Abstract
A majority of studies involving prodrugs are directed to overcome low bioavailability of the parent drug. The aim of this study is to increase the bioavailability of acyclovir (ACV) by designing a novel prodrug delivery system which is more lipophilic, and at the same time site specific. In this study, a lipid raft has been conjugated to the parent drug molecule to impart lipophilicity. Simultaneously a targeting moiety that can be recognized by a specific transporter/receptor in the cell membrane has also been tethered to the other terminal of lipid raft. Targeted lipid prodrugs i.e., biotin-ricinoleicacid-acyclovir (B-R-ACV) and biotin-12hydroxystearicacid-acyclovir (B-12HS-ACV) were synthesized with ricinoleicacid and 12hydroxystearicacid as the lipophilic rafts and biotin as the targeting moiety. Biotin-ACV (B-ACV), ricinoleicacid-ACV (R-ACV) and 12hydroxystearicacid-ACV (12HS-ACV) were also synthesized to delineate the individual effects of the targeting and the lipid moieties. Cellular accumulation studies were performed in confluent MDCK-MDR1 and Caco-2 cells. The targeted lipid prodrugs B-R-ACV and B-12HS-ACV exhibited much higher cellular accumulation than B-ACV, R-ACV and 12HS-ACV in both cell lines. This result indicates that both the targeting and the lipid moiety act synergistically towards cellular uptake. The biotin conjugated prodrugs caused a decrease in the uptake of [3H] biotin suggesting the role of sodium dependent multivitamin transporter (SMVT) in uptake. The affinity of these targeted lipid prodrugs towards SMVT was studied in MDCK-MDR1 cells. Both the targeted lipid prodrugs B-R-ACV (20.25 ± 1.74 µM) and B-12HS-ACV (23.99 ± 3.20 µM) demonstrated higher affinity towards SMVT than B-ACV (30.90 ± 4.19 µM). Further, dose dependent studies revealed a concentration dependent inhibitory effect on [3H] biotin uptake in the presence of biotinylated prodrugs. Transepithelial transport studies showed lowering of [3H] biotin permeability in the presence of biotin and biotinylated prodrugs, further indicating a carrier mediated translocation by SMVT. Overall, results from these studies clearly suggest that these biotinylated lipid prodrugs of ACV possess enhanced affinity towards SMVT. These prodrugs appear to be potential candidates for the treatment of oral and ocular herpes virus infections, because of higher expression of SMVT on intestinal and corneal epithelial cells. In conclusion we hypothesize that our novel prodrug design strategy may help in higher absorption of hydrophilic parent drug. Moreover, this novel prodrug design can result in higher cell permeability of hydrophilic therapeutics such as genes, siRNA, antisense RNA, DNA, oligonucleotides, peptides and proteins.
Keywords: acyclovir, targeted lipid prodrugs, biotin, SMVT, affinity, drug delivery
1. INTRODUCTION
Acyclovir [ACV, 2-amino-9-((2-hydroxyethoxy) methyl)-1H-purin-6(9H)-one] is a guanosine analogue with excellent antiviral activity. Since its discovery, ACV has been indicated for the treatment of herpes simplex virus (HSV) and herpes zoster (shingles) infections. The compound is phosphorylated by viral thymidine kinases to ACV monophosphate, which is subsequently converted by cellular kinases to active ACV triphosphate. ACV triphosphate is then incorporated into viral DNA, inhibiting the activity of DNA polymerase and leading to chain termination (Piret and Boivin, 2011; Reardon and Spector, 1989; Wilson et al., 2009). ACV suffers from limited aqueous solubility and low oral bioavailability (15–30%), resulting in poor accumulation at the target site. A wide variety of strategies have been investigated to enhance cellular absorption amongst which transporter targeted prodrug strategy has been a promising approach. In our laboratory, the functional characteristics of various transporters have been exploited for targeted drug delivery. These include amino acid transporters (Anand et al., 2004; Jain-Vakkalagadda et al., 2003), peptide transporter (PEPT1 and PEPT2) (Anand et al., 2003a; Anand and Mitra, 2002), sodium dependent multivitamin transporter (SMVT) (Janoria et al., 2009; Janoria et al., 2006; Luo et al., 2006), sodium dependent vitamin C transporter (SVCT1 and SVCT2) (Luo et al., 2008; Talluri et al., 2006), riboflavin transporter (Hariharan et al., 2006), nucleoside and nucleobase transporters (Majumdar et al., 2003a; Majumdar et al., 2003b). Valacyclovir (VACV), an amino acid ester prodrug of ACV has been shown to increase the oral bioavailability of ACV by 3 to 5-fold (Balimane et al., 1998; de Vrueh et al., 1998; Guo et al., 1999; Jacobson, 1993; Perry and Faulds, 1996; Soul-Lawton et al., 1995; Talluri et al., 2008).
SMVT is primarily responsible for the uptake of vitamins such as biotin, pantothenic acid and lipoate in epithelial cells (Chatterjee et al., 1999; Prasad et al., 1999; Vadlapudi et al., 2012). Biotin, a water soluble vitamin is essential for normal cellular growth. Absorption of biotin by SMVT is a pH dependent process. Significant amount of biotin absorption through SMVT has been reported in major tissues such as cornea, retina, kidney, intestine, liver and placenta (Balamurugan et al., 2003b; Janoria et al., 2009; Janoria et al., 2006; Luo et al., 2006; Prasad and Ganapathy, 2000; Prasad et al., 1998; Said, 1999; Said et al., 1987). SMVT has been utilized for delivery of poorly permeable drugs by conjugating biotin as the targeting moiety (Gunaseelan et al., 2004; Minko et al., 2002; Ramanathan et al., 2001b). Ramanathan et al. utilized this transporter to improve the intestinal absorption of peptides. Interaction of the PEG-biotin conjugates with SMVT has also been reported (Ramanathan et al., 2001b). Other conjugates like CPT-PEG-biotin showed enhanced anticancer activity relative to the parent drug camptothecin (CPT) in multidrug-resistant human ovarian carcinoma cells (Minko et al., 2002). Biotin conjugated nonapeptide R.I.-K (biotin)-Tat9 exhibited three times higher permeability in comparison to non-biotinylated R.I.-K-Tat9 across Caco-2 cell monolayers (Ramanathan et al., 2001a).
The apparent affinity constant (Km) values of SMVT substrates are usually in the low micro molar range (Ma et al., 1994; Said, 1999; Said et al., 1992; Said et al., 1994; Said et al., 1998; Said et al., 1987). Such low Km values result in the saturation of the transporter which limits the dose of drug molecule that can be delivered through this carrier. To overcome this constraint, we have investigated the possibility of utilizing lipid-raft based drug conjugates to maximize the amount of drug transport. Biological membranes are lipophilic and hence only relatively lipophilic molecules can penetrate such membranes. It has been shown that the lipid moieties conjugated to drugs enhanced the absorption of drug resulting in higher oral bioavailability (Trevaskis et al., 2008). Acyclovir diphosphate dimyristoylglycerol (ACVDP-DG), a lipid prodrug of ACV was shown to be very active against HSV1 and HSV2, ACV-resistant strains of HSV and human cytomegalovirus (CMV) (Hostetler et al., 1993). Such lipid prodrugs had shown prolonged antiviral activity against HSV-1 retinitis in a rabbit model (Taskintuna et al., 1997). 1-O-hexadecylpropanediol-3-P-ACV, an orally bioavailable lipid prodrug of ACV is highly effective against acute HSV-1 infection in mice. It was also found to be active against CMV infections in vitro due to its ability to bypass thymidine kinases (Beadle et al., 2000). Recently, hexadecyloxypropyl esters of cidofovir and (S)-HPMPA have been synthesized. These lipid prodrugs were readily absorbed and converted intracellularly to their respective diphosphates following oral administration. These prodrugs are also orally active in animal models of viral infection (Beadle, 2007).
Previous work from our laboratory suggested that lipid prodrug diffuses readily across the cell membrane by facilitated diffusion whereas transporter/receptor targeted prodrug translocates compounds across the cell membrane via active transport. Both approaches have individually shown marginal improvement in cellular uptake. However, our current approach combines both lipid and transporter/receptor targeted delivery to generate synergistic effect. The lipid raft facilitates enhanced interaction of prodrug with membrane transporters/receptors probably assisting docking of the targeted ligand into the binding domain of transporter/receptor protein. The net effect is rapid translocation of the cargo across cell membrane. Biotinylated lipid prodrugs with different lipid rafts conjugated to ACV have been synthesized. These prodrugs include biotin-Ricinoleicacid-ACV (B-R-ACV) and biotin-12Hydroxystearicacid-ACV (B-12HS-ACV). To delineate the individual effects of the targeting and the lipid moieties, biotin ACV (B-ACV), ricinoleicacid-ACV(R-ACV) and 12hydroxystearicacid-ACV (12HS-ACV) have also been synthesized. Emphasis was placed on the rate of cellular accumulation of these prodrugs for enhanced absorption of the parent drug, ACV. These compounds were evaluated for their ability to be translocated by SMVT across Caco-2 and MDCK-MDR1 cell lines. These model in vitro cell lines have been extensively employed to delineate compounds that may be recognized by SMVT (Balamurugan et al., 2003a; Luo et al., 2006).
