Abstract
Allergic Bronchopulmonary Aspergillosis (ABPA) is often difficult to treat and results in morbidity associated with chronic airway changes. This study assessed the requirement for B cells and their products in the allergic pulmonary phenotype in a murine model of fungal allergic asthma that mimics ABPA. C57BL/6 and muMT mice (assumed to lack peripheral B cells), were sensitized with Aspergillus fumigatus extract and challenged with two inhalation exposures of live conidia to induce airway disease. Airway hyperresponsiveness after methacholine challenge, peribronchovascular inflammation, goblet cell metaplasia, and fibrotic remodeling of the airways was similar between muMT mice and their wild type counterparts (C57BL/6). Surprisingly, even in the absence of the mu-chain, these muMT mice produced IgE and IgG antibodies, although the antibodies induced did not have specificity for A. fumigatus antigens. In contrast, IgA was not detected in either the lavage fluid or serum of muMT mice that had been exposed to A. fumigatus. Our findings also reveal the existence of CD19+/CD9+/IgD+ B-1 cells in the lungs of the muMT animals. These data show the muMT mice to have a developmental pathway independent of the canonical mu-chain route that allows for their survival upon antigenic challenge with A. fumigatus conidia, although this pathway does not seem to allow for the normal development of antigen-specific repertoires. Additionally, the study shows that IgA is required for neither clearance nor containment of A. fumigatus in the murine lung, since fungal outgrowth was not observed in the muMT animals after multiple inhalation exposures to live conidia.
INTRODUCTION
Allergic asthma is characterized by reversible airway obstruction due to the recruitment of leukocytes to the lung in response to an inhaled allergen (1). Increased mucus production in the airways, smooth muscle mass around the large airways, and peribronchial collagen deposition further narrows the airway lumen and restricts normal airway compliance contributing to airway obstruction (2–6). Sensitization to fungi with production of IgE and/or colonization by fungal species often signals a disease course that is particularly difficult to treat and results in chronic architecture changes in the lung causing long-term morbidity (7).
Aspergillus fumigatus has a number of characteristics that make it an ideal aeroallergen and opportunistic pathogen of humans. Its small conidia are ubiquitous in indoor and outdoor environments and can remain airborne for long periods of time (8). The size and shape of the conidia are such that they may be inhaled deep into the lung tissue, past the mucociliary elevator that clears many particulates from the airways (9). Holding an environmental niche as a carbon and nitrogen recycler in compost piles, it can take advantage of a wide range of substrates and can grow at the high internal body temperature that discourages most fungal species (10).
Resident plasma cells have been observed in the lungs of both human asthma sufferers and mice under experimental allergic airways protocols (11). Secretory IgA is recognized as an integral part of the innate mucosal response that protects the upper respiratory tract (12–14), and selective IgA deficiency in clinic patients is associated with an increased prevalence of atopy (15, 16). The IgG subtype IgG1, which is a Th2-elicited antibody, is cytophillic to mast cells (17); and IgG2a, which is produced by Th1-activated B cells, plays a role in host protection against fungal growth (18). As instigators of humoral immunity, B lymphocytes provide specificity to allergens in the production of IgE Abs that enable mast cell degranulation (19). IgE has long been recognized as a perpetrator of asthma exacerbations, and anti-IgE therapies have been used successfully for treatment (20–22). During asthma exacerbations, B cells in all stages of activation and differentiation are found in increased numbers in the blood of asthmatic patients (23). B cells are also present in the bronchial mucosa of asthmatics (24). While allergen-specific antibodies (Abs) are recognized as contributing factors in the immunopathology of an aberrant response against an innocuous allergen such as pollen or animal dander (25), they have also been suggested to be part of the successful clearance of fungus from the airways (26, 27).
The aim of the current study was to determine the extent to which B cells impact the development and maintenance of the phenotype of the allergic lung. We used mice that, due to a homozygous targeted disruption of the membrane exon of the Ig mu-chain, are deficient of peripheral B cells, known as muMT (28). Using an Aspergillus fumigatus murine inhalation model developed in our laboratory to mimic human fungal asthma (29), we compared the effects of repeated A. fumigatus inhalation in C57BL/6 wild type controls and muMT animals. We found that the absence of the mu-chain did not alter the pulmonary pathology that results from inhalation of A. fumigatus in allergic animals: AHR, peribronchial inflammation, epithelial changes, and collagen deposition were equivalent to wild type controls. Surprisingly, we found that repeated A. fumigatus conidia exposure resulted in elevated IgE, IgG1 (in bronchoalveolar lavage, BAL), and IgG2a production in sensitized muMT mice, although IgA was undetectable in the muMT animals. This has implications both for the role of the B cell in the allergic lung and for IgA in the response to fungal allergens. We report for the first time that, even in the absence of the immunoglobulin mu-chain, IgG1 (only in BAL), IgG2a, and IgE isotypes were produced in animals after exposure to fungal antigens, but IgA was not made. The Abs produced after fungal exposure showed no antigen specificity for A. fumigatus. Our findings also reveal the previously unreported presence of B-1 cells (CD19+/CD9+/IgD+) in the lungs of muMT mice, even in the complete absence of B-2 cells. Taken together, we show for the first time that muMT mice have B-1 cells in the lungs and that these animals produce selected isotypes through a mu-independent pathway in the context of the fungal allergen-exposed lung.
MATERIALS AND METHODS
Experimental animals
C57BL/6 and muMT mice (5–9 weeks of age) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Animals were housed on Alpha-dri™ paper bedding (Shepherd Specialty Papers, Watertown, TN, USA) in micro filter-topped cages (Ancare, Bellmore, NY, USA) in a specific pathogen-free facility with ad libitum access to food and water. The study described was performed in accordance with the Office of Laboratory Animal Welfare guidelines and was approved by the North Dakota State University Institutional Animal Care and Use Committee.
Antigen preparation and A. fumigatus culture
Soluble A. fumigatus antigen extract was purchased from Greer Laboratories (Lenoir, NC, USA) and fungal culture stock (strain NIH 5233) was purchased from American Type Culture Collection (Manassas, VA, USA). The A. fumigatus culture was reconstituted in 5ml PBS, and 60-μl aliquots were stored at 4°C until use. All experiments that utilized A. fumigatus were conducted with prior approval of the Institutional Biological Safety Committee of North Dakota State University.
