Introduction
One of the most pervasive disorders of pregnancy is preeclampsia, which is defined as new-onset hypertension presenting after the 20th week of gestation, and is accompanied by increasing levels of proteinuria and often edema (1). The overall prevalence of preeclampsia is ~8% with higher incidence in specific ethnic subpopulations, notably African-Americans (2–3). The disorder is predominantly a complication of primiparous women, which have a three-fold higher incidence rate than multiparous women. There are a number of known factors which convey elevated risk of developing preeclampsia, notably elevated BMI, and a previous incidence of preeclampsia, as women who experienced preeclampsia in prior pregnancies are seven times more likely to exhibit preeclamptic symptoms in subsequent pregnancies than those with no history of preeclampsia (4). It has also been shown that high BMI can double the risk of preeclampsia, and the scale of obesity correlates directly with the increase risk of developing the disorder (5). While the link between these risk factors appear logical, there are also a number of other risk factors (maternal age, induced abortions, interpregnancy interval, and socioeconomic factors) whose links to preeclampsia are not as clear (6). More research is warranted into the mechanistic effect of these factors in the etiology of preeclampsia.
There are currently no definitive treatment options for the resolution of preeclampsia. Available interventions are confined to magnesium sulfate for the prophylaxis of seizures and the administration of one of various anti-hypertensive agents, though normalization of blood pressure is not normally possible, and the disease continues to progress (1). Indeed the only effective resolution of preeclampsia is delivery of the placenta, as delivery of the fetus alone is not sufficient (7–8). It is this fact which suggested the pathological origins of the development of preeclampsia. While the underlying molecular mechanisms which lie at the root of preeclampsia are not clear, it is believed that a major causative agent is placental insufficiency resulting from inadequate remodeling of the maternal vasculature (9). During normal pregnancy, fetally derived cytotrophoblasts invade the maternal spiral arteries of the uterus, replace the maternal endothelium, and undergo differentiation into an endothelial-like phenotype. This causes a conversion of the high-resistance, small-diameter vessels into high-capacitance, low-resistance vessels and ensures adequate delivery of maternal blood to the developing uteroplacental unit (7, 10). In the preeclamptic patient, unknown errors in this carefully orchestrated scheme lead to inadequate delivery of blood to the developing uteroplacental unit, and create hypoxia and chronic ischemia within the placenta (11). In response the placenta produces pathogenic factors which enter the maternal blood stream and are responsible for the clinical manifestations of the disorder. Among the factors known to be released, anti-angiogenic and autoimmune/inflammatory factors have received a great deal of attention. Interestingly, recent evidence suggests that these agents have a final common pathway-activation of the endothelin-1 system.
There are several lines of evidence which suggest a pathogenic role for ET-1 in the preeclamptic patient. A number of studies looking at circulating levels of ET-1 in preeclamptic women have generally demonstrated significantly higher levels of circulating ET-1 in the plasma of preeclamptic patients when compared to healthy controls, with some suggestion that genetic polymorphism could play a role in variation of ET-1 levels. (12–16). However, circulating levels of ET-1 are not necessarily good indicators of ET-1 production in the tissues due the fact that ET-1 secretion is directional, with a larger proportion of the peptide being released on the basolateral side of the endothelium, and tissue levels may not be accurately reflected in the levels of ET-1 in the circulation. Studies indicating elevations in ET-1 in preeclamptic placental cells are perhaps more telling, though this is not a universal finding (17–18). Finally, one report has indicated an increase in endothelin converting enzyme (ECE) in the circulation of preeclamptic patients compared to normal pregnant controls, suggesting a potential mechanism for enhanced local production of ET-1 (19). This data supports earlier studies demonstrating increased ECE production by endothelial cells exposed to preeclamptic serum (20). There are also indications that matrix metalloproteinase-2 (MMP-2), which can also cleave big endothelin into ET-1, is significantly elevated in preeclamptic women (21–22). These findings suggest at least a correlation between ET-1 and preeclampsia in humans, and warrant further study.
