Abstract
Adoptive immunotherapy using cultured T cells holds promise for the treatment of cancer and infectious disease. Ligands immobilized on surfaces fabricated from hard materials such as polystyrene plastic are commonly employed for T cell culture. The mechanical properties of a culture surface can influence the adhesion, proliferation, and differentiation of stem cells and fibroblasts. We therefore explored the impact of culture substrate stiffness on the ex vivo activation and expansion of human T cells. We describe a simple system for the stimulation of the TCR/CD3 complex and the CD28 receptor using substrates with variable rigidity manufactured from poly(dimethylsiloxane) (PDMS), a biocompatible silicone elastomer. We show that softer (Young’s Modulus [E] < 100 kPa) substrates stimulate an average 4-fold greater IL-2 production and ex vivo proliferation of human CD4+ and CD8+ T cells compared with stiffer substrates (E >2 MPa). Mixed peripheral blood T cells cultured on the stiffer substrates also demonstrate a trend (non-significant) towards a greater proportion of CD62Lneg, effector-differentiated CD4+ and CD8+ T cells. Naïve CD4+ T cells expanded on softer substrates yield an average 3-fold greater proportion of IFN-γ producing TH1-like cells. These results reveal that the rigidity of the substrate used to immobilize T cell stimulatory ligands is an important and previously unrecognized parameter influencing T cell activation, proliferation and TH differentiation. Substrate rigidity should therefore be a consideration in the development of T cell culture systems as well as when interpreting results of T cell activation based upon solid-phase immobilization of TCR/CD3 and CD28 ligands.
Keywords: T cells, Cell Activation, Cell Proliferation, Cytokines, Human
Introduction
Adoptive immunotherapy holds great potential as a therapeutic modality for the treatment of a variety of diseases including cancer and chronic viral infections[1]. Central to these therapeutic approaches are controllable platforms for ex vivo activation of T cells, and several cell-based, and artificial substrate systems, have been described[2]. Agonist antibodies to CD3 and CD28 immobilized on rigid materials like polystyrene plastic, and glass, are widely used in many of these systems for the activation and expansion of T cells. These artificial culture substrates are also widely used in basic studies of T cell activation forming the foundation for much of our knowledge of T signal transduction[3].
The outcome of ex vivo culture for many types of adherent cells is increasingly recognized to depend upon the mechanical properties of the culture substrate. Fibroblast spreading and focal adhesion formation is highly dependent upon the force generated by the fibroblast, as well as the elasticity of the material to which they attach[4, 5]. The differentiation of pluripotent mesenchymal stem cells is directly linked to the stiffness of the culture substrate[6]. Similarly, expanding myogenic stem cells on soft hydrogel materials leads to enhanced self-renewal and improved engraftment into mice[7].
T cells are unlikely to encounter a stimulatory surface with the stiffness of plastic in vivo, and the stiffness of the solid supports used for ex vivo culture of T cells may have important influences on their activation, proliferation, and differentiation. It has long been recognized that anti-CD3ε agonist antibodies such as OKT3 and peptide/MHC complexes require immobilization on solid supports for robust T cell activation [8]. T cell cytoskeleton integrity and contractility also appear vital for T cell activation [9-11], and models wherein forces applied by the T cell cytoskeleton to ligand-bound TCR modulate and/or trigger TCR/CD3 signaling have been proposed [9, 12]. More recent studies have provided direct evidence for force as a mediator of TCR signal transduction [13, 14]. The demonstration that the immunotyrosine-based activation motif (ITAM) in the CD3ε chain can be activated by conformational changes in ITAM interaction with the inner leaflet of the plasma membrane provides at least one possible mechanism by which force might be able to mediate signals through the TCR/CD3 complex [15]. In addition to the direct role of force in TCR/CD3 complex signal transduction, many proteins involved in TCR and co-stimulatory receptor signal transduction directly or indirectly interact with the actin cytoskeleton[16]. The structure and dynamics of the actin cytoskeleton, which is affected by attachment substrate stiffness[17], has been reported to play an important role in supporting and regulating signal transduction at the immune synapse[18, 19].
We describe a novel system for stimulating T cells under conditions of variable substrate stiffness based upon a biocompatible polymer. We show that softer substrates used for immobilizing T cell ligands significantly enhance T cell activation and expansion. We also demonstrate effects of rigidity on TH cell differentiation. These results have implications for the ex vivo culture of T cells that forms the foundation for many adoptive immunotherapy approaches currently being pursued in clinical trials. They also suggest that the mechanical properties of the substrate used for immobilizing T cell surface receptor ligands, especially TCR/CD3 and CD28 receptor ligands, should be considered when interpreting basic studies of signal transduction and T cell activation.
Materials and Methods
Fabrication of silicone-based culture surfaces
PDMS surfaces were fabricated by mixing dimethylsiloxane monomer (Dow Corning Sylgard 184) with its corresponding cross-linking agent according to manufacturer instructions. The ratio of crosslinking agent to base polymer was varied from 1:5 to 1:50. PDMS elastomer slabs of > 1 mm were cured at 60°C for 2 hours in multiwell plates prior to use in T cell culture experiments. Young’s Modulus (E) of PDMS prepared at each ratio of crosslinking agent to elastomer base was estimated using a custom-built indentation apparatus. Slabs of PDMS (32 mm × 43 mm) with a thickness of ~10 mm were deformed using a flat, cylindrical head, which makes a no-slip contact with the PDMS surface. A calibrated mass was applied to this head, producing a deflection of the PDMS slab. Hertzian contact between the head and PDMS was assumed[20], which allows estimation of the material’s Young’s modulus from the head diameter (D), deflection (h), weight (m), gravitational constant (g), and Poisson ratio (ν), assumed to be 0.5 for PDMS using the following equation:
Coating of PDMS surfaces with antibodies for T cell stimulation
Cured PDMS elastomer was incubated with a goat anti-mouse IgG (Cappel, MP Biomedicals) in PBS overnight at 4°C. Unless otherwise indicated, a concentration of 1 μg/mL was used. Following PBS rinsing, a 1hr incubation with a blocking buffer containing 5% BSA was performed. After washing, agonist monoclonal antibodies to human CD3ε (5 μg/mL, OKT3, Roche Pharmaceuticals) and human CD28 (5 μg/mL, clone 9.3, kind gift of Dr. Carl June) were captured by incubation in PBS for 2 hrs followed by washing before use.
