Abstract
Trace amine-associated receptors (TAARs) are vertebrate olfactory receptors. However, ligand recognition properties of TAARs remain poorly understood, as most are ‘orphan receptors’ without known agonists. Here, we identify the first ligands for many rodent TAARs, and classify these receptors into two subfamilies based on phylogeny and binding preference for primary or tertiary amines. Some mouse and rat orthologs have similar response profiles, although independent Taar7 gene expansions led to highly related receptors with altered ligand specificities. Using chimeric TAAR7 receptors, we identified an odor contact site in transmembrane helix III that functions as a selectivity filter. Homology models based on the β2 adrenergic receptor structure indicate close proximity of this site to the ligand. Gain-of-function mutations at this site created olfactory receptors with radically altered odor recognition properties. These studies provide new TAAR ligands, valuable tools to study receptor function, and general insights into the molecular pharmacology of GPCRs.
The initial event in mammalian olfaction is the detection of odor molecules by dedicated sensory neurons in the nose. Olfactory sensory neurons, in particular, use two families of G Protein-Coupled Receptors (GPCRs), Odorant Receptors (ORs) and Trace Amine-Associated Receptors (TAARs), to effectively convert chemical signals from the environment into electrical signals that are transmitted to the brain (1, 2). In addition, rare olfactory sensory neurons use a non-canonical odor detection mechanism that relies on membrane guanylate cyclase-D instead of GPCRs (1).
The olfactory system uses a combinatorial coding scheme, in which each receptor detects multiple odors and each odor activates multiple receptors (3). Consistent with this scheme, many olfactory receptors are broadly tuned to detect a large number of structurally related chemicals (4, 5), although some are narrowly tuned for particular odors (6). While many OR agonists have now been identified (4-8), our current understanding of the ligand specificity among olfactory receptors is based on studies involving only a small number of ORs (5, 9-11). The odor binding pocket in these ORs is composed of highly variable amino acid side chains in transmembrane (TM) helices III, V, and VI (5, 9, 10).
In contrast, the structural basis for odorant recognition by TAARs remains uncharacterized, mainly due to a lack of identified agonists. The TAARs are an evolutionarily conserved family of receptors found in diverse vertebrates, including 15 in mouse (mTAARs), 17 in rat (rTAARs), 6 in human, and 112 in zebrafish (12-17). TAARs do not share sequence similarity with ORs but instead are distantly related to biogenic amine receptors, a medically important class of GPCRs (12-17). In mammals, most TAARs retain amine recognition motifs conserved in biogenic amine receptors (12, 18), including an aspartic acid in TM helix III that forms a salt bridge with the ligand amino group. These observations suggested that rodent TAARs would be amine receptors, but ligands remained largely unknown.
We previously identified the first ligands for mTAAR3, mTAAR4, mTAAR5, and mTAAR7f, and each indeed detects a different combination of volatile amines (19). In addition, ligands were reported for TAAR1, the only receptor in this family which is not an olfactory receptor, and rTAAR4 (then called TA-2) (19-22). Identified TAAR agonists include biogenic amines secreted into urine, a rich source of chemosignals for rodents (19, 23, 24). A TAAR4 agonist, 2-phenylethylamine, is a carnivore odor that repels rodents (23), and a TAAR5 agonist, trimethylamine, is a sexually dimorphic mouse odor (19). The biosynthesis of these naturally occurring TAAR ligands can be dynamic, varying with age, sex, or physiological state (19, 24). Furthermore, some TAAR ligands trigger innate behavioral responses in mice (23, 25).
Here, we set out to identify agonists for additional mouse and rat TAARs. We examined odor response profiles using a previously established reporter gene assay based on cAMP-dependent odor transduction in olfactory sensory neurons (19, 23). Briefly, TAAR plasmids were transfected into HEK293 cells along with a cAMP-dependent reporter gene encoding secreted alkaline phosphatase (CRE-SEAP). TAARs were expressed both in unmodified form and as fusion proteins with an N-terminal sequence of bovine rhodopsin (‘Rho tag’) that promotes cell surface expression of some chemosensory receptors (11). Transfected cells were incubated with test chemicals, and phosphatase activity was quantified with a fluorescent substrate as a reporter for TAAR activation. In initial experiments, we tested 38 different odorant mixtures containing 244 structurally diverse test chemicals (2–5 μM) for the ability to activate each mTAAR. Subsequently, we tested 73 amines that included known mTAAR agonists and related chemicals for the ability to activate each rTAAR. Test chemicals are listed in Supporting Information.