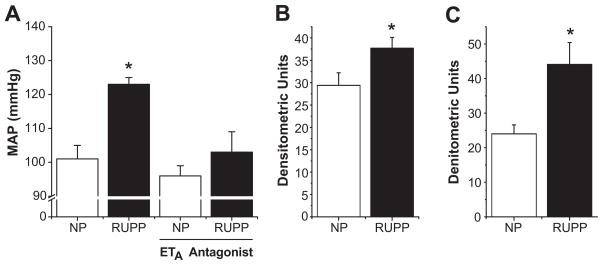
As studies on pregnant women are at best complicated, a number of experimental animal models have been utilized to examine the etiology and development of preeclampsia. One which we and others have used with great success is the reduced uterine perfusion pressure (RUPP) model, which mechanically restricts blood flow to the placenta, leading to hypoxia and ischemia. This model has been used in species ranging from rats to non-human primates, and mimics a number of the pathological features of human preeclampsia, including hypertension, angiogenic imbalance, renal injury, proteinuria, and endothelial dysfunction (23–25). In studies where human endothelial cells were exposed to serum from RUPP rats in vitro, it was shown that RUPP sera significantly induced production of ET-1 from the endothelial cells when compared to those exposed to serum from normal pregnant animals, suggesting that circulating factors produced by placental ischemia are responsible for increased vascular ET-1 production (26). Initial in vivo studies suggested that both the renal cortex and medulla of RUPP rats express significantly higher levels of the ET-1 precursor, preproendothelin, at the mRNA level when compared to normal pregnant controls (Figure 1b–c). Crucially, when an ETA receptor antagonist was administered to the RUPP rats, the associated hypertension was abolished (Figure 1a), and there was a trend for increased renal function (27). These studies suggest that placental ischemia induces factors which activate the production of ET-1 in the vasculature, which acts through ETA receptors to mediate the hypertension seen in this model. Whether similar ETA antagonism could prove effective in preeclamptic women remains to be seen.
Figure 1.
Effect of ETA antagonism on RUPP induced hypertension. As seen in panel A, RUPP treated animals exhibit significant hypertension when compared to normal pregnant controls. This hypertensive response is completely blocked by administration of an ETA selective antagonist. In both the renal cortex (B) and medulla (C), RUPP treatment significantly increases the expression of preproendothelin relative to β-actin mRNA as determined by RNAse protection assays. (* = p<0.05). Adapted from reference (27).
sFlt-1
One of the most intensely studied pathways in the manifestation of preeclampsia is the vascular endothelial growth factor (VEGF) signaling pathway. VEGF, besides its role in angiogenesis has an important role in the maintenance of proper endothelial cell function and health. This signaling pathway came to prominence with the discovery of elevated circulating and placental levels of the soluble form of the VEGF receptor Flt-1, denominated sFlt-1 (28–29). sFlt-1 acts as a direct inhibitor of VEGF by binding to the protein and preventing it from being available for its normal function (30). Subsequent studies looking at the regulation of sFlt-1 in cell culture and placental tissue in vitro have demonstrated that sFlt-1 is released from placental villi and trophoblast cells in response to reduced oxygen tensions similar to that seen in the ischemic placenta (31–33). A promising recent pilot study demonstrated that sFlt-1 could be removed from the maternal circulation by apheresis safely, and that this therapy reduced both blood pressure and proteinuria, with a trend toward increased gestational duration (34).
A number of groups have also reported that artificial elevation of sFlt-1 in animal models, either by direct infusion or by viral overexpression, leads to a hypertension, renal injury, and proteinuria, all symptomatic of preeclampsia (29, 35–41). These results are supported by clinical data showing that patients receiving the anti-VEGF antibody therapy Bevacizumab, which functions in much the same way as sFlt-1, exhibit marked proteinuria and hypertension (42). Kappers et al also reported that Sunitinib a tyrosine kinase inhibitor of the vascular endothelial growth factor receptor induces a reversible rise in BP in patients and in rats associated with activation of the endothelin-1 system and generalized microvascular dysfunction (43). Finally, the same group recently reported that VEGF inhibition with Sunitinib in pigs results in endothelin mediated hypertension in pigs (44). This suggests another potential mechanism whereby VEGF blockade could increase BP is by enhancing ET-1 synthesis.