Primary Cell Preparation and Cell Culture
Primary peripheral blood lymphocytes were obtained under approval by the University of Pennsylvania Institutional Review Board. Purified total T cells, CD4+ T cells or CD8+ T cells were isolated using RosetteSep isolation kits (Stem Cell Technologies). Naïve CD4+ T cells were obtained by further depletion of CD45RO+ cells using human CD45RO-specific magnetic microbeads, LD selection columns and a VarioMACS system (Miltenyi Biotec). Lymphocytes were cultured in either X-VIVO 15 or RPMI (Lonza) supplemented with 5% human serum (GemCell) or 10% Fetal Bovine Serum (Hyclone), respectively, with 10 mM HEPES, L-glutamine, penicillin G and streptomycin. 4.5 μm beads with immobilized anti-human CD3 and anti-human CD28 (a kind gift of Dr. Bruce Levine, University of Pennsylvania) were used in some experiments at a ratio of 3 beads to 1 cell. T cells were maintained in culture at a concentration of 0.8 - 1.0 × 106 cells/mL by regular counting on a Multisizer III particle counter (Beckman-Coulter). In some experiments, cells were also counted by flow cytometry using CountBright beads (BD Biosciences) and monoclonal antibodies to human CD4 and CD8.
Quantitative real-time RT-PCR (qRT-PCR) analyses for IL-2 mRNA
Total RNA was isolated from cells using an RNeasy kit (Qiagen). cDNA was generated by reverse transcription using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA). cDNA was amplified with a predesigned primer-probe set for hIL-2 (Hs00174114_m1; Applied Biosystems). A β-actin specific primer-probe set (Hs999999 03_m1; Applied Biosystems) was used as a normalization control. qRT-PCR assays were performed on a 7500 Fast Real-Time PCR system thermal cycler (Applied Biosystems) using the comparative Ct model. In experiments where the stability of IL-2 mRNA was evaluated, CD4+ lymphocytes were seeded at 0.5 × 106 cells/well. After 6 hrs, these cultures were treated with/without cyclosporine A (10−6 M; Sigma-Aldrich) or Actinomycin D (5 μg/mL, Sigma-Aldrich) to inhibit de novo IL-2 mRNA transcription. Cells were harvested as indicated, and IL-2 mRNA was determined by qRT-PCR analyses as described above.
Antibodies and Flow Cytometry
At the indicated time following activation, cells were stained with a panel of monoclonal antibodies to CD3, CD4, and CD8, CD45RA, CD45RO, CD62L, CCR7, CD27 and LIVE/DEAD Aqua dye (Invitrogen). Flow cytometry was performed using an LSRII (BD Biosciences, San Jose, CA), and data were analyzed using FlowJo software (Treestar, Inc., Ashland, OR). In experiments in which proliferation was assessed using CFSE dilution, cells were stained with 5 μM CFSE for 5 min, washed twice and re-suspended in culture medium prior to initiation of the culture as indicated. Flow cytometry was performed on cells on day 3, and data were analyzed using the Proliferation module as implemented in FlowJo.
Statistical Analysis
Student’s t test for paired data, Wilcoxon Rank Sum or a one-way analysis of variance (ANOVA) were performed using GraphPad Prism version 4.0a (GraphPad Software Inc.). A p-value of < 0.05 was considered statistically significant.
Results
PDMS as a substrate with controllable rigidity for T cell activation and culture
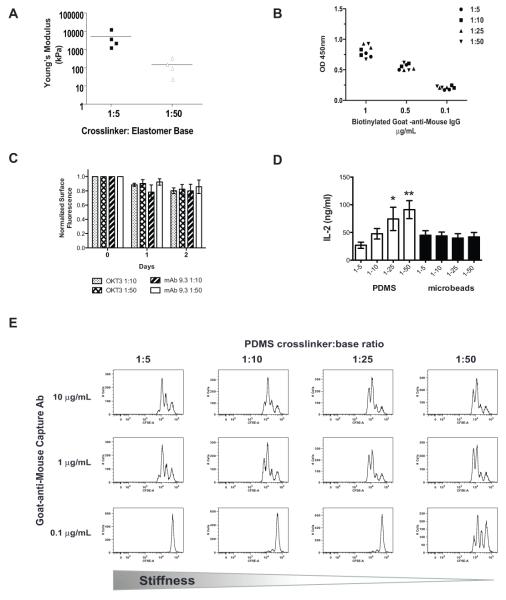
PDMS, a biocompatible organosilicon polymer commonly used as a lubricant, anti-caking agent in foods and anti-bloating agent was selected as a substrate for antibody immobilization. Following crosslinking of the base polymer, PDMS forms an elastomeric material with a highly hydrophobic surface [21]. Proteins, including antibody, passively adsorb to this hydrophobic surface. Alteration of the crosslinking-agent-to-base-polymer stoichiometry in the commonly used Sylgard 184 preparation of PDMS provides a simple method for varying the elastic modulus of PDMS from a Young’s modulus of > 2.3MPa (stiff) to a range of 50-100 kPa (soft) (Fig. 1A). Prepared this way, this material has been used to study the effects of substrate rigidity on fibroblast focal adhesion formation [4]. Adsorption of anti-CD3 (OKT3) and anti-CD28 (clone 9.3) antibodies to the surface of PDMS provides a system for activation of T cells on substrates with varying elastic modulus, analogous to standard immobilization on more rigid polystyrene tissue culture plastic or glass. Quantitative measurement of enzymatically-coupled primary capture antibody (Fig. 1B) as well as fluorescently-labeled OKT3 and clone 9.3 (data not shown) demonstrate that the amount of antibody adsorbed on PDMS surfaces with varying elastic modulus is equivalent despite changes in the ratio of base polymer to crosslinking agent. Both OKT3 and clone 9.3 also demonstrated stable binding over the course of 48 hours with < 20% loss of antibody at 37°C in complete culture medium independent of the crosslinker ratio (Fig. 1C). Clone 9.3 appeared to demonstrate a slightly more rapid loss from stiff surfaces compared soft surfaces; however, the quantity of bound clone 9.3 was not significantly different between the PDMS surfaces at 48 hours, after which T cells are typically transfer to uncoated culture vessels for log-phase ex vivo expansion using planar activating substrates.