Allergen sensitization and challenge by airborne delivery system
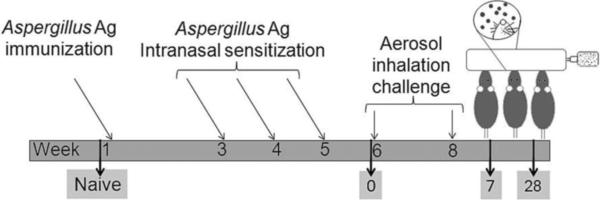
Animals were sensitized per Hogaboam's published protocol (30), with the exception that alum was used as the adjuvant. Mice were sensitized globally with 10μg of A. fumigatus antigen (Greer Laboratories) in 0.1 ml normal saline (NS) mixed with 0.1ml of Imject Alum ( Pierce, Rockford, IL, USA) which was injected subcutaneously (0.1 ml) and intraperitoneally (0.1ml). After two weeks, mice were given a series of three intranasal, weekly 20-μg doses of A. fumigatus antigen in 20μl of NS. Animals were challenged as previously described with a 10-min nose-only aerosol exposure to live A. fumigatus conidia (29). Each anesthetized mouse was placed supine with its nose in an inoculation port inhaling the live fungal conidia for 10 min. Two weeks after the first allergen challenge, mice were subjected to a second 10-min aerosol fungal challenge. Naïve animals from both the groups were neither sensitized nor challenged. After the second allergen exposure, the mice were separated into groups of five for analysis at day 0 (sensitized, but not challenged) or days 7 or 28 after the second aerosol challenge. Day 7 after challenge had been previously determined to be the peak of B cell recruitment into the allergic lungs, and leukocyte inflammation was assessed at this time point. Airway wall remodeling can be seen as early as 7 days after the second aerosol challenge in this model, and the changes to the lung architecture continue to accrue through at least day 28 after the second inhalation of fungal conidia. The day-28 time point was chosen to assess epithelial changes, as well as peribronchial fibrosis. The experimental protocol is depicted in Figure 1.
Figure 1. Sensitization, challenge, and analysis schedule for the A. fumigatus murine model of allergic asthma.
Mice are first sensitized with immunizations and intranasal inoculations of fungal antigens. They are then exposed to 2 nose-only inhalation doses of live Aspergillus conidia 2 weeks apart. Groups of animals are assessed at various time points after allergen challenge (by convention, time points are named for their day after challenge, represented here by days 7 and 28 time points). Day 0 controls denote sensitized animals that did not received the inhalation exposure to fungus.
Airway hyperresponsiveness measurement
Mice were anesthetized using sodium pentobarbital (Butler, Columbus OH; 0.1mg/0.01kg of mouse body weight), intubated, and ventilated with a Harvard pump ventilator (Harvard Apparatus, Reno, NV, USA) to assess allergic airway responses. Restrained plethysmography (Buxco, Troy, NY, USA) was used to assess airway hyperresponsiveness. Before performing readings, the system was first calibrated and the stroke volume set at 225 with the stroke/min set at 150. The value for baseline airway resistance was measured for each animal before an intravenous injection of acetyl-β-methacholine (420μg/kg) was administered to determine AHR at each time point.
Sample collection
Approximately 500 μl of blood was removed from each mouse via ocular bleed and centrifuged at 13,000 ×g for 10 min to yield serum. Serum was stored at −20°C until use. Bronchoalveolar lavage (BAL) was performed on each mouse with 1.0 ml sterile normal saline (NS). The BAL contents were centrifuged at 2000 ×g for 10 min to separate cells from fluid. The BAL fluid was stored at −20°C until use, and cells were used immediately for morphometric analysis. Left lungs were harvested and fixed in 10% neutral buffered formalin for histological analysis.
Morphometric and histological analysis
BAL cells were cytospun (Shandon Scientific, Runcorn, UK) onto microscope slides and differentially stained (Quick-Dip stain, Mercedes Medical, Sarasota, FL, USA). Cells from five, random high-powered fields (HPFs) were counted to determine the mean number of each cell type per HPF in the airway lumen of each mouse.
Formalin-fixed, paraffin-embedded lungs were cut longitudinally across the coronal plane in 5-μm sections and stained with hematoxylin and eosin (H&E) to assess inflammation and with periodic acid Schiff's (PAS) stain (Richard-Allan Scientific, Kalamazoo, MI, USA) for the analysis of goblet cells.
Evaluation of collagen thickness
Gomori's trichrome (Richard-Allan Scientific, Kalamazoo, MI, USA) was used to stain histological sections to assess collagen deposition in naïve and allergic mice as described previously by Hoselton et.al (29). For each sample, at least 50 discrete points were measured at 50-μm intervals along the largest lateral bronchiolar branch visible on the histological section (the second or third lateral branch). A perpendicular line was drawn from the point on the basement membrane through the full thickness of the collagen immediately below. The mean collagen thickness was reported for each sample, and the mean of the means was reported for each group.
Quantification of serum and BAL IgE, IgG1, IgG2a, and IgA
The total IgE (BD OptEIA, San Diego, CA, USA), IgG1 (Immunology Consultants Laboratory, Portland, OR, USA), IgG2a (BD OptEIA, San Diego, CA, USA), and IgA (Bethyl laboratories, TX, USA) in serum and BAL were quantified via specific ELISA according to manufacturer's guidelines. Serum samples were diluted in PBS 1:100 for IgE, 1:500 and 1:5000 for IgG1 and IgG2a, and 1:500 for IgA. BAL samples were pooled and diluted 1:5 for IgE and IgG2a, 1:2 for IgG1, or undiluted for IgA. The detection limits for the kits were1.6 ng/ml for IgE, 6.25 ng/ml for IgG1, 3.1 ng/ml for IgG2a, and 15.625 ng/ml for IgA.
A. fumigatus-specific antibody detection
ELISA plates were coated with 100 μl/well of a 20-μg/ml sample of A. fumigatus antigen (Greer Laboratories) diluted in coating buffer (pH 9.6, 15 mM Na2CO3, and 35 mM NaHCO3) and incubated overnight at 4°C. The next day, the plates were washed three times with PBS containing 0.05% tween-20, and 200 μl of blocker (3% BSA in coating buffer) was added to each well. Plates were incubated in the dark for 2 h at room temperature and washed 3 times with PBS-tween. After blocking, 100 μl/well of serially diluted serum or BAL from C57BL/6 and muMT mice diluted in PBS-tween/1% BSA (10−1 to 10−8 for serum and 1:2 to 1:64 for BAL fluid) was added to each well and incubated for 1 h. Plates were washed 5 times with PBS-tween, and 100 μl/well of diluted goat anti-mouse Ig-HRP (Southern Biotech, Birmingham, AL, USA) secondary antibody was added. Following a 1-h incubation, the plates were again washed 5 times, and 100 μl per well of TMB substrate (BD Biosciences, San Jose, CA, USA) was added. The absorbance was read at 650 nm using a Synergy HT microplate reader (BioTek, Winooski, VT, USA). In addition, serum and BAL samples were tested to check the specificity of individual subclasses of antibody (IgG1 and IgE) for A. fumigatus. For this, rat anti-mouse IgG1-AKP (1:5000 dilution, BD Pharmingen, San Jose, CA, USA) and goat anti-mouse IgE-HRP (1:500 dilution, Thermo Scientific, Rochester, NY, USA) secondary Abs were used in place of Ig-HRP. The absorbance was read at 650 nm when TMB was used as a substrate and at 405 nm when p-Nitrophenyl phosphate disodium salt hexahydrate was used as a substrate (Sigma-Aldrich Corp., St. Louis, MO, USA) using a Synergy HT microplate reader.
