean difference [MD] −1.08, 95 % CI [−2.08, −0.09]) and fast insulin levels (MD −19.82, 95 % CI [−34.07, −5.58]). However, this polymorphism did not display an impact on HOMA-IR in PCOS patients.
Conclusions
Ala variant would decrease the risk of PCOS and result in lower BMI and fast insulin levels in a European population, but had no impact on HOMA-IR in PCOS patients. Further studies are required to elucidate these associations more clear.
Keywords: Polycystic ovary syndrome, PPAR-γ, Meta-analysis, Body mass index, Fast insulin, Insulin sensitivity
Introduction
The polycystic ovary syndrome (PCOS) is a common endocrinopathy affecting an estimated 5–10 % of adult women and diagnosed frequently during adolescence [1–3]. PCOS is primarily characterized by anovulation, hyperandrogenism, insulin resistance and polycystic ovaries by ultrasonography in most patients [4, 5]. Although the pathogenesis of PCOS remains elusive, both the genetic and environmental factors are involved in the development of this condition [6]. Evidence from different familial and monozygotic twins’ studies indicates that the genetic factors play an important role [7–9]. To date, a large number of candidate genes have been studied concerning their potential roles in the etiology of PCOS, such as CYP11a, CYP17, CYP19, LHR, FSHR, IRS-1/2, INSR, and PPAR-γ. Among them, the peroxisome proliferator activated receptor gamma (PPAR-γ) gene was presumed to be involved in this disease.
PPAR-γ is a ligand-activated transcription factor that belongs to the nuclear hormone receptor superfamily. It is expressed in adipose tissue, macrophages, intestines, ovaries and plays a crucial role in the regulation of energy storage, insulin sensitivity, adipocyte differentiation and lipid metabolism [10]. PPAR-γ was demonstrated to be up-regulated in the ovaries of PCOS patients [11], which was consistent with other animal studies [12–14]. Recently, many researchers focused on the association between Pro12Ala polymorphism (rs1801282) in PPAR-γ and PCOS, which may facilitate to elucidate the pathogenesis of this condition. However, the results were inconsistent.
To deal with these contradictory issues, we conducted a meta-analysis to examine the influence of the Pro12Ala polymorphism on the susceptibility of PCOS and body mass index (BMI), fast insulin levels, homeostasis model assessment of insulin resistance (HOMA-IR) in PCOS patients.
Materials and methods
Literature search and data extraction
A literature search of the PubMed, EMBASE, MEDLINE and Cochrane Central Register of Controlled Trials (CENTRAL) databases was conducted to identify all the articles about the association between PPAR-γ Pro12Ala polymorphism and PCOS before November 2011, using the keywords “(polycystic ovary syndrome or PCOS) and (peroxisome proliferator-activated receptor or PPAR or PPARgamma or PPARγ) and (gene or polymorphism or variant or mutation)”. Besides, all references cited in these studies and previously published review articles were reviewed to identify additional eligible researches. The search was not restricted by language. Eligible studies must meet the following criteria: (1) the study had at least one of following data: (I) genotype or allele frequency, (II) BMI, (III) fast insulin, (IV) HOMA-IR; (2) for the comparison of allele and genotype, eligible study should use an unrelated case–control design, and genotype distributions in the control group were consistent with Hardy–Weinberg equilibrium (P > 0.01). Two authors (J.H. and J.L.) independently screened the titles and abstracts to exclude obviously irrelevant reports. All full text copies of studies meeting the inclusion criteria were retrieved and examined for the eligibility. Disagreements resolved by discussion between the authors.
Data extraction
Another two authors (L.W. and F.L.) independently extracted data using the checklist for data collection adapted from the Cochrane Handbook [15]. All disagreements about eligibility were resolved during a consensus with a third reviewer (X.L.). The data extracted included: author, year of publication, ethnicity of the population. For binary outcomes, the information on the numbers of cases and controls, allele and genotype distributions of Pro12Ala polymorphism was extracted. For continuous outcomes (BMI, fast insulin and HOMA-IR), information on the numbers of patients with different genotypes, mean values and standard differences (SD) was also extracted. The outcomes of fast insulin were converted from μIU/ml to pmol/l (where needed, pmol/l = μIU/ml multiply 6.965). The ethnic descents were categorized as European, Asian, African and others.
Statistical analysis
The effect measure of allele and genotype was odds ratio (OR) and its corresponding 95 % confidence interval (CI). For BMI, fast insulin and HOMA-IR, mean difference (MD) and 95 % CI were adopted. Since the Ala/Ala homozygote is extremely rare, Pro/Ala and Ala/Ala were referred to X/Ala for the pooled analysis. The association between the risk allele and PCOS was evaluated by comparing Ala allele to Pro allele. Besides, the impact of Pro12Ala polymorphism on BMI, fast insulin and HOMA-IR was also examined (patients with X/A genotype versus patients with Pro/Pro genotype). Forest plots were generated by using RevMan5.1 software (The Nordic Cochrane Centre, The Cochrane Collaboration, 2011).