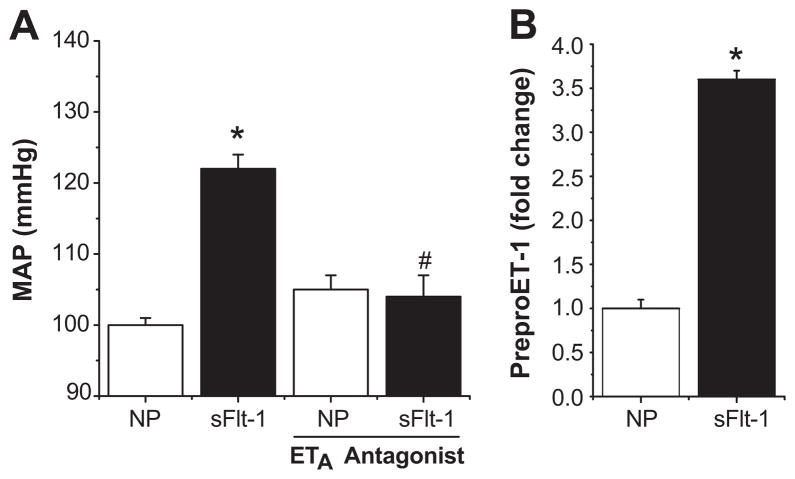
Several reports have been made into the role of the ET-1 system in models of sFlt-1 overexpression in pregnancy (29, 35–36). In support of ET-1 as a mediator of sFlt-1 induced hypertension, our group has recently reported that continuous infusion of sFlt-1 in pregnant rats directly increased the level of Et-1 in the renal cortex, and resulted in an increase in the mean arterial pressure of ~20mmHg (Figure 2). Tellingly, with co-administration of an ETA receptor antagonist, the hypertension associated with this model was completely abolished, strongly supporting ET-1 as an important mediator of sFlt-1 induced hypertension (36).
Figure 2.
sFlt-1 induces hypertension through ET-1 induction. Infusion of sFlt-1 significantly increases MAP in pregnant rats. This hypertensive response is completely blunted with administration of an ETA selective antagonist (A). Production of preproendothelin mRNA is significantly increased in the renal cortex by sFlt-1 infusion (B). (* = p<0.05 compared to controls, # = p<0.05 compared to sFlt-1 infused rats) Adapted from reference (36).
Autoimmune Factors
One of the earliest and most persistent theories about the origins of preeclampsia view it as a disorder of immunity. In fact there is a growing realization that autoimmunity plays a pivotal role in the symptomatic phase of the disorder and perhaps the early etiology of the disease as well (45–47). The autoimmune components of preeclampsia can be compartmentalized into two headings: the production of auto-antibodies and the innate immune response. In the first category, much attention has been focused on the production of agonistic angiotensin II type-1 receptor auto antibodies (AT1-AA) which have been found in the circulation of women with preeclampsia, and verified in several experimental models. Even more clearly characterized is the innate inflammatory response mediated by inflammatory cytokines. The importance of these two components has begun to be elucidated and several experimental approaches have been used to understand their role in the development of preeclampsia.