Flow cytometry
Minced lungs from naïve animals and at days 0 (sensitized, but not challenged) and 7 were subjected to collagenase IV (Sigma-Aldrich, St. Louis, MO, USA) digestion and red blood cell lysis. For collagenase digestion, minced lung sections were treated with 0.04% collagenase IV in DMEM and were incubated at 37°C for 1 h with gentle agitation. For flow cytometry analysis, the cells were suspended in PBS with 1% BSA (Sigma Aldrich, St. Louis, MO, USA) to a final concentration of 1 × 107 cells/ml. Fc receptors were blocked with anti-mouse CD16/CD32 (1 μg /1 × 106 cells) for 10 min on ice. The following Abs were used for phenotypic characterization of B lymphocytes using flow cytometry: PerCP-Cy5.5-Anti-CD19, PE-anti-CD23, Alexa flour 647-Anti-CD9, FITC-anti-IgD, FITC-anti-IgM, FITC-anti-IgG, FITC-anti-IgE, FITC-anti-IGA, (all Abs were purchased from eBiosciences, San Diego, CA, USA). The samples were pre-incubated with combinations of directly labeled Abs for 30 min in the dark and then washed with PBS 1% BSA twice before the samples were analyzed using an Accuri® C6 Flow Cytometer (Accuri Cytometers Inc., Ann Arbor, MI, USA) or a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). A minimum of 50,000 events were acquired and the data was analyzed using FlowjoTM software (Tree Star, Inc., Ashland, OR, USA).
Statistical analysis
Allergic C57BL/6 wild type and muMT animals were compared to each other and to their respective naïve controls at each time point. An unpaired, Student's two tailed t test with Welch's correction was used to determine statistical significance with Prism Graph Pad software (San Diego, CA, USA). p=0.01-0.05 (*), p = 0.001-0.01(**), and p <0.001 (***) indicates statistical difference when each of the mouse strains were compared to their naïve controls. Where appropriate, a bracket indicates statistical difference between the C57BL/6 and muMT mice. p < 0.05 (#). All results are expressed as the mean ± SEM.
RESULTS
Airborne fungal challenge results in airway hyperresponsiveness in muMT mice after sensitization to A. fumigatus
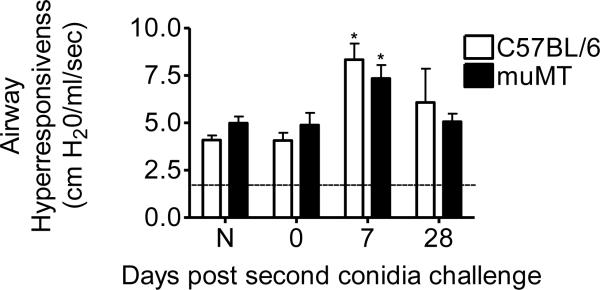
In the present study, airway physiology of both murine groups (i.e. C57BL/6 and muMT animals) was monitored before allergen challenge at day 0 and at days 7 and 28 post second conidia inhalation (Fig. 2). Airway response measurements from all study animals were used to determine the baseline mean for airway hyperresponsiveness prior to methacholine challenge (Fig. 2, dotted line, 1.78 ± 0.05 cm H20/ml/s). Sensitized animals from wild type and muMT groups that were not challenged with spore inhalation showed no difference in airway resistance values when compared to naïve animals in their respective groups. At day 7 after 2 conidia challenges, airway hyperresponsiveness was significantly increased in both murine groups as compared to naive controls (Fig. 2); however, there was no difference in the AHR values of muMT animals as compared to the C57BL/6 wild type animals. By day 28 after the second conidia challenge, AHR values for both murine groups returned to baseline, irrespective of the presence or absence of the mu-gene.
Figure 2. Inhalation of A. fumigatus increases airway hyperresponsiveness (AHR) in C57BL/6 and muMT mice.
Baseline response was obtained prior to methacholine challenge (mean value of 1.78 ± 0.05 cm H2O/ml/s is indicated by the dotted line). Peak increases in airway resistance were recorded after i.v. methacholine injection (420 μg/kg). AHR was increased following allergen challenge and the trend was quite similar in both the murine groups throughout the course of the study. Data analyzed using an unpaired, Student's two tailed t test with Welch's correction. All values expressed as the mean ± S.E.M. n = 3–5 mice/group, *p < 0.05was considered as statistically significant when compared to the respective naïve controls.
Leukocytes are recruited to the allergic airways after fungal conidia challenge in muMT mice
Leukocyte recruitment to the lungs of allergen-sensitized animals that had inhaled conidia was evaluated using H&E-stained lung sections and morphometric analysis of BAL cells. Naïve animals from both groups exhibited no pulmonary inflammation (Fig. 3A&B). Similarly, sensitized C57BL/6 and muMT animals that did not inhale spores (day 0) showed no evidence of inflammation (Fig. 3C&D, respectively). However, upon allergen challenge, both C57BL/6 and muMT animals actively recruited inflammatory cells to the lungs. Allergic animals exhibited prominent perivascular and peribronchial leukocyte inflammation 7 days after the second spore challenge (Fig. 3E&F). The pattern of perivascular and peribronchial inflammation was similar in the C57BL/6 and muMT animals at day 7, and inflammation was largely resolved in both strains by day 28 post challenge (Fig. 3G&H).
Figure 3. Inhalation of A. fumigatus conidia increases pulmonary inflammation in C57BL/6 and muMT mice.
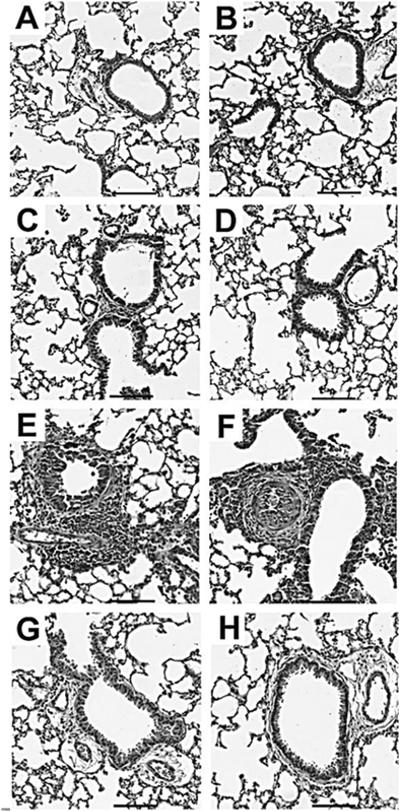
Representative photomicrographs of H&E stained lung sections of allergen-challenged C57BL/6 (left column) and muMT (right column) mice. Naïve and day 0 mice in both the groups did not show inflammation (Fig. 3A–D). Peribronchovascular inflammation was prominent at day 7 post second conidia challenge in both groups (Fig. 3E&F) and subsided well into day 28 (Fig.3G&H). Scale Bar = 100μm.