Between-study heterogeneity was tested by Q-statistic test and I2 metric [16, 17]. The I2 metric is independent of the number of studies in the meta-analysis, and ranges between 0 % and 100 % (I2 < 25 %: low heterogeneity; 25 % < I2 < 50 %: moderate heterogeneity; 50 % < I2 < 75 %: high heterogeneity; I2 > 75 %: extreme heterogeneity). If there was heterogeneity between studies (I2 > 50 %), random-effect model was adopted. In the absence of heterogeneity, both the random-effect and fixed-effect models were appropriate.
Egger’s test and Begg’s test were undertaken to detect publication bias using Stata software 11.0 (Stata Corporation, College Station, Texas). Log (OR)/the standard error of log (OR) and MD/the standard error of MD were adopted to yield funnel plots. Hardy–Weinberg equilibrium was examined by the chi-square test with a web program (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl).
Results
Quality control
A total of 82 studies were identified. Only 22 articles were concerned the influence of Pro12Ala polymorphism on the susceptibility, BMI, fast insulin and insulin resistance of PCOS [18–39]. Among them, five articles were excluded for: (1) participants derived from the same cohort [20, 21, 30]; (2) the study did not use an unrelated case–control design [26]; (3) genotype distribution of controls was not consistent with Hardy–Weinberg equilibrium [32]. Ultimately, 17 studies (including 2,149 patients and 2,124 controls) were enrolled in this meta-analysis. The data extracted were listed in Tables 1 and 2.
Table 1.
Characteristics and Allele, genotype distributions of the studies enrolled in this meta-analysis
| Author(year) | Country | Criteria for PCOS | Case/Control(n) | Allele | Genotype | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | ||||||||
| Ala | Pro | Ala | Pro | X/Ala | Pro/Pro | X/Ala | Pro/Pro | ||||
| Hara (2002) [18] | USA | NICHD criteria | 124/N/A | 21 | 227 | N/A | N/A | 21 | 103 | N/A | N/A |
| Korhonen (2003) [19] | Finland | Anovulation and polycystic ovaries | 135/115 | 34 | 236 | 44 | 186 | 31 | 104 | 39 | 76 |
| Orio (2004) [22] | Italy | NICHD criteria | 120/120 | 7 | 233 | 5 | 235 | 7 | 113 | 5 | 115 |
| Tok (2005) [23] | Turkey | Hyperandrogenism, oligoamenorrhoea and polycystic ovaries | 60/60 | 6 | 114 | 13 | 107 | 6 | 54 | 13 | 47 |
| Hahn (2005) [24] | Germany | NICHD criteria | 102/104 | N/A | N/A | N/A | N/A | 23 | 79 | 24 | 80 |
| Haap (2005) [25] | Germany | Rotterdam criteria | 53/546 | 11 | 95 | 145 | 947 | 10 | 43 | 139 | 407 |
| Wang (2006) [27] | China | Rotterdam criteria | 201/147 | 18 | 384 | 12 | 282 | 18 | 183 | 11 | 136 |
| Yilmaz (2006) [28] | Turkey | Rotterdam criteria | 100/100 | 15 | 185 | 22 | 178 | 15 | 85 | 22 | 78 |
| Antoine (2007) [29] | USA | NICHD criteria | 267/168 | 56 | 478 | 39 | 297 | 54 | 213 | 34 | 134 |
| Dragojevič (2008) [39] | Slovenia | NICHD criteria | 69/N/A | 19 | 119 | N/A | N/A | 19 | 50 | N/A | N/A |
| Koika (2009) [31] | Greek | NICHD criteria | 156/56 | 21 | 291 | 10 | 102 | 20 | 136 | 8 | 48 |
| Xita (2009) [33] | Greek | NICHD criteria | 180/140 | 30 | 330 | N/A | N/A | 30 | 150 | N/A | N/A |
| San-Millán (2010) [34] | Spain | NICHD criteria | 161/113 | N/A | N/A | N/A | N/A | 20 | 141 | 21 | 92 |
| Chae (2010) [35] | Korea | Rotterdam criteria | 184/256 | 15 | 353 | 29 | 483 | 13 | 171 | 26 | 230 |
| Christopoulos (2010) [36] | Greek | Rotterdam criteria | 183/148 | 20 | 346 | 19 | 277 | 17 | 166 | 17 | 131 |
| Bidzińska-Speichert (2011) [37, 38] | Poland | Rotterdam criteria | 54/51 | 25 | 83 | 27 | 75 | 19 | 35 | 21 | 30 |
N/A not available; NICHD National Institute of Child Health and Human Development
Table 2.