2. MATERIALS AND METHODS
2.1. MATERIALS
ACV is a gift from GlaxoSmithKline (Research Triangle Park, NC). Biotin, ricinoleicacid and 12-hydroxystearic acid were purchased from Sigma-Aldrich (St. Louis, MO). [3H] Biotin (60Ci/mmol) was procured from Perkin-Elmer Life Science, Inc. (Boston, MA). Madin-Darby canine kidney cells transfected with human mdr1 gene (MDCK-MDR1) were a generous gift from Drs. Schinkel and P. Borst (The Netherlands Cancer Institute, Amsterdam). Human colon carcinoma derived cells (Caco-2) were obtained from American Type Culture Collection (Manassas, VA). The growth medium, Dulbecco’s modified Eagle’s medium (DMEM) and TrypLE™ Express were obtained from Invitrogen (Carlsbad, CA). Nonessential amino acids, penicillin, streptomycin, sodium bicarbonate and HEPES were purchased from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA). Culture flasks (75cm2 growth area), 12-well plates (3.8cm2 growth area per well) and 96-well plates (0.32cm2 growth area per well) were procured from Costar (Cambridge, MA). The buffer components and solvents were obtained from Fisher Scientific Co. (Fair Lawn, NJ). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and used without further purification.
2.2. SYNTHESIS
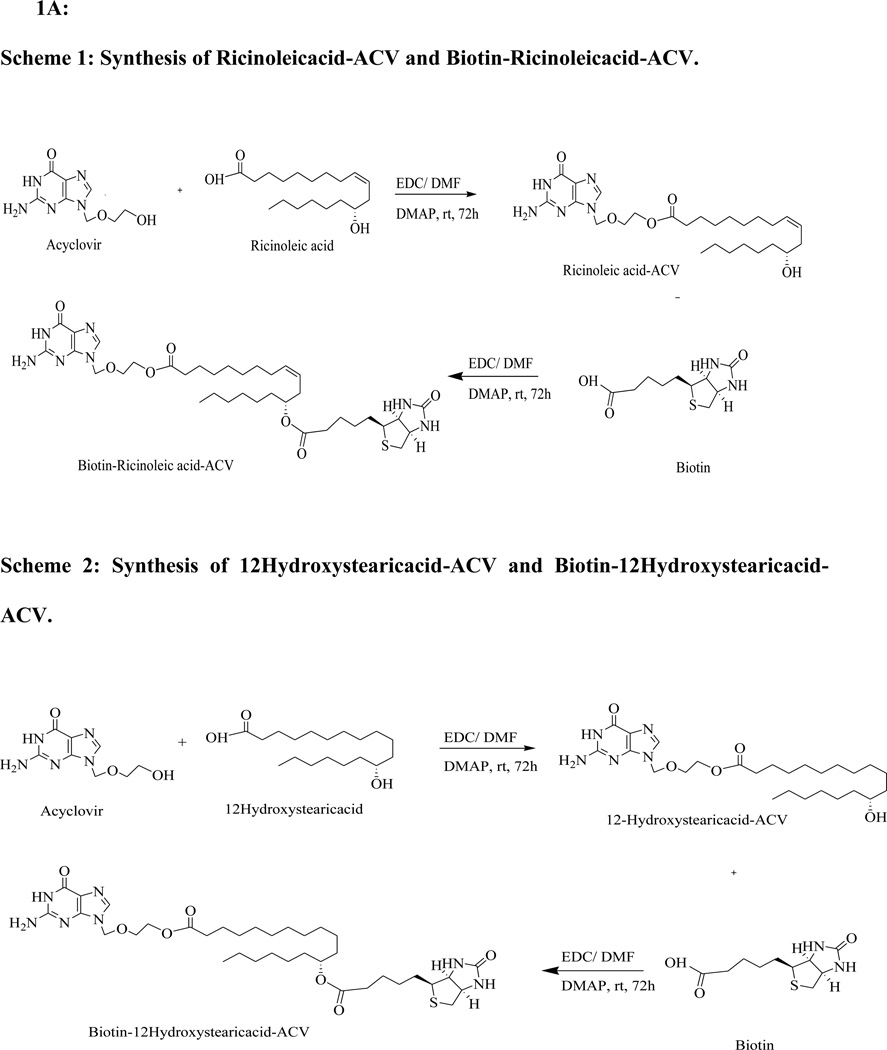
Synthesis of Ricinoleicacid-ACV(R-ACV)
Ricinoleicacid (100mg, 0.33mmol) is dissolved in dimethyl formamide (DMF) (2ml), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) (126mg, 0.66mmol) is added and stirred for 1 h at room temperature to activate the carboxyl group of ricinoleicacid. In a separate reaction flask, ACV (110mg, 0.49mmol) is dissolved in DMF, 4-dimethylaminopyridine (DMAP) (60mg, 0.49mmol) is added and stirred for 10 min at room temperature to activate the hydroxyl group of ACV. This mixture is then added to the reaction vessel containing ricinoleicacid through a syringe and is stirred continuously for 72 h. Small portions of the reaction mixture are taken out and injected into LC/MS to ensure the complete conversion of the starting material to the product. The reaction mixture is then filtered and evaporated at room temperature under reduced pressure to generate crude product. The product R-ACV is then purified by silica gel column chromatography using 6% methanol/dichloromethane (MeOH/DCM) as eluent. The yield is approximately 47%. The synthetic scheme has been summarized in scheme 1.
Fig 1.
Fig 1A:
Scheme 1: Synthesis of Ricinoleicacid-ACV and Biotin-Ricinoleicacid-ACV.
Scheme 2: Synthesis of 12Hydroxystearicacid-ACV and Biotin-12Hydroxystearicacid-ACV.
Scheme 3: Synthesis of Biotin-ACV.
Fig 1B: The percent yield, mass and NMR (both 1HNMR and 13CNMR) spectra for all the synthesized prodrugs.
Synthesis of Biotin-Ricinoleicacid-ACV (B-R-ACV)
R-ACV (30mg, 0.05mmol) is dissolved in DMF; DMAP (10mg, 0.075mmol) is added and stirred for 10 min at room temperature. In a separate reaction flask, biotin (29mg, 0.11mmol) is dissolved in DMF (1ml); EDC (23mg, 0.11mmol) is added and stirred for 1 h. This mixture is added into the reaction containing R-ACV through a syringe and is stirred continuously for 72 h. The complete conversion of the starting material to product is ensured by injecting small portion of the reaction mixture into LC/MS. The reaction mixture is filtered and evaporated under reduced pressure to generate crude product. The product B-R-ACV is purified by silica gel column chromatography using 20% MeOH/DCM as eluent. The yield is approximately 79%. The synthetic scheme has been summarized in scheme 1.
Synthesis of 12Hydroxystearicacid-ACV (12HS-ACV) and Biotin-12Hydroxystearicacid-ACV (B-12HS-ACV)
12HS-ACV and B-12HS-ACV are synthesized following the same procedure used for the synthesis of R-ACV and B-R-ACV respectively. The yield of B-12HS-ACV is approximately 78%. The synthetic scheme has been summarized in scheme 2.
Synthesis of Biotin-ACV (B-ACV)
Biotin (100mg, 0.40mmol) is dissolved in DMF (2ml), EDC (152mg, 0.80mmol) is added and stirred for 1 h. In a separate reaction flask ACV (184mg, 0.80mmol) is dissolved in DMF, DMAP (58mg, 0.48mmol) is added and stirred for 10 min at room temperature. This mixture is then added into the reaction containing biotin through a syringe and is stirred continuously for 72 h. The complete conversion of the starting material to product is ensured and the solvent is evaporated to yield the crude product. The product B-ACV is purified by silica gel column chromatography using 10% MeOH/DCM as eluent. The yield is approximately 78%. The synthetic scheme has been summarized in scheme 3.
All the reactions are run under inert atmosphere. The prodrugs are characterized by 1H NMR, 13C NMR and LC/MS. 1H and 13C NMR spectra are recorded using tetra methyl silane as an internal standard on Varian Mercury 400 Plus spectrometer. Chemical shifts (δ) are reported in parts per million relative to the NMR solvent signal (CD3OD, 3.31 ppm for proton and 49.15 ppm for carbon NMR spectra; DMSO-d6, 2.51ppm for proton and 39.30 ppm for carbon NMR). A hybrid triple quadrupole linear ion trap mass spectrometer (QTRAP® LC/MS/MS mass spectrometer (API 3200, Applied Biosystems/MDS Sciex, Foster City, CA, USA) under enhanced mass (EMS) mode is used for carrying out the mass analysis. Electrospray ionization (ESI) is utilized as an ion source and operated in positive and negative ion mode.