Figure 1. A T cell culture surface with controlled elastic modulus can be generated using variably cross-linked PDMS.
(A) The elastic modulus of PDMS was measured as described in the Materials and Methods. Horizontal bars represent the mean of four independent batches of PDMS. (B) PDMS surfaces were coated with the indicated concentration of biotinylated goat-anti-mouse IgG. Adsorbed antibody was detected by incubation with horseradish peroxidase conjugated to streptavidin and TMB followed by measurement of the optical density (OD) at 450 nm. Data presented is representative of two independent experiments. Symbols indicated replicate wells performed within the experiment. (C) Fluorescently-conjugated antibodies against CD3 (OKT3) and CD28 (9.3) were simultaneously applied to PDMS surfaces pre-coated with goat-anti-mouse IgG at 5 mg/mL followed by washing and blocking. The fluorescent signal intensity at the surface for each antibody was measured by fluorescence microscopy, and normalized to the signal intensity observed on the 1:10 PDMS surface. Surfaces were stored for 2 days in serum-containing culture medium at 37°C, 5% CO2, and the surface fluorescence was measured at the indicated time points. Bars represent mean change in fluorescence intensity with the 95% confidence interval for 3 independent experiments with 5 replicates per experiment. (D) Supernatants were collected from primary human CD4+ T cells grown in X-VIVO 15 culture medium on PDMS surfaces coated with OKT3 and clone 9.3 for 24 hrs. IL-2 was measured by ELISA. Cells were stimulated with anti-CD3/anti-CD28-coated microbeads and cultured in wells containing uncoated PDMS as a control. Data were analyzed by a Repeated-measures one-way ANOVA and a Neuman-Keuls multiple comparison test for post-hoc analysis. Values are means ± S.D. from 4 independent experiments (*P< 0.05 for PDMS 1-5 vs 1-25; ** P<0.05 for PDMS1-5 vs 1-50 and 1-10vs 1-50). (E) CFSE-labeled T cells were stimulated on the indicated PDMS surface coated with anti-CD3 and anti-CD28 for 3 days. CFSE content was assessed by flow cytometry.
Initial evaluation of T cell activation demonstrated that softer PDMS stimulatory substrate increased IL-2 secretion (Fig. 1D). Since the stiffer PDMS substrates contain more crosslinking agent, we considered the possibility that one of the components in the crosslinking agent may be toxic, leading to non-specific inhibition of T cell activation and IL-2 secretion. In order to evaluate this possibility, we simultaneously stimulated T cells with antibody-coated magnetic microbeads in the presence of PDMS with variable rigidity. Unlike T cells stimulated with antibodies immobilized on the PDMS substrate, microbead-stimulated IL-2 secretion was comparable across the different PDMS surfaces, arguing against a toxic effect of PDMS elastomer or its crosslinking agent (Fig. 1D).
Since antibody density is an important factor affecting T cell activation and proliferation, we evaluated the ability of primary human peripheral blood CD4+ T cells to undergo proliferation in response to varying density of OKT3 and clone 9.3 on PDMS surfaces. We observed both antibody density-dependent and stiffness-dependent effects on T cell proliferation. Using a carboxyfluoroscein succimidyl ester (CFSE) dilution assay, greater proliferation was reproducibly observed at 72 hours on softer surfaces. The difference in proliferation became more pronounced as the coating concentration of the goat-anti-mouse capture antibody decreased below 1 μg/mL with the proliferation completely lost on harder surfaces at coated with antibodies at low concentration (0.1 μg/mL Fig. 1E).
Polyclonal expansion of peripheral blood T cells is enhanced by culture on softer substrates
The demonstration that anti-CD3 and anti-CD28 immobilized on PDMS substrates with a lower elastic modulus stimulate greater IL-2 secretion and short-term proliferation suggested that manipulation of substrate rigidity could be a useful parameter in the ex vivo expansion of T cells. We therefore evaluated the ability of PDMS substrates to support more long-term proliferation of T cells. We observed a graded increase in overall polyclonal expansion of naïve CD4+ T cells across PDMS substrates with decreasing Young’s elastic modulus (Fig. 2A). On average, a 4-fold increase in overall culture yield is observed when comparing the softest PDMS substrate (1:50 cross-linker to base ratio) to the stiffest PDMS substrate (1:5 cross-linker to base ratio). The difference in overall expansion is primarily due to a more prolonged log-phase growth rather than a difference in the rate of proliferation (Fig 2B). This enhanced log-phase expansion correlates with a more prolonged blast-phase as shown by the increase in mean cellular volume during the course of the culture (Fig 2C). Similar to IL-2 secretion (Fig. 1D), CD4+ T cells cultured on PDMS surfaces, but activated by anti-CD3 and anti-CD28 immobilized on microbeads rather than PDMS, demonstrate comparable proliferation supporting the non-toxic nature of PDMS and the dependency on PDMS immobilization for the observed enhancement in the T cell proliferative response (Fig. 2D). Experiments were also performed using soluble anti-CD3 and anti-CD28 antibodies with goat-anti-mouse IgG coated PDMS surfaces to create an equilibrium binding state that avoids the loss of antibody from pre-coated surfaces. A similar ~4-fold enhanced CD4+ T cell expansion was observed with the softer surfaces supporting an effect of PDMS rigidity rather than differential antibody binding between the PDMS surfaces (Supplemental Fig. 1).
Figure 2. Human naïve CD4+ T cell proliferation is enhanced by softer surfaces.