Parameters of BMI, HOMA-IR and fasting insulin in patients with PCOS
| First author | BMI (kg/m2) | HOMA-IR (mUmM/l) | Fast insulin (pmol/l) | n |
|---|---|---|---|---|
| Hara | 33.9 ± 8.7 | 5.18 ± 6.09 | 132.0 ± 123.7 | 21 |
| 36.1 ± 8.1 | 6.54 ± 5.48 | 165.0 ± 121.7 | 103 | |
| Orio | 30.2 ± 5.4 | 4.6 ± 0.4 | 149.7 ± 47.4 | 7 |
| 30.3 ± 5.5 | 4.4 ± 0.6 | 153.9 ± 45.3 | 113 | |
| Tok | 27.33 ± 4.82 | 1.88 ± 1.2 | 64.7 ± 38.9 | 6 |
| 24.56 ± 4.78 | 2.91 ± 1.18 | 97.8 ± 38.4 | 54 | |
| Hahn | 30.0 ± 7.6 | 2.9 ± 1.9 | 70.0 ± 52.6 | 22 |
| 30.0 ± 9.4 | 4.5 ± 4.1 | 110.0 ± 100.6 | 79 | |
| Wang | 22.6 ± 2.9 | N/A | N/A | 18 |
| 22.4 ± 3.8 | N/A | N/A | 183 | |
| Yilmaz | 21.78 ± 6.65 | 2.56 ± 0.62 | 102.7 ± 42.8 | 15 |
| 24.7 ± 6.78 | 3.55 ± 1.50 | 133.4 ± 50.1 | 85 | |
| Antoine | 34.5 ± 9.9 | 2.74 ± 1.68 | 174.5 ± 127.9 | 54/40a |
| 34.9 ± 9.1 | 2.31 ± 1.36 | 147.2 ± 110.6 | 213/148a | |
| Dragojevič | 25.2 ± 5.2 | 2.18 ± 1.67 | 77.3 ± 57.8 | 19 |
| 28.9 ± 7.2 | 2.49 ± 1.53 | 84.3 ± 48.8 | 50 | |
| Koika | 24.4 ± 3.49 | 2.08 ± 1.19 | 63.5 ± 30.8 | 20 |
| 25.76 ± 6.69 | 2.35 ± 2.56 | 99.4 ± 42.1 | 136 | |
| Xita | 26.6 ± 6.9 | 3.3 ± 2.2 | N/A | 30 |
| 28.2 ± 7.6 | 3.2 ± 2.4 | N/A | 150 | |
| Chae | 24.1 ± 5.0 | 3.8 ± 2.0 | 120.5 ± 57.0 | 13 |
| 22.0 ± 4.8 | 2.9 ± 2.2 | 88.5 ± 55.7 | 171 | |
| San-Millán | N/A | 3.9 ± 3.6 | 114 ± 99 | 20 |
| N/A | 3.2 ± 2.4 | 98 ± 68 | 141 | |
| Bidzińska- | 28.50 ± 8.48 | N/A | N/A | 19 |
| Speichert | 26.02 ± 6.69 | N/A | N/A | 35 |
Values are mean ± SD
For each study, the parameters of patients with X/Ala and Pro/Pro genotype are expressed in the first and second lines, respectively
aFigure before the slash represents the number of patients for “BMI”, that after the slash represents the number of patients for “HOMA-IR” and “fast insulin”
The power of our sample size to detect the correlation between this SNP and PCOS achieved 100 % (α = 0.05, effect size index = 0.1). For the continuous data, the power of our sample size to detect the association between this SNP and BMI, fast insulin and HOMA-IR in PCOS patients was 82.0 %, 70.5 % and 76.8 %, respectively (α = 0.05, effect size index = 0.2).