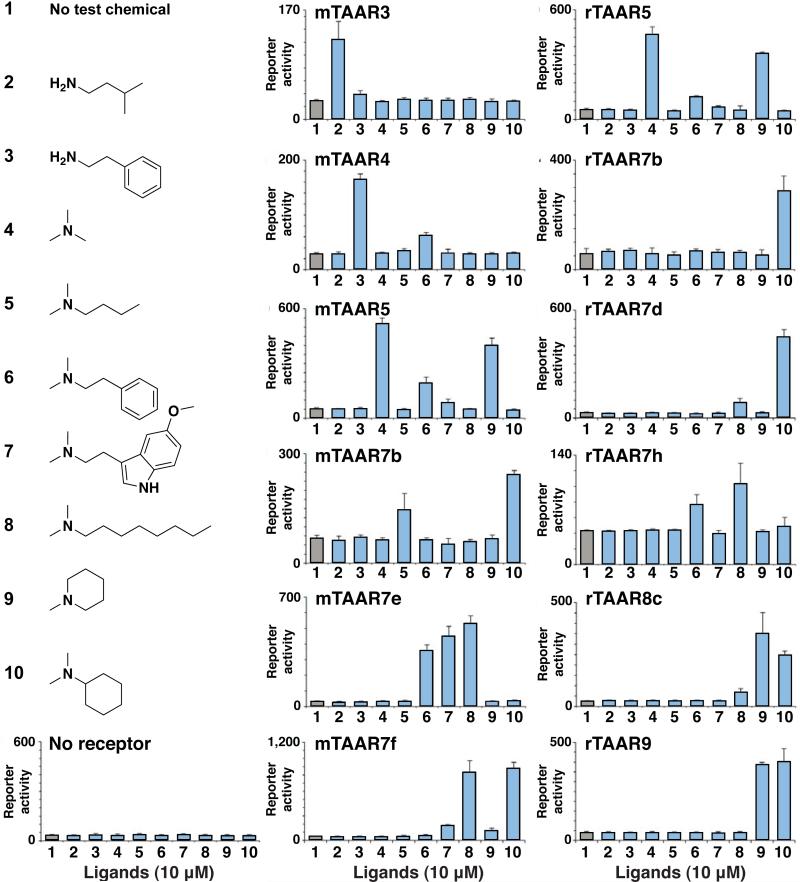
Using this strategy, we identified ligands for nine additional olfactory TAARs that were previously orphan receptors (Figure 1), including mTAAR7b, mTAAR7e, rTAAR3, rTAAR5, rTAAR7b, rTAAR7d, rTAAR7h, rTAAR8c, and rTAAR9. The first ligands for mTAAR7b and mTAAR7e were identified in previous unpublished work (SDL and Linda B. Buck). Responding TAARs were functional with or without a ‘Rho tag’ except for mTAAR4 and rTAAR5, which required a ‘Rho tag’, and mTAAR3 and rTAAR7b which did not work with a ‘Rho tag’. Each of these nine TAARs was activated by volatile amines, while other chemicals (0/202) lacking amino groups did not activate any mTAARs examined. Ligand preferences were similar between mouse and rat orthologs, in cases where ligands were identified for both receptors. Amines that activated mTAAR3, mTAAR4, and rTAAR3 were primary amines that could be derived from natural amino acids by a single decarboxylation reaction. In contrast, ten other TAARs were activated by tertiary amines, including several N,N-dimethylated amines. Identified ligands elicited half maximal TAAR responses at concentrations (EC50) that ranged from 100 nM to 30 μM (Supplementary Figure 1), comparable to the agonist sensitivity of ORs in similar assays (8, 10). Interestingly, several TAAR ligands were natural products secreted by animals, including various amino acid derivatives and the serotonin metabolite 5-methoxy-N,N-dimethyltryptamine whose production patterns in urine are dynamic and vary with physiological state (26, 27).
Figure 1.