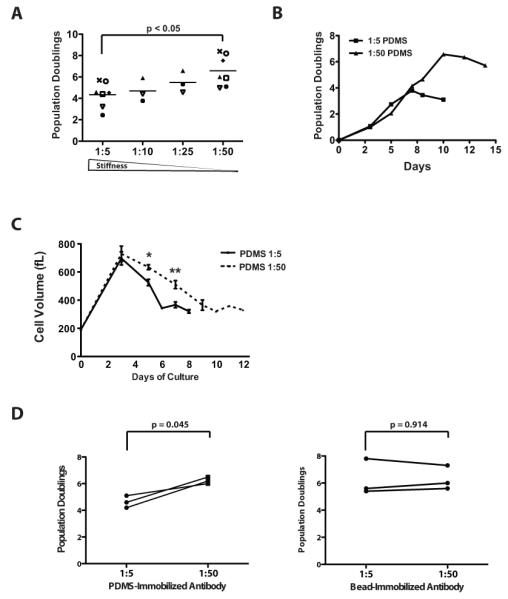
(A) CD45RO-depleted CD4+ T cells were stimulated on anti-CD3 and anti-CD28 coated PDMS surfaces of varying rigidity for three days prior to transfer to uncoated culture vessels. Cell enumeration was performed every other day beginning on day 3 until the number of cells in the culture ceased increasing and the mean cell volume is below 350 fL. The maximum number of population doublings is plotted with the horizontal bars representing the mean and each symbol representing a separate donor. Data were analyzed by a one-way ANOVA (p = 0.0261). All groups were compared using a Newman-Keuls multiple comparison test with the difference between the 1:5 and 1:50 group statistically significant as indicated. (B) CD45RO-depleted CD4+ T cells were stimulated as in panel A. Cells were enumerated at the indicated time points. Data presented is representative of 7 independent experiments with separate donors. (C) The mean cell volume of a mixed population of T cells expanded on the PDMS surfaces as indicated. Data were analyzed by a one-way ANOVA and a Newman-Keuls multiple comparison test for post-hoc analysis. Values represent means ± S.D. from 6 independent healthy donors (*P<0.05; **p<0.05). (D) CD4+ T cells were stimulated with anti-CD3 and anti-CD28 antibodies immobilized on either PDMS or 4.5 μm microbeads as indicated in the figure with each line representing the results from a separate donor. Culture was performed as in panel A. Data represent the maximum number of population doublings achieved following a single round of stimulation. The number of population doublings for cells cultured on soft or stiff PDMS surfaces was compared under each stimulation condition using a paired, two-tailed Student’s t-test with the indicated p-value.
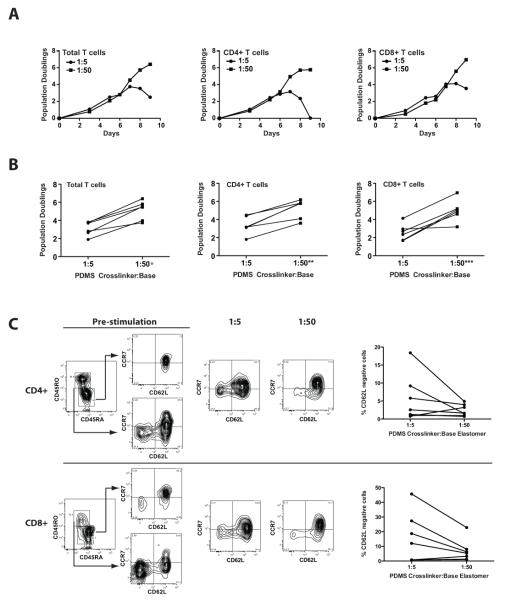
Given the significant enhancement in polyclonal expansion of naïve CD4+ T cells, we evaluated the ex vivo expansion of a mixed population of CD4+ and CD8+ peripheral blood T cells, a population of cells that is more reflective of the starting population currently being employed for many adoptive immunotherapy studies. Consistently, the softer surface also supported an average 4-fold increase in overall T cell expansion compared with stiffer substrate (Fig 3A and 3B). This effect was observed in both CD4+ and CD8+ T cells. Since the increase in expansion for a mixed population of cells could be explained by differential expansion of individual T cell subsets, we evaluated the surface phenotype of cells prior to expansion and at the end of the log phase of expansion. Table 1 shows that the ratio of CD4 to CD8 T cells were comparable between both conditions of rigidity following expansion. The surface expression of CD62L, a cell surface marker routinely used to distinguish memory from effector T cell subsets also exhibited a trend (non-significant, p=0.0745 for CD8+ T cells and p=0.1821 for CD4+ T cells) toward more CD62L-negative cells following expansion on stiffer surfaces, as shown in Fig 3C. We further evaluated the phenotypic and functional characteristics of T cells expanded on soft and stiff PDMS surfaces to examine regulatory T cells, effector function and T cell exhaustion. Neither T cells derived from cultures on soft or stiff PDMS surfaces exhibit a regulatory phenotype based upon flow cytometric markers of regulatory T cells (Supplementary Figure 2). The Treg cell-specific demethylated region (TSDR) in the FOXP3 locus promoter was hypomethylated following expansion of CD4+ T cells on both PDMS surfaces. CD4+CD25+ and CD4+CD25- T cells expanded from either PDMS surfaces also failed to suppress conventional T cell proliferation when cocultured with PBMC’s from a separate donor. Although the effector phenotype of the T cells was highly variable between donors, the frequency of cells expressing IFN-γ and TNF-α following activation (Supplementary Figure 3A & 3B) and perforin (< 5% positive in CD4+ T cells and > 70% positive in CD8+ T cells, data not shown) are largely similar between cells cultured on soft and stiff surfaces. The cells also showed similar low expression of markers of T cell exhaustion including 2B4, Lag-3, and PD-1 (Supplementary Figure 3C & 3D).
Figure 3. The expansion of mixed human peripheral blood T cells is enhanced on soft substrates.
(A) Representative growth curves for total T cells, CD4+ and CD8+ T cells expanded on 1:5 and 1:50 PDMS coated with OKT3 and clone 9.3 in X-VIVO 15 culture medium. CD4+ and CD8+ T cells were enumerated by flow cytometry as described in the materials and methods. (B) The overall number of population doublings achieved for total T cells, CD4+, and CD8+ T cells stimulated by 1:5 or 1:50 PDMS coated with OKT3 and clone 9.3. Results are derived from 6 separate experiments using T cells from independent healthy donors. The 1:5 and 1:50 conditions were compared using a Paired, two-tailed Student’s t-test (*P<0.0008 for total T cells grown on a soft surface relative to hard; **P<0.0013 for CD4+ T cells grown on a soft surface relative to hard; ***P<0.0036 for CD8+ T cells grown on a soft surface relative to hard). (C) Representative data on the expression of the memory markers CD62L and CCR7 in T cell subsets prior to expansion and following expansion on PDMS substrates. The graphs to the right of the flow cytometry plots show a summary of the percent of CD4+ or CD8+ T cells lacking CD62L expression at the end of the culture period. All cultures began with a mixed population of human peripheral blood CD4+ and CD8+ T cells from 7 separate healthy donors.