Publication bias and heterogeneity
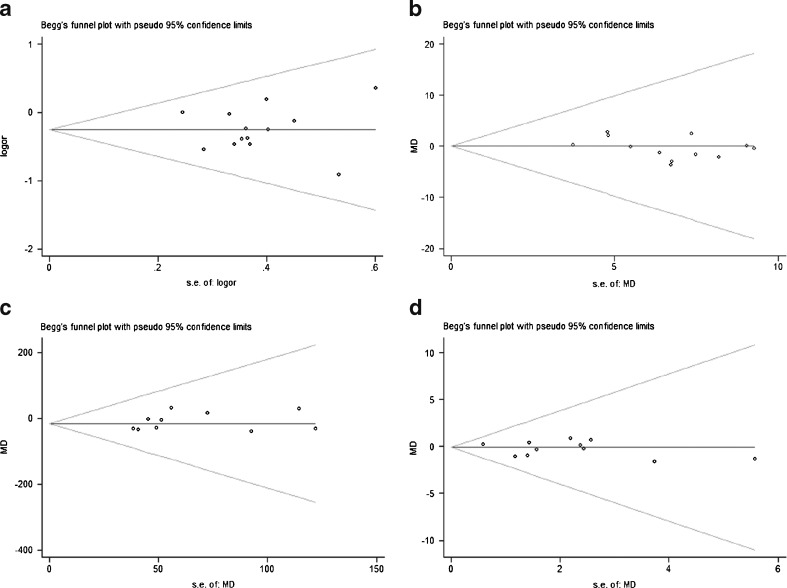
No publication bias was detected in all the five comparisons (Fig. 1). The heterogeneity examined is listed as follows: P = 0.79, I2 = 0 % for allele; P = 0.36, I2 = 9 % for genotype; P = 0.13, I2 = 33 % for BMI; P < 0.01, I2 = 65 % for fast insulin; P < 0.01, I2 = 73 % for HOMA-IR. According to the I2 metric, the fixed-effect model was used for the allele, genotype and BMI comparisons. The random-effect model was adopted for the other two analyses.
Fig. 1.
Begg’s funnel plots for the test of publication bias. a–d represent the funnel plots for comparisons of genotype, BMI, fast insulin and HOMA-IR in order
Meta-analysis
Significant association was found between Pro12Ala and the susceptibility of PCOS (OR 0.74, 95 % CI [0.61, 0.90], P = .002 for allele; OR 0.70, 95 % CI [0.57, 0.86], P = .0006 for genotype). This association also existed in the European subgroup (OR 0.72, 95 % CI [0.58, 0.89], P = .002 for allele; OR 0.68, 95 % CI [0.54, 0.84], P = .0005 for genotype) (Fig. 2).
Fig. 2.
Forest plots of the association between Pro12Ala polymorphism and PCOS. Weight, OR and its corresponding 95 % CI of each study are indicated. a Association of the alleles and PCOS. Events and Total indicate the numbers of Ala and Pro allele, respectively. b Association of the genotypes and PCOS. Events and Total indicate the numbers of X/Ala and Pro/Pro genotypes, respectively
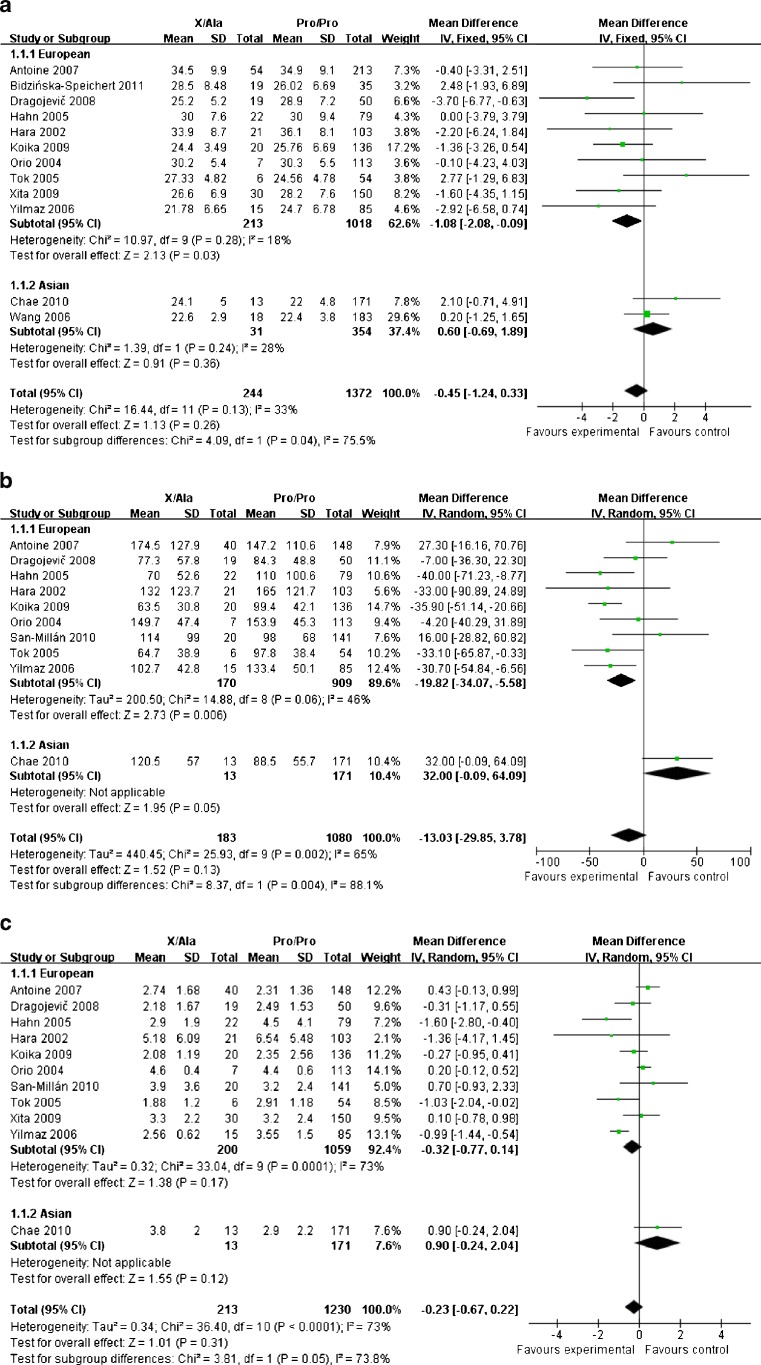
For the comparisons of BMI and fast insulin, the results of total group analysis indicated that no association existed (MD −0.45, 95 % CI [−1.24, 0.33], P = .26; MD −13.03, 95 % CI [−29.85, 3.78], P = .13, respectively). However, these parameters were significantly lower in patients carried X/Ala genotype in the European subgroup (MD −1.08, 95 % CI [−2.08, −0.09], P = .03 for BMI; MD −19.82, 95 % CI [−34.07, −5.58], P = .006 for fast insulin). Pro12Ala polymorphism did not display an impact on HOMA-IR in PCOS patients neither in the total group (MD −0.23, 95 % CI [−0.67, 0.22], P = .31) nor in the European subgroup (MD −0.32, 95 % CI [−0.77, 0.14], P = .17) (Fig. 3).
Fig. 3.
Forest plots of the association between Pro12Ala polymorphism and BMI (a), fast insulin (b), HOMA-IR (c) in PCOS patients. Weight, MD and its corresponding 95 % CI of each study are indicated. Total indicates the numbers of patients in each study
Discussion