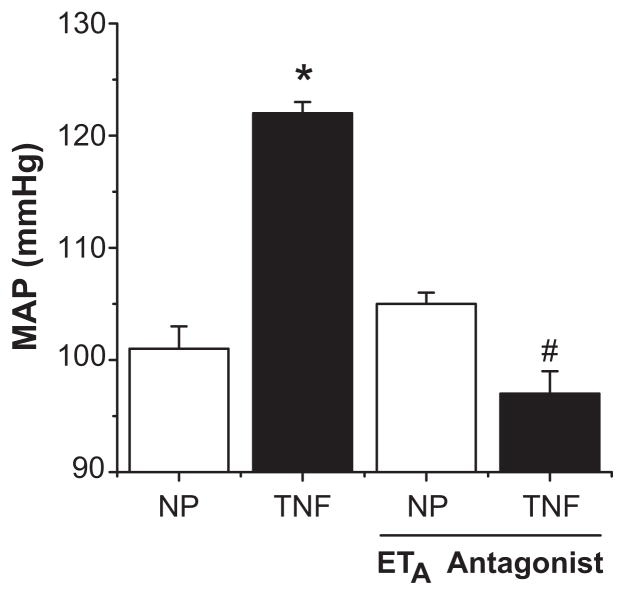
One well-characterized component of the innate immune response to preeclampsia is the production of tumor necrosis factor-α (TNF-α) which is elevated in both preeclamptic women and rodents undergoing chronic placental ischemia (48–50). Prior studies in vitro demonstrated that production of ET-1 by endothelial cells could be driven by exposure to TNF-α (51). Studies from our group have demonstrated that administration of the soluble TNF-α receptor Etanercept is capable of attenuating the hypertension associated with placental ischemia in pregnant rats. This treatment is associated with reduced expression of the preproendothelin in the renal cortex and medulla as well as the placenta itself (50). It has also been shown that infusion of TNF-α directly induced hypertension in pregnant rats, producing an approximate 20mmHg increase in mean arterial pressure in late gestation. This is associated with a significant increase in the expression of preproendothelin in the maternal vasculature, placenta, and kidney. As in the RUPP model, co-administration of an ETA receptor antagonist in these animals completely abolished the associated hypertension (Figure 3) (52). Together, these data suggest that TNF-α is an important component of the hypertensive response to placental ischemia, and that ET-1, acting through the ETA receptor is a crucial mediator of TNF-α induced hypertension.
Figure 3.
TNF-α induces hypertension through ET-1 induction. Infusion of TNF-α significantly increases MAP in pregnant rats. This hypertensive response is completely blunted with administration of an ETA selective antagonist. (* = p<0.05 compared to controls, # = p<0.05 compared to TNF-α infused rats) Adapted from reference (52).
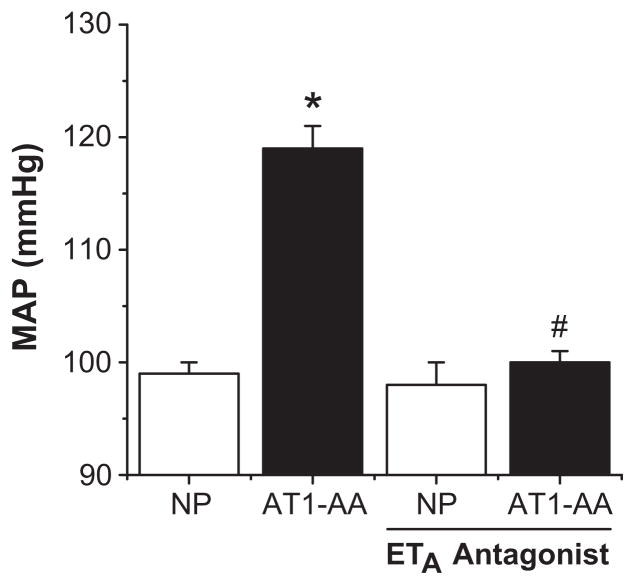
A relatively recent addition to the immune component of preeclampsia is the agonist AT1-AA. These antibodies were originally isolated just over a decade ago in preeclamptic women, and have since been found in the circulation of rats undergoing placental ischemia (53–54). Interestingly, these antibodies appear to be induced by the production of TNF-α, as infusion of TNF-α to pregnant rats also results in production of the antibody at levels comparable to that seen in pregnant women and the RUPP rat (54). It has also been demonstrated that infusion of the AT1-AA directly into pregnant rats results in moderate hypertension that is associated with increased preproendothelin expression in both the renal cortex and placenta. Again, administration of an ETA receptor antagonist abrogated the hypertensive response to the AT1-AA, suggesting its importance in the manifestation of AT1-AA induced hypertension (Figure 4) (55). However, the pathogenic importance of these antibodies remains to be fully elucidated, as their presence has been noted post-partum in a subset of preeclamptic patients with no discernible phenotype (56–57). It is likely then that these antibodies act more as modulators of the hypertensive phenotype begun by other pathogenic factors rather than first-cause agents directly, possibly by enhancing the response of the ET-1 system. Further studies are needed to determine how these antibodies interact with the other pathogenic agents in preeclampsia to produce the clinical phenotype.