Thirteen TAARs detect volatile amines. HEK293 cells were transfected with TAAR and reporter plasmids, incubated with ligands (10 μM), and assayed for reporter activity (triplicates ± s.d.). Test conditions were 1 no ligand, 2 isoamylamine, 3 2-phenylethylamine, 4 trimethylamine, 5 N,N-dimethylbutylamine, 6 N,N-dimethyl-2-phenylethylamine, 7 5-methoxy-N,N-dimethyltryptamine, 8 N,N-dimethyloctylamine, 9 N-methylpiperidine, or 10 N,N-dimethylcyclohexylamine. Twelve TAARs indicated and rTAAR3 (Supplementary Figure 1) responded to at least one ligand shown, but no responses were observed in control cells transfected with reporter plasmid alone. Based on these data, 6/14 mouse olfactory TAARs and 7/16 rat olfactory TAARs respond selectively to amines.
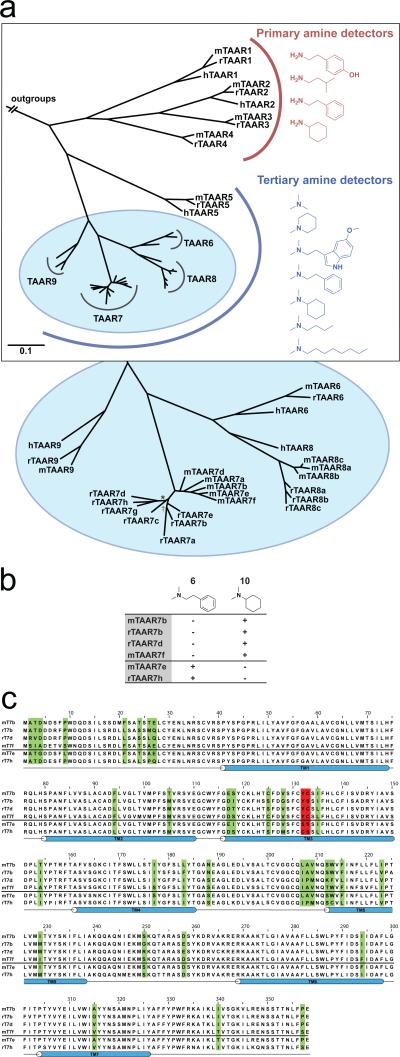
We noted that TAARs could be clustered into two groups based on whether they detected primary or tertiary amines. Interestingly, these two groups mapped to distinct branches of the TAAR phylogenetic tree (Figure 2, panel a). This phylogeny was constructed by Bayesian analysis of all Taar nucleotide sequences in the mouse, human, and rat genomes. Unlike vomeronasal receptors, which are rapidly evolving (28), TAAR orthologs are highly conserved in sequence and gene number between species, as well as in ligand binding preference when determined (Figure 1, Figure 2, and Supplementary Figure 2). Exceptions are lineage-specific expansions of the TAAR7 and TAAR8 sub-families, which occurred independently in mouse and rat. Our analysis suggests that the last common ancestor of rat and mouse likely had one TAAR8 and one TAAR7.
Figure 2.
Functional evolution of the TAAR family. (a) TAAR phylogenetic tree constructed by Bayesian analysis of all Taar nucleotide sequences in the mouse, human, and rat genomes. TAARs cluster into two groups, which exhibit distinct binding preferences for primary or tertiary amines. All tree nodes have a posterior probability above 0.9, except * (0.87), and † (0.86). (b) TAAR7s respond (+) or do not respond (-) to ligands 6 and 10 (10 μM). (c) Alignment of the amino acid sequences of six TAAR7s with identified ligands. Four TAARs with sequences shown above the line (mTAAR7b, rTAAR7b, rTAAR7d, mTAAR7f) respond to ligand 10 but not ligand 6, whereas two TAARs with sequences shown below the line (mTAAR7e, rTAAR7h) respond to ligand 6 but not ligand 10. Residues that vary in 2 or more receptors are colored in green, mutated positions (see Figure 3) are colored in red, and TM segments are shown in blue.
The rapid expansion of the TAAR7 subfamily led to the evolution of highly related olfactory receptors with distinct response profiles. Based on this observation, we reasoned that the TAAR7 subfamily could provide a unique opportunity to study how evolutionary changes in receptor sequence drive changes in odor binding preference.