It has been demonstrated that both ovarian and metabolic dysfunctions play critical roles in the pathophysiology of PCOS [40], but the underlying mechanism is still elusive. In the last decade, tens of studies were focused on the association between PPAR-γ Pro12Ala polymorphism and PCOS. But the results were varied and no convincing evidence had been provided. Though San-Millán et al. [34] had done an earlier meta-analysis, only 9 studies were enrolled and no analysis of BMI was conducted. In this meta-analysis, 17 researches were eligible and were stratified into two subgroups (European and Asian) to avoid the heterogeneity of ethnicity. We found Pro12Ala polymorphism was significantly associated with the susceptibility of PCOS either in the total group or in the European subgroup. The common Pro allele would increase the risk of PCOS, whereas the Ala variant would be protective against this disease, which was consistent with the meta-analysis of San-Millán. Besides, BMI and fast insulin levels were showed to be lower in patients carried X/Ala genotype in European subgroup. However, no association between this polymorphism and HOMA-IR was yielded.
Though PCOS is clinically heterogeneous, obese and insulin resistance are very common and play a central pathogenic role. Since PPAR-γ is mainly expressed in adipose tissue, the Pro12Ala polymorphism was originally studied to examine its potential impact on obesity. A meta-analysis comprising 32,000 non diabetic subjects revealed positive correlation between this polymorphism and BMI in the subgroup of European and Asian, though no correlation was found in global comparison [41]. In another study, Masud et al. [42] reported Ala carriers had higher BMI than none-carriers in the subgroup of BMI ≥ 27 kg/m2; no correlation was found in total group and in the subgroup of BMI < 27 kg/m2. However, our meta-analysis indicated that PCOS patients with Ala variant had lower BMI only in European subgroup and was not related to the degrees of BMI.
Deeb et al. [43] found that Ala variant of PPAR-γ displayed lower transactivation capacity. This minor allele may lead to less efficient stimulation of PPAR-γ target genes and predispose to lower levels of adipose tissue mass accumulation, which may be responsible for improved insulin sensitivity. Tőnjes et al. [41] reported that fasting insulin levels was significantly lower in nondiabetic individuals with Ala/Ala genotype compared with those subjects with Pro/Pro genotype, while the pooled X/Ala genotype was associated with significantly decreased fast glucose and HOMA-IR only in the obese subgroup (BMI > 30 kg/m2). It seemed that the Pro12Ala polymorphism had an apparent effect on HOMA-IR only in markedly obese individuals. However, since most the studies enrolled in our meta-analysis had no information about the degrees of BMI, we failed to make this comparison in an obese subgroup.