Figure 4.
AT1-AA induces hypertension through ET-1 induction. Infusion of AT1-AA significantly increases MAP in pregnant rats, an effect which is blocked by an ETA selective antagonist. (* = p<0.05 compared to controls, # = p<0.05 compared to AT1-AA infused rats) Adapted from reference (55).
Perspective
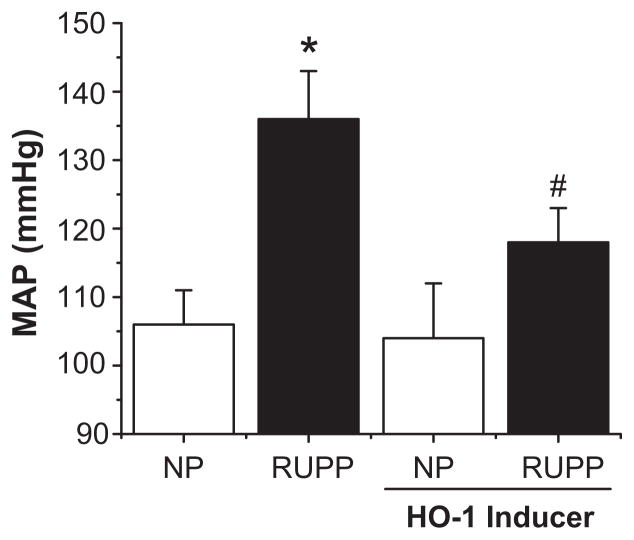
Given the myriad of experimental models of preeclampsia (placental ischemia, sFlt-1 infusion, TNF-α infusion, and AT1-AA infusion) which have proven susceptible to ETA antagonism, could the ET-1 system be a therapeutic approach in managing the hypertension associated with preeclampsia? Excitement at this approach has been tempered by work showing that genetic knockout of the ETA receptor leads to birth defects and eventual embryonic lethality in mice (58). As a result, administration of endothelin receptor antagonists is contraindicated in pregnancy (59). However, studies which have examined pharmacological antagonism of the ETA receptor during pregnancy in rats have identified specific windows of development in early and mid-gestation in which the agents caused phenotypes similar to that seen in the knockout. Administration of the ETA antagonist only during late gestation was not performed, and it is entirely possible that ETA receptor antagonists might prove safe and efficacious in later pregnancy, when the symptoms of preeclampsia are most severe (60). We have also recently demonstrated that induction of heme oxygenase-1 in both placental ischemia and sFlt-1-induced hypertension significantly blunts blood pressure, in part by reducing vascular production of preproendothelin-1, suggesting another mechanism through which the ET-1 system could be targeted for the management of preeclampsia (Figure 5) (61–62). Alternatively, development of ETA receptor antagonists which do not cross the placental barrier would circumvent these problems altogether. Further work to determine the transmission of existing agents as well as the development of new antagonists could provide a truly effective therapy for the management of hypertension in the preeclamptic patient.
Figure 5.
HO-1 induction blocks RUPP induced hypertension. RUPP treated rats exhibit significant elevations in MAP when compared to normal pregnant controls. This hypertensive response is significantly attenuated by systemic induction of heme oxygenase-1, which acts in part by reduction of vascular ET-1. (* = p<0.05 compared to normal controls, # = p<0.05 compared to RUPP animals). Adapted from reference (61).
