Abstract
The antimicrobial agents triclosan (TCS), triclocarban (TCC) and their associated transformation products are of increasing concern as environmental pollutants due to their potential adverse effects on humans and wildlife, including bioaccumulation and endocrine-disrupting activity. Analysis by tandem mass spectrometry of 24 paired freshwater bed sediment samples (top 10 cm) collected by the U.S. Geological Survey near 12 wastewater treatment plants (WWTPs) in Minnesota revealed TCS and TCC concentrations of up to 85 and 822 ng/g dry weight (dw), respectively. Concentrations of TCS and TCC in bed sediments collected downstream of WWTPs were significantly greater than upstream concentrations in 58% and 42% of the sites, respectively. Dichloro- and non-chlorinated carbanilides (DCC and NCC) were detected in sediments collected at all sites at concentrations of up to 160 and 1.1 ng/g dw, respectively. Overall, antimicrobial concentrations were significantly higher in lakes than in rivers and creeks, with relative abundances decreasing from TCC > TCS > DCC > NCC. This is the first statewide report on the occurrence of TCS, TCC and TCC transformation products in freshwater sediments. Moreover, the results suggest biological or chemical TCC dechlorination products to be ubiquitous in freshwater environments of Minnesota, but whether this transformation occurs in the WWTP or bed sediment remains to be determined.
Keywords: Antimicrobial, Reductive Dechlorination, Personal Care Products, Surface Water, Pollution
1. Introduction
Triclosan (5-chloro-2-(2,4-dichlorophenoxy)phenol; TCS) and triclocarban (3,4,4′-trichlorocarbanilide; TCC) are widely used as antimicrobial agents in personal care products at levels of up to 2% and 0.3% (w/w), respectively [1, 2]. Both antimicrobials can be absorbed through the skin during intended use of antimicrobial personal care products and both have been detected in human plasma, urine, and milk [3-6]. Concentrations as low as 0.07 and 0.61 mg/L of TCS have been shown to disturb metabolic systems, immune function, and hormone production in rats and humans, respectively [7-10]. The acceptable daily intake for TCS is proposed to be as low as 1.9 × 10-4 mg kg-1 day-1 for humans [11]. These studies raise concern about the impacts of TCS on fetal and infant development. TCC is known to interfere with mammalian reproduction and can cause the blood disorder, methemoglobinemia, in humans [12-14]. It is also associated with the amplification of transcriptional activity of steroid sex hormones in the estrogen and androgen receptors of humans [15]. Both TCS and TCC have been shown to bioaccumulate in aquatic species [16-18]. Historical discharge of these antimicrobials in aquatic environments has resulted in the detection of concentrations as high as 80 μg/L TCS in fish bile and 39.9 ng/g TCC in fish muscle tissue [18, 19]. Other studies associate TCS with estrogenic effects, reduced testosterone levels, and endocrine disruption in aquatic animals at concentrations between 0.03 and 29 μg/L [20-23].
TCS and TCC are some of the most frequently detected chemicals in streams across the U.S. [24, 25]. Influent wastewater concentrations as high as 4,700 ng/L for TCS and 6,100 ng/L for TCC have been reported [26, 27]. TCS and TCC have low solubility in water (1.97 – 4.6 mg/L for TCS and 0.65 – 1.55 mg/L for TCC at 25°C) and high n-octanol-water partition coefficients (log KOW of 4.8 for TCS and 4.9 for TCC pH 7.0) and as a result tend to accumulate in biosolids and sediments at μg/g levels [24]. A mass balance analysis of these chemicals in a conventional wastewater treatment plant showed that, owing to their hydrophobic nature, about 31% (TCS) and 76% (TCC) of the influent mass accumulated in the processed wastewater sludge [26-28]. Concentrations as high as 30,000 ng/g for TCS and 51,000 ng/g for TCC were detected in biosolids [26, 27]. Land application of these biosolids and wastewater effluent discharge into surface water forms a secondary pathway of environmental exposure to these antimicrobials.