Table I: Phenotypic analysis of mixed lymphocytes following expansion on stimulatory PDMS surfaces
|
Start of culture |
PDMS 1:5 | PDMS 1:50 | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Donor | CD4+(%) | CD8+ (%) | CD4+ (%) | CD8+ (%) | CD4+ (%) | CD8+ (%) |
| 1 | 44.2 | 53.7 | 25.5 | 70.0 | 56 | 43.7 |
| 2 | 65.9 | 33.8 | 37.6 | 62.4 | 45.4 | 53.5 |
| 3 | 48.6 | 49.5 | 27.6 | 70.7 | 20.3 | 78.9 |
| 4 | 65.5 | 29.5 | 94.6 | 6.11 | 81 | 17.9 |
| 5 | 73.7 | 25.4 | 90.5 | 9.39 | 83 | 16.8 |
| 6 | 67.8 | 31.8 | 82.4 | 17.3 | 78.8 | 20.7 |
These findings show that a simple decrease in elastic modulus of the substrate used to immobilize T cell activating antibodies can significantly enhance the T cell activation and proliferative response to these signals. Furthermore, the effects of stiffness are not subset dependent, with similar effects observed in both CD4+ and CD8+ T cells.
Softer substrates stimulate greater IL-2 secretion primarily through enhanced transcription
Autocrine production of IL-2 promotes clonal expansion of T cells. Correlating with the enhanced proliferation observed in T cells stimulated with softer substrates, we observed a 4-fold increase in IL-2 production between mixed T cells stimulated on softer compared with stiffer substrates (Fig. 4A). The regulation of IL-2 gene expression is well described to occur at both the level of transcriptional activation and post-transcriptional RNA stability [22]. We therefore investigated the effect of substrate rigidity on IL-2 mRNA expression and stability. To address IL-2 mRNA stability, we took advantage of the specific inhibition of IL-2 transcription afforded by the calcineurin inhibitor, cyclosporine A (CsA)[23], in addition to the less-specific transcriptional inhibitor, actinomycin D. While the expression of IL-2 mRNA was significantly higher at 6 hours on softer substrates (Fig 4B), the kinetics of mRNA decay following transcriptional arrest by CsA were strikingly similar on both soft and rigid substrates (Fig. 4C). Comparable results were also obtained with actinomycin D (data not shown). These findings indicate that the enhanced IL-2 secretion by T cells stimulated on softer substrates is primarily due to enhanced transcription at the IL-2 gene rather than a difference in the post-transcriptional regulation of IL-2 mRNA stability.
Figure 4. Softer substrates increase IL-2 expression independent of mRNA stability.
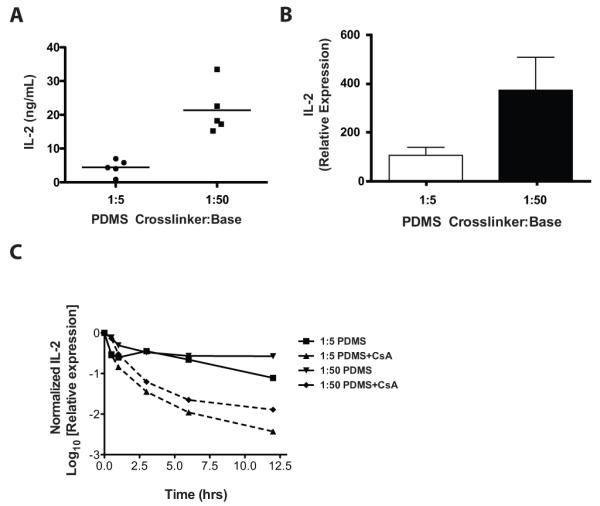
(A) Cellular supernatants were collected from mixed populations of human T cells grown on PDMS surfaces coated with OKT3 and clone 9.3 for 24 hrs in RPMI culture medium. IL-2 was measured by a human IL-2 specific ELISA. The results shown are derived from 5 separate healthy donors. (B) CD4+ T cells were stimulated on PDMS substrates coated with OKT3 and clone 9.3. Cells were harvested after 20-24 hrs and IL-2 mRNA expression was measured by qRT-PCR analysis following normalization to β-actin as described in the materials and methods. Values represent the means ± S.D. from four independent experiments. (C) T cells were stimulated on PDMS substrates coated with OKT3 and clone 9.3. After 6 hrs, cyclosporine A was added (t=0 hrs) to the culture to inhibit IL-2 transcription. IL-2 mRNA was measured by qRT-PCR analysis with normalization to the t=6 hrs mRNA expression for each PDMS condition.