Acknowledgments
Sources of Funding
This work was supported by NIH grants HL51971, HL108618-01 1T32HL105324–01, and a postdoctoral fellowship from the American Heart Association (11POST7840039)
Footnotes
Conflicts of Interest
None
References
- 1.Turner JA. Diagnosis and management of pre-eclampsia: an update. Int J Womens Health. 2010;2:327–337. doi: 10.2147/IJWH.S8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 3.Knuist M, Bonsel GJ, Zondervan HA, Treffers PE. Risk factors for preeclampsia in nulliparous women in distinct ethnic groups: a prospective cohort study. Obstet Gynecol. 1998;92:174–178. doi: 10.1016/s0029-7844(98)00143-4. [DOI] [PubMed] [Google Scholar]
- 4.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaillard R, Steegers EA, Hofman A, Jaddoe VW. Associations of maternal obesity with blood pressure and the risks of gestational hypertensive disorders. The Generation R Study. J Hypertens. 2011;29:937–944. doi: 10.1097/HJH.0b013e328345500c. [DOI] [PubMed] [Google Scholar]
- 6.Trogstad L, Magnus P, Stoltenberg C. Pre-eclampsia: Risk factors and causal models. Best Pract Res Clin Obstet Gynaecol. 2011;25:329–342. doi: 10.1016/j.bpobgyn.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Hladunewich M, Karumanchi SA, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol. 2007;2:543–549. doi: 10.2215/CJN.03761106. [DOI] [PubMed] [Google Scholar]
- 8.Shembrey MA, Noble AD. An instructive case of abdominal pregnancy. Aust N Z J Obstet Gynaecol. 1995;35:220–221. doi: 10.1111/j.1479-828x.1995.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 9.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- 10.Khong Y, Brosens I. Defective deep placentation. Best Pract Res Clin Obstet Gynaecol. 2011;25:301–311. doi: 10.1016/j.bpobgyn.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Rajakumar A, Doty K, Daftary A, Harger G, Conrad KP. Impaired oxygen-dependent reduction of HIF-1alpha and -2alpha proteins in pre-eclamptic placentae. Placenta. 2003;24:199–208. doi: 10.1053/plac.2002.0893. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RN, Varma M, Teng NN, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J Clin Endocrinol Metab. 1990;71:1675–1677. doi: 10.1210/jcem-71-6-1675. [DOI] [PubMed] [Google Scholar]
- 13.Baksu B, Davas I, Baksu A, Akyol A, Gulbaba G. Plasma nitric oxide, endothelin-1 and urinary nitric oxide and cyclic guanosine monophosphate levels in hypertensive pregnant women. Int J Gynaecol Obstet. 2005;90:112–117. doi: 10.1016/j.ijgo.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Nishikawa S, Miyamoto A, Yamamoto H, Ohshika H, Kudo R. The relationship between serum nitrate and endothelin-1 concentrations in preeclampsia. Life Sci. 2000;67:1447–1454. doi: 10.1016/s0024-3205(00)00736-0. [DOI] [PubMed] [Google Scholar]
- 15.Maltaris T, Scalera F, Schlembach D, Hoffmann I, Mueller A, Binder H, Goecke T, Hothorn T, Schild RL, Beckmann MW, Dittrich R. Increased uterine arterial pressure and contractility of perfused swine uterus after treatment with serum from pre-eclamptic women and endothelin-1. Clin Sci (Lond) 2005;109:209–215. doi: 10.1042/CS20040340. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal PK, Jain V, Srinivasan R, Jha V. Maternal EDN1 G5665T polymorphism influences circulating endothelin-1 levels and plays a role in determination of preeclampsia phenotype. J Hypertens. 2009;27:2044–2050. doi: 10.1097/HJH.0b013e32832f7f3f. [DOI] [PubMed] [Google Scholar]
- 17.Faxen M, Nasiell J, Lunell NO, Blanck A. Differences in mRNA expression of endothelin-1, c-fos and c-jun in placentas from normal pregnancies and pregnancies complicated with preeclampsia and/or intrauterine growth retardation. Gynecol Obstet Invest. 1997;44:93–96. doi: 10.1159/000291494. [DOI] [PubMed] [Google Scholar]