2.3 CELL CULTURE
Caco-2 (passage numbers 25–30) and MDCK-MDR1 cells (passage numbers 10–15) are utilized in the studies. Cells are grown at 37°C in an incubator with 95% air and 5% CO2. Cells are maintained in 75cm2 culture flasks using DMEM with 29mM sodium bicarbonate, 20mM HEPES, 100µg/ml streptomycin, 100µg/ml of penicillin, 10% FBS (Heat Inactivated) and 1% nonessential amino acids at pH 7.4. The medium is changed every alternate day. After reaching 80% confluency, cells are passaged using TrypLE™ Express (superior replacement for trypsin). Cells are subsequently plated in 12-well uptake plates at a density 250,000 cells/well and in 96-well plates at a density 10,000 cells/well. Cells are grown in a similar way and utilized for further studies.
2.4 PREPARATION OF DRUG SOLUTIONS
Concentrated stock solutions of all the drugs/prodrugs are prepared in dimethyl sulfoxide (DMSO). Test solutions are then prepared by adding aliquots from the respective stock solution and diluted with Dulbecco’s Phosphate Buffered Saline (DPBS) (130mM NaCl, 0.03mM KCl, 7.5mM Na2HPO4, 1.5mM KH2PO4, 1mM CaCl2, 0.5mM MgSO4, 20mM HEPES and 5mM glucose) to achieve required concentration when needed. The final concentration of DMSO in all the drug solutions does not exceed 1% v/v.
2.5 CELLULAR ACCUMULATION STUDIES
Cellular accumulation studies are performed on MDCK-MDR1 and Caco-2 cell monolayers after growing them for 7 and 21 days, respectively. The medium is aspirated and cells are rinsed 3 times with DPBS. Uptake is initiated by adding 1ml drug solution into each well and incubated for a period of 30 min. After incubation, drug solutions are removed and uptake is terminated with ice cold stop solution (200 mM KCl and 2 mM HEPES). Cells are lysed overnight at −80°C in 500µl cremophore water (2 drops of cremophore gel in 50 ml of deionized water) in each well. Samples are then analyzed with LC-MS/MS and the rate of uptake is normalized to the protein content of each well. The amount of protein in the cell lysate is estimated with BioRad protein estimation kit (BioRad, Hercules, CA) using bovine serum albumin as an internal standard. A similar procedure is adopted to determine cellular accumulation of the prodrugs in the presence of excess biotin to delineate the interaction of the prodrugs with SMVT (substrate specificity).
2.6 SATURATION KINETICS
Saturation kinetics of [3H] biotin in the presence of varying concentrations (0.1–100µM) of unlabeled biotin and prodrugs is determined on MDCK-MDR1 cells according to a previously published method (Anand et al., 2003b; Suresh et al., 2010). Briefly, various concentrations of the prodrugs are prepared in DPBS spiked with 0.5µCi/ml of [3H] biotin. The medium is aspirated and cells are rinsed thrice with DPBS. Uptake is initiated by adding 1ml of drug solution. After 30 min, solutions are removed and uptake process is terminated with ice cold stop solution. Cells are lysed overnight with 1ml of lysis solution (0.1% w/v, Triton X-100 in 0.3N sodium hydroxide) at room temperature. Subsequently, aliquots (500 µl) of cell lysate are withdrawn from each well and transferred to scintillation vials containing 3ml scintillation cocktail (Fisher Scientific). Samples are then analyzed by liquid scintillation spectrophotometer with a Beckman scintillation counter (Model LS-6500, Beckman Instruments, Inc.). The rate of uptake is normalized to protein content of each well. The data is fitted to Michaelis-Menten equation to calculate the apparent affinity constant (Km) and maximum velocity of uptake (Vmax).
2.7 DOSE DEPENDENT INHIBITION STUDIES
Dose dependent inhibition studies of [3H] Biotin on MDCK-MDR1 cells are studied in the presence of various concentrations (0.1–100µM) of unlabeled biotin and biotinylated prodrugs. The study is performed using the similar procedure described earlier in section 2.6. The data is fitted to calculate the half maximal inhibitory concentration (IC50) following a previously published method (Kwatra et al., 2010; Vadlapatla et al., 2011).
2.8 PERMEABILITY STUDIES
Permeability of [3H] biotin (0.5µCi/ml) across monolayers of MDCK-MDR1 cells in absence and presence of 50µM concentration of unlabeled biotin and prodrugs B-ACV, B-R-ACV, and B-12HS-ACV has been determined. This study is performed to ascertain whether these prodrugs are recognized by SMVT and thus share the same transporter for their translocation. Cells were grown on transwell inserts in 12-well plates. Prior to an experiment, medium is removed and monolayers are washed 3 times with DPBS pH 7.4 and equilibrated for 30 min at 37°C. Volumes of apical and basal chambers are 0.5 and 1.5ml, respectively. Test solutions containing [3H] biotin alone and with respective prodrugs are added on donor side and the receiving chamber contains only DPBS. Transport experiment is conducted over a period of 3 h. Samples (200µl) are withdrawn from the receiving chamber at predetermined time points (0, 15, 30, 45, 60, 90, 120, 150 and 180 min) and replaced with equal volume of DPBS to maintain sink conditions. Samples are transferred to scintillation vials containing 3mL of scintillation fluid (Fisher Scientific), and radioactivity is analyzed with a Beckman Scintillation Coulter Model LS-6500, Beckman Instruments, Inc.).
2.9 CYTOTOXICITY MEASUREMENTS
Cytotoxicity assay is carried out with Cell Titer 96® Aqueous Non-Radioactive Cell Proliferation Assay Kit (Promega, Madison, WI). MDCK-MDR1 and Caco-2 cells are grown on 96 well plates. Sterile drug solutions (100µM) are made in the culture medium using 0.22µm nylon sterile membrane filters. Aliquots of prodrugs in culture medium (100µl) have been added to each well and incubated for 48 h. Proliferation of the cells in the presence of ACV and its prodrugs is compared with a positive control (medium without drug) and a negative control (medium without cells). After 48 h of incubation, 20µl dye solution is added to each well and incubated for 3 h allowing the dye to react. The amount of farmazan formed is measured with a 96-well micro titer plate reader (SpectraFluor Plus, Tecan, Maennedorf, Switzerland), absorbance set at 490 nm wavelength. As the amount of farmazan formed is directly proportional to the viability of the cells, the toxicity of these prodrugs can be estimated.
2.10 DATA ANALYSIS
All the non-radioactive samples were analyzed with LC-MS/MS. A fast and sensitive LC-MS/MS method has been developed in multiple reaction monitoring (MRM) with electrospray (ES) positive ionization mode for detection of ACV and targeted lipid prodrugs. QTRAP® LC/MS/MS mass spectrometer (API 3200, Applied Biosystems/MDS Sciex, Foster City, CA, USA) is employed to analyze samples from non-radioactive cellular accumulation and inhibition studies. Chromatographic separation is achieved on XTerra® RP8 Column, 5 µm, 4.6 × 50 mm (Waters Corporation, Milford, MA) with an isocratic mobile phase. The mobile phase consists of 70% acetonitrile, 30% water and 0.1% formic acid which are pumped at a flow rate of 0.2ml/min. Precursor ions of the analytes as well as internal standard are determined from spectra obtained during the infusion of standard drug/prodrug solutions with an infusion pump connected directly to the ESI source. Each of these precursor ions is subjected to collision-induced dissociation to determine their respective product ions. MRM transitions at m/z [M+H]+ generated were 226.4/152.2 for ACV, 452.3/301.3 for B-ACV, 506.3/488.6 for R-ACV, 732.3/257.4 for B-R-ACV, 507.34/488.5 for 12HS-ACV, 735.6/257.3 for B-12HS-ACV and 256/152 for GCV. Peak areas for all components are automatically integrated by Analyst™ software and peak-area ratios (area of analytes to area of internal standard) are plotted against concentration by weighted linear regression (1/concentration). The analytical data resulted from prodrugs with MRM method shows a significant linearity. This method generates rapid and reproducible results.
Stocks and stock dilutions of ACV and respective prodrugs are prepared similarly following a previously published procedure (Earla et al., 2010). Samples are extracted by liquid-liquid extraction method. Ganciclovir (GCV) is used as an internal standard to ensure reproducibility and reliability of the method. Samples are thawed at room temperature. Two hundred microliter sample along with 20µl of GCV (5µg/ml) is extracted with 1ml of organic solvent containing 2:3 ratios of isopropanol (IPA) and dichloromethane (DCM). The samples are vortexed for approximately 2 min and centrifuged at 12000 g for 15 min at 4°C. Organic layer (850µl) is transferred into eppendorf tubes and evaporated to dryness under speed vacuum with a Speedvac (SAVANT Instruments, Inc., Holbrook, NY). The residue is then reconstituted in 100µL of mobile phase, vortexed for 30 sec and transferred into pre-labeled vials with silanized inserts. Subsequently, 15µl of the resulting solution is injected onto LC-MS/MS. Appropriate calibration standards of ACV and its novel prodrugs are prepared by spiking known analyte concentrations to blank cell homogenate obtained from cultured cells following similar procedure. A calibration curve is generated using calibration standards.