Softer substrates enhance naïve CD4+ T cell Th1-like differentiation
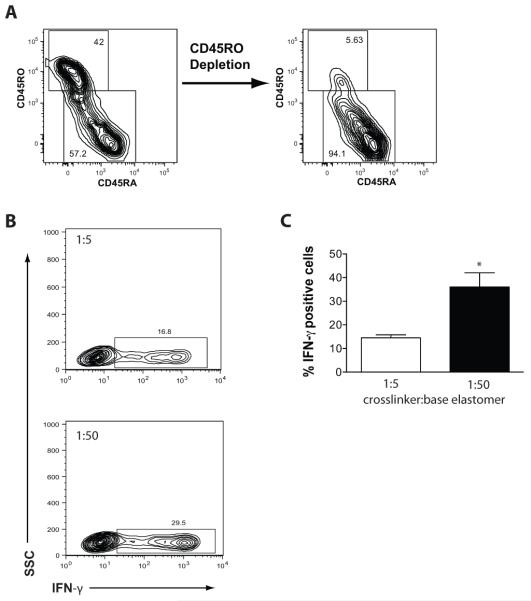
The impact of softer substrates on T cell activation and proliferation led us to consider whether substrate rigidity might also alter CD4+ T cell differentiation since the “strength” of the TCR signal has been reported to affect helper T cell differentiation. The anti-CD3 and anti-CD28 conditions of activation used in our study have been reported to generate cells of a primarily Th1-like phenotype[24]. We therefore evaluated the frequency of IFN-γ-producing T cells following expansion of naïve CD4+ T cells. Naïve CD4+ T cells isolated by magnetic bead separation were highly enriched for cells with a CD45RA+, CD62L+ phenotype (Fig. 5A). Following expansion on either the soft or stiff surfaces, the cells exhibited comparable proportions of CD62L+ and CCR7+ cells (data not shown); however, the cells expanded on soft surfaces exhibited a 3-fold increase in the proportion of cells capable of producing IFN-γ (Fig. 5B and 5C). Combined with the observed enhancement in expansion, softer surfaces produce an ~1-log greater number of IFN-γ-producing T cells. Greater than 80% of T cells produce IFN-γ, independent of culture surface in the presence of exogenous IL-12, demonstrating that T cells expanded on stiff substrates are capable of differentiation (data not shown). Based upon these results, we conclude that substrate rigidity might be a useful parameter for controlling CD4+ T cell expansion, particularly in mixed T cell cultures where the cytokine milieu may be difficult to control.
Figure 5. Stimulatory substrate rigidity influences the frequency of IFN-γ producing cells derived from naïve CD4+ T cell cultures.
(A) CD45RO-depletion generates CD4+ T cells with a homogenous CD45RA+ naïve phenotype. Data are representative of the minimum purity from experiments with three independent donors. (B) Naïve CD4+ T cells were stimulated on OKT3 and clone 9.3 coated PDMS surfaces in RPMI culture medium and expanded until termination of log phase growth. The T cells were restimulated with PMA and ionomycin for 5 hrs in presence of GolgiStop followed by paraformaldehyde fixation, permeabilization and staining for intracellular IFN-γ. The flow cytometry plots shown are representative of 3 independent donors. (C) The mean percentage of IFN-γ positive cells (± SEM) from three independent experiments with separate donors as analyzed in panel B. The 1:5 and 1:50 conditions were significantly different when compared using a two-tailed Student’s t-test (*p = 0.0219).
Discussion
This report is the first study to our knowledge demonstrating that the elastic modulus of a cell culture surface influences the activation, proliferation, and differentiation of T cells in ways that could be advantageous for adoptive immunotherapy. Previous studies established that T cells discriminate between surface-bound TCR/CD3 complex ligands and their corresponding soluble forms[8, 12]. Our work extends this current body of knowledge, providing evidence that the mechanical properties of the underlying surface used for immobilization is also important, potentially modifying the forces that are generated by T cells and/or the forces sensed within the immune synapse affecting the T cell activation process.
PDMS was selected for our studies due to its established biocompatibility and stability along with the ease by which its mechanical properties can be modified through variation of the crosslinker-to-base-polymer ratio. It is possible that additional material properties of PDMS change with the variation in crosslinker ratio. Protein adsorption to a surface is highly dependent upon both the hydrophobicity and electrostatic properties of the surface[25]. PDMS is well known to be highly hydrophobic, with a surface hydrophobicity, as measured by the contact angle of water, that changes little with variation in the crosslinker-to-base-polymer ratio[26]. We demonstrate comparable antibody adsorption and stability across the crosslinker-to-base ratios used in our study, which is largely consistent with other studies of PDMS surface hydrophobicity and passive adsorption of protein[27]. Brown et al., using a layer-by-layer polyanion coating on PDMS with variable stiffness, reported some differences in surface roughness and water contact angle following their surface treatment. These differences appeared to impact vascular smooth muscle cell spreading in the absence of serum; however, cell spreading was similar when serum was present [28].
Physical forces at the TCR/CD3 complex have been linked to intracellular signaling events. Dynamic imaging studies of T cells interacting with supported planar bilayers and solid glass supports demonstrate that TCR microclusters and signaling complexes initially form at the periphery of the immune synapse [19]. This peripheral area of the immune synapse is rich in actin and myosin, with force exerted on many adhesive receptor-ligand interactions by actin-driven, lamellipodial extension of the T cell membrane as well as myosin-driven contraction of the actin network[9]. The importance of actin-driven force in the generation of TCR signal transduction is highlighted by studies demonstrating that inhibition of actin polymerization affects T cell activation [12, 19, 29, 30]. Inhibition of myosin light chain kinase by blebbistatin or the depletion of myosin IIA has also been demonstrated to severely inhibit T cell receptor signaling, supporting a critical role for cell-generated force in the T cell activation process[10].
Changes in the stiffness of the TCR/CD3 ligand support substrate would be expected to dampen the forces applied to individual adhesive receptor-ligand interactions. The range of force necessary to induce signal transduction by the TCR compared with the force necessary to disrupt the mechanical linkage between the receptor and its ligand is currently unknown; however, bond strength and bond lifetime change with the application of a loading force[31]. While the bond between avidin and biotin (Kd of ~10−15) is one of the strongest non-covalent associations, with a lifetime (1/koff) on the order of ~109 seconds, this bond’s lifetime is reduced dramatically under load to <1 sec with a pN-range load[32]. Much lower forces are therefore expected to have significant effects on the lifetime of bonds between antibodies or TCRs and their ligands, which are several orders of magnitude weaker in their binding affinity. While the softer surfaces used in our studies have sufficient stiffness to trigger TCR signaling, the net effect of the softer surface may be to provide prolongation of receptor-ligand binding and signaling leading to more effective stimulation. Unfortunately, the testing of this hypothesis requires the ability to evaluate dynamic signaling in the context of measuring the lifespan of receptor-ligand interactions and/or molecular-scale forces, both of which are currently technically challenging in the context of living cells.