- 18.Napolitano M, Miceli F, Calce A, Vacca A, Gulino A, Apa R, Lanzone A. Expression and relationship between endothelin-1 messenger ribonucleic acid (mRNA) and inducible/endothelial nitric oxide synthase mRNA isoforms from normal and preeclamptic placentas. J Clin Endocrinol Metab. 2000;85:2318–2323. doi: 10.1210/jcem.85.6.6623. [DOI] [PubMed] [Google Scholar]
- 19.Ajne G, Wolff K, Fyhrquist F, Carlstrom K, Hemsen-Mortberg A, Nisell H. Endothelin converting enzyme (ECE) activity in normal pregnancy and preeclampsia. Hypertens Pregnancy. 2003;22:215–224. doi: 10.1081/PRG-120024025. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa S, Miyamoto A, Yamamoto H, Ohshika H, Kudo R. Preeclamptic serum enhances endothelin-converting enzyme expression in cultured endothelial cells. Am J Hypertens. 2001;14:77–83. doi: 10.1016/s0895-7061(00)01244-9. [DOI] [PubMed] [Google Scholar]
- 21.Narumiya H, Zhang Y, Fernandez-Patron C, Guilbert LJ, Davidge ST. Matrix metalloproteinase-2 is elevated in the plasma of women with preeclampsia. Hypertens Pregnancy. 2001;20:185–194. doi: 10.1081/PRG-100106968. [DOI] [PubMed] [Google Scholar]
- 22.Myers JE, Merchant SJ, Macleod M, Mires GJ, Baker PN, Davidge ST. MMP-2 levels are elevated in the plasma of women who subsequently develop preeclampsia. Hypertens Pregnancy. 2005;24:103–115. doi: 10.1081/PRG-200059836. [DOI] [PubMed] [Google Scholar]
- 23.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 24.Abitbol MM, Ober MB, Gallo GR, Driscoll SG, Pirani CL. Experimental toxemia of pregnancy in the monkey, with a preliminary report on renin and aldosterone. Am J Pathol. 1977;86:573–590. [PMC free article] [PubMed] [Google Scholar]
- 25.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977–984. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 26.Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006;47:615–618. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- 27.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37:485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 28.Signore C, Mills JL, Qian C, Yu K, Lam C, Epstein FH, Karumanchi SA, Levine RJ. Circulating angiogenic factors and placental abruption. Obstet Gynecol. 2006;108:338–344. doi: 10.1097/01.AOG.0000216014.72503.09. [DOI] [PubMed] [Google Scholar]
- 29.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu FT, Stefanini MO, Mac Gabhann F, Kontos CD, Annex BH, Popel AS. A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. J Cell Mol Med. 2010;14:528–552. doi: 10.1111/j.1582-4934.2009.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 32.Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, Kozuma S, Taketani Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838–4845. doi: 10.1210/en.2004-0533. [DOI] [PubMed] [Google Scholar]
- 33.Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, Zamudio S, Caniggia I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1085–1093. doi: 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thadhani R, Kisner T, Hagmann H, Bossung V, Noack S, Schaarschmidt W, Jank A, Kribs A, Cornely OA, Kreyssig C, Hemphill L, Rigby AC, Khedkar S, Lindner TH, Mallmann P, Stepan H, Karumanchi SA, Benzing T. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation. 2011;124:940–950. doi: 10.1161/CIRCULATIONAHA.111.034793. [DOI] [PubMed] [Google Scholar]
- 35.Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens. 2009;22:564–568. doi: 10.1038/ajh.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension. 2010;55:394–398. doi: 10.1161/HYPERTENSIONAHA.109.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, Lam A, O’Young G, Sannajust F, Stathis P, Schreiner G, Karumanchi SA, Protter AA, Pollitt NS. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50:686–692. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki H, Ohkuchi A, Matsubara S, Takei Y, Murakami M, Shibuya M, Suzuki M, Sato Y. Effect of recombinant placental growth factor 2 on hypertension induced by full-length mouse soluble fms-like tyrosine kinase 1 adenoviral vector in pregnant mice. Hypertension. 2009;54:1129–1135. doi: 10.1161/HYPERTENSIONAHA.109.134668. [DOI] [PubMed] [Google Scholar]
