Abstract
Polyreactivity is well known as a property of natural IgM produced by B-1 cells. We demonstrate that polyreactive IgM is also generated during infection of mice with Ehrlichia muris, a tick-borne intracellular bacterial pathogen. The polyreactive IgM bound self and foreign antigens, including single- and double-stranded DNA, insulin, thyroglobulin, lipopolysaccharide, influenza virus, and Borrelia burgdorferi. Production of polyreactive IgM during infection was antigen-driven, not due to polyclonal B cell activation, as the majority of polyreactive IgM recognized ehrlichial antigen(s), including an immunodominant outer membrane protein-19 (OMP-19). Monoclonal polyreactive IgM derived from T cell-independent spleen plasmablasts, which was germline-encoded, also bound cytoplasmic and nuclear antigens in HEp-2 cells. Polyreactive IgM protected immunocompromised mice against lethal bacterial challenge infection. Serum from human ehrlichiosis patients also contained poly- and self-reactive IgM. We propose that polyreactivity increases IgM efficacy during infection, but may also exacerbate or mollify the response to foreign and self antigens.
Introduction
Polyreactivity, the ability of an immunoglobulin to bind two or more unrelated antigens, is a characteristic of natural antibodies produced by B-1 cells. Polyreactive natural antibodies, commonly germline or near-germline encoded, exhibit structural flexibility, which allows for more promiscuous binding to antigens (1–3). Polyreactive IgM is thought to aid in the neutralization of pathogens, prior to the development of high affinity, antigen-specific antibodies (1, 4, 5). Polyreactivity is characterized by low affinity interactions with antigen, although IgM polyvalency allows for higher avidity interactions. Although polyreactive antibody is found in normal serum, it is often bound to self antigen, from which it must be dissociated in order to be detected (2, 6).
Polyreactivity, as a property of an immunoglobulin molecule, is distinguished here from apparent polyreactivity, a property of polyclonal serum. The latter may occur via non-antigen-specific antibody production, often in response to stimulation of B cells by parasitic membrane proteins, or bacterial LPS and/or CpG motifs (7–11). Polyreactivity is not a unique property of IgM, as it has been observed in IgG induced by some viral infections, and in IgA secreted by intestinal plasmablasts (12–14). Although acute viral infections can drive the production of polyreactive IgM, such antibodies have not been reported to be induced by bacterial infection (15).
Various models have been proposed to explain how natural and immune polyreactive antibodies function. Polyreactivity may serve to increase the overall affinity of an antibody by enhancing binding to a pathogen during instances where homotypic bivalent binding is not possible (described as heteroligation); such a model may explain why antibody elicited during HIV-1 infection is broadly polyreactive (12). Alternately, naturally-occurring polyreactive IgM may serve to facilitate the clearance of apoptotic cells and/or autoantigen-containing immune complexes, thereby contributing to immune surveillance (16–19).
Our previous studies described the induction of T cell-independent (TI) IgM by a large population of CD11c-expressing spleen plasmablasts during infection with the intracellular bacterium Ehrlichia muris (20). The plasmablasts are responsible for the generation of nearly all the IgM produced in the spleen during acute infection, and IgM is sufficient for both short- and long-term protection against ehrlichial infection (20, 21). In the present study, we demonstrate that the IgM produced by the spleen plasmablasts is polyreactive, a characteristic that has not been described for infection-induced IgM. We demonstrate that the polyreactive IgM is pathogen-specific, but also binds a number of unrelated self and foreign antigens, and is produced during human ehrlichiosis. We propose that polyreactive IgM serves to facilitate host defense, but also may modify host responses to self. These studies highlight a novel aspect of the host humoral response that may be characteristic of many bacterial infections that induce strong TI immunity.
Materials and Methods
Animals
The mice used in the studies were obtained from The Jackson Laboratory (Bar Harbor, ME), or were bred under microisolator conditions at the Wadsworth Center Animal Care Facility, in accordance with institutional guidelines for animal welfare. Sex-matched C57BL/6, RAG1-deficient (B6.129S7-Rag1tm1Mom/J), MHC II-deficient (B6.129S2-H2dIAb1-Ea/J), and CD1d-deficient (B6.C129S-Cd1tm1Gru) mice 6–12 weeks of age were used for the studies. As institutional guidelines do not allow the use of death as an endpoint in animal studies, mice were humanely sacrificed when judged to be moribund.
Infections and immunizations
Mice were infected intraperitoneally with 5×104 copies of E. muris, as previously described (22). Mice were administered purified recombinant OMP-19 in alum (Imject, Thermo Scientific, Rockford, IL), prepared according to the manufacturer’s instructions.
Quantification of bacteria
Bacterial copy number was determined by probe-based PCR, using primers and probes for the E. muris dsb gene, as described previously (23). The PCR products were analyzed with an Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). The copy number of the E. muris dsb gene was determined using known quantities of dsb amplicon as standards. We have made the simplifying assumption that bacterial copy number and numbers of viable bacteria were equivalent in our experimental model.
ELISA
Microtiter plates (Immulon, VWR) were adsorbed overnight with 10μg/mL recombinant E. muris or E. chaffeensis OMP-19, or the following antigens: 10μg/mL single-stranded bovine DNA, 5μg/mL porcine insulin, 10μg/mL thyroglobulin, 10μg/mL lipopolysaccharide from Salmonella enterica (all from Sigma); 10μg/mL double-stranded DNA (EMD Biosciences); heat-inactivated influenza virus (A/PR/8; generously provided by Dr. L. Haynes, Trudeau Institute, Saranac Lake, NY); or Borrelia burgdorferi (a gift from Dr. T. Sellati, Albany Medical College, Albany, NY). The microtiter plates were blocked with 20% fetal bovine serum in PBS. Primary antibodies were detected with alkaline phosphatase-conjugated anti-IgM or anti-IgG secondary antibodies (Southern Biotech), followed by incubation with p-nitrophenyl phosphate (Sigma). Absorbance was measured at 405nm; endpoint titers were defined as the highest dilution that generated an A405 value greater than 0.1.