[3H] Biotin accumulated inside the cell monolayers in the presence of various concentrations of prodrugs are calculated according to Eq. 1.
| Eq. 1 |
CPMsample and CPMdonor denote average values of Counts per Minute (CPM) counts of sample and donor (n=4) respectively; Cdonor represents the concentration of donor used and Csample represents the concentration of sample.
The saturation kinetics of biotin uptake is calculated by a classic Michaelis-Menten equation (Eq. 2).
| Eq. 2 |
V is the total rate of uptake, Vmax is the maximum uptake rate for the carrier-mediated process and Km is the Michaelis-Menten constant. Data is fitted to Eq. 2 using nonlinear least squares regression analysis program (KaleidaGraph 3.5).
For dose dependent inhibition studies, the inhibitory effect of [3H] biotin by unlabeled biotin and biotinylated prodrugs is described by Eq. 3.
| Eq. 3 |
X represents the logarithm of the concentration used; Y is the cellular accumulation of [3H] biotin. Data is fitted to Eq. 3 with a transformed nonlinear regression curve analysis program (GraphPad Prism Version 4.0; GraphPad Software, Inc., San Diego, CA).
2.11 STATISTICAL ANALYSIS
All the experiments are conducted at least in quadruplicate (n = 4), and the results were expressed as mean ± standard deviation (SD). Statistical comparison of mean values is performed with a Student’s t test. A P-value of less than 0.05 is considered to be statistically significant.
3. RESULTS
3.1. SYNTHESIS
The synthetic schemes are provided in Fig. 1A. The percent yield, mass and NMR (both 1HNMR and 13CNMR) spectra for all the synthesized prodrugs are given in Fig. 1B.
3.2. CELLULAR ACCUMULATION STUDIES
Cellular accumulation of B-R-ACV, B-ACV, R-ACV and ACV is performed on MDCK-MDR1 cell monolayers. The results showed 9.5 times increase in the uptake of B-R-ACV compared to parent drug, ACV. B-ACV and R-ACV showed 6 times and 4 times increase in the uptake, respectively (Fig. 2). With these results, B-12HS-ACV was included along with other prodrugs for cellular accumulation studies on human intestinal Caco-2 cells following similar procedure. Compared to ACV, the uptake of B-R-ACV and B-12HS-ACV increased by 10 and 8.3 times respectively, whereas the uptake of B-ACV, R-ACV and 12HS-ACV was higher by 3.5, 1.4 and 1.3 times respectively (Fig. 3).
Fig 2.
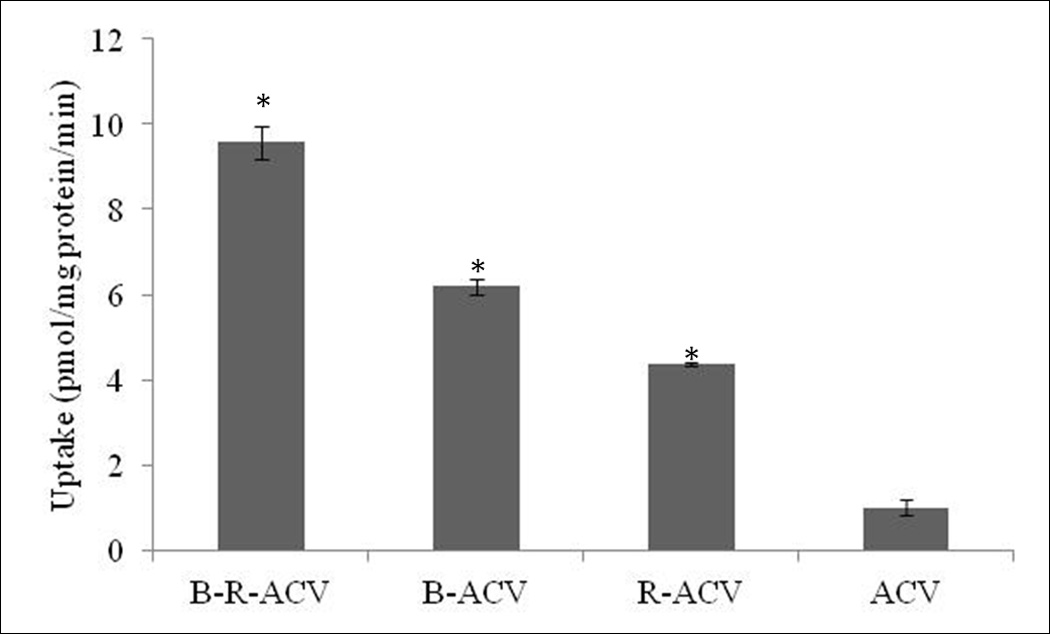
Cellular accumulation of B-R-ACV, B-ACV, R-ACV and ACV on MDCK-MDR1 cells. Values represent mean ± standard deviation (n= 4) of three independent experiments. A P-value of less than 0.05 was considered to be statistically significant.
Fig 3.

Cellular accumulation of B-R-ACV, B-12HS-ACV, B-ACV, R-ACV, 12HS-ACV and ACV on Caco-2 cells. Values represent mean ± standard deviation (n= 4) of three independent experiments. A P-value of less than 0.05 was considered to be statistically significant.
3.3 INHIBITION STUDY (SUBSTRATE SPECIFICITY)
To investigate the involvement of SMVT, the substrate specificity of the transporter is examined by inhibiting the uptake of B-R-ACV, B-12HS-ACV and B-ACV in presence of biotin on MDCK-MDR1 cells. The results show that the uptake is significantly lower in the presence of unlabeled biotin (Fig. 4). This result indicates that SMVT transporter may be involved in the cellular uptake of B-R-ACV, B-12HS-ACV and B-ACV.
Fig 4.
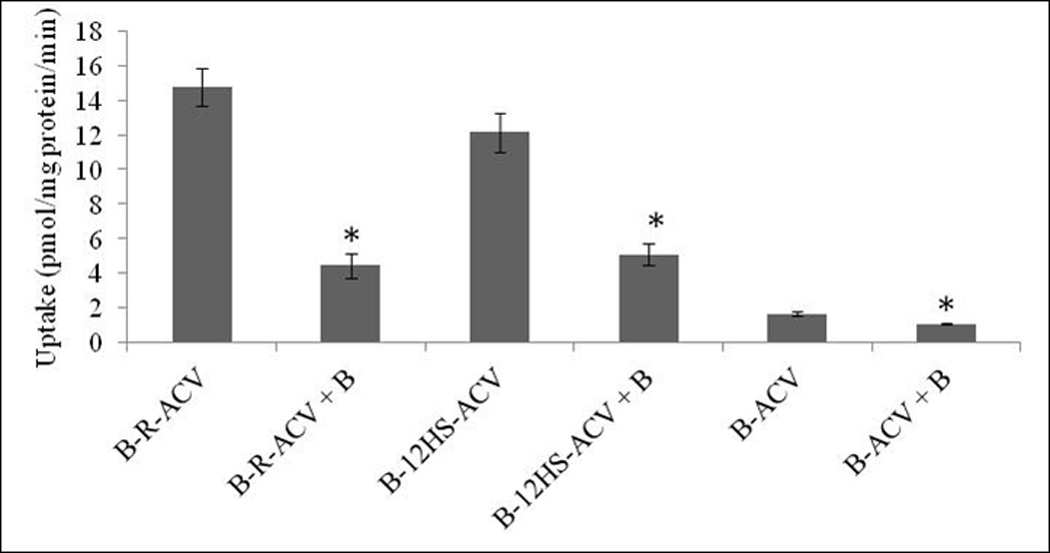
Cellular accumulation of B-R-ACV, B-12HS-ACV and B-ACV in the presence of excess biotin on MDCK-MDR1 cells. Values represent mean ± standard deviation (n= 4) of three independent experiments. A P-value of less than 0.05 was considered to be statistically significant.
3.4 SATURATION KINETICS
Uptake of [3H] biotin in MDCK-MDR1 cells is studied to determine the saturation kinetics using varying concentrations (0.1–100µM) of biotin, B-ACV, B-R-ACV and B-12HS-ACV, respectively. The results from [3H] biotin uptake as a function of concentration of biotinylated prodrugs suggest a saturable component with a significant change in apparent affinity constant (Km) (Fig 5). Transformation of the data from the concentration dependent uptake resulted in a Lineweaver-Burk plot (R2 = 0.99). The concentration dependent uptake kinetics of [3H] biotin in the presence of all the biotinylated prodrugs denotes a single, saturable carrier model.