While mechanical force appears to play a central role in the activation and maintenance of TCR signal transduction, it may also modify downstream signal transduction by the TCR and other receptors affecting immune synapse formation and stability. Focal adhesions (FA) in cells such as fibroblasts depend upon mechanical tension applied to adhesion sites for their assembly[4, 5, 33, 34]. Many of the same proteins that regulate FA assembly, such as Pyk2, FAK, p130Cas, paxillin, vinculin and talin are also present within T cells at the immunological synapse (IS)[35-39]. The presence of these proteins, and other mechano-sensitive proteins within the IS, provide mechanism(s) by which forces at the synapse, modified by the substrate rigidity, could influence T cell signal transduction, synapse formation and stability and the activation process.
It is possible that the observed effect of modifying the rigidity of T cell activating substrates could also be relevant in vivo. Cytoskeletal changes within dendritic cells are reported to alter the process of T cell activation[40-43]. MHC molecules and co-stimulatory ligands such as CD80 also appear to be anchored to the dendritic cell cytoskeleton[44, 45]. Since the actin cytoskeleton represents a gel with viscoelastic properties not unlike PDMS, modification of the cytoskeleton may be a mechanism used by dendritic cells to alter synapse formation, dynamics and ultimately the T cell activation process. Cells attached to solid substrates assume a cytoskeletal rigidity that is proportional to the rigidity of their attachment substrate[17]. Although yet to be evaluated, the pliancy and tension of the surrounding ECM within a lymph node likely change quite dramatically during the course of an immune response as the lymph node undergoes marked hyperplasia and distention. Studies to evaluate the dynamic mechanical properties of the ECM within a lymph node or lymphoid tissue during an immune response are currently lacking, but should be an area of future study.
Our observation that substrate rigidity affects the TH-cell differentiation of T cells could be explained by a number of factors. Although cytokines certainly play a critical role, differentiation of TH-cells towards different fates also depends upon TCR ligand density and duration of signaling [46-49]. Cell-ECM interactions have also been linked to the control of gene expression in cells. The PDZ-domain-containing transcription factors YAP and TAZ were recently demonstrated to be important nuclear mediators of ECM-stiffness-induced mesenchymal cell differentiation[50]. These pathways or other mechanosensitive pathways could affect lymphocyte differentiation in ways that have not been previously considered, and may depend upon the mechanical properties of culture systems employed to study T cells.
In addition to the fundamental importance of our findings to the basic study of T cells, a culture system with altered mechanical properties may also have useful applications to the field of adoptive T cell immunotherapy. Anti-CD3 and anti-CD28 antibodies immobilized either on planar plastic surfaces or plastic microbeads are a commonly employed system for activating T cells ex vivo[3, 24]. Efficient expansion of T cells, especially from patients with cancer, represents a significant challenge. The observation of enhanced expansion on softer surfaces with retention of a mostly CD62Lhi memory-like phenotype suggests that a softer substrate might increase the feasibility of adoptive immunotherapy for more patients, especially in challenging diseases like leukemia where few peripheral blood T cells are available in the circulation for expansion. The increased frequency of IFN-γ-producing cells observed with softer substrates also suggests that T cells expanded on a softer surface may have improved function following adoptive therapy in cancer. Th1-differentiated, IFN-γ-producing cells have been shown in pre-clinical immunotherapy models to be important for efficacy[51].
In summary, these data highlight a novel role for the elastic modulus of a T cell culture surface, a previously unrecognized culture parameter for lymphocytes. Using PDMS elastomers we demonstrate that stimulatory substrate rigidity can be controlled to effect changes in T cells of biologic importance. Although not directly evaluated in this study, these data also support the role of force in T cell activation by their antigen receptor. We provide evidence that PDMS, a biocompatible polymer, could be used as a platform for the ex vivo culture of T cells for adoptive immunotherapy with potential advantages over currently used rigid plastic surfaces.
Supplementary Material
Acknowledgments
We would like to thank Dr. Carl June at the University of Pennsylvania and Dr. Michael Dustin at New York University for their helpful discussions and critiques of the manuscript. We would also like to thank Nicholas Vena, Christina Fang, and Trevor Cassidy for their technical assistance with these studies. In addition, we would like to thank Korey Demers, Carolina Martinez, and Michael Betts for assistance with flow cytometry.
Footnotes
This work was funded by the National Institutes of Health (NIH) Common Fund Nanomedicine program (PN2 EY016586).
References
- 1.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117(6):1466–76. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117(5):1204–12. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruisbeek A, Shevach E, Thornton A. Proliferative Assays for T Cell Function. In: Coligan JE, editor. Current protocols in immunology. John Wiley and Sons; New York: p. v. loose leaf. [DOI] [PubMed] [Google Scholar]
- 4.Balaban NQ, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3(5):466–72. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 5.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88(1):39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 6.Engler AJ, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert PM, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078–81. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geppert TD, Lipsky PE. Accessory cell independent proliferation of human T4 cells stimulated by immobilized monoclonal antibodies to CD3. J Immunol. 1987;138(6):1660–6. [PubMed] [Google Scholar]
- 9.Sims TN, et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129(4):773–85. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Ilani T, et al. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat Immunol. 2009;10(5):531–9. doi: 10.1038/ni.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valitutti S, et al. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995;181(2):577–84. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Z, et al. Surface-anchored monomeric agonist pMHCs alone trigger TCR with high sensitivity. PLoS Biol. 2008;6(2):e43. doi: 10.1371/journal.pbio.0060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim ST, et al. The alphabeta T cell receptor is an anisotropic mechanosensor. J Biol Chem. 2009;284(45):31028–37. doi: 10.1074/jbc.M109.052712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YC, et al. Cutting Edge: mechanical forces acting on T cells immobilized via the TCR complex can trigger TCR signaling. J Immunol. 2010;184(11):5959–63. doi: 10.4049/jimmunol.0900775. [DOI] [PubMed] [Google Scholar]