ELISPOT analyses
Nitrocellulose plates (Millipore) were coated with antigen, as for ELISA; the plates were blocked with IMDM+10% FBS, and splenocytes were applied at a concentration of 5×105 cells/mL in IMDM supplemented with 2mM L-glutamine, 100 U/mL penicillin, 100U/mL streptomycin, 50μM 2-mercaptoethanol, and 10% FBS. Cells were incubated for 18 hr at 37°C in 5% CO2. Bound antibody was detected using alkaline phosphatase-conjugated goat anti-mouse IgM, followed by the substrate 5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium (Amresco). Spots were counted using a CTL ImmunoSpot Analyzer (Cellular Technology Ltd., Shaker Heights, OH), and data were analyzed using CTL ImmunoSpot software.
Hybridoma production
Splenocytes were harvested from uninfected C57BL/6 mice, or from infected mice on day 14 post-infection. The tissue was disrupted using razor blades, and the cells were disaggregated using a 70μm cell strainer (BD Falcon). Erythrocytes were removed by hypotonic lysis using ammonium chloride. Splenocytes were fused to the myeloma cell line SP2/0, using standard methods. Hybridoma supernatants were screened for IgM production by ELISA, and IgM-secreting cultures were screened for reactivity to OMP-19, ssDNA, dsDNA, insulin, thyroglobulin, LPS, and influenza virus. Hybridomas that produced specific antibodies were cloned by limiting dilution.
Production of IgM in vivo
Antibody-secreting myeloma cells (3×106 cells/mouse) were implanted subcutaneously in RAG-deficient mice, and the mice were challenged with E. muris 3 days later. On day 10 post-infection, mice were sacrificed, sera were harvested, and bacterial copy number in the spleen was determined by quantitative RT-PCR.
Dot immunoblot assay
Polyvinylidene fluoride membranes (Millipore) were spotted with cell-free ehrlichiae isolated from the DH-82 cell line, or with a DH-82 cell lysate, using a vacuum apparatus (Bio-Dot Apparatus, BioRad). Bacteria were liberated from host cells by repeated passage through a 23-gauge needle, followed by differential centrifugation, to separate the ehrlichiae from host cellular debris. A cell lysate from uninfected DH-82 cells was similarly prepared. Hybridoma supernatants were applied to the membranes, and bound IgM was detected using an HRP-conjugated goat anti-mouse antibody (Southern Biotech). An ECL chemiluminescence kit (Pierce, Rockford, IL) was used to detect the secondary antibody.
Analysis of human samples
Sera from human ehrlichiosis patients, or normal human controls, were generously provided by Dr. S. Wong of the Wadsworth Center, and by Dr. H. Prince of Focus Diagnostics. The use of human serum samples was approved by the Wadsworth Center Institutional Review Board.
HEp-2 immunofluorescence assays
Human sera were diluted 1:80 and applied to HEp-2 cell-coated microscope slides (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Serum antibodies were detected with biotin-conjugated goat anti-human IgM or IgG followed by a streptavidin-Alexa Fluor 488 conjugate (Invitrogen, Eugene, OR). For hybridomas, supernatants were applied to microscope slides and antibody binding was detected with biotin-conjugated goat anti-mouse IgM. Cells were visualized using a Zeiss Axioskop2 epifluorescence microscope equipped with a Hamamatsu ORCA-ER camera, and analyzed using OpenLab software.
Analysis of Ig heavy chain genes
Hybridoma variable regions were sequenced as previously described, with minor modifications (24). Ig gene sequence analyses and alignments were performed using IgBLAST (http://www.ncbi.nlm.nih.gov/igblast/).
Statistical analyses
Statistical analyses were performed using an unpaired Student’s t test or Fisher’s Exact 2×4 test (Prism GraphPad Software, La Jolla CA).
Results
Ehrlichia infection induces a T cell-independent polyreactive IgM response
Our previous studies have revealed an important role for T cell-independent (TI) IgM in mediating short- and long-term immunity during ehrlichial infection. IgM is produced during infection by a population of extrafollicular splenic CD11c-expressing plasmablasts (20), among which 10–20% recognized an immunodominant antigen, outer membrane protein-19 (OMP-19). IgM production was highest during acute infection, but was maintained for at least as long as 366 days, at least in part by a population of bone marrow IgM-producing plasmablasts/plasma cells (21). In the course of these studies, we observed that the IgM elicited during E. muris infection was highly polyreactive. The IgM bound double- and single-stranded DNA, insulin, and thyroglobulin (Figure 1, A–D). These self antigens are commonly used to assay polyreactivity, because their distinct structures reduce the likelihood that binding is due to cross reactivity (i.e., binding to related epitopes on different antigens; 14, 25). Polyreactive IgM was not detected in normal sera from C57BL/6 mice. Polyreactive IgM was observed beginning on days 7–9 post-infection, was maximal on day 14, and declined thereafter, reaching modest titers that were maintained for at least 396 days post-infection. We also observed reactivity of immune sera to whole influenza virus A/PR/8 and Borrelia burgdorferi, pathogens to which the mice had not been previously exposed (Figure 1, E–F). Self- and foreign-reactive IgM-secreting B cells were detected in the spleens of infected mice, indicating that the splenic CD11c-positive plasmablasts cells we have described previously were partly or wholly responsible for the generation of the polyreactive IgM (Figure 1, G–H; 20). Polyreactivity was not detected as a property of IgG at any point during infection (data not shown).
FIGURE 1. Ehrlichia muris infection induces a long-term polyreactive IgM response.
(A–F) Serum IgM was tested for reactivity against the indicated antigens by ELISA on the indicated days post-infection. The data represent antibody titers detected in individual mice; the horizontal lines indicate the mean titers. Titers lower than 10 were considered background. (G, H) ELISPOT analysis was used to determine the frequency and number of antigen-specific IgM-secreting B cells in the spleen on day 14 post-infection. Spots produced by cells from uninfected mice (which were negligible) were subtracted from values obtained for infected mice. The data are representative of at least three experiments. A student’s t test was used to calculate statistical significance; *p < 0.05.