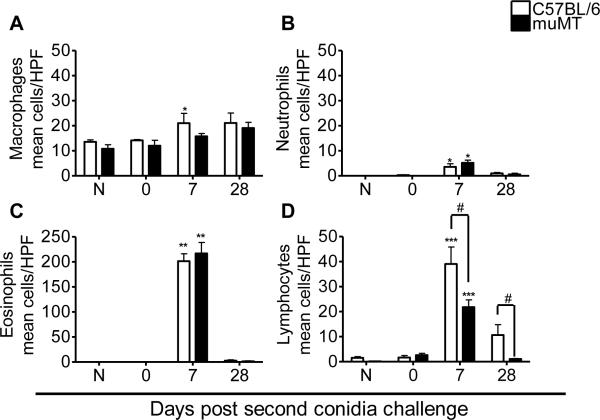
Morphometric analysis of monocyte/macrophage lineage cells, neutrophils, eosinophils, and lymphocytes was performed to estimate the relative makeup of the cellular inflammation and to monitor leukocyte egress into the airway lumen (Fig. 4). In naïve and sensitized animals that were not challenged (day 0), alveolar macrophages were the dominant cell type (Fig. 4A). Neutrophils, lymphocytes, and particularly eosinophils were prominent cell types identified in the BAL 7 days after the second conidia challenge (Fig. 4B, C, &D). Eosinophils were the most numerous cell type counted (Fig. 4C) in the BAL of both C57BL6 and muMT mice at day 7 after the second conidia exposure, emphasizing the polarization of the immune response in favor of allergy after multiple inhalations of conidia. At day 28 post challenge, macrophages were again the major cellular component of the BAL compartment with very few neutrophils (Fig. 4A&B). The inflammation pattern was similar between C57BL/6 and muMT animals, with eosinophils dominating at day 7 in both murine groups when they were compared to their naïve controls.
Figure 4. Effect of A. fumigatus conidia inhalation on inflammatory leukocytes in the allergic lung.
Airway inflammation was marked by the presence of macrophages, neutrophils, eosinophils, and lymphocytes in naïve, allergic C57BL/6, and muMT mice. The inflammation pattern was similar in both C57BL/6 and muMT mice throughout the course of the study. Data analyzed using an unpaired, Student's two tailed t test with Welch's correction. All values expressed as the mean ± S.E.M. n =4 – 5 mice/group, * p< 0.05; ** p < 0.01; ***p <0.001 was considered as statistically significant when compared to the respective naïve controls. # p <0.05 was considered statistically significant when muMT were compared to C57BL/6 animals.
Inhalation of fungal conidia changes the airway architecture in allergic C57BL/6 mice and allergic muMT mice
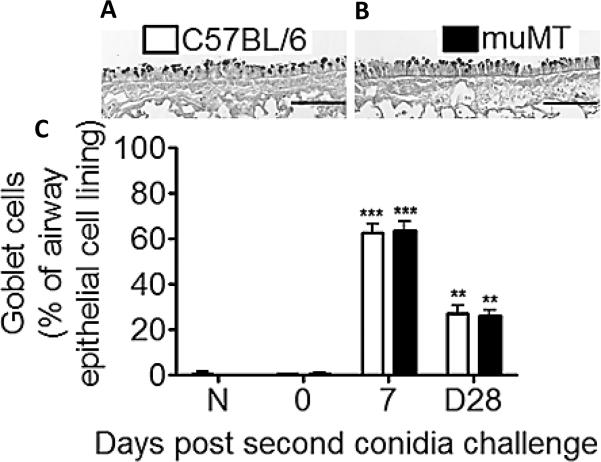
In the present study, goblet cells were assessed by counting PAS-stained cells and representing them as a percentage of total epithelial cells lining the second or third lateral airways in each histological section. Goblet cell metaplasia was not observed in the day 0 (sensitized, but not challenged) animals of either the C57BL/6 or muMT groups (Fig. 5C). Challenge with A. fumigatus conidia resulted in a marked increase in the percentage of goblet cells lining the airways (Fig. 5C). As compared to sensitized animals that did not receive inhaled conidia, the number of goblet cells was increased dramatically (~65% of total) but equally in both groups 7 days post challenge (Fig. 5A,B,&C). By day 28 after the second conidia challenge, fewer goblet cells were noted in the allergic lungs of both the C57BL/6 and the muMT group as compared to the day-7 time point, although there was no difference in the number of goblet cells between the wild type and the muMT groups (Fig. 5C ~27% of the total epithelial cells for each).
Figure 5. Inhalation of A. fumigatus conidia increases goblet cell metaplasia in C57BL/6 and muMT mice.
Representative photomicrographs of PAS stained whole lung sections of C57BL/6 and muMT mice show that goblet cells (GCs) and mucus were evident in the airways at Day 7 post second conidia challenge (Fig. 5A&B). GC numbers were reported as the percent of total epithelial cells along segments of airway epithelium lining the large lateral branches of the bronchi (Fig. 5C). Scale bars in A and B = 100μm.
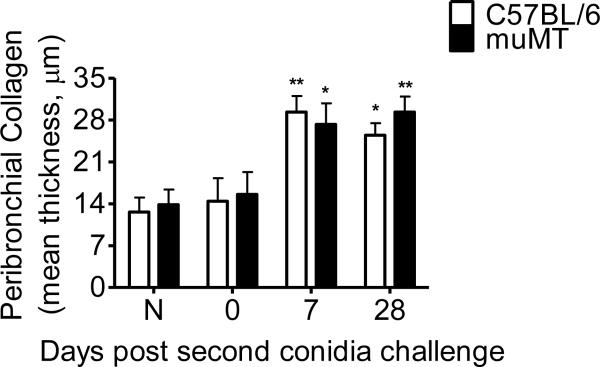
Collagen accumulation in the peribronchial space of allergic C57BL/6 or muMT animals was significantly increased at both day 7 and day 28 after the second conidia inhalation as compared to sensitized animals that had not been exposed to inhaled conidia (Fig. 6). In contrast to the pattern seen in goblet cell metaplasia, collagen accumulation did not diminish over the time course of this study. This phenomena has been seen and extended in other studies by our laboratory in both BALB/c and C57BL/6 mice (29, 31–33).
Figure 6. Effect of A. fumigatus conidia inhalation on peribronchial collagen thickness.
Gomorri's trichome stain was used to visualize subepithelial collagen deposition in histological sections. Peribronchial collagen thickness was similar in both C57BL/6 and muMT mice throughout the course of the study. Approximately 50 discrete points were measured at 50-μm intervals along the largest lateral bronchiolar branch visible on the histological section (L2 or L3). A perpendicular line was drawn from the point on the basement membrane through the full thickness of the collagen immediately below. The mean collagen thickness was reported for each sample. Data analyzed using an unpaired, Student's two tailed t test with Welch's correction. All values expressed as the mean ± S.E.M. n =3–5 mice/group, * p< 0.05; ** p < 0.01; was considered as statistically significant when compared to their respective naïve controls.