Due to their trichlorinated aromatic structure, both compounds are fairly resistant to biodegradation and can persist for extended periods, even decades, in the environment [29]. Significant levels of TCS and TCC are being discharged every day from WWTPs across the United States, resulting in the contamination of receiving surface water and sediments. One study reported significant levels of TCS and TCC in dated estuarine sediment cores in Chesapeake Bay, Maryland and Jamaica Bay, New York [29]. Another study reported TCS in dated sediment cores from Lake Greifensee, Switzerland, the concentration of which trended with the use of TCS in the study area [30]. However, there is a lack of data on antimicrobial compound occurrence and levels in freshwater sediments. Yet, such information is essential in understanding the relevance of point vs. non-point sources, environmental loading, geographic variability and the fate of these compounds under WWTP and environmental conditions.
Though TCC is very persistent in the environment, the detection of lower chlorinated carbanilides like dichlorocarbanilide (DCC), monochlorocarbanilide (MCC), and non-chlorinated carbanilide (NCC) in the environment suggests possible reductive dechlorination. One study in a tributary to the Chesapeake Bay, Maryland observed significant quantities of DCC, MCC, and NCC in aged, deep estuarine sediments suggesting dechlorination of TCC in situ [29]. Results showed that dechlorination products of TCC occurred only in strictly anaerobic zones of deep sediments; no significant amounts of dechlorination products were observed in partially oxygenated bed sediments [29]. Although the specific mechanism of TCC dechlorination is yet to be identified, the results from that study suggested possible involvement of dehalorespiring microorganisms in the reductive dechlorination of TCC. Hence, more research focusing on the lesser chlorinated carbanilides in the environment is necessary to understand the occurrence and relevance of this putative in situ mechanism for attenuation of TCC, a compound that is more abundant and more persistent than the intensely studied antimicrobial compound TCS. Moreover, the environmental fate and toxicity of the dechlorination products of TCC are unknown.
During 2009, the U.S. Geological Survey (USGS), St. Cloud State University, the University of St. Thomas, and the Minnesota Pollution Control Agency conducted a statewide study of Minnesota for the occurrence of endocrine active chemicals, pharmaceuticals, and other chemicals in surface waters, wastewater treatment plant (WWTP) effluents, and bed sediments [31]. In the present study, bed sediment samples collected upstream and downstream from 12 WWTPs by the USGS were analyzed for the antimicrobial compounds TCS, TCC and its lesser chlorinated congeners (DCC and NCC). To the best of our knowledge, this is the first statewide study to find these antimicrobial compounds and their putative transformation products in freshwater bed sediments.
2. Methods
2.1. Chemicals
High purity standards of non-labeled TCS, TCC and NCC, and High Pressure Liquid Chromatography (HPLC) grade solvents were purchased from Sigma Aldrich (Milwaukee, WI). A DCC standard was purchased from Oakwoods products, Inc. (West Columbia, SC). Isotope-labeled standards 13C13-TCC and 13C12-TCS were purchased from Wellington Laboratories Inc. (Guelph, Canada).
2.2. Study area
Sediment grab samples were collected from the upper 10 cm of the bed surface at sites upstream and downstream from 12 WWTPs (Figure 1) using methods described elsewhere [31]. All the samples were paired as upstream and downstream in relation to the associated WWTP. Samples were collected from rivers (Mississippi, Sauk, South Fork of the Crow, and Grindstone), creeks (Center, Okabena) and lakes (Pepin, Superior, Shagawa). The land use, wastewater treatment techniques, and surface water receiving the WWTP discharge varied widely in their characteristics at different sampling sites [31]. Site characteristics and other physicochemical properties (pH, temperature, etc.) at the time of sampling are provided as supplemental information (Table S1 – S2).
Figure 1.
Map of Minnesota showing the different sampling locations. Each symbol represents two adjacent sites located upstream and downstream of wastewater treatment plant (WWTP) effluent discharge locations.