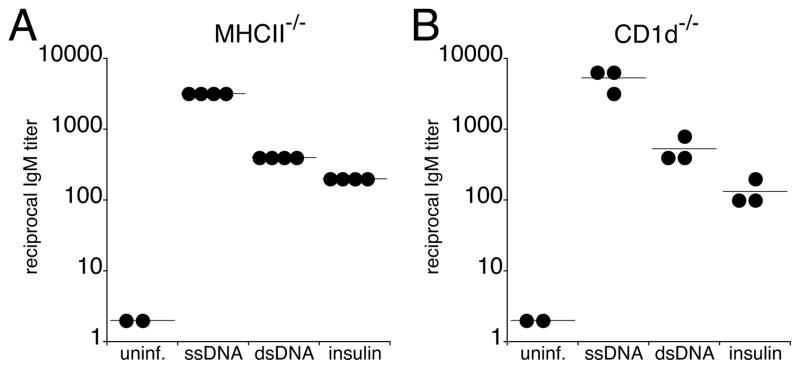
Our previous study demonstrated that the IgM response induced during ehrlichial infection occurred independently of CD4 T cells (20). Consistent with these findings, we detected self-reactive IgM during infection of MHCII- and CD1d-deficient mice (26), indicating that neither CD4 T cells nor CD1d-restricted cells (i.e., NK T cells) were required for the generation of polyreactive IgM (Figure 2, A and B).
FIGURE 2.
Polyreactive IgM was produced independent of CD4+ T cells or CD1d+ NKT cells.
Sera were obtained from MHCII-deficient (A) and CD1d-deficient (B) mice on day 14 post-infection. IgM reactivity to self antigens was determined by ELISA. The data are representative of two experiments.
Polyreactivity is associated with OMP-19-specificity
Although our data suggested that the infection-induced IgM was polyreactive, we could not discount the possibility that E. muris stimulated the production of non-polyreactive IgM of unrelated specificities, via polyclonal B cell activation, or by BCR ligation by a B cell superantigen (27). To address whether polyreactivity was a property of monoclonal IgM, we generated B cell hybridomas from spleen B cells of infected mice on day 14 post-infection. We first tested whether the hybridomas produced pathogen-specific IgM, using a dot immunoblot assay to detect IgM binding to intact bacteria. Of 329 IgM-secreting hybridomas isolated, nearly 85% (72/85) produced ehrlichial-specific antibodies (Figure 3A–B). These findings were confirmed by immunofluorescence microscopy of ehrlichia-infected splenocytes (data not shown). Among all IgM-secreting hybridomas, 30% (96/329) bound OMP-19 (Figure 3C); these data are reflective of our published data which reported a similar frequency of OMP-19-specific IgM-producing cells in the spleen, as detected by ELISPOT assay (20). The non-OMP-19-reactive ehrlichia-specific hybridomas produced IgM that bound to yet unidentified antigen(s) that were not detected by western analysis, indicating that the antibodies bound conformational epitopes, or non-protein antigens. Since the majority of monoclonal IgM was pathogen-specific, our data indicate that non-specific B cell activation does not play a major role in the generation of the polyreactive IgM.
FIGURE 3.
Polyreactivity is correlated with OMP-19 specificity.
Antigen specificity of IgM-secreting hybridomas generated from mouse splenocytes on day 14 post-infection. (A) Dot immunoblot analysis was used to identify ehrlichia-specific IgM; a representative selection of hybridomas is shown. In total, 85% (72/85) of the hybridomas produced ehrlichia-specific IgM. (B) A representative dot blot demonstrating that the IgM bound cell-free E. muris, but not lysates generated from uninfected cells. (C) A schematic summarizing the binding specificities of IgM produced by hybridomas generated from infected and uninfected mice is shown; the IgM were segregated on the basis of OMP-19 binding specificity. The number of antibodies evaluated in each group is indicated by the number in the center of each pie chart; numbers in the sectors represent the frequency of antibodies that recognized the indicated number of unrelated antigens (dsDNA, ssDNA, insulin, LPS, and influenza virus; depicted by shading). The differences in the frequencies of polyreactive B cell hybridomas within the OMP-19-specific and non-OMP-19-specific IgM groups from infected mice, relative to the uninfected IgM group, were statistically significant (p < 0.001 and P < 0.0001, respectively, as determined using a χ2 test). (D) Polyreactive IgM were not generated following immunization. Mice were immunized with recombinant OMP-19 in alum, and serum reactivity to the indicated antigens was determined on day 14 post-immunization.
To test whether the monoclonal IgM exhibited polyreactivity, the hybridoma supernatants were tested for reactivity to the panel of self and foreign antigens utilized in the studies described in Figure 1. Over 20% of the OMP-19-specific IgM was highly polyreactive (i.e., they bound to 3–5 unrelated antigens), and greater than 75% of the OMP-19-specific IgM bound at least 1 unrelated antigen (Figure 3C). In contrast, among the non-OMP-19-specific IgM, only 36.5% exhibited polyreactivity; moreover, none of the non-OMP-19-specific IgM bound all 5 antigens examined, and only a few (3%) were highly polyreactive (Figure 3C). In contrast, IgM produced by hybridomas generated from uninfected mice were largely non-polyreactive, as only 2.9% (2/68) of the antibodies bound two unrelated antigens (Figure 3C).
Because polyreactivity was correlated with OMP-19 specificity, we addressed whether polyreactivity was an intrinsic property of OMP-19-specific IgM. Immunization with recombinant OMP-19 failed to induce polyreactive IgM (Figure 3D), indicating that polyreactivity was not a consequence of OMP-19 recognition, per se. These data provide additional support for the conclusion that polyreactive IgM was elicited during infection by pathogen-specific B cells, and was not generated by natural antibody-producing cells.