We identified three amines, 6 (N,N-dimethylphenylethylamine), 7 (5-methoxy-N,N-dimethyltryptamine), and 10 (N,N-dimethylcyclohexylamine) that activated different TAAR7 paralogs in mouse and rat. Two receptors (mTAAR7e and rTAAR7h) were activated by 6 but not 10, while four receptors (mTAAR7b, mTAAR7f, rTAAR7b, and rTAAR7d) were activated by 10 but not 6. We aligned the sequences of responding TAAR7s to identify amino acid variations that correlated with differences in odor responses (Figure 2, panels b and c). These sequences were highly related (>87% identical), and most amino acids were conserved (found in >5/6 analyzed sequences). Of the few amino acid variations identified, only residues 1323.37 and 1333.38 varied in accordance with ligand response profiles (superscripts indicate the TM number and relative TM position of particular residues, as defined by the Ballesteros & Weinstein indexing method (18). This method defines the most conserved residue in each TM helix across all GPCRs as position 50, and positions of other residues are defined in relation to this position. For example, residue 1323.37 is 13 amino acids away from the most conserved residue of TM helix III, Arg1453.50). Interestingly, residues 1323.37 and 1333.38 are immediately adjacent on TM helix III, and in close proximity to Asp1273.32, the conserved amine-contact site of biogenic amine receptors (18). Furthermore, a key odor contact site of a eugenol-detecting OR, Ser1133.40, occupies a similar position in TM helix III (10). Based on these observations, we reasoned that amino acid variations at positions 1323.37 and 1333.38 could contribute to selective TAAR responses.
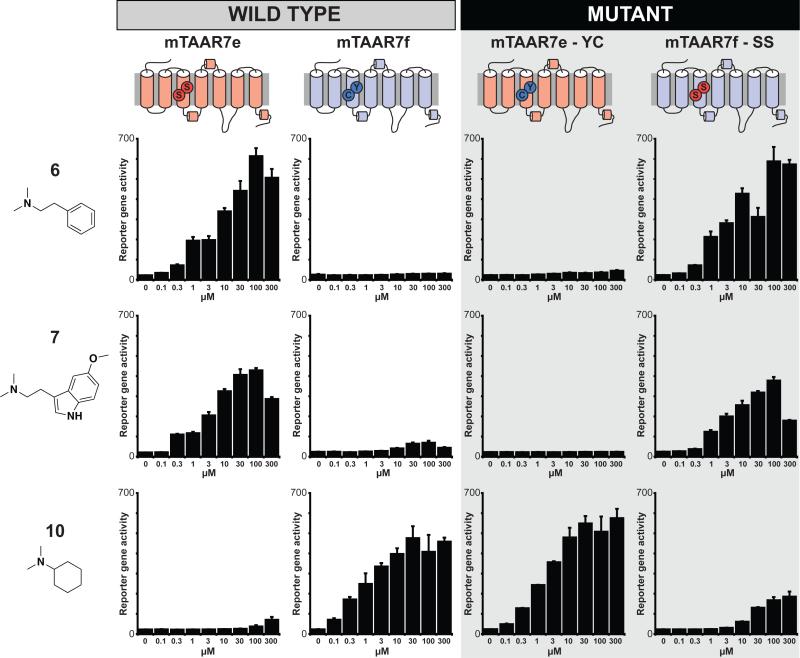
To test this hypothesis, we created mutant receptors in which amino acids at positions 1323.37 and 1333.38 of mTAAR7e were swapped into mTAAR7f and vice versa. Position 1323.37 is a tyrosine in mTAAR7f and the other three receptors that detect 10, but a serine in mTAAR7e and a cysteine in rTAAR7h, the two receptors that detect 6. Furthermore, position 1333.38 is a cysteine in mTAAR7f but a serine in mTAAR7e and rTAAR7h. We altered positions 1323.37 and 1333.38 by mutation of mTAAR7e (mTAAR7eS132Y, S133C or ‘mTAAR7e-YC’) and mTAAR7f (mTAAR7fY132S, C133S or ‘mTAAR7f-SS’) and examined odor responses of these mutants using the cellular reporter gene assay (Figure 3).
Figure 3.
Altering TAAR responses by mutation of an odor selectivity filter. Sequences of mTAAR7e were swapped into mTAAR7f and vice versa by exchanging residues 1323.37 and 1333.38 of mTAAR7e (‘mTAAR7e-YC’) and mTAAR7f (‘mTAAR7f-SS’). Odor responses of these mutant receptors are shown using the cellular reporter gene assay for ligands 6, 7, and 10 at concentrations indicated (triplicates ± sem).