In conclusion, this meta-analysis indicated that Ala allele would decrease the susceptibility of PCOS and result in lower BMI and fast insulin levels in European patients with PCOS. No correlation between this polymorphism and HOMA-IR was detected. This conclusion supported the idea that Pro12Ala polymorphism played a role in the etiology of PCOS and implied that populations without Ala allele may need to pay more attention to the early prevention and diagnosis of this disease. However, there are some limitations that should not be neglected in this study. Firstly, there are high heterogeneities in the meta-analysis of fast insulin and HOMA-IR, which may decrease the reliability of the results. PCOS is a heterogeneous disease, but we did not classify our patients due to the clinical phenotypes. Secondly, since most of the original studies derived from European population and the metabolic parameters seemed to be different in PCOS patients with different ethnic descents [44–47], our findings may not be extended to other descent. Thirdly, due to the limited numbers of original researches enrolled, our results are not conclusive. Further studies are needed to elucidate these associations more clear.
Acknowledgments
We thank the authors of the original studies who responded to request for data.
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
Capsule
This meta-analysis indicated that Ala variant would decrease the risk of PCOS; X/Ala genotype lowered BMI and fast insulin levels, but had no impact on HOMA-IR in PCOS patients.
References
- 1.Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18:671–83. doi: 10.1016/j.bpobgyn.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Hart R, Norman R. Polycystic ovarian syndrome–prognosis and outcomes. Best Pract Res Clin Obstet Gynaecol. 2006;20:751–78. doi: 10.1016/j.bpobgyn.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Franks S. Polycystic ovary syndrome in adolescents. Int J Obes (Lond) 2008;32:1035–41. doi: 10.1038/ijo.2008.61. [DOI] [PubMed] [Google Scholar]
- 4.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–61. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 5.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 6.Franks S, McCarthy MI, Hardy K. Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl. 2006;29:278–85. doi: 10.1111/j.1365-2605.2005.00623.x. [DOI] [PubMed] [Google Scholar]
- 7.Legro RS, Driscoll D, Strauss JF, 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998;95:14956–60. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battaglia C, Regnani G, Mancini F, Iughetti L, Flamigni C, Venturoli S. Polycystic ovaries in childhood: a common finding in daughters of PCOS patients. A pilot study. Hum Reprod. 2002;17:771–6. doi: 10.1093/humrep/17.3.771. [DOI] [PubMed] [Google Scholar]
- 9.Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91:2100–4. doi: 10.1210/jc.2005-1494. [DOI] [PubMed] [Google Scholar]
- 10.Auwerx J. PPARgamma, the ultimate thrifty gene. Diabetologia. 1999;42:1033–49. doi: 10.1007/s001250051268. [DOI] [PubMed] [Google Scholar]
- 11.Jansen E, Laven JS, Dommerholt HB, Polman J, Rijt C, Hurk C, et al. Abnormal gene expression profiles in human ovaries from polycystic ovary syndrome patients. Mol Endocrinol. 2004;18:3050–63. doi: 10.1210/me.2004-0074. [DOI] [PubMed] [Google Scholar]
- 12.Komar CM. Peroxisome proliferator-activated receptors (PPARs) and ovarian function–implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod Biol Endocrinol. 2005;3:41. doi: 10.1186/1477-7827-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovekamp-Swan T, Chaffin CL. The peroxisome proliferator-activated receptor gamma ligand troglitazone induces apoptosis and p53 in rat granulosa cells. Mol Cell Endocrinol. 2005;233:15–24. doi: 10.1016/j.mce.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Froment P, Gizard F, Defever D, Staels B, Dupont J, Monget P. Peroxisome proliferator-activated receptors in reproductive tissues: from gametogenesis to parturition. J Endocrinol. 2006;189:199–209. doi: 10.1677/joe.1.06667. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S (editors). Cochrane handbook for systematic reviews of interventions version 5.0.2. The Cochrane Collaboration: 2009. Available from http://www.cochrane-handbook.org.