Fig 5.
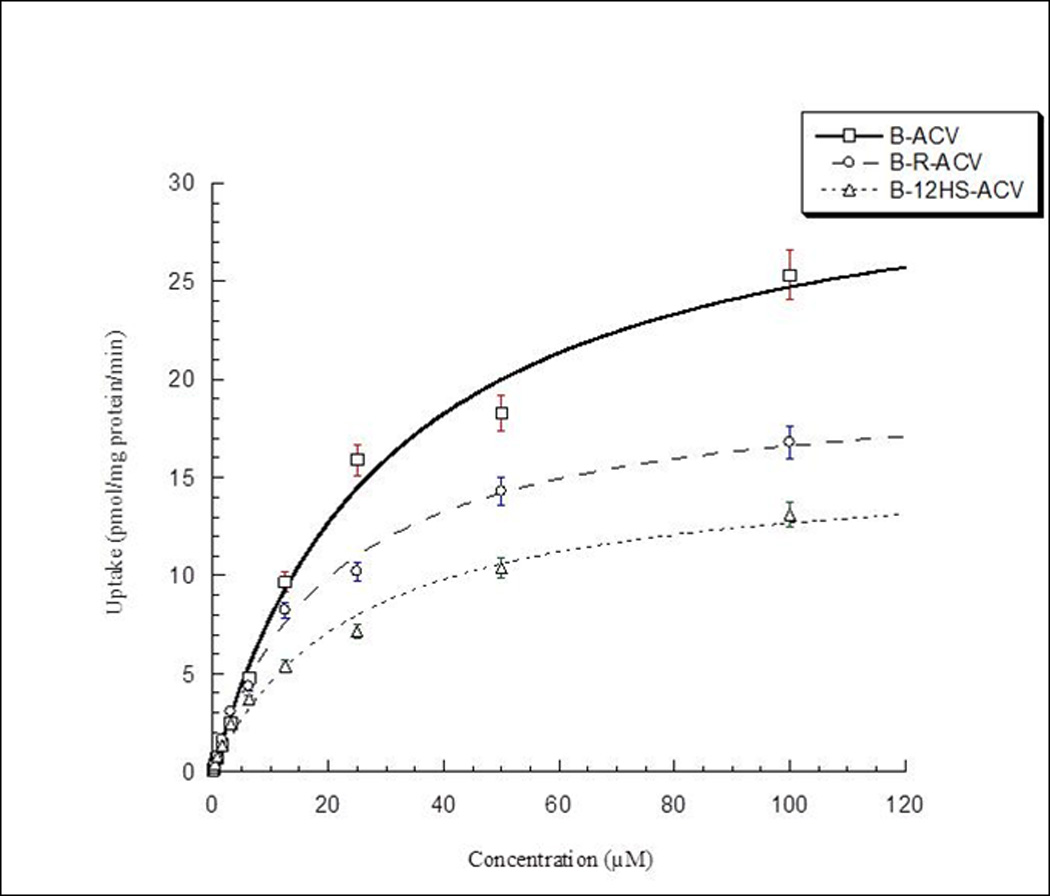
Saturation kinetics across MDCK-MDR1 cells in the presence of B-R-ACV, B-12HS-ACV and B-ACV. Values represent mean ± standard deviation (n= 4) of three independent experiments. A P-value of less than 0.05 was considered to be statistically significant.
3.5 DOSE-DEPENDENT INHIBITION OF [3H] BIOTIN UPTAKE
Dose dependent inhibition of [3H] biotin uptake in MDCK-MDR1 cells has been studied with varying concentrations (0.1–100µM) of biotin, B-ACV, B-R-ACV and B-12HS-ACV, respectively. IC50 values of B-R-ACV and B-12HS-ACV from the dose–response curves are calculated to be 8.04 ± 0.07µM and 8.17 ± 0.09µM, respectively. These values appear to be lower relative to B-ACV (14.84 ± 0.10µM). IC50 value of biotin used as control is found to be 2.93±0.06µM (Figs 6A–6D).
Fig 6.
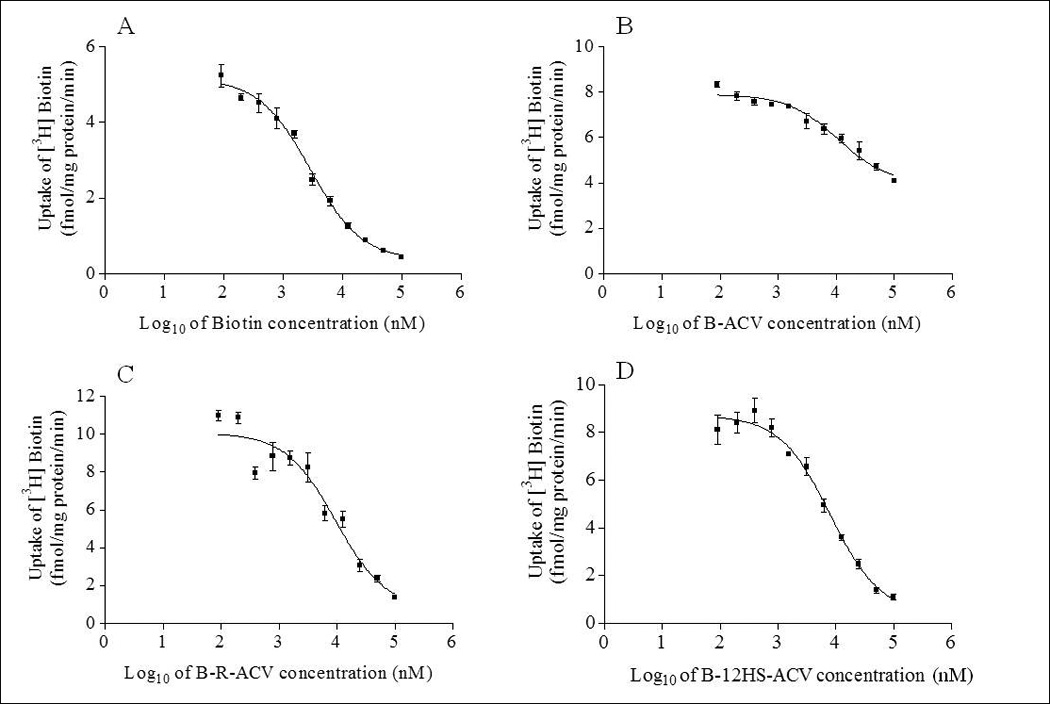
Dose dependent inhibition of [3H] biotin uptake in MDCK-MDR1 cells in the presence of varying concentrations (0.1–100µM) of A) biotin, B) B-ACV, C) B-R-ACV and D) B-12HS-ACV. Values represent mean ± standard deviation (n= 4) of three independent experiments.
3.7. PERMEABILITY STUDIES
Transport of [3H] biotin (0.5µCi/ml) across MDCK-MDR1 monolayers was assessed. From the transport data, cumulative amount transported is plotted against time. Unlabeled biotin and all the biotinylated ACV prodrugs appears to inhibit the transport of [3H] biotin, a substrate extensively studied for its translocation by SMVT (Fig. 7A). Biotinylated lipid prodrugs of ACV (B-R-ACV and B-12HS-ACV) exhibited higher inhibition in [3H] biotin transport than that of B-ACV suggesting the synergistic involvement of SMVT transporter and the lipid moiety in mediating cellular permeation across MDCK-MDR1. Permeabilities of [3H] biotin are 4.74 × 10−6 cm/sec in comparison to 0.57 × 10−6 cm/sec, 2.96 × 10−6 cm/sec, 1.90 × 10−6 cm/sec, 2.10 × 10−6 cm/sec in presence of 50µM concentration of unlabeled biotin and prodrugs B-ACV, B-R-ACV, and B-12HS-ACV, respectively (Fig. 7B).
Fig. 7.