Although many IgM bound to a wide range of unrelated antigens (Table I; i.e., Em504.1 and Em720.1), others exhibited quasi-specificity; that is, they bound only to particular antigens in the panel tested (e.g., compare Em622 and Em749). These data suggested that the polyreactive antibodies were structurally distinct. This was confirmed by nucleotide sequence analysis of the Ig heavy chain genes encoding regions of 6 highly polyreactive IgM, which revealed that the genes utilized distinct V, D, and J segments (Table II). Moreover, the IgM were all encoded by germline Ig genes, consistent with their TI, extrafollicular origin (20).
Table 1.
Antigen reactivity of polyreactive IgM
| Hybridoma | Antigen
|
|||||
|---|---|---|---|---|---|---|
| OMP-19 | dsDNA | ssDNA | Insulin | LPS | Influenza | |
| Em400 | +1 | − | − | + | + | − |
| Em504.1 | + | + | + | + | + | + |
| Em622 | + | + | + | − | − | − |
| Em638 | + | − | + | + | − | − |
| Em720.1 | + | + | + | + | + | + |
| Em749 | + | − | − | − | + | + |
Antigen reactivity, as determined by ELISA, where + indicates A405 > 0.25, and − indicates A405 < 0.25.
Table 2.
Heavy chain gene utilization by infection-induced polyreactive IgM
| Hybridoma | VH | DH | JH |
|---|---|---|---|
| Em504.1 | J558.84.1901 | DSP2.9 | JH3 |
| Em702.1 | 7183.20.37 | DFL16.1 | JH4 |
| Em703.1 | J558.67.166 | DFL16.1 | JH2 |
| Em720.1 | J558.18.108 | DQ52-C57BL/6 | JH3 |
| Em785.1 | J558.31.121 | DFL16.1 | JH3 |
Sequence alignments were performed using IgBLAST(http://www.ncbi.nlm.nih.gov/igblast/).
Polyreactive IgM is sufficient to protect against ehrlichial infection
We next tested whether monoclonal polyreactive IgM was sufficient to protect mice against E. muris infection in immunodeficient mice. IgM was produced in vivo by administering polyreactive IgM-producing hybridoma cells subcutaneously into RAG-deficient mice (28, 29). This approach generated relatively high serum IgM titers in the recipient mice within 7 days (1:100–1:6400). The recipient mice survived for at least two weeks following myeloma cell administration, which provided sufficient time to test whether the IgM was sufficient to control acute E. muris infection. The recipient mice were infected with E. muris 3 days following myeloma cell administration, and splenic bacterial colonization was determined 10 days later on day 13 post-infection. Mice implanted with irrelevant IgM-secreting myeloma cells exhibited splenic bacteremia comparable to infected mice that had not been administered hybridoma cells. In contrast, mice implanted with polyreactive OMP-19-specific IgM-secreting hybridoma cells exhibited low to no bacterial infection (Figure 4A). Moreover, the reduction in bacterial colonization was directly correlated with high serum OMP-19-specific IgM in the recipient mice (Figure 4B; i.e., mice with the highest IgM titers exhibited the lowest infections). Although most polyreactive IgM tested in the assay were OMP-19 specific, a non-OMP-19-specific ehrlichial-specific IgM (Em412) also reduced bacterial infection below the limit of detection. These data indicate that polyreactive IgM is sufficient to mediate host defense.
FIGURE 4.
Polyreactive IgM effectively reduced bacterial infection in the spleen.
(A) Antibody-secreting hybridoma cells were implanted subcutaneously in RAG-deficient mice three days prior to infection; spleen cells were harvested on day 10 post-infection, and bacterial burden was quantitated. (B) Serum OMP-19-specific antibody titers were measured in mice implanted with hybridomas. An irrelevant IgM hybridoma was derived from spleen B cells obtained from uninfected mice; Ec56.5 is an OMP-19 IgG2a that has been described previously (54); Em412 is an ehrlichia-specific non-OMP-19-binding IgM; Em504.1, Em553.2, Em662.2, Em702.1, and Em720.1 are polyreactive IgM-secreting hybridomas. The data are representative of three experiments. A student’s t test was used to calculate statistically significant differences in bacterial burden, relative to mice that received no tumor; **p < 0.01.
Ehrlichial infection induces IgM autoantibodies
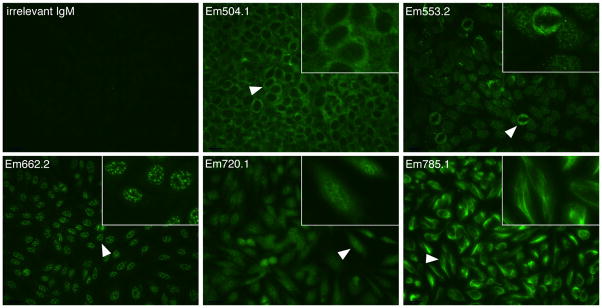
We next addressed whether the polyreactive IgM bound nuclear and cytoplasmic antigens. Antinuclear antibodies are characteristic of systemic lupus erythematosus and other autoimmune disorders. Non-polyreactive irrelevant IgM generated from uninfected C57BL/6 mice did not bind HEp-2 cells (Figure 5, irrelevant IgM). In contrast, all of the polyreactive IgM tested bound HEp-2 cells, although each of the IgM exhibited distinct binding specificities, underscoring our observation that many of the IgM were quasi-specific. Anti-cytoplasmic (Figure 5; mAb Em504.1), anti-nuclear (mAbs Em553.2 and Em662.2), mixed nuclear and cytoplasmic (mAb Em720.1), and anti-cytoskeletal specificities (mAb Em785.1) were detected. Thus, the polyreactive IgM generated during infection was also autoreactive.
FIGURE 5.
Polyreactive IgM binds distinct nuclear and cytoplasmic determinants.
An irrelevant IgM and polyreactive IgM were tested for binding to HEp-2 cells by immunofluorescence assay. The polyreactive IgM exhibited anti-cytoplasmic (mAb Em504.1), antinuclear (mAbs Em553.2 and Em662.2), anti-nuclear + cytoplasmic (mAb Em702.1), and anti-cytoskeletal specificities (mAb Em785.1). A higher magnification of the regions indicated by white arrowheads is shown in the inset of each panel. Original magnification, 40X.