- 16.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Hara M, Alcoser SY, Qaadir A, Beiswenger KK, Cox NJ, Ehrmann DA. Insulin resistance is attenuated in women with polycystic ovary syndrome with the Pro(12)Ala polymorphism in the PPARgamma gene. J Clin Endocrinol Metab. 2002;87:772–5. doi: 10.1210/jc.87.2.772. [DOI] [PubMed] [Google Scholar]
- 19.Korhonen S, Heinonen S, Hiltunen M, Helisalmi S, Hippelainen M, Koivunen R, et al. Polymorphism in the peroxisome proliferator-activated receptor-gamma gene in women with polycystic ovary syndrome. Hum Reprod. 2003;18:540–3. doi: 10.1093/humrep/deg128. [DOI] [PubMed] [Google Scholar]
- 20.Orio F, Jr, Matarese G, Biase S, Palomba S, Labella D, Sanna V, et al. Exon 6 and 2 peroxisome proliferator-activated receptor-gamma polymorphisms in polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:5887–92. doi: 10.1210/jc.2002-021816. [DOI] [PubMed] [Google Scholar]
- 21.San-Millán JL, Corton M, Villuendas G, Sancho J, Peral B, Escobar-Morreale HF. Association of the polycystic ovary syndrome with genomic variants related to insulin resistance, type 2 diabetes mellitus, and obesity. J Clin Endocrinol Metab. 2004;89:2640–6. doi: 10.1210/jc.2003-031252. [DOI] [PubMed] [Google Scholar]
- 22.Orio F, Jr, Palomba S, Cascella T, Biase S, Labella D, Russo T, et al. Lack of an association between peroxisome proliferator-activated receptor-gamma gene Pro12Ala polymorphism and adiponectin levels in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5110–5. doi: 10.1210/jc.2004-0109. [DOI] [PubMed] [Google Scholar]
- 23.Tok EC, Aktas A, Ertunc D, Erdal EM, Dilek S. Evaluation of glucose metabolism and reproductive hormones in polycystic ovary syndrome on the basis of peroxisome proliferator-activated receptor (PPAR)-gamma2 Pro12Ala genotype. Hum Reprod. 2005;20:1590–5. doi: 10.1093/humrep/deh769. [DOI] [PubMed] [Google Scholar]
- 24.Hahn S, Fingerhut A, Khomtsiv U, Khomtsiv L, Tan S, Quadbeck B, et al. The peroxisome proliferator activated receptor gamma Pro12Ala polymorphism is associated with a lower hirsutism score and increased insulin sensitivity in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2005;62:573–9. doi: 10.1111/j.1365-2265.2005.02261.x. [DOI] [PubMed] [Google Scholar]
- 25.Haap M, Machicao F, Stefan N, Thamer C, Tschritter O, Schnuck F, et al. Genetic determinants of insulin action in polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2005;113:275–81. doi: 10.1055/s-2005-837665. [DOI] [PubMed] [Google Scholar]
- 26.Yilmaz M, Ergun MA, Karakoc A, Yurtcu E, Yetkin I, Ayvaz G, et al. Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-gamma gene in first-degree relatives of subjects with polycystic ovary syndrome. Gynecol Endocrinol. 2005;21:206–10. doi: 10.1080/09513590500231593. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Wu X, Cao Y, Yi L, Fan H, Chen J. Polymorphisms of the peroxisome proliferator-activated receptor-gamma and its coactivator-1alpha genes in Chinese women with polycystic ovary syndrome. Fertil Steril. 2006;85:1536–40. doi: 10.1016/j.fertnstert.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 28.Yilmaz M, Ergun MA, Karakoc A, Yurtcu E, Cakir N, Arslan M. Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-gamma gene in women with polycystic ovary syndrome. Gynecol Endocrinol. 2006;22:336–42. doi: 10.1080/09513590600733357. [DOI] [PubMed] [Google Scholar]
- 29.Antoine HJ, Pall M, Trader BC, Chen YD, Azziz R, Goodarzi MO. Genetic variants in peroxisome proliferator-activated receptor gamma influence insulin resistance and testosterone levels in normal women, but not those with polycystic ovary syndrome. Fertil Steril. 2007;87:862–9. doi: 10.1016/j.fertnstert.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knebel B, Janssen OE, Hahn S, Jacob S, Gleich J, Kotzka J, et al. Increased low grade inflammatory serum markers in patients with Polycystic ovary syndrome (PCOS) and their relationship to PPARgamma gene variants. Exp Clin Endocrinol Diabetes. 2008;116:481–6. doi: 10.1055/s-2008-1058085. [DOI] [PubMed] [Google Scholar]
- 31.Koika V, Marioli DJ, Saltamavros AD, Vervita V, Koufogiannis KD, Adonakis G, et al. Association of the Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma2 with decreased basic metabolic rate in women with polycystic ovary syndrome. Eur J Endocrinol. 2009;161:317–22. doi: 10.1530/EJE-08-1014. [DOI] [PubMed] [Google Scholar]