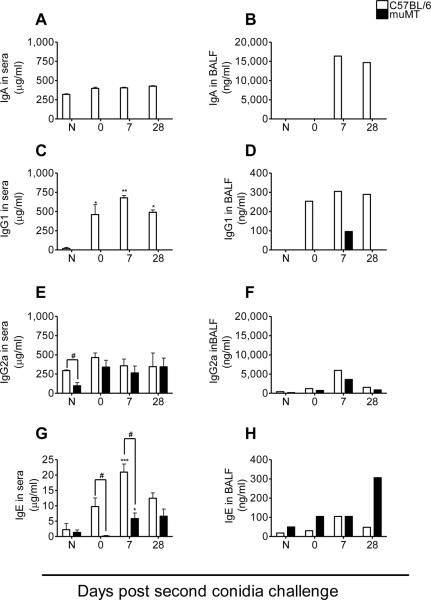
Fungal inhalation resulted in increased serum IgA, IgG1, IgG2a, and IgE levels in allergic C57BL/6 mice, while muMT mice exhibited elevated IgG1 in BAL, IgG2a and IgE in serum
In the present study, inhalation of A. fumigatus conidia resulted in an increase in the BAL IgA from C57BL/6 mice at day 7 after 2 conidia inhalations. IgA Abs were not detected in either serum or the BAL fluid of muMT mice (Fig. 7A&B). IgG1 was detected in the BAL fluid of allergic muMT mice 7 days after 2 exposures to conidia, but was not found in the serum (Fig. 7C&D). Although serum IgG2a levels in naïve muMT animals were significantly lower than wild type, sensitization with fungal antigens stimulated its production to levels equivalent to those of wild type, and the mu-deficient animals matched IgG2a levels throughout the rest of the study (Fig. 7E). IgG2a was also detected in the BAL fluid of the muMT mice (Fig. 7F). IgE was elevated in A. fumigatus-sensitized and challenged C57BL/6 and muMT animals (Fig. 7G&H). Even though there was a significant difference in the IgE levels of C57BL/6 and muMT mice, the production of IgE was significantly higher at day 7 post second conidia challenge in both the murine groups when they were compared to their respective naïve controls, suggesting that isotype switching to an allergic phenotype was possible even in the muMT mice. However, IgE production in C57BL/6 mice was 4X higher than muMT levels at day 7 after the second inhalation.
Figure 7. Inhalation of A. fumigatus conidia induces muMT mice to produce IgG1 (only in BAL), IgE and IgG2a in serum and bronchoalveolar lavage (BAL) fluid.
The Ab levels of C57BL/6 and muMT mice were compared to naïve animals and to each other at each time point. ELISA's indicated that the muMT mice produced antibodies in response to A. fumigatus allergen challenge. Data analyzed using an unpaired, student's two tailed t test with Welch's correction. All values expressed as the mean ± S.E.M. n = 4–5 mice/group, * p< 0.05; *** p <0.001 was considered significant as compared to naïve controls. # p <0.05 was considered statistically significant when muMT were compared to C57BL/6 animals. No statistics are shown for Ig ELISAs for BAL fluid as the samples from each time point were pooled and run as a single sample.
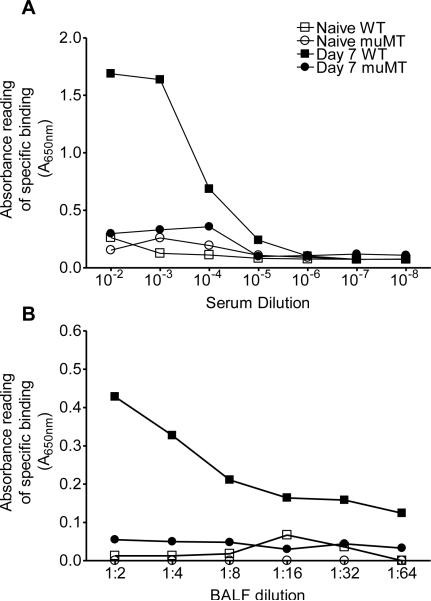
To investigate the extent to which the antibodies produced as a result of fungal sensitization and inhalation exposure were specific to A. fumigatus, serial dilutions of serum and BAL samples from C57BL/6 and muMT mice were collected at day 7 after the second conidia exposure and analyzed against the sensitizing antigen. The specificity of antibodies to A. fumigatus at day 7 post second conidia challenge (when the antibody levels are higher in serum and BAL) of both C57BL/6 and muMT mice are shown in figure 8. The serum and BAL antibodies produced in the C57BL/6 mice were specific to A. fumigatus (closed box), while the ones produced in muMT mice (open box) appeared to be non-specific and the values were comparable to those of naïve control animals (Fig.8A&B). When the specificity of individual subclasses of Abs (IgE and IgG1) for A. fumigatus was tested, we observed similar results (data not shown).
Figure 8. Inhalation of A. fumigatus conidia induces specific serum and BAL antibody production in C57BL/6 mice, but not in muMT mice.
Serum and BAL samples from the C57BL/6 and muMT mice were pooled and serial dilution was used to evaluation antibody titers. (A) The specificity of total serum antibody from C57BL/6 (Day 7 WT) and muMT (day 7 muMT) for A. fumigatus was evaluated against A. fumigatus Ag. (B) The specificity of total BAL antibody from C57BL/6 (Day 7 WT) and muMT (day 7 muMT) for A. fumigatus Ag. C57BL/6 mice produced specific antibodies for A. fumigatus, while none were detected in muMT mice.
CD19+/CD9+/IgD+ B-1 cells are present in the lungs of muMT mice despite a lack of the Ig mu chain
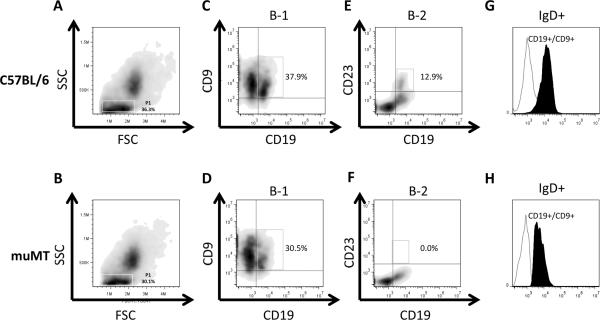
It has previously been shown that muMT mice on the BALB/c, but not C57BL/6 background, display an incomplete block in B cell development and harbor mature B cells in secondary lymphoid organs (34, 35). Although all muMT animals used here were on the C57BL/6 background, we considered the possibility that A. fumigatus exposure might overcome the B cell developmental block (28, 35). In the present study, after inhalation with A. fumigatus conidia, CD19+ B cells were detected in the lungs of muMT mice at day 7 after the second conidia inhalation and their numbers were fewer than the WT controls (Fig. 9C,D,E&F). When evaluated, CD19+ B cells were also detected in the lungs of naïve muMT mice (data not shown).
Figure 9. CD19+/CD9+/IgD+ B-1 cells are present in the lungs of muMT mice despite a sustained block in B cell development.
Lungs harvested from C57BL/6 and muMT mice at day 7 post second conidia challenge were analyzed for the presence of B-1, B-2, and IgD+ B-1 cells. (A and B) The forward scatter (FSC) and side scatter (SSC) plot of the cells isolated from the lungs of C57BL/6 and muMT mice. (C and D) The percentage of CD19+/CD9+ B-1 cells in the lungs of C57BL/6 and muMT mice (gated on population P1 in the FSC-SSC plot). (E&F) The percentage of CD19+/ CD23+ B-2 cells in the lungs of C57BL/6 and muMT mice (gated on population P1 in the FSC-SSC plot). (G&H) Histogram overlay of CD19−/CD9+ (non B1 cells, open histogram) and the CD19+/CD9+ (B1 cells, filled histogram) cell populations showing the presence of IgD on the surface of B-1 cells in the lungs of C57BL/6 and muMT mice.