- 15.Xu C, et al. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell. 2008;135(4):702–13. doi: 10.1016/j.cell.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–59. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 17.Tee SY, et al. Cell shape and substrate rigidity both regulate cell stiffness. Biophys J. 2011;100(5):L25–7. doi: 10.1016/j.bpj.2010.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez TS, et al. HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity. 2006;24(6):741–52. doi: 10.1016/j.immuni.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202(8):1031–6. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sneddon I. The Relation between Load and Penetration in the Axisymmetric Boussinesq Problem for a Punch of Arbitrary Profile. Int J Eng Sci. 1965;3:47–57. [Google Scholar]
- 21.Androit M, et al. Silicones in Industrial Applications. In: Jaeger RD, Gleria M, editors. Inorganic polymers. Nova Science Publishers; New York: 2007. pp. 61–161. [Google Scholar]
- 22.Lindsten T, et al. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244(4902):339–43. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- 23.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357(6380):695–7. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 24.Levine BL, et al. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159(12):5921–30. [PubMed] [Google Scholar]
- 25.Nakanishi K, Sakiyama T, Imamura K. On the adsorption of proteins on solid surfaces, a common but very complicated phenomenon. J Biosci Bioeng. 2001;91(3):233–44. doi: 10.1263/jbb.91.233. [DOI] [PubMed] [Google Scholar]
- 26.Mata A, Fleischman AJ, Roy S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed Microdevices. 2005;7(4):281–93. doi: 10.1007/s10544-005-6070-2. [DOI] [PubMed] [Google Scholar]
- 27.Gray DS, Tien J, Chen CS. Repositioning of cells by mechanotaxis on surfaces with micropatterned Young’s modulus. J Biomed Mater Res A. 2003;66(3):605–14. doi: 10.1002/jbm.a.10585. [DOI] [PubMed] [Google Scholar]
- 28.Brown XQ, Ookawa K, Wong JY. Evaluation of polydimethylsiloxane scaffolds with physiologically-relevant elastic moduli: interplay of substrate mechanics and surface chemistry effects on vascular smooth muscle cell response. Biomaterials. 2005;26(16):3123–9. doi: 10.1016/j.biomaterials.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Rivas FV, et al. Actin cytoskeleton regulates calcium dynamics and NFAT nuclear duration. Mol Cell Biol. 2004;24(4):1628–39. doi: 10.1128/MCB.24.4.1628-1639.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bunnell SC, et al. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity. 2001;14(3):315–29. doi: 10.1016/s1074-7613(01)00112-1. [DOI] [PubMed] [Google Scholar]
- 31.Evans E. Probing the relation between force--lifetime--and chemistry in single molecular bonds. Annu Rev Biophys Biomol Struct. 2001;30:105–28. doi: 10.1146/annurev.biophys.30.1.105. [DOI] [PubMed] [Google Scholar]
- 32.Merkel R, et al. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature. 1999;397(6714):50–3. doi: 10.1038/16219. [DOI] [PubMed] [Google Scholar]
- 33.Riveline D, et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153(6):1175–86. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfenson H, et al. Actomyosin-generated tension controls the molecular kinetics of focal adhesions. J Cell Sci. 2011;124(Pt 9):1425–32. doi: 10.1242/jcs.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berg NN, Ostergaard HL. T cell receptor engagement induces tyrosine phosphorylation of FAK and Pyk2 and their association with Lck. J Immunol. 1997;159(4):1753–7. [PubMed] [Google Scholar]
- 36.Simonson WT, Franco SJ, Huttenlocher A. Talin1 regulates TCR-mediated LFA-1 function. J Immunol. 2006;177(11):7707–14. doi: 10.4049/jimmunol.177.11.7707. [DOI] [PubMed] [Google Scholar]
- 37.Collins M, Bartelt RR, Houtman JC. T cell receptor activation leads to two distinct phases of Pyk2 activation and actin cytoskeletal rearrangement in human T cells. Mol Immunol. 2010;47(9):1665–74. doi: 10.1016/j.molimm.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Robertson LK, Mireau LR, Ostergaard HL. A role for phosphatidylinositol 3-kinase in TCR-stimulated ERK activation leading to paxillin phosphorylation and CTL degranulation. J Immunol. 2005;175(12):8138–45. doi: 10.4049/jimmunol.175.12.8138. [DOI] [PubMed] [Google Scholar]
- 39.Nolz JC, et al. WAVE2 regulates high-affinity integrin binding by recruiting vinculin and talin to the immunological synapse. Mol Cell Biol. 2007;27(17):5986–6000. doi: 10.1128/MCB.00136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aldinucci A, et al. Inhibition of immune synapse by altered dendritic cell actin distribution: a new pathway of mesenchymal stem cell immune regulation. J Immunol. 2010;185(9):5102–10. doi: 10.4049/jimmunol.1001332. [DOI] [PubMed] [Google Scholar]
- 41.Benvenuti F, et al. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305(5687):1150–3. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- 42.Al-Alwan MM, et al. Cutting edge: dendritic cell actin cytoskeletal polarization during immunological synapse formation is highly antigen-dependent. J Immunol. 2003;171(9):4479–83. doi: 10.4049/jimmunol.171.9.4479. [DOI] [PubMed] [Google Scholar]
- 43.Al-Alwan MM, et al. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166(3):1452–6. doi: 10.4049/jimmunol.166.3.1452. [DOI] [PubMed] [Google Scholar]
- 44.Doty RT, Clark EA. Subcellular localization of CD80 receptors is dependent on an intact cytoplasmic tail and is required for CD28-dependent T cell costimulation. J Immunol. 1996;157(8):3270–9. [PubMed] [Google Scholar]
- 45.Tseng SY, Liu M, Dustin ML. CD80 cytoplasmic domain controls localization of CD28, CTLA-4, and protein kinase Ctheta in the immunological synapse. J Immunol. 2005;175(12):7829–36. doi: 10.4049/jimmunol.175.12.7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Constant S, et al. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182(5):1591–6. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosken NA, et al. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182(5):1579–84. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogers PR, Huston G, Swain SL. High antigen density and IL-2 are required for generation of CD4 effectors secreting Th1 rather than Th0 cytokines. J Immunol. 1998;161(8):3844–52. [PubMed] [Google Scholar]
- 49.Rogers PR, Croft M. Peptide dose, affinity, and time of differentiation can contribute to the Th1/Th2 cytokine balance. J Immunol. 1999;163(3):1205–13. [PubMed] [Google Scholar]
- 50.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 51.Nishimura T, et al. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol. 2000;46(Suppl):S52–61. doi: 10.1007/pl00014051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.