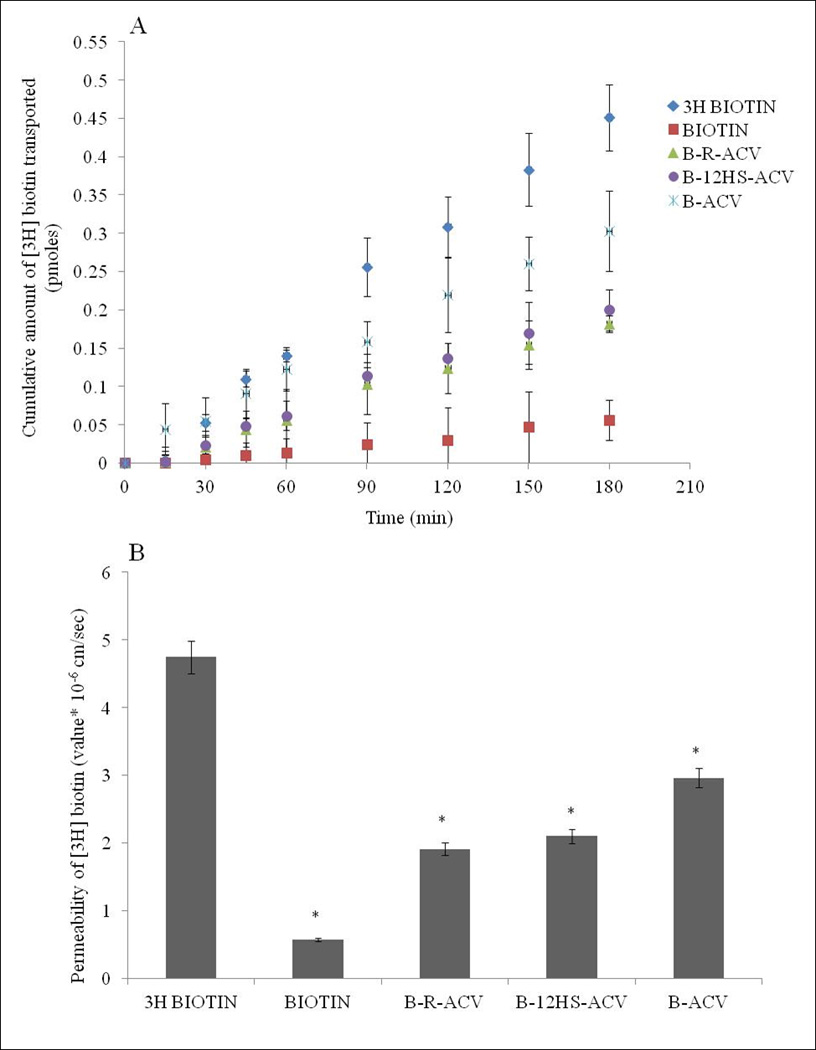
A) Transepithelial transport of [3H] biotin in MDCK-MDR1 cells in the absence and presence of 50µM unlabeled biotin, B-ACV, B-R-ACV, and B-12HS-ACV. B) Comparison of permeabilities (cm/sec) of [3H] biotin alone, in the presence of 50µM unlabeled biotin, B-ACV, B-R-ACV, and B-12HS-ACV on MDCK-MDR1 cells. Data represents mean ± standard deviation (n= 4–6) of four independent experiments. A P-value of less than 0.05 was considered to be statistically significant.
3.7 CYTOTOXICITY ASSAY
Cytotoxicity assay was performed on MDCK-MDR1 and Caco-2 cell monolayers for a period of 48 h to evaluate the cytotoxic effect of all the prodrugs studied. Neither ACV nor its targeted lipid prodrugs demonstrate any cytotoxic effect at the examined concentration (100µM). The results from this assay clearly suggest that all the targeted and non-targeted lipid prodrugs studied may be comparatively less cytotoxic with respect to ACV (Fig. 8).
Fig. 8.
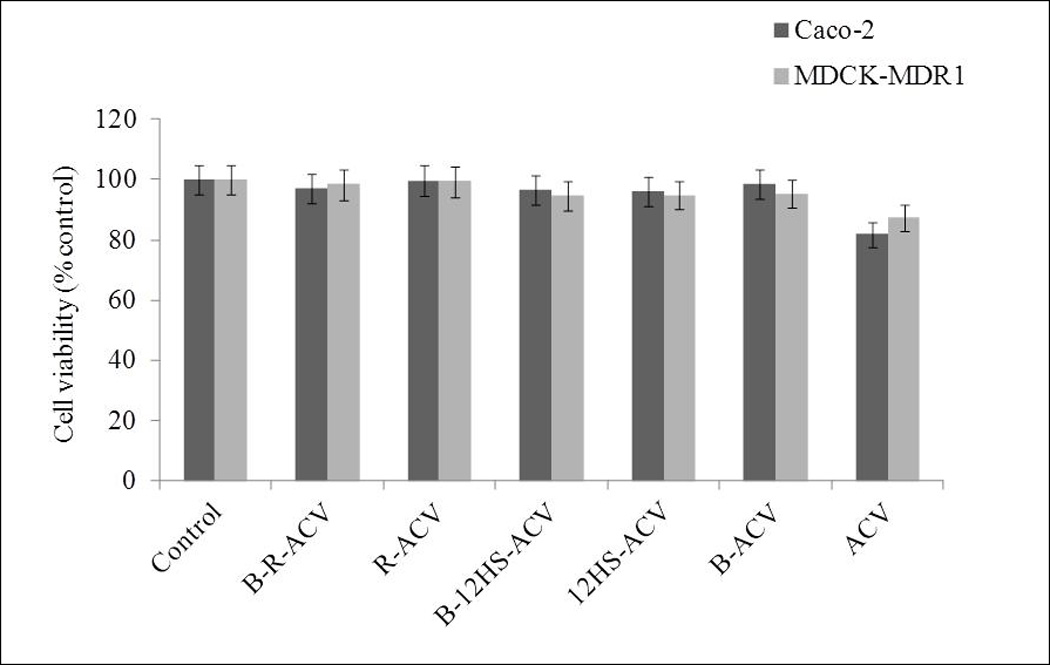
Cytotoxicity assay in the presence of B-R-ACV, R-ACV, B-12HS-ACV, 12HS-ACV, B-ACV and ACV on Caco-2 and MDCK-MDR1 cells for 48 h. Data represent mean percentage of viable cells ± standard deviation (n= 4) of three independent experiments.
4. DISCUSSION
DNA, RNA and other nucleotide-based therapeutic agents are highly hydrophilic and encounter resistance in crossing lipophilic cellular membrane. Despite advances in drug delivery technology, there still remains a need to develop newer technologies, especially for hydrophilic nucleotide-based therapeutic agents such as ACV. Therefore, we have developed novel drug conjugates in which therapeutic agents are linked to a substrate for a membrane transporter/receptor via a lipophilic raft. In this unique combination, we hypothesize that the lipid raft facilitates interaction of prodrug with cell membrane probably assisting in docking of the targeted ligand into the binding domain of transporter protein. The net effect is rapid translocation of the cargo across cell membrane. The synthesis has been carried out by conjugating the compounds via esterification reactions. NMR data confirms the structure of all the prodrugs synthesized (Figs 1A and 1B).
To test the hypothesis that SMVT can be a target for drug delivery, cellular accumulation studies have been carried out in two different cell lines (MDCK-MDR1 and Caco-2). The results are consistent in both the cell lines which demonstrate significantly higher cellular accumulation of biotinylated lipid prodrugs (B-R-ACV and B-12HS-ACV) relative to only biotinylated (B-ACV) or only lipid conjugated compounds (R-ACV and 12HS-ACV). Such rise in intracellular drug accumulation may presumably be due to a combined effect of higher transcellular diffusion due to enhanced lipophilicity and significant carrier-mediated transport by SMVT (Figs 2–3).
MDCK-MDR1 cells have been selected for inhibition, saturation kinetics and dose dependent studies because it has been shown as an alternative to Caco-2 cell line for high throughput screening in drug discovery (Tang and Borchardt, 2002; Tang et al., 2002; Tang et al., 2004). Specific interaction of B-R-ACV, B-12HS-ACV and B-ACV with SMVT (substrate specificity) has been examined by inhibiting the cellular uptake of these prodrugs across MDCK-MDR1 cell monolayers in the presence of excess biotin. Accumulation of B-R-ACV, B-12HS-ACV and B-ACV are significantly lowered supporting the hypothesis that these compounds are transported primarily by SMVT (Fig 4).
The results from concentration dependent (saturation kinetics) studies reveal lower Km values of B-R-ACV (20.25 ± 1.74µM) and B-12HS-ACV (23.99 ± 3.20µM) relative to B-ACV (30.90 ± 4.19µM) demonstrating an improved affinity of targeted lipid prodrugs towards SMVT (Fig 5). The apparent affinity constant (Km) values of all the biotinylated prodrugs are found to be slightly higher relative to biotin (12.25 ± 0.79µM) indicating that the prodrugs may not have similar affinity for SMVT relative to biotin itself. Lower Km values of B-R-ACV and B-12HS-ACV may be attributed to the presence of lipid raft between the targeting moiety and the drug, which may assist in enhancing the binding affinity of these prodrugs to SMVT. The data is then transformed into Lineweaver-Burk plots to determine the nature of the inhibition process i.e. competitive or non-competitive. The transformations suggest the inhibition to be competitive, revealing that these prodrugs may share a common site for biotin in SMVT structure (data not shown). This data further confirms the hypothesis that the lipoidal linker is further enhancing the affinity of substrate towards SMVT.