Autoreactive IgM was detected in sera from human ehrlichiosis patients
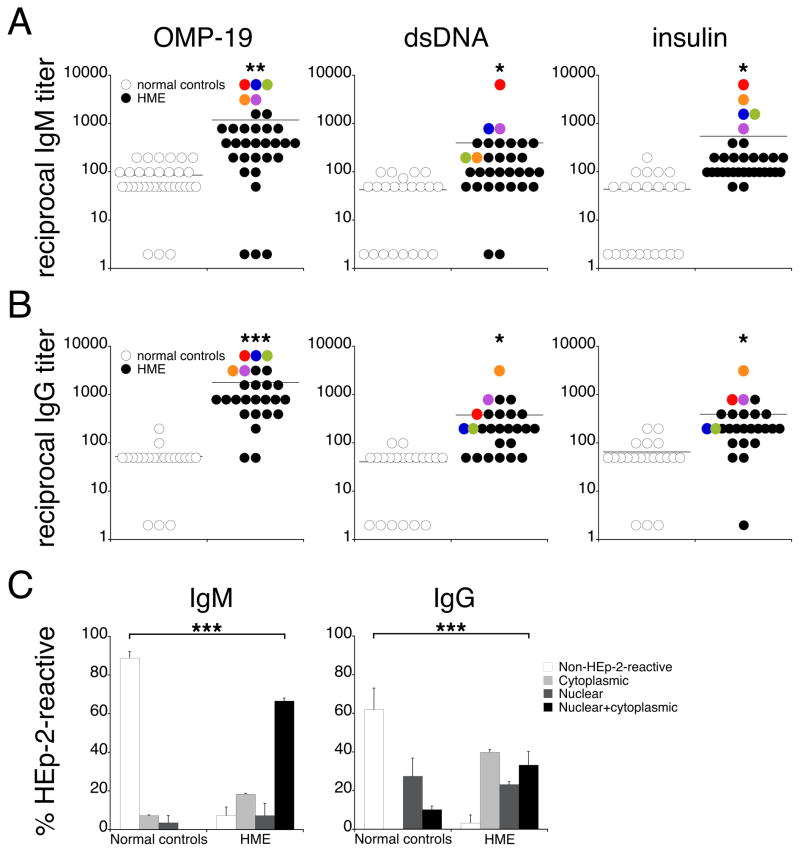
Although autoimmunity has not been reported to be associated with ehrlichiosis in humans, our finding from the mouse model led us to address whether IgM from human ehrlichial patients exhibited poly-and/or autoreactivity. Sera from 32 human monocytic ehrlichiosis (HME) patients, and normal controls, were evaluated for reactivity to E. chaffeensis OMP-19, dsDNA, and insulin. Sera from HME patients exhibited nearly 10-fold higher titers against each of these antigens, relative to healthy human control sera (Figure 6A). Moreover, autoreactivity in HME patients was directly correlated with OMP-19 specificity: the patients with the highest anti-OMP-19 IgM titers also exhibited high reactivity to dsDNA and insulin (Figure 6A). We also addressed whether patient sera exhibited autoreactivity, by assay for HEp-2 cell-reactive IgM. The majority of normal control sera (90%) contained non-HEp-2-reactive IgM; in contrast, most HME patients produced autoreactive IgM that exhibited mixed nuclear and cytoplasmic specificity (Figure 6C). HME patients also produced autoreactive IgG, which was also correlated with high anti-OMP-19 reactivity (Figure 6B). Although anti-nuclear IgG specificities were observed in both HME patients and normal controls, the overall frequency of autoreactive IgG was significantly higher in HME patients (Figure 6C; 97% vs. 38%). Although it was not possible to determine whether the monoclonal Igs detected in ehrlichial patients were polyreactive, the data from the mouse suggest such an interpretation, and raise the possibility that ehrlichial as well as other bacterial infections may play a role in the generation or exacerbation of autoimmunity in humans, via the induction of polyreactive IgM and/or IgG.
FIGURE 6.
Sera from human monocytic ehrlichiosis patients exhibit apparent polyreactivity.
Sera from human ehrlichiosis patients (HME) or normal controls were assayed for IgM (A) and IgG (B) reactivity to Ehrlichia chaffeensis OMP-19, dsDNA, and insulin, by ELISA. Data from five representative patients are indicated in the plots by pseudo-coloring. The differences between HME patients and normal controls were statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001; student’s t test). (C) Sera from HME patients (n = 27–30) and normal controls (n = 27–29) were tested for autoreactivity by HEp-2 assay. Bar graphs summarize the frequency of patients exhibiting cytoplasmic, nuclear, or nuclear + cytoplasmic specificities. The differences in the frequencies of patients exhibiting self-reactivity were statistically significant (***p < 0.001; Fisher’s Exact 2x4 test). The error bars indicate the SD.
Discussion
Although polyreactive antibody is often considered to be a property of natural antibody produced by B-1 cells, or is a consequence of polyclonal B cell activation, our study shows that polyreactive IgM can be produced by antigen-specific B cells in response to bacterial infection. We also demonstrate the production of poly- and/or autoreactive IgM by human ehrlichiosis patients. Our findings from the mouse model may be relevant to human disease, and suggest that polyreactive IgM may modulate autoimmunity.