Given the fact that the Abs produced in the muMT mice were not specific for A. fumigatus, we looked for the presence of B-1 lymphocytes in the lungs as these cells are known to produce Abs in a non-specific manner and they predominate in the pleural and peritoneal cavities (36). In addition to B-1 cells, we also looked for B-2 cells in the lungs of muMT mice as these conventional B-2 cells form a major population of lymphocytes which is present in the body (37, 38). The CD19+ B cell population in the lungs of C57BL/6 WT mice expressed either CD9 (as B-1 cells are CD9+) (36, 39) or CD23 (as B-2 cells are CD23+ and have low to no expression of CD9) (38, 40), showing the presence of both B-1 and B-2 B cells (Fig. 9C&E). On the contrary, CD19+ B cells that were present in the lungs of muMT mice did not express CD23 indicating the absence of B-2 lymphocytes (Fig. 9F). Similar to the B-1 population in C57BL/6 WT mice, the muMT CD19+ B cells expressed CD9, illustrating the presence of B-1 lymphocytes (36) (Fig. 9D).
It has been shown that IgD can substitute for IgM if it is expressed early in the B cell development process (41). As such, we looked for the expression of IgD on the CD19+/CD9+ cells that were present in the lungs of C57BL/6 WT and muMT mice using flow cytometry. IgD was expressed on the CD19+/CD9+ cells present in the lungs of C57BL/6 and muMT mice at day 7 post second conidia challenge (Fig. 9G&H). As expected, IgM positive cells were not detected in either naïve or A. fumigatus challenged muMT mice (data not shown). These data demonstrate that in the muMT mice, IgD can substitute for IgM early in B-1 cell development.
DISCUSSION
In the current study, we show that the localized production of IgG1, IgG2a, and IgE is elicited in muMT mice in response to systemic fungal sensitization and inhalational challenge in an experimental allergic asthma model. In addition to the localized production, our work demonstrates that mu-deficient mice produced systemic IgG2a and IgE Abs after exposure to A. fumigatus extract antigens followed by inhalation of A. fumigatus conidia. However, when tested in binding assays with the Aspergillus antigens that were used to sensitize the animals, the Ab isotypes from the muMT animals proved to be non-specific, while the antibodies produced by the mu-sufficient controls were specific.
In the present work using a fungal trigger to elicit allergic airways disease, the characteristic signs and symptoms of allergic airway disease were present. AHR, pulmonary inflammation, excessive mucus production, and serum IgE in muMT mice were comparable to C57BL/6 controls. In contrast, studies using a repeated aerosol exposure to OVA showed reduced lung inflammation and mucus hypersecretion in muMT mice as compared to controls (42, 43). In addition, OVA-challenged mice failed to develop AHR, suggesting a possible role of B cells in the development of AHR in response to OVA (42, 43). So, in addition to the type of Ab that can be elicited in muMT animals, the type of immune response is also different with different antigenic stimuli, dissecting further the role of B cell activation in response to fungal pathogens/allergens.
IgM and IgA immunoglobulins share a number of similarities. IgA is related more closely to IgM than other isotypes, with the mu and alpha chains sharing a characteristic long secretory segment (44). In addition, both IgA and IgM can form multimers in conjunction with the J chain and both can be secreted at the mucosal surfaces coupled to the polymeric Ig receptor (45). Some investigators have speculated that expression of IgA may act as a surrogate for membrane IgM in B cell development in some instances (45). Secretory IgA has been recognized as an integral part of the innate mucosal response that protects the upper respiratory tract (12, 13), and selective IgA deficiency in clinic patients has been associated with an increased prevalence of atopy (15, 16). In experimental conditions, muMT mice infected with Salmonella did produce IgA (45), and we expected that, if any isotype was made in the muMT mice in response to fungal stimulation, IgA would be that isotype. However, we found no IgA in the serum or the BAL of muMT mice. This suggests that: 1) other Ab isotypes can substitute for IgM in B cell development, 2) that the type of antigenic stimulus dictates isotype development in the muMT mouse, even when the context of the exposure (mucosal delivery) is similar (although not identical), and 3) that IgA is not necessary for fungal containment in this model.
In previous work using muMT mice on a C57BL/6 background, a very sensitive method to detect low levels of FcεRI-bound IgE on basophils showed that IgE was made in muMT mice after prolonged exposure to Heligmosomoides polygyrus, Tricuris muris, or Schistosoma mansoni gut parasites (46). In fungal sensitization and challenge, our results show a similar capacity for an IgE response in muMT mice after treatment with Aspergillus Ags. In the current study, a robust IgE response was readily quantified by ELISA, and elevated IgE levels were sustained throughout the study. Our findings show for the first time that a conserved mucosal humoral response, which is not mediated through IgM, may significantly impact the response to, not only gut parasites, but inhaled fungal pathogens as well. Interestingly, in studies in which ovalbumin (OVA) was used as a surrogate for clinically relevant inhaled allergens, no IgE was produced in muMT mice (3, 42).
In addition to IgE, we report for the first time IgG1 production in the BAL and IgG2a production in the serum and BAL of muMT mice on a C57BL/6 background. IgG1, which is associated with Th2-type responses elicited by IL-4, was elevated only in the BAL and only after fungal challenge, suggesting a localized production of this Ab isotype. IgG2a, which plays an important role in fungal opsonization and clearance (18), was elevated throughout the study in both C57BL/6 and muMT mice. This further supports the fact that antigenic stimulus dictates isotype development in the muMT mice and that without the benefit of the mu gene a small percentage of pre-B cells can escape elimination, switch to downstream immunoglobulin heavy chains, and respond to antigens (35, 47).
The canonical pathway of B cell ontogeny requires surface expression of the mu Ig chain at an early pre-B cell stage (48). Indeed, until recently only B cells which express IgM were believed to migrate from the bone marrow to the peripheral lymphoid organs (35), and membrane-bound IgM expression was thought to be essential for B cell maturation and differentiation to Ab-producing cells. However, recent research using muMT mice has shown that the expression of the mu Ig chain is not an absolute requirement for B cell survival (35, 45, 46, 49). These genetically altered animals have been useful tools in understanding the complex biological processes associated with different diseases.
Although Ab-binding ELISAs were not attempted in the helminth infection study, the IgE was functional in that it elicited IL-4 production by basophils (46). In their study, the Ab was produced at a low concentration and Ab-producing B cells could not be detected in the central or peripheral lymph organs. In the current study, while muMT mice were able to produce Abs after sensitization to and challenge with fungal Ags, our results show that the Abs produced by the mu-deficient animals had no affinity for A. fumigatus Ags as compared to wild type. Together, these results suggest a tissue-centric Ab production, which mandated the assessment of B cell populations in the lung.
In investigating the potential source of Abs in muMT mice, no other study has examined the presence of CD19+/IgD+ cells in the lungs. Since we did not find CD19+/IgD+ or CD19+/IgM+ cells in the bone marrow or spleens of naïve mice (data not shown), we hypothesized that tissue-resident B-1 cells act as the source of Abs in muMT mice. We were able to detect IgD-expressing CD19+/CD9+ B-1 cells in the lungs of muMT mice using flow cytometry, supporting a tissue resident B-1 cell as a source for localized Ab production. These observations are consistent with the notion that B cells can receive switching signals in peripheral sites (45, 46, 50, 51), a process that may occur in the allergic lung.