The half-maximal inhibitory concentration (IC50) for inhibition of [3H] biotin uptake by the prodrug is a further indication of the enhanced affinity of the test compound for the SMVT transporter. A comparison of IC50 values clearly suggests that biotin exhibits much higher affinity towards SMVT relative to biotinylated prodrugs. The targeted lipid prodrugs B-R463 ACV and B-12HS-ACV appear to have comparatively lower IC50 values of 8.04 ± 0.07µM and 8.17 ± 0.09µM, respectively relative to biotinylated prodrug B-ACV (IC50 value of 14.84 ± 0.10µM) (Fig 6). These values suggest that the presence of a lipid raft in targeted lipid prodrugs further aids in enhancing the inhibitory potential of the compound compared to non-lipidated biotin conjugated prodrug. The results from this study strongly correlates to the one obtained from the saturation kinetic studies.
[3H] biotin transport results are consistent with a previous report published from our laboratory using MDCK-MDR1 cells (Luo et al., 2006). [3H] biotin permeability is assessed in the presence of biotin and various prodrugs (Fig. 7A). Inhibition of [3H] biotin transport probably indicates an interaction of these newly synthesized biotinylated prodrugs with SMVT. A significantly elevated enhancement in the inhibition of [3H] biotin permeability by B-R-ACV and B-12HS-ACV compared to B-ACV may be attributed to our current approach which combines both lipid and transporter targeted delivery to generate a synergistic transport effect Fig. 7B). The results from cytotoxicity assay showed that all of these prodrugs are safe and exhibit much lower cytotoxicity relative to ACV itself (Fig. 8).
In conclusion, results from this study clearly demonstrate that targeted lipid prodrugs of ACV exhibit higher affinity towards SMVT. Cellular accumulation of these prodrugs is mainly mediated by SMVT as biotin uptake can be significantly inhibited. The lipid raft facilitates enhanced interaction of prodrug with membrane proteins probably assisting docking of the targeted ligand into the binding domain of transporter/receptor protein. The net effect is rapid translocation of the cargo across cell membrane. This novel technology may also allow for enhanced plasma membrane uptake of various hydrophilic therapeutic agents such as nucleosides, nucleotides, oligonucleotides or antisense oligonucleotides and peptides.
ACKNOWLEDGEMENTS
This work has been supported by NIH grant R01EY009171-16.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anand B, Nashed Y, Mitra A. Novel dipeptide prodrugs of acyclovir for ocular herpes infections: Bioreversion, antiviral activity and transport across rabbit cornea. Curr Eye Res. 2003a;26:151–163. doi: 10.1076/ceyr.26.3.151.14893. [DOI] [PubMed] [Google Scholar]
- Anand BS, Katragadda S, Nashed YE, Mitra AK. Amino acid prodrugs of acyclovir as possible antiviral agents against ocular HSV-1 infections: interactions with the neutral and cationic amino acid transporter on the corneal epithelium. Curr Eye Res. 2004;29:153–166. doi: 10.1080/02713680490504614. [DOI] [PubMed] [Google Scholar]
- Anand BS, Mitra AK. Mechanism of corneal permeation of L-valyl ester of acyclovir: targeting the oligopeptide transporter on the rabbit cornea. Pharm Res. 2002;19:1194–1202. doi: 10.1023/a:1019806411610. [DOI] [PubMed] [Google Scholar]
- Anand BS, Patel J, Mitra AK. Interactions of the dipeptide ester prodrugs of acyclovir with the intestinal oligopeptide transporter: competitive inhibition of glycylsarcosine transport in human intestinal cell line-Caco-2. J Pharmacol Exp Ther. 2003b;304:781–791. doi: 10.1124/jpet.102.044313. [DOI] [PubMed] [Google Scholar]
- Balamurugan K, Ortiz A, Said HM. Biotin uptake by human intestinal and liver epithelial cells: role of the SMVT system. Am J Physiol Gastrointest Liver Physiol. 2003a;285:G73–G77. doi: 10.1152/ajpgi.00059.2003. [DOI] [PubMed] [Google Scholar]
- Balamurugan K, Ortiz A, Said HM. Biotin uptake by human intestinal and liver epithelial cells: role of the SMVT system. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2003b;285:G73–G77. doi: 10.1152/ajpgi.00059.2003. [DOI] [PubMed] [Google Scholar]
- Balimane PV, Tamai I, Guo A, Nakanishi T, Kitada H, Leibach FH, Tsuji A, Sinko PJ. Direct evidence for peptide transporter (PepT1)-mediated uptake of a nonpeptide prodrug, valacyclovir. Biochem Biophys Res Commun. 1998;250:246–251. doi: 10.1006/bbrc.1998.9298. [DOI] [PubMed] [Google Scholar]
- Beadle JR. Synthesis of cidofovir and (S)-HPMPA ether lipid prodrugs. Curr Protoc Nucleic Acid Chem. 2007;Chapter 15(Unit 15):12. doi: 10.1002/0471142700.nc1502s29. [DOI] [PubMed] [Google Scholar]
- Beadle JR, Kini GD, Aldern KA, Gardner MF, Wright KN, Rybak RJ, Kern ER, Hostetler KY. Synthesis and antiviral evaluation of 1-O-hexadecylpropanediol-3-P-acyclovir: efficacy against HSV-1 infection in mice. Nucleosides Nucleotides Nucleic Acids. 2000;19:471–479. doi: 10.1080/15257770008033022. [DOI] [PubMed] [Google Scholar]
- Chatterjee NS, Kumar CK, Ortiz A, Rubin SA, Said HM. Molecular mechanism of the intestinal biotin transport process. Am J Physiol. 1999;277:C605–C613. doi: 10.1152/ajpcell.1999.277.4.C605. [DOI] [PubMed] [Google Scholar]
- de Vrueh RL, Smith PL, Lee CP. Transport of L-valine-acyclovir via the oligopeptide transporter in the human intestinal cell line, Caco-2. J Pharmacol Exp Ther. 1998;286:1166–1170. [PubMed] [Google Scholar]
- Earla R, Boddu SH, Cholkar K, Hariharan S, Jwala J, Mitra AK. Development and validation of a fast and sensitive bioanalytical method for the quantitative determination of glucocorticoids--quantitative measurement of dexamethasone in rabbit ocular matrices by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal. 2010;52:525–533. doi: 10.1016/j.jpba.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaseelan S, Debrah O, Wan L, Leibowitz MJ, Rabson AB, Stein S, Sinko PJ. Synthesis of Poly(ethylene glycol)-Based Saquinavir Prodrug Conjugates and Assessment of Release and Anti-HIV-1 Bioactivity Using a Novel Protease Inhibition Assay. Bioconjugate Chemistry. 2004;15:1322–1333. doi: 10.1021/bc0498875. [DOI] [PubMed] [Google Scholar]
- Guo A, Hu P, Balimane PV, Leibach FH, Sinko PJ. Interactions of a nonpeptidic drug, valacyclovir, with the human intestinal peptide transporter (hPEPT1) expressed in a mammalian cell line. J Pharmacol Exp Ther. 1999;289:448–454. [PubMed] [Google Scholar]
- Hariharan S, Janoria KG, Gunda S, Zhu X, Pal D, Mitra AK. Identification and functional expression of a carrier-mediated riboflavin transport system on rabbit corneal epithelium. Curr Eye Res. 2006;31:811–824. doi: 10.1080/02713680600899655. [DOI] [PubMed] [Google Scholar]
- Hostetler KY, Parker S, Sridhar CN, Martin MJ, Li JL, Stuhmiller LM, van Wijk GM, van den Bosch H, Gardner MF, Aldern KA, et al. Acyclovir diphosphate dimyristoylglycerol: a phospholipid prodrug with activity against acyclovir-resistant herpes simplex virus. Proc Natl Acad Sci U S A. 1993;90:11835–11839. doi: 10.1073/pnas.90.24.11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MA. Valaciclovir (BW256U87): the L-valyl ester of acyclovir. J Med Virol Suppl. 1993;1:150–153. doi: 10.1002/jmv.1890410529. [DOI] [PubMed] [Google Scholar]
- Jain-Vakkalagadda B, Dey S, Pal D, Mitra AK. Identification and functional characterization of a Na+-independent large neutral amino acid transporter, LAT1, in human and rabbit cornea. Invest Ophthalmol Vis Sci. 2003;44:2919–2927. doi: 10.1167/iovs.02-0907. [DOI] [PubMed] [Google Scholar]
- Janoria KG, Boddu SH, Wang Z, Paturi DK, Samanta S, Pal D, Mitra AK. Vitreal pharmacokinetics of biotinylated ganciclovir: role of sodium-dependent multivitamin transporter expressed on retina. J Ocul Pharmacol Ther. 2009;25:39–49. doi: 10.1089/jop.2008.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoria KG, Hariharan S, Paturi D, Pal D, Mitra AK. Biotin uptake by rabbit corneal epithelial cells: role of sodium-dependent multivitamin transporter (SMVT) Curr Eye Res. 2006;31:797–809. doi: 10.1080/02713680600900206. [DOI] [PubMed] [Google Scholar]