Polyreactivity is not a universal property of IgM molecules, although a significant portion of circulating natural IgM has been reported to bind multiple antigens (30, 31). In our studies, we did not detect polyreactive IgM in uninfected mice, indicating that the IgM we characterized were not derived from natural antibody-secreting B cells. Other studies have also described the production of antibodies of irrelevant specificity during infection; however, it has been unclear whether the apparent polyreactivity observed in other studies was a consequence of polyclonal, or antigen-specific, B cell activation (7, 10, 11, 32, 33). For example, during influenza infection, polyreactive antibody is secreted by B-1a cells; however, these cells were apparently activated in a polyclonal fashion, as the majority of antibody produced was non-antigen-specific (5, 34). In contrast, during ehrlichial infection, most B cells were triggered via their BCRs, because nearly 85% of hybridomas we generated produced ehrlichia-specific IgM, and were capable of reducing bacterial infection in vivo. However, we cannot exclude the possibility that polyreactive antibody production was triggered through dual BCR-TLR signaling (8, 9, 35), even though the ehrlichiae do not encode classical TLR ligands (with the possible exception of CpG DNA). In most cases, polyreactivity was associated with ehrlichial OMP-19 specificity, suggesting that this protein drives a polyreactive response. The mechanism(s) responsible for the apparent association of polyreactivity with a particular antigen specificity are not yet understood.
Why does ehrlichial infection induce such a robust polyreactive response? The polyreactive IgM analyzed in our study was derived from a TI CD11c-positive spleen plasmablast population, which we have shown to be responsible for IgM production during acute infection. Although we have not resolved the B cell lineage of these plasmablasts, they exhibit surface expression of several cell surface antigens characteristic of B-1 cells, a subset of B cells that has been shown to secrete polyreactive natural IgM, and that can respond to pathogens in a TI fashion (20, 36, 37). Thus, polyreactivity may be a property of unmutated IgM produced in response to infection by a subset of B-1 cells that are elicited at particularly high frequency by ehrlichial infection. If this explanation is correct, it would suggest that other infections that induce B-1 cell responses also generate pathogen-specific polyreactive IgM.
Several hypotheses exist to explain how polyreactive antibodies function. In the context of HIV infection, polyreactivity has been suggested to act by increasing the relative affinity of IgG for virions, by facilitating the binding of the antibody to two distinct antigens on the viral envelope, a mechanism termed heteroligation (12, 38). Similarly, polyreactivity, coupled with polyvalency, may allow IgM to bridge two or more distinct antigens on the ehrlichiae, thereby enhancing the avidity of the antibody-pathogen interaction, and promoting pathogen clearance by professional APCs (30, 39, 40). Another possibility is that polyreactivity may facilitate the IgM-mediated uptake and clearance of apoptotic cells and self-antigens, thereby reducing the availability of these potentially harmful antigens to other immune cells (16, 41, 18). Indeed, we have observed that polyreactive IgM binds apoptotic cells (D.D.J. and G.M.W., unpublished observations). Thus, it is possible that polyreactive IgM serves to aid in the clearance of apoptotic debris associated with ehrlichial infection, thereby maintaining tissue homeostasis and reducing inflammation (30). Alternately, an antigen-specific, polyreactive IgM-expressing B cell may retain the capacity to respond to a number of unrelated antigens (42). This raises the possibility that a polyreactive B cell may provide a degree of protection against pathogens that have not yet been encountered by the host. Thus, polyreactivity, as a property of IgM, is more likely to benefit the host, rather than cause harm.
Sera from HME patients were also apparently polyreactive. Although it was not determined whether polyreactivity was a property of monoclonal IgM in patients, our findings from the mouse model suggest that this was the case. Whereas we only detected polyreactive IgM in mice, apparently polyreactive IgG was detected in the sera of HME patients. This difference may be a consequence of subsequent exposure to antigens or pathogens in humans, which can drive autoreactive IgM-expressing B cells to class switch to IgG-secreting plasma cells (43–45). These findings suggest that the induction of polyreactive IgG in human patients may contribute to the development of autoimmunity. Autoantibodies are produced during infection of humans with a related rickettsial pathogen, Anaplasma phagocytophilum (46), and have been observed in Ehrlichia canis-infected dogs (47, 48). Thus, autoantibody production may be a common feature of rickettsial infections, and may be a consequence of induced polyreactivity. Although a correlation between human ehrlichial infections and autoimmunity has not been reported, this remains a possibility that warrants further investigation. How or if polyreactive IgM may contribute to autoimmunity in mice or humans is not understood.
Anti-dsDNA IgM has been shown to be associated with reduced pathology in various models of autoimmunity (49–51, 17). Another study reported a protective effect of IgM anti-β2 glycoprotein I antibodies in SLE patients (52). In contrast, class switching of autoreactive IgM-expressing B cells can lead to the production of pathogenic IgG antibodies (43–45). In the context of parasitic infection, non-specific IgM production during leishmaniasis has been suggested to exacerbate disease (53). Although the aforementioned studies did not address a role for polyreactive IgM, our findings indicate that ehrlichial infection drives the production of polyreactive and autoreactive IgM in mice and humans, and suggest that polyreactivity may be associated with the exacerbation or amelioration of autoimmune disease.
Acknowledgments
This work was supported by U.S. Department of Health and Human Services, NIH, NIAID, grant R01AI064678 to G.M.W.
We thank Maura Jones, Olga Martin, and Wei Du, for excellent technical assistance; we also thank the Wadsworth Center Genomics Core Facility, and Dr. Karen Chave, of the Northeast Biodefense Center Expression Core Facility, for production of recombinant OMP-19 (grant U54-AI057158 to Dr. Ian Lipkin). We also thank Dr. Susan Wong (Wadsworth Center), and Dr. Harry Prince (Focus Laboratories, Cypress, CA) for the human serum samples, Dr. Timothy Sellati (Albany Medical College, Albany, NY) for Borrelia burgdorferi, Dr. Laura Haynes (Trudeau Institute, Saranac Lake, NY) for influenza A/PR/8, and Dr. Lawrence Wysocki (National Jewish Health) for technical advice.