Interestingly, this modification caused a dramatic reversal in odor responsiveness (Figure 3). mTAAR7e-YC had the same ligand selectivity profile as mTAAR7f rather than mTAAR7e. These effects were striking, as mTAAR7e-YC had >1,000-fold enhanced affinity for 10 and >1,000-fold decreased affinity for 6 or 7. Furthermore, the reciprocal mutant, mTAAR7f-SS, had mTAAR7e-like responses, displaying >1,000-fold increases in affinity for both ligands 6 and 7 and ~1006fold reduced affinity for ligand 10. These data provide strong evidence that residues 1323.37 and 1333.38 are part of the TAAR ligand binding pocket and form an important selectivity filter that imparts selective odor responses.
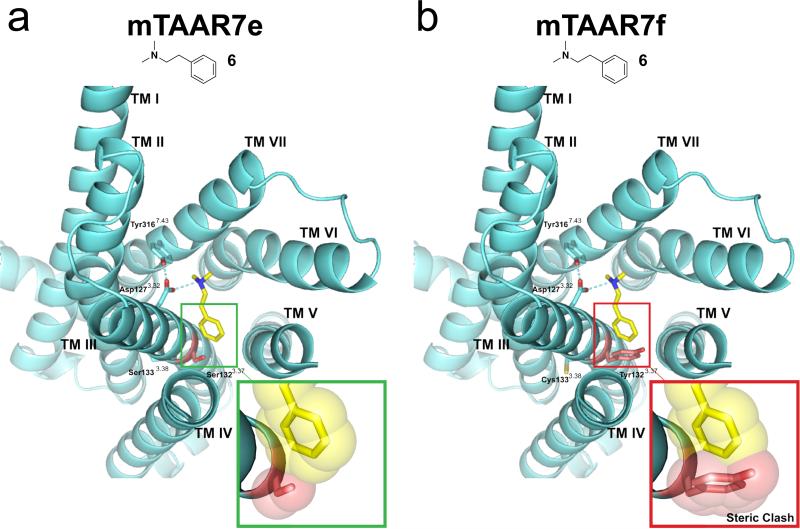
To gain additional insights into the structure of the odor-binding pocket in TAARs, we created homology models of mTAAR7e and mTAAR7f (Figure 4, Supplementary Figures 3 and 4). We based our model on the X-ray crystal structure of the human β2 adrenergic receptor (β2AR, PDB ID: 3P0G) (29). Although several other GPCR structural templates are now available (30), β2AR was selected since it is 25% identical to mTAAR7e and mTAAR7f, binds similar amine ligands, and aligns without gaps in 6 out of 7 TM helices. Based on these models, mTAAR7e and mTAAR7f have the canonical bundle of 7 α-helices followed by the intracellular helix VIII that runs parallel to the membrane axis. In addition, we observed other conserved motifs of class A GPCRs (31), such as a disulfide bridge between extracellular loop 2 (ECL2) and the extracellular end of helix III (Cys205 and Cys1203.25), as well as the D/ERY sequence of the “ionic lock” motif at the cytoplasmic end of helix III (31). Surprisingly, we also observed a short α-helix in ECL2 (Glu193-Thr200) in mTAAR7e and mTAAR7f, a motif that is not common among most GPCRs, but is present in β1AR and β2AR (31).
Figure 4.
Homology modeling of mTAAR7e and mTAAR7f provides a molecular basis for selective odor recognition. Predicted structures (cyan) of mTAAR7e (a) and mTAAR7f (b) bound to N,N-dimethylphenylethylamine (6) (yellow). GPCR transmembrane helices are numbered from TM I to VII and side chains of key residues that line the ligand binding site are displayed. Hydrogen bonds are shown as dotted cyan lines. Inserts represent a magnified view of ligand 6 interacting with residue 1323.37 of mTAAR7e and mTAAR7f. Van der Waals radii are shown with a transparent space-filling model, and predict a steric clash of ligand 6 with residue Tyr1323.37 of mTAAR7f but not Ser1323.37of mTAAR7e.