- 32.Gu BH, Baek KH. Pro12Ala and His447His polymorphisms of PPAR-gamma are associated with polycystic ovary syndrome. Reprod Biomed Online. 2009;18:644–50. doi: 10.1016/S1472-6483(10)60008-9. [DOI] [PubMed] [Google Scholar]
- 33.Xita N, Lazaros L, Georgiou I, Tsatsoulis A. The Pro12Ala polymorphism of the PPAR-gamma gene is not associated with the polycystic ovary syndrome. Hormones (Athens) 2009;8:267–72. doi: 10.1007/BF03401274. [DOI] [PubMed] [Google Scholar]
- 34.San-Millán JL, Escobar-Morreale HF. The role of genetic variation in peroxisome proliferator-activated receptors in the polycystic ovary syndrome (PCOS): an original case–control study followed by systematic review and meta-analysis of existing evidence. Clin Endocrinol (Oxf) 2010;72:383–92. doi: 10.1111/j.1365-2265.2009.03679.x. [DOI] [PubMed] [Google Scholar]
- 35.Chae SJ, Kim JJ, Choi YM, Kim JM, Cho YM, Moon SY. Peroxisome proliferator-activated receptor-gamma and its coactivator-1alpha gene polymorphisms in Korean women with polycystic ovary syndrome. Gynecol Obstet Invest. 2010;70:1–7. doi: 10.1159/000279309. [DOI] [PubMed] [Google Scholar]
- 36.Christopoulos P, Mastorakos G, Gazouli M, Deligeoroglou E, Katsikis I, Diamanti-Kandarakis E, et al. Peroxisome proliferator-activated receptor-gamma and -delta polymorphisms in women with polycystic ovary syndrome. Ann N Y Acad Sci. 2010;1205:185–91. doi: 10.1111/j.1749-6632.2010.05647.x. [DOI] [PubMed] [Google Scholar]
- 37.Bidzińska-Speichert B, Lenarcik A, Tworowska-Bardzinska U, Slezak R, Bednarek-Tupikowska G, Milewicz A, et al. Pro12Ala PPAR gamma2 gene polymorphism in women with polycystic ovary syndrome. Ginekol Pol. 2011;82:426–9. [PubMed] [Google Scholar]
- 38.Bidzińska-Speichert B, Lenarcik A, Tworowska-Bardzinska U, Slezak R, Bednarek-Tupikowska G, Milewicz A. Pro12Ala PPAR gamma2 gene polymorphism in PCOS women: the role of compounds regulating satiety. Gynecol Endocrinol. 2012;28:195–8. doi: 10.3109/09513590.2011.593670. [DOI] [PubMed] [Google Scholar]
- 39.Dragojevič J, Marc J, Mlinar B. Association between Pro12Ala and His477His polymorphisms in PPARG gene and insulin resistance in patients with polycystic ovary syndrome. Biochemia Medica. 2008;18:342–50. [Google Scholar]
- 40.Nardo LG, Patchava S, Laing I. Polycystic ovary syndrome: pathophysiology, molecular aspects and clinical implications. Panminerva Med. 2008;50:267–78. [PubMed] [Google Scholar]
- 41.Tőnjes A, Scholz M, Loeffler M, Stumvoll M. Association of Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma with Pre-diabetic phenotypes: meta-analysis of 57 studies on nondiabetic individuals. Diabetes Care. 2006;29:2489–97. doi: 10.2337/dc06-0513. [DOI] [PubMed] [Google Scholar]
- 42.Masud S, Ye S. Effect of the peroxisome proliferator activated receptor-gamma gene Pro12Ala variant on body mass index: a meta-analysis. J Med Genet. 2003;40:773–80. doi: 10.1136/jmg.40.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deeb SS, Fajas L, Nemoto M, Pihlajamaki J, Mykkanen L, Kuusisto J, et al. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet. 1998;20:284–7. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- 44.Wijeyaratne CN, Balen AH, Barth JH, Belchetz PE. Clinical manifestations and insulin resistance (IR) in polycystic ovary syndrome (PCOS) among South Asians and Caucasians: is there a difference? Clin Endocrinol (Oxf) 2002;57:343–50. doi: 10.1046/j.1365-2265.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- 45.Palep-Singh M, Picton HM, Vrotsou K, Maruthini D, Balen AH. South Asian women with polycystic ovary syndrome exhibit greater sensitivity to gonadotropin stimulation with reduced fertilization and ongoing pregnancy rates than their Caucasian counterparts. Eur J Obstet Gynecol Reprod Biol. 2007;134:202–7. doi: 10.1016/j.ejogrb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Palep-Singh M, Picton HM, Yates ZR, Barth J, Balen AH. Polycystic ovary syndrome and the single nucleotide polymorphisms of methylenetetrahydrofolate reductase: a pilot observational study. Hum Fertil (Camb) 2007;10:33–41. doi: 10.1080/14647270600950157. [DOI] [PubMed] [Google Scholar]
- 47.Glintborg D, Mumm H, Hougaard D, Ravn P, Andersen M. Ethnic differences in Rotterdam criteria and metabolic risk factors in a multiethnic group of women with PCOS studied in Denmark. Clin Endocrinol (Oxf) 2010;73:732–8. doi: 10.1111/j.1365-2265.2010.03873.x. [DOI] [PubMed] [Google Scholar]