References
- 1.Zhou ZH, Zhang Y, Hu YF, Wahl LM, Cisar JO, Notkins AL. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe. 2007;1:51–61. doi: 10.1016/j.chom.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Notkins AL. Polyreactivity of antibody molecules. Trends Immunol. 2004;25:174–179. doi: 10.1016/j.it.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 3.James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science. 2003;299:1362–1367. doi: 10.1126/science.1079731. [DOI] [PubMed] [Google Scholar]
- 4.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel RM. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 5.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casali P, Notkins AL. CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol Today. 1989;10:364–368. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- 7.Montes CL, Acosta-Rodriguez EV, Merino MC, Bermejo DA, Gruppi A. Polyclonal B cell activation in infections: infectious agents’ devilry or defense mechanism of the host? J Leukoc Biol. 2007;82:1027–1032. doi: 10.1189/jlb.0407214. [DOI] [PubMed] [Google Scholar]
- 8.Leadbetter EA, I, Rifkin R, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 9.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 10.Castillo-Mendez SI, Zago CA, Sardinha LR, Freitas do Rosario AP, Alvarez JM, D’Imperio Lima MR. Characterization of the spleen B-cell compartment at the early and late blood-stage Plasmodium chabaudi malaria. Scand J Immunol. 2007;66:309–319. doi: 10.1111/j.1365-3083.2007.01972.x. [DOI] [PubMed] [Google Scholar]
- 11.Bermejo DA, Amezcua Vesely MC, Khan M, Acosta Rodriguez EV, Montes CL, Merino MC, Toellner KM, Mohr E, Taylor D, Cunningham AF, Gruppi A. Trypanosoma cruzi infection induces a massive extrafollicular and follicular splenic B-cell response which is a high source of non-parasite-specific antibodies. Immunology. 2011;132:123–133. doi: 10.1111/j.1365-2567.2010.03347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mouquet H, Scheid JF, Zoller MJ, Krogsgaard M, Ott RG, Shukair S, Artyomov MN, Pietzsch J, Connors M, Pereyra F, Walker BD, Ho DD, Wilson PC, Seaman MS, Eisen HN, Chakraborty AK, Hope TJ, Ravetch JV, Wardemann H, Nussenzweig MC. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunziker L, Recher M, Macpherson AJ, Ciurea A, Freigang S, Hengartner H, Zinkernagel RM. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat Immunol. 2003;4:343–349. doi: 10.1038/ni911. [DOI] [PubMed] [Google Scholar]
- 14.Benckert J, Schmolka N, Kreschel C, Zoller MJ, Sturm A, Wiedenmann B, Wardemann H. The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J Clin Invest. 2011;121:1946–1955. doi: 10.1172/JCI44447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuuminen T, Hedman K, Soderlund-Venermo M, Seppala I. Acute parvovirus B19 infection causes nonspecificity frequently in Borrelia and less often in Salmonella and Campylobacter serology, posing a problem in diagnosis of infectious arthropathy. Clin Vaccine Immunol. 2011;18:167–172. doi: 10.1128/CVI.00367-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litvack ML, Post M, Palaniyar N. IgM promotes the clearance of small particles and apoptotic microparticles by macrophages. PLoS ONE. 2011;6:e17223. doi: 10.1371/journal.pone.0017223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gronwall C, Akhter E, Oh C, Burlingame RW, Petri M, Silverman GJ. IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin Immunol. 2012;142:390–398. doi: 10.1016/j.clim.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverman GJ. Regulatory natural autoantibodies to apoptotic cells: pallbearers and protectors. Arthritis Rheum. 2011;63:597–602. doi: 10.1002/art.30140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Notley CA, Brown MA, Wright GP, Ehrenstein MR. Natural IgM is required for suppression of inflammatory arthritis by apoptotic cells. J Immunol. 2011;186:4967–4972. doi: 10.4049/jimmunol.1003021. [DOI] [PubMed] [Google Scholar]
- 20.Racine R, Chatterjee M, Winslow GM. CD11c expression identifies a population of extrafollicular antigen-specific splenic plasmablasts responsible for CD4 T-independent antibody responses during intracellular bacterial infection. J Immunol. 2008;181:1375–1385. doi: 10.4049/jimmunol.181.2.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Racine R, McLaughlin M, Jones DD, Wittmer ST, MacNamara KC, Woodland DL, Winslow GM. IgM production by bone marrow plasmablasts contributes to long-term protection against intracellular bacterial infection. J Immunol. 2011;186:1011–1021. doi: 10.4049/jimmunol.1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bitsaktsis C, Nandi B, Racine R, MacNamara KC, Winslow G. T-Cell-independent humoral immunity is sufficient for protection against fatal intracellular ehrlichia infection. Infect Immun. 2007;75:4933–4941. doi: 10.1128/IAI.00705-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevenson HL, Jordan JM, Peerwani Z, Wang HQ, Walker DH, Ismail N. An intradermal environment promotes a protective type-1 response against lethal systemic monocytotropic ehrlichial infection. Infect Immun. 2006;74:4856–4864. doi: 10.1128/IAI.00246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo W, Smith D, Aviszus K, Detanico T, Heiser RA, Wysocki LJ. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. J Exp Med. 2010;207:2225–2237. doi: 10.1084/jem.20092712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohler F, Hug E, Eschbach C, Meixlsperger S, Hobeika E, Kofer J, Wardemann H, Jumaa H. Autoreactive B cell receptors mimic autonomous pre-B cell receptor signaling and induce proliferation of early B cells. Immunity. 2008;29:912–921. doi: 10.1016/j.immuni.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Leadbetter EA, Brigl M, Illarionov P, Cohen N, Luteran MC, Pillai S, Besra GS, Brenner MB. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA. 2008;105:8339–8344. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pospisil R, Mage RG. CD5 and other superantigens as ‘ticklers’ of the B-cell receptor. Immunol Today. 1998;19:106–108. doi: 10.1016/s0167-5699(97)01215-2. [DOI] [PubMed] [Google Scholar]