In summary, we provide conclusive evidence that B-1 cells can impact asthma pathophysiology in the absence of conventional B-2 lymphocytes. From these studies, we report two significant conclusions. First, the route of the pathogenic/allergenic challenge as well as the type of antigen has a significant impact on the generation of Ab responses in muMT mice lacking the normal pathway for B-2 cell maturation, as different types of antigen yield very different outcomes. The second major finding is that as a B-2 cell KO mouse, muMT animals may be very useful to determine the role of B-1 cells in response to various pulmonary insults. Future studies may include elucidating the mechanism for B-1 isotype switching in the lung and B-1 cells' contribution to protective responses, which would have important implications for experimental analysis and for understanding normal B-1 and B-2 cell activation in health and disease.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Pawel Borowicz (North Dakota State University) and the Advanced Imaging and Microscopy Laboratory at North Dakota State University for imaging support using the Zeiss Z1 AxioObserver inverted microscope. We also thank Dr. Jodie Haring from the Core Biology Facility at NDSU for technical assistance with flow cytometry.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of North Dakota State University.
Funding source: These studies and the core facility used were funded by grants from the NIH (1R15AI69061: J. Schuh and 2P20RR015566: M. Sibi); Major Research Instrumentation Program grant from NSF (MRI-R2 grant No.0959512: A. Grazul-Bilska and J. Schuh) and an NSF doctoral fellowship funded through ND EPSCoR (EPS-0814442)
References
- 1.Boulet LP. Irreversible airway obstruction in asthma. Curr Allergy Asthma Rep. 2009;9:168–173. doi: 10.1007/s11882-009-0025-2. [DOI] [PubMed] [Google Scholar]
- 2.Bradley BL, Azzawi M, Jacobson M, Assoufi B, Collins JV, Irani AMA, Schwartz LB, Durham SR, Jeffery PK, Kay AB. Eosinophils, T-Lymphocytes, Mast-Cells, Neutrophils, and Macrophages in Bronchial Biopsy Specimens from Atopic Subjects with Asthma - Comparison with Biopsy Specimens from Atopic Subjects without Asthma and Normal Control Subjects and Relationship to Bronchial Hyperresponsiveness. Journal of Allergy and Clinical Immunology. 1991;88:661–674. doi: 10.1016/0091-6749(91)90160-p. [DOI] [PubMed] [Google Scholar]
- 3.Korsgren M, Erjefalt JS, Korsgren O, Sundler F, Persson CG. Allergic eosinophil-rich inflammation develops in lungs and airways of B cell-deficient mice. J Exp Med. 1997;185:885–892. doi: 10.1084/jem.185.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frigas E, Motojima S, Gleich GJ. The eosinophilic injury to the mucosa of the airways in the pathogenesis of bronchial asthma. Eur Respir J Suppl. 1991;13:123s–135s. [PubMed] [Google Scholar]
- 5.Seminario MC, Gleich GJ. The role of eosinophils in the pathogenesis of asthma. Curr Opin Immunol. 1994;6:860–864. doi: 10.1016/0952-7915(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 6.Kumar RK, Foster PS. Murine model of chronic human asthma. Immunology and cell biology. 2001;79:141–144. doi: 10.1046/j.1440-1711.2001.00981.x. [DOI] [PubMed] [Google Scholar]
- 7.Denning DW, O'Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006;27:615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 8.Tsuji S, Ogawa K. [Chronic pulmonary aspergillosis] Nihon rinsho. Japanese journal of clinical medicine. 2011;69:1462–1467. [PubMed] [Google Scholar]
- 9.Cramer RA, Rivera A, Hohl TM. Immune responses against Aspergillus fumigatus: what have we learned? Curr Opin Infect Dis. 2011;24:315–322. doi: 10.1097/QCO.0b013e328348b159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knutsen AP, Slavin RG. Allergic bronchopulmonary aspergillosis in asthma and cystic fibrosis. Clin Dev Immunol. 2011;2011:843763. doi: 10.1155/2011/843763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bice DE, Gray RH, Evans MJ, Muggenburg BA. Identification of plasma cells in lung alveoli and interstitial tissues after localized lung immunization. J Leukoc Biol. 1987;41:1–7. doi: 10.1002/jlb.41.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K, Maruya M, Kawamoto S, Fagarasan S. Roles of B-1 and B-2 cells in innate and acquired IgA-mediated immunity. Immunological reviews. 2010;237:180–190. doi: 10.1111/j.1600-065X.2010.00941.x. [DOI] [PubMed] [Google Scholar]
- 13.Mogi G. Secretory immunoglobulin A in oral and respiratory passages in man. Ann Otol Rhinol Laryngol. 1975;84(Suppl 20):1–23. doi: 10.1177/0003489475084S2001. [DOI] [PubMed] [Google Scholar]
- 14.Mantis NJ, Rol N, Corthesy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal immunology. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Asperen PP, Gleeson M, Kemp AS, Cripps AW, Geraghty SB, Mellis CM, Clancy RL. The relationship between atopy and salivary IgA deficiency in infancy. Clin Exp Immunol. 1985;62:753–757. [PMC free article] [PubMed] [Google Scholar]
- 16.Ostergaard PA. Clinical and immunological features of transient IgA deficiency in children. Clin Exp Immunol. 1980;40:561–565. [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan C, Valdez JC, Chandrasekaran R, Eibel H, Mikecz K, Glant TT, Finnegan A. Th1 and Th2 cytokines regulate proteoglycan-specific autoantibody isotypes and arthritis. Arthritis Res. 2002;4:54–58. doi: 10.1186/ar383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherjee S, Lee SC, Casadevall A. Antibodies to Cryptococcus neoformans glucuronoxylomannan enhance antifungal activity of murine macrophages. Infect Immun. 1995;63:573–579. doi: 10.1128/iai.63.2.573-579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeimpekoglou K. The Role of B lymphocytes in lung disease. PNEUMON. 2008;21:196–199. [Google Scholar]
- 20.Laitinen LA, Laitinen A, Haahtela T. Airway mucosal inflammation even in patients with newly diagnosed asthma. Am Rev Respir Dis. 1993;147:697–704. doi: 10.1164/ajrccm/147.3.697. [DOI] [PubMed] [Google Scholar]
- 21.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–1071. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 22.Rolinck-Werninghaus C, Wahn U, Hamelmann E. Anti-IgE therapy in allergic asthma. Curr Drug Targets Inflamm Allergy. 2005;4:551–564. doi: 10.2174/156801005774322162. [DOI] [PubMed] [Google Scholar]
- 23.Poryadin GV, Zhuravleva NE, Salmasi JM, Kazimirsky AN, Semenova LY, Polner SA, Chervinskaya TA. Immunological mechanisms of recovery from an acute stage in patients with atopic bronchial asthma. Russ J Immunol. 2002;7:259–264. [PubMed] [Google Scholar]
- 24.Zhu J, Qiu YS, Figueroa DJ, Bandi V, Galczenski H, Hamada K, Guntupalli KK, Evans JF, Jeffery PK. Localization and upregulation of cysteinyl leukotriene-1 receptor in asthmatic bronchial mucosa. American journal of respiratory cell and molecular biology. 2005;33:531–540. doi: 10.1165/rcmb.2005-0124OC. [DOI] [PubMed] [Google Scholar]