Next, we examined the putative ligand contact sites in the structural models of mTAAR7e and mTAAR7f. Our models suggested that the ligand amino group forms a salt bridge with Asp1273.32, which itself is anchored by a hydrogen bond to the hydroxyl group of Tyr31-7.43 (Figure 4, Supplementary Figures 3 and 4). Asp1273.32 is conserved among many GPCRs and a similar salt bridge between receptor and ligand was also found in crystal structures of β1AR, β2AR, and the H1 histamine receptor (31, 32). The model shows that Tyr1323.37 of mTAAR7f extends into the ligand binding pocket where it sterically blocks both ligands 6 and 7 (Figure 4, Supplementary Figures 3 and 4). In contrast, Ser1323.37 of mTAAR7e does not sterically interfere with ligand 6 and may even stabilize ligand 7 through formation of a hydrogen bond between the hydroxyl group of its sidechain and the indole nitrogen of the aromatic ligand moiety (Figure 4, Supplementary Figures 3 and 4). We did not detect any additional amino acid variations in or near the odor binding pockets of mTAAR7e and mTAAR7f, other than positions 1323.37 and 1333.38. Based on these structural models, and the dramatic functional change caused by mutation of these residues (Figure 3), we conclude that these two residues are critical determinants of ligand selectivity differences between these two receptors.
Here, we show how neofunctionalization of the TAAR7 family occurred during evolution by gene duplication and subsequent mutation. The olfactory system uses such evolutionary mechanisms to generate large repertoires of sensory receptors with divergent recognition properties, and these mechanisms are enabled by the inherent flexibility of olfactory system development. Minimal requirements for incorporation of a new GPCR into olfactory circuits include (1) obtaining proper gene regulation, and (2) coupling to the correct G protein. For this reason, sensory neurons expressing foreign GPCRs, such as the β2AR (33), can be readily incorporated into the system and can couple to unique neural circuits in the brain. Also for this reason, gene duplication events followed by subsequent mutation of one duplicate is a powerful mechanism to achieve receptor diversity (34). Here, we observe recent expansion of the TAAR7 family in rodents, and subsequent incorporation of specific mutations that alter odor responses. Through this process, evolutionary mechanisms have sculpted the TAAR7 subfamily, leading to rapid and functional expansion of the olfactory receptor repertoire.
METHODS
Detailed methods for chemicals tested, TAAR functional assays, phylogenetic analysis, and homology modeling, are provided in Supporting Information.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank J. Lemon for assistance with the functional assay, M. Fujita and S. Edwards for advice on the phylogenetic analysis, as well as S. Katritch for help with the sequence alignments and models. This work was supported by a grant from the National Institute On Deafness And Other Communicative Disorders (Award Number R01DC010155) to SDL and the PSI:Biology GPCR Network (U54 GM094-18) to RCS. DMF and DW are supported by a Boehringer Ingelheim Fonds PhD Fellowship.
Footnotes
Supporting Information Available: This material is available free of charge via the Internet.
REFERENCES
- 1.Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Annu Rev Physiol. 2009;71:115–140. doi: 10.1146/annurev.physiol.70.113006.100608. [DOI] [PubMed] [Google Scholar]
- 2.Su CY, Menuz K, Carlson JR. Olfactory perception: receptors, cells, and circuits. Cell. 2009;139:45–59. doi: 10.1016/j.cell.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 4.Mombaerts P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci. 2004;5:263–278. doi: 10.1038/nrn1365. [DOI] [PubMed] [Google Scholar]
- 5.Reisert J, Restrepo D. Molecular tuning of odorant receptors and its implication for odor signal processing. Chem Senses. 2009;34:535–545. doi: 10.1093/chemse/bjp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller A, Zhuang H, Chi Q, Vosshall LB, Matsunami H. Genetic variation in a human odorant receptor alters odour perception. Nature. 2007;449:468–472. doi: 10.1038/nature06162. [DOI] [PubMed] [Google Scholar]