- 28.Neal LM, O’Hara J, Brey RN, 3rd, Mantis NJ. A monoclonal immunoglobulin G antibody directed against an immunodominant linear epitope on the ricin A chain confers systemic and mucosal immunity to ricin. Infect Immun. 2010;78:552–561. doi: 10.1128/IAI.00796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apter FM, Michetti P, Winner LS, 3rd, Mack JA, Mekalanos JJ, Neutra MR. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun. 1993;61:5279–5285. doi: 10.1128/iai.61.12.5279-5285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaveri SV, Silverman GJ, Bayry J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J Immunol. 2012;188:939–945. doi: 10.4049/jimmunol.1102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elkon K, Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol. 2008;4:491–498. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koga T, Nishihara T, Fujiwara T, Nisizawa T, Okahashi N, Noguchi T, Hamada S. Biochemical and immunobiological properties of lipopolysaccharide (LPS) from Bacteroides gingivalis and comparison with LPS from Escherichia coli. Infect Immun. 1985;47:638–647. doi: 10.1128/iai.47.3.638-647.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoda LK, Kegerreis KA, Suarez CE, Roditi I, Corral RS, Bertot GM, Norimine J, Brown WC. DNA from protozoan parasites Babesia bovis, Trypanosoma cruzi, and T. brucei is mitogenic for B lymphocytes and stimulates macrophage expression of interleukin-12, tumor necrosis factor alpha, and nitric oxide. Infect Immun. 2001;69:2162–2171. doi: 10.1128/IAI.69.4.2162-2171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 35.Fields ML, Metzgar MH, Hondowicz BD, Kang SA, Alexander ST, Hazard KD, Hsu AC, Du YZ, Prak EL, Monestier M, Erikson J. Exogenous and endogenous TLR ligands activate anti-chromatin and polyreactive B cells. J Immunol. 2006;176:6491–6502. doi: 10.4049/jimmunol.176.11.6491. [DOI] [PubMed] [Google Scholar]
- 36.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, Leong JM. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol. 2003;170:3819–3827. doi: 10.4049/jimmunol.170.7.3819. [DOI] [PubMed] [Google Scholar]
- 38.Mouquet H, Warncke M, Scheid JF, Seaman MS, Nussenzweig MC. Enhanced HIV-1 neutralization by antibody heteroligation. Proc Natl Acad Sci USA. 2012;109:875–880. doi: 10.1073/pnas.1120059109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rapaka RR, Ricks DM, Alcorn JF, Chen K, Khader SA, Zheng M, Plevy S, Bengten E, Kolls JK. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungusPneumocystis murina. J Exp Med. 2010;207:2907–2919. doi: 10.1084/jem.20100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramaniam KS, Datta K, Quintero E, Manix C, Marks MS, Pirofski LA. The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. J Immunol. 2010;184:5755–5767. doi: 10.4049/jimmunol.0901638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Khanna S, Goodyear CS, Park YB, Raz E, Thiel S, Gronwall C, Vas J, Boyle DL, Corr M, Kono DH, Silverman GJ. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J Immunol. 2009;183:1346–1359. doi: 10.4049/jimmunol.0900948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisen HN, Chakraborty AK. Evolving concepts of specificity in immune reactions. Proc Natl Acad Sci USA. 2010;107:22373–22380. doi: 10.1073/pnas.1012051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Jacobi AM, Wang T, Berlin R, Volpe BT, Diamond B. Polyreactive autoantibodies in systemic lupus erythematosus have pathogenic potential. J Autoimmun. 2009;33:270–274. doi: 10.1016/j.jaut.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, Gonzalez JB, Pascual V, Stichweh D, Wardemann H, Nussenzweig MC. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci USA. 2008;105:9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venkatesh J, Yoshifuji H, Kawabata D, Chinnasamy P, Stanevsky A, Grimaldi CM, Cohen-Solal J, Diamond B. Antigen is required for maturation and activation of pathogenic anti-DNA antibodies and systemic inflammation. J Immunol. 2011;186:5304–5312. doi: 10.4049/jimmunol.1000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong SJ, Thomas JA. Cytoplasmic, nuclear, and platelet autoantibodies in human granulocytic ehrlichiosis patients. J Clin Microbiol. 1998;36:1959–1963. doi: 10.1128/jcm.36.7.1959-1963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waner T, Harrus S, Jongejan F, Bark H, Keysary A, Cornelissen AW. Significance of serological testing for ehrlichial diseases in dogs with special emphasis on the diagnosis of canine monocytic ehrlichiosis caused by Ehrlichia canis. Vet Parasitol. 2001;95:1–15. doi: 10.1016/s0304-4017(00)00407-6. [DOI] [PubMed] [Google Scholar]
- 48.Harrus S, Waner T, Weiss DJ, Keysary A, Bark H. Kinetics of serum antiplatelet antibodies in experimental acute canine ehrlichiosis. Vet Immunol Immunop. 1996;51:13–20. doi: 10.1016/0165-2427(95)05516-9. [DOI] [PubMed] [Google Scholar]
- 49.Werwitzke S, Trick D, Kamino K, Matthias T, Kniesch K, Schlegelberger B, Schmidt RE, Witte T. Inhibition of lupus disease by anti-double-stranded DNA antibodies of the IgM isotype in the (NZB × NZW)F1 mouse. Arthritis Rheum. 2005;52:3629–3638. doi: 10.1002/art.21379. [DOI] [PubMed] [Google Scholar]
- 50.Witte T. IgM antibodies against dsDNA in SLE. Clin Rev Allergy Immunol. 2008;34:345–347. doi: 10.1007/s12016-007-8046-x. [DOI] [PubMed] [Google Scholar]
- 51.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci USA. 2000;97:1184–1189. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehrani T, Petri M. IgM anti-beta2 glycoprotein I is protective against lupus nephritis and renal damage in systemic lupus erythematosus. J Rheumatol. 2011;38:450–453. doi: 10.3899/jrheum.100650. [DOI] [PubMed] [Google Scholar]
- 53.Deak E, Jayakumar A, Cho KW, Goldsmith-Pestana K, Dondji B, Lambris JD, McMahon-Pratt D. Murine visceral leishmaniasis: IgM and polyclonal B-cell activation lead to disease exacerbation. Eur J Immunol. 2010;40:1355–1368. doi: 10.1002/eji.200939455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li JS, Chu F, Reilly A, Winslow GM. Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J Immunol. 2002;169:1419–1425. doi: 10.4049/jimmunol.169.3.1419. [DOI] [PubMed] [Google Scholar]