- 39.Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, da Costa Gonzalves AC, Grone HJ, Ahmed A, Weich HA. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension. 2010;55:380–385. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sunderland N, Hennessy A, Makris A. Animal models of pre-eclampsia. Am J Reprod Immunol. 2010 doi: 10.1111/j.1600-0897.2010.00929.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 43.Kappers MH, Smedts FM, Horn T, van Esch JH, Sleijfer S, Leijten F, Wesseling S, Strevens H, Jan Danser AH, van den Meiracker AH. The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension. 2011;58:295–302. doi: 10.1161/HYPERTENSIONAHA.111.173559. [DOI] [PubMed] [Google Scholar]
- 44.Kappers MH, de Beer VJ, Zhou Z, Danser AH, Sleijfer S, Duncker DJ, van den Meiracker AH, Merkus D. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension. 2012;59:151–157. doi: 10.1161/HYPERTENSIONAHA.111.182220. [DOI] [PubMed] [Google Scholar]
- 45.Xia Y, Kellems RE. Is preeclampsia an autoimmune disease? Clin Immunol. 2009;133:1–12. doi: 10.1016/j.clim.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol Aspects Med. 2007;28:192–209. doi: 10.1016/j.mam.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Schiessl B. Inflammatory response in preeclampsia. Mol Aspects Med. 2007;28:210–219. doi: 10.1016/j.mam.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 1994;170:1752–1757. discussion 1757–1759. [PubMed] [Google Scholar]
- 49.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102:20–25. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 50.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52:1161–1167. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marsden PA, Brenner BM. Transcriptional regulation of the endothelin-1 gene by TNF-alpha. Am J Physiol. 1992;262:C854–861. doi: 10.1152/ajpcell.1992.262.4.C854. [DOI] [PubMed] [Google Scholar]
- 52.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46:82–86. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- 53.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension. 2008;52:1168–1172. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54:905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hubel CA, Wallukat G, Wolf M, Herse F, Rajakumar A, Roberts JM, Markovic N, Thadhani R, Luft FC, Dechend R. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension. 2007;49:612–617. doi: 10.1161/01.HYP.0000256565.20983.d4. [DOI] [PubMed] [Google Scholar]
- 57.Stepan H, Faber R. Elevated sFlt1 level and preeclampsia with parvovirus-induced hydrops. N Engl J Med. 2006;354:1857–1858. doi: 10.1056/NEJMc052721. [DOI] [PubMed] [Google Scholar]
- 58.Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- 59.Kingman M, Ruggiero R, Torres F. Ambrisentan, an endothelin receptor type A-selective endothelin receptor antagonist, for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2009;10:1847–1858. doi: 10.1517/14656560903061275. [DOI] [PubMed] [Google Scholar]
- 60.Taniguchi T, Muramatsu I. Pharmacological knockout of endothelin ET(A) receptors. Life Sci. 2003;74:405–409. doi: 10.1016/j.lfs.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 61.George EM, Cockrell K, Aranay M, Csongradi E, Stec DE, Granger JP. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension. 2011;57:941–948. doi: 10.1161/HYPERTENSIONAHA.111.169755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.George EM, Arany M, Cockrell K, Storm MV, Stec DE, Granger JP. Induction of heme oxygenase-1 attenuates sFlt-1-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1495–1500. doi: 10.1152/ajpregu.00325.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]