- 7.Nara K, Saraiva LR, Ye X, Buck LB. A large-scale analysis of odor coding in the olfactory epithelium. J Neurosci. 31:9179–9191. doi: 10.1523/JNEUROSCI.1282-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito H, Chi Q, Zhuang H, Matsunami H, Mainland JD. Odor coding by a Mammalian receptor repertoire. Sci Signal. 2009;2:ra9. doi: 10.1126/scisignal.2000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato A, Touhara K. Mammalian olfactory receptors: pharmacology, G protein coupling and desensitization. Cell Mol Life Sci. 2009;66:3743–3753. doi: 10.1007/s00018-009-0111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katada S, Hirokawa T, Oka Y, Suwa M, Touhara K. Structural basis for a broad but selective ligand spectrum of a mouse olfactory receptor: mapping the odorant-binding site. J Neurosci. 2005;25:1806–1815. doi: 10.1523/JNEUROSCI.4723-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krautwurst D, Yau KW, Reed RR. Identification of ligands for olfactory receptors by functional expression of a receptor library. Cell. 1998;95:917–926. doi: 10.1016/s0092-8674(00)81716-x. [DOI] [PubMed] [Google Scholar]
- 12.Zucchi R, Chiellini G, Scanlan TS, Grandy DK. Trace amine-associated receptors and their ligands. Br J Pharmacol. 2006;149:967–978. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewin AH. Receptors of mammalian trace amines. AAPS J. 2006;8:E138–145. doi: 10.1208/aapsj080116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain A, Saraiva LR, Korsching SI. Positive Darwinian selection and the birth of an olfactory receptor clade in teleosts. Proc Natl Acad Sci U S A. 2009;106:4313–4318. doi: 10.1073/pnas.0803229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindemann L, Hoener MC. A renaissance in trace amines inspired by a novel GPCR family. Trends Pharmacol Sci. 2005;26:274–281. doi: 10.1016/j.tips.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005;85:372–385. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Liberles SD. Trace amine-associated receptors are olfactory receptors in vertebrates. Ann N Y Acad Sci. 2009;1170:168–172. doi: 10.1111/j.1749-6632.2009.04014.x. [DOI] [PubMed] [Google Scholar]
- 18.Shi L, Javitch JA. The binding site of aminergic G protein-coupled receptors: the transmembrane segments and second extracellular loop. Annu Rev Pharmacol Toxicol. 2002;42:437–467. doi: 10.1146/annurev.pharmtox.42.091101.144224. [DOI] [PubMed] [Google Scholar]
- 19.Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- 20.Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, Wettstein JG, Pinard A, Buchy D, Gassmann M, Hoener MC, Bettler B. The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci U S A. 2009;106:20081–20086. doi: 10.1073/pnas.0906522106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, Hoener MC. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci U S A. 108:8485–8490. doi: 10.1073/pnas.1103029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrero DM, Lemon JK, Fluegge D, Pashkovski SL, Korzan WJ, Datta SR, Spehr M, Fendt M, Liberles SD. Detection and avoidance of a carnivore odor by prey. Proc Natl Acad Sci U S A. 108:11235–11240. doi: 10.1073/pnas.1103317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrero DM, Liberles SD. The secret codes of mammalian scents. Wiley Interdiscip Rev Syst Biol Med. 2:23–33. doi: 10.1002/wsbm.39. [DOI] [PubMed] [Google Scholar]
- 25.Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, Mori K, Sakano H. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- 26.Franzen F, Gross H. Tryptamine, N,N-dimethyltryptamine, N,N- dimethyl-5-hydroxytryptamine and 5-methoxytryptamine in human blood and urine. Nature. 1965;206:1052. doi: 10.1038/2061052a0. [DOI] [PubMed] [Google Scholar]
- 27.Sitaram BR, Lockett L, Blackman GL, McLeod WR. Urinary excretion of 5-methoxy-N,N-dimethyltryptamine, N,N-dimethyltryptamine and their N-oxides in the rat. Biochem Pharmacol. 1987;36:2235–2237. doi: 10.1016/0006-2952(87)90159-6. [DOI] [PubMed] [Google Scholar]
- 28.Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, Schnapp A, Konetzki I, Sunahara RK, Gellman SH, Pautsch A, Steyaert J, Weis WI, Kobilka BK. Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature. 469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katritch V, Cherezov V, Stevens RC. Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol Sci. 33:17–27. doi: 10.1016/j.tips.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimamura T, Shiroishi M, Weyand S, Tsujimoto H, Winter G, Katritch V, Abagyan R, Cherezov V, Liu W, Han GW, Kobayashi T, Stevens RC, Iwata S. Structure of the human histamine H1 receptor complex with doxepin. Nature. 475:65–70. doi: 10.1038/nature10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feinstein P, Bozza T, Rodriguez I, Vassalli A, Mombaerts P. Axon guidance of mouse olfactory sensory neurons by odorant receptors and the beta2 adrenergic receptor. Cell. 2004;117:833–846. doi: 10.1016/j.cell.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Roth C, Rastogi S, Arvestad L, Dittmar K, Light S, Ekman D, Liberles DA. Evolution after gene duplication: models, mechanisms, sequences, systems, and organisms. J Exp Zool B Mol Dev Evol. 2007;308:58–73. doi: 10.1002/jez.b.21124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.