- Kwatra D, Vadlapatla RK, Vadlapudi AD, Pal D, Mitra AK. Interaction of gatifloxacin with efflux transporters: a possible mechanism for drug resistance. Int J Pharm. 2010;395:114–121. doi: 10.1016/j.ijpharm.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Kansara VS, Zhu X, Mandava NK, Pal D, Mitra AK. Functional characterization of sodium-dependent multivitamin transporter in MDCK-MDR1 cells and its utilization as a target for drug delivery. Mol Pharm. 2006;3:329–339. doi: 10.1021/mp0500768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Wang Z, Kansara V, Pal D, Mitra AK. Activity of a sodium-dependent vitamin C transporter (SVCT) in MDCK-MDR1 cells and mechanism of ascorbate uptake. Int J Pharm. 2008;358:168–176. doi: 10.1016/j.ijpharm.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TY, Dyer DL, Said HM. Human intestinal cell line Caco-2: a useful model for studying cellular and molecular regulation of biotin uptake. Biochim Biophys Acta. 1994;1189:81–88. doi: 10.1016/0005-2736(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Gunda S, Mitra A. Functional expression of a sodium dependent nucleoside transporter on rabbit cornea: Role in corneal permeation of acyclovir and idoxuridine. Curr Eye Res. 2003a;26:175–183. doi: 10.1076/ceyr.26.3.175.14895. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Tirucherai GS, Pal D, Mitra AK. Functional differences in nucleoside and nucleobase transporters expressed on the rabbit corneal epithelial cell line (SIRC) and isolated rabbit cornea. AAPS PharmSci. 2003b;5:E15. doi: 10.1208/ps050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minko T, Paranjpe PV, Qiu B, Lalloo A, Won R, Stein S, Sinko PJ. Enhancing the anticancer efficacy of camptothecin using biotinylated poly(ethylene glycol) conjugates in sensitive and multidrug-resistant human ovarian carcinoma cells. Cancer Chemother Pharmacol. 2002;50:143–150. doi: 10.1007/s00280-002-0463-1. [DOI] [PubMed] [Google Scholar]
- Perry CM, Faulds D. Valaciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in herpesvirus infections. Drugs. 1996;52:754–772. doi: 10.2165/00003495-199652050-00009. [DOI] [PubMed] [Google Scholar]
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad PD, Ganapathy V. Structure and function of mammalian sodium-dependent multivitamin transporter. Curr Opin Clin Nutr Metab Care. 2000;3:263–266. doi: 10.1097/00075197-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Prasad PD, Wang H, Huang W, Fei YJ, Leibach FH, Devoe LD, Ganapathy V. Molecular and functional characterization of the intestinal Na+-dependent multivitamin transporter. Arch Biochem Biophys. 1999;366:95–106. doi: 10.1006/abbi.1999.1213. [DOI] [PubMed] [Google Scholar]
- Prasad PD, Wang H, Kekuda R, Fujita T, Fei YJ, Devoe LD, Leibach FH, Ganapathy V. Cloning and functional expression of a cDNA encoding a mammalian sodium-dependent vitamin transporter mediating the uptake of pantothenate, biotin, and lipoate. J Biol Chem. 1998;273:7501–7506. doi: 10.1074/jbc.273.13.7501. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Pooyan S, Stein S, Prasad PD, Wang J, Leibowitz MJ, Ganapathy V, Sinko PJ. Targeting the sodium-dependent multivitamin transporter (SMVT) for improving the oral absorption properties of a retro-inverso Tat nonapeptide. Pharm Res. 2001a;18:950–956. doi: 10.1023/a:1010932126662. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Qiu B, Pooyan S, Zhang G, Stein S, Leibowitz MJ, Sinko PJ. Targeted PEG-based bioconjugates enhance the cellular uptake and transport of a HIV-1 TAT nonapeptide. J Control Release. 2001b;77:199–212. doi: 10.1016/s0168-3659(01)00474-6. [DOI] [PubMed] [Google Scholar]
- Reardon JE, Spector T. Herpes simplex virus type 1 DNA polymerase. Mechanism of inhibition by acyclovir triphosphate. 1989;264:7405–7411. [PubMed] [Google Scholar]
- Said HM. Cellular uptake of biotin: mechanisms and regulation. J Nutr. 1999;129:490S–493S. doi: 10.1093/jn/129.2.490S. [DOI] [PubMed] [Google Scholar]
- Said HM, Hoefs J, Mohammadkhani R, Horne DW. Biotin transport in human liver basolateral membrane vesicles: a carrier-mediated, Na+ gradient-dependent process. Gastroenterology. 1992;102:2120–2125. doi: 10.1016/0016-5085(92)90341-u. [DOI] [PubMed] [Google Scholar]
- Said HM, Ma TY, Kamanna VS. Uptake of biotin by human hepatoma cell line, Hep G2: a carrier-mediated process similar to that of normal liver. J Cell Physiol. 1994;161:483–489. doi: 10.1002/jcp.1041610311. [DOI] [PubMed] [Google Scholar]
- Said HM, Ortiz A, McCloud E, Dyer D, Moyer MP, Rubin S. Biotin uptake by human colonic epithelial NCM460 cells: a carrier-mediated process shared with pantothenic acid. Am J Physiol. 1998;275:C1365–C1371. doi: 10.1152/ajpcell.1998.275.5.C1365. [DOI] [PubMed] [Google Scholar]
- Said HM, Redha R, Nylander W. A carrier-mediated, Na+ gradient-dependent transport for biotin in human intestinal brush-border membrane vesicles. Am J Physiol. 1987;253:G631–G636. doi: 10.1152/ajpgi.1987.253.5.G631. [DOI] [PubMed] [Google Scholar]
- Soul-Lawton J, Seaber E, On N, Wootton R, Rolan P, Posner J. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother. 1995;39:2759–2764. doi: 10.1128/aac.39.12.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh K, Xiadong Z, Ravi TS, Mitra AK. Small Neutral Amino Acid Ester Prodrugs of Acyclovir Targeting Amino Acid Transporters on the Cornea: Possible Antiviral Agents Against Ocular HSV-1 Infections. Ophthalmology and Eye Diseases. 2010;2:43. [PMC free article] [PubMed] [Google Scholar]
- Talluri RS, Katragadda S, Pal D, Mitra AK. Mechanism of L-ascorbic acid uptake by rabbit corneal epithelial cells: evidence for the involvement of sodium-dependent vitamin C transporter 2. Curr Eye Res. 2006;31:481–489. doi: 10.1080/02713680600693629. [DOI] [PubMed] [Google Scholar]
- Talluri RS, Samanta SK, Gaudana R, Mitra AK. Synthesis, metabolism and cellular permeability of enzymatically stable dipeptide prodrugs of acyclovir. Int J Pharm. 2008;361:118–124. doi: 10.1016/j.ijpharm.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Borchardt RT. Characterization of the efflux transporteRs) responsible for restricting intestinal mucosa permeation of the coumarinic acid-based cyclic prodrug of the opioid peptide DADLE. Pharm Res. 2002;19:787–793. doi: 10.1023/a:1016196514217. [DOI] [PubMed] [Google Scholar]
- Tang F, Horie K, Borchardt RT. Are MDCK cells transfected with the human MDR1 gene a good model of the human intestinal mucosa? Pharm Res. 2002;19:765–772. doi: 10.1023/a:1016140429238. [DOI] [PubMed] [Google Scholar]
- Tang F, Ouyang H, Yang JZ, Borchardt RT. Bidirectional transport of rhodamine 123 and Hoechst 33342, fluorescence probes of the binding sites on P-glycoprotein, across MDCK-MDR1 cell monolayers. J Pharm Sci. 2004;93:1185–1194. doi: 10.1002/jps.20046. [DOI] [PubMed] [Google Scholar]
- Taskintuna I, Banker AS, Flores-Aguilar M, Bergeron-Lynn G, Aldern KA, Hostetler KY, Freeman WR. Evaluation of a novel lipid prodrug for intraocular drug delivery: effect of acyclovir diphosphate dimyristoylglycerol in a rabbit model with herpes simplex virus-1 retinitis. Retina. 1997;17:57–64. doi: 10.1097/00006982-199701000-00011. [DOI] [PubMed] [Google Scholar]
- Trevaskis NL, Charman WN, Porter CJ. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev. 2008;60:702–716. doi: 10.1016/j.addr.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlapatla RK, Vadlapudi AD, Kwatra D, Pal D, Mitra AK. Differential effect of P-gp and MRP2 on cellular translocation of gemifloxacin. Int J Pharm. 2011;420:26–33. doi: 10.1016/j.ijpharm.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlapudi AD, Vadlapatla RK, Mitra AK. Sodium Dependent Multivitamin Transporter (SMVT): A Potential Target For Drug Delivery. Curr Drug Targets. 2012 doi: 10.2174/138945012800675650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SS, Fakioglu E, Herold BC. Novel approaches in fighting herpes simplex virus infections. Expert Rev Anti Infect Ther. 2009;7:559–568. doi: 10.1586/eri.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]