- 25.Knutsen AP. Immunopathology and immunogenetics of allergic bronchopulmonary aspergillosis. J Allergy (Cairo) 2011;2011:785983. doi: 10.1155/2011/785983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Rapaka RR, Ricks DM, Alcorn JF, Chen K, Khader SA, Zheng M, Plevy S, Bengten E, Kolls JK. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. J Exp Med. 2010;207:2907–2919. doi: 10.1084/jem.20100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flicker S, Gadermaier E, Madritsch C, Valenta R. Passive immunization with allergen-specific antibodies. Curr Top Microbiol Immunol. 2011;352:141–159. doi: 10.1007/82_2011_143. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 29.Hoselton SA, Samarasinghe AE, Seydel JM, Schuh JM. An inhalation model of airway allergic response to inhalation of environmental Aspergillus fumigatus conidia in sensitized BALB/c mice. Medical mycology : official publication of the International Society for Human and Animal Mycology. 2010;48:1056–1065. doi: 10.3109/13693786.2010.485582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogaboam CM, Blease K, Mehrad B, Steinhauser ML, Standiford TJ, Kunkel SL, Lukacs NW. Chronic airway hyperreactivity, goblet cell hyperplasia, and peribronchial fibrosis during allergic airway disease induced by Aspergillus fumigatus. Am J Pathol. 2000;156:723–732. doi: 10.1016/S0002-9440(10)64775-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samarasinghe AE, Hoselton SA, Schuh JM. The absence of VPAC2 leads to aberrant antibody production in Aspergillus fumigatus sensitized and challenged mice. Peptides. 2011;32:131–137. doi: 10.1016/j.peptides.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samarasinghe AE, Hoselton SA, Schuh JM. A comparison between intratracheal and inhalation delivery of Aspergillus fumigatus conidia in the development of fungal allergic asthma in C57BL/6 mice. Fungal biology. 2011;115:21–29. doi: 10.1016/j.funbio.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samarasinghe AE, Hoselton SA, Schuh JM. Spatio-temporal localization of vasoactive intestinal peptide and neutral endopeptidase in allergic murine lungs. Regulatory peptides. 2010;164:151–157. doi: 10.1016/j.regpep.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasan M, Polic B, Bralic M, Jonjic S, Rajewsky K. Incomplete block of B cell development and immunoglobulin production in mice carrying the muMT mutation on the BALB/c background. Eur J Immunol. 2002;32:3463–3471. doi: 10.1002/1521-4141(200212)32:12<3463::AID-IMMU3463>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 35.Orinska Z, Osiak A, Lohler J, Bulanova E, Budagian V, Horak I, Bulfone-Paus S. Novel B cell population producing functional IgG in the absence of membrane IgM expression. Eur J Immunol. 2002;32:3472–3480. doi: 10.1002/1521-4141(200212)32:12<3472::AID-IMMU3472>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 36.Won WJ, Kearney JF. CD9 is a unique marker for marginal zone B cells, B1 cells, and plasma cells in mice. J Immunol. 2002;168:5605–5611. doi: 10.4049/jimmunol.168.11.5605. [DOI] [PubMed] [Google Scholar]
- 37.Natasha Kushnir NAB, Zuercher Adrian W., Coffin Susan E., Moser Charlotte A., Offit Paul A., Cebra John J. B2 but Not B1 Cells Can Contribute to CD4+ T-Cell-Mediated Clearance of Rotavirus in SCID Mice. Journal of Virology. 2001:5482–5490. doi: 10.1128/JVI.75.12.5482-5490.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliveira FL, Aguiar AM, Borojevic R, El-Cheikh MC. IgE expression on the surface of B1 and B2 lymphocytes in experimental murine schistosomiasis. Braz J Med Biol Res. 2005;38:1033–1042. doi: 10.1590/s0100-879x2005000700006. [DOI] [PubMed] [Google Scholar]
- 39.Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Curr Opin Immunol. 2001;13:195–201. doi: 10.1016/s0952-7915(00)00204-1. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Clarke SH. Evidence for a ligand-mediated positive selection signal in differentiation to a mature B cell. J Immunol. 2003;171:6381–6388. doi: 10.4049/jimmunol.171.12.6381. [DOI] [PubMed] [Google Scholar]
- 41.Lutz C, Ledermann B, Kosco-Vilbois MH, Ochsenbein AF, Zinkernagel RM, Kohler G, Brombacher F. IgD can largely substitute for loss of IgM function in B cells. Nature. 1998;393:797–801. doi: 10.1038/31716. [DOI] [PubMed] [Google Scholar]
- 42.Jungsuwadee P, Benkovszky M, Dekan G, Stingl G, Epstein MM. Repeated aerosol allergen exposure suppresses inflammation in B-cell-deficient mice with established allergic asthma. Int Arch Allergy Immunol. 2004;133:40–48. doi: 10.1159/000075252. [DOI] [PubMed] [Google Scholar]
- 43.Hamelmann E, Vella AT, Oshiba A, Kappler JW, Marrack P, Gelfand EW. Allergic airway sensitization induces T cell activation but not airway hyperresponsiveness in B cell-deficient mice. Proc Natl Acad Sci U S A. 1997;94:1350–1355. doi: 10.1073/pnas.94.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warr GW, Magor KE, Higgins DA. IgY: clues to the origins of modern antibodies. Immunol Today. 1995;16:392–398. doi: 10.1016/0167-5699(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 45.Macpherson AJ, Lamarre A, McCoy K, Harriman GR, Odermatt B, Dougan G, Hengartner H, Zinkernagel RM. IgA production without mu or delta chain expression in developing B cells. Nat Immunol. 2001;2:625–631. doi: 10.1038/89775. [DOI] [PubMed] [Google Scholar]
- 46.Perona-Wright G, Mohrs K, Taylor J, Zaph C, Artis D, Pearce EJ, Mohrs M. Cutting edge: Helminth infection induces IgE in the absence of mu- or delta-chain expression. J Immunol. 2008;181:6697–6701. doi: 10.4049/jimmunol.181.10.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waisman A, Croxford AL, Demircik F. New tools to study the role of B cells in cytomegalovirus infections. Med Microbiol Immunol. 2008;197:145–149. doi: 10.1007/s00430-008-0088-z. [DOI] [PubMed] [Google Scholar]
- 48.Melchers F, ten Boekel E, Seidl T, Kong XC, Yamagami T, Onishi K, Shimizu T, Rolink AG, Andersson J. Repertoire selection by pre-B-cell receptors and B-cell receptors, and genetic control of B-cell development from immature to mature B cells. Immunological reviews. 2000;175:33–46. [PubMed] [Google Scholar]
- 49.Melamed D, Miri E, Leider N, Nemazee D. Unexpected autoantibody production in membrane Ig-mu-deficient/lpr mice. J Immunol. 2000;165:4353–4358. doi: 10.4049/jimmunol.165.8.4353. [DOI] [PubMed] [Google Scholar]
- 50.KleinJan A, Vinke JG, Severijnen LW, Fokkens WJ. Local production and detection of (specific) IgE in nasal B-cells and plasma cells of allergic rhinitis patients. Eur Respir J. 2000;15:491–497. doi: 10.1034/j.1399-3003.2000.15.11.x. [DOI] [PubMed] [Google Scholar]
- 51.Samitas K, Lotvall J, Bossios A. B cells: from early development to regulating allergic diseases. Archivum immunologiae et therapiae experimentalis. 2010;58:209–225. doi: 10.1007/s00005-010-0073-2. [DOI] [PubMed] [Google Scholar]