2.3. Sample preparation and analysis
Sediment aliquots were stored at -20°C until use and dried at 103°C prior to solvent extraction. Dryness was established through gravimetric monitoring of the sediment samples. 13C13 –TCC and 13C12 – TCS surrogates were then spiked to the dried sediments to correct for analyte recovery. Analytes were extracted from sediments by adding 3 mL of organic solvent (50:50 mix of acetone/methanol containing 10 mM acetic acid) per g of dried sediment and by placing the capped extraction vial horizontally on a rotary shaker for 3 hours at 150 rpm. The sample was decanted, the organic extracts were concentrated to dryness, reconstituted with 1.5 mL acetonitrile, filtered (0.2 μm PTFE, 13 mm syringe filters, VWR International, LLC, PA), diluted to 50% (v/v) water content, and analyzed using isotope dilution liquid chromatography negative electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS). Mass spectrometric analyses were carried out on an API 4000 instrument (Applied Biosystems, Framingham, MA, USA), coupled to a Shimadzu Prominence HPLC (Shimadzu Scientific Instruments, Inc., Columbia, MD, USA) and controlled by Analyst 1.5 software (Applied Biosystems, Framingham, MA, USA). Separation was carried out using an IBD C18 column (5 μm particle size, 2.1 × 150 mm; Restek Corporation, Bellefonte, PA). The isocratic mobile phase consisted of 60% acetonitrile and 40% water flowing at a rate of 400 μL/min with a total runtime of 12 min. Analytes were introduced into the mass spectrometer using an electrospray ionization probe operating in negative mode. Multiple reaction monitoring (MRM) was used for qualitative analysis. Optimized conditions for the ionization and fragmentation of the analytes are specified in supplementary table (Table S3). All concentrations are reported on dry weight (dw) basis. Quality assurance and quality control protocols were followed as described previously [1].
The tandem mass spectrometry method yielded method detection limits (MDLs) of 0.0003, 0.22, 0.01, and 0.003 ng/g dw for TCC, TCS, DCC, and NCC, respectively. The respective limits of quantifications (LOQs) were 0.002, 1.12, 0.04, and 0.01 ng/g dw. All MDLs were determined based on EPA guidelines described in 40 CFR 136, Appendix B [32]. The loss of TCS and TCC from sediments during extraction was corrected for using isotope labeled internal standards, and the calculated average recoveries were 81 ± 20% and 88 ± 23%, respectively. Because no labeled standards were available for DCC or NCC, their recoveries were determined from spiking experiments described elsewhere [29]. Average recovery rates were found to be 68 ± 5% and 79 ± 11% for DCC and NCC, respectively, but were not corrected for.
2.4. Statistical analysis
In order to determine the interrelations in the analytical dataset of detected antimicrobial concentrations and various physicochemical properties of the studied locations, a principal component analysis (PCA) was performed using version 19 of the IBM SPSS software package (IBM, Armonk, New York, U.S.A.).
3. Results and Discussion
3.1. Antimicrobial contamination of bed sediments
TCS concentration in surface sediments varied from 0.4 to 85 ng/g dw (n = 24) and was detected above LOQ in all samples analyzed (Figure 2). Concentrations of TCC ranged between 5 and 822 ng/g dw; about one order of magnitude higher than TCS in 63% of samples analyzed (Figure 2). The ratio of TCC to TCS levels in sediments varied between 3:1 (Melrose) and 58:1 (Lake City), showing the abundance of TCC contamination over TCS in freshwater sediments. DCC and NCC were detected in all the samples above LOQ at concentrations ranging from 0.05 to 160 ng/g dw and 0.021 to 1.14 ng/g dw, respectively (Figure 2). DCC and NCC were detected, on average, one order and three orders of magnitude lower than TCC, respectively. The relative abundance of these antimicrobials in bed sediments were in the order TCC>TCS>DCC>NCC in 92% of the sites. Antimicrobial concentrations in sediment from Shagawa Lake (Ely) and St. Louis Bay at Lake Superior (Duluth) in the area of influence of WWTP discharge locations were more than three times higher than those observed in river and creek sediments. The downstream site of Duluth (St. Louis Bay at Lake Superior) near the WWTP had the greatest concentrations of TCS, TCC, and DCC with concentrations of 85, 822, and 160 ng/g dw, respectively.
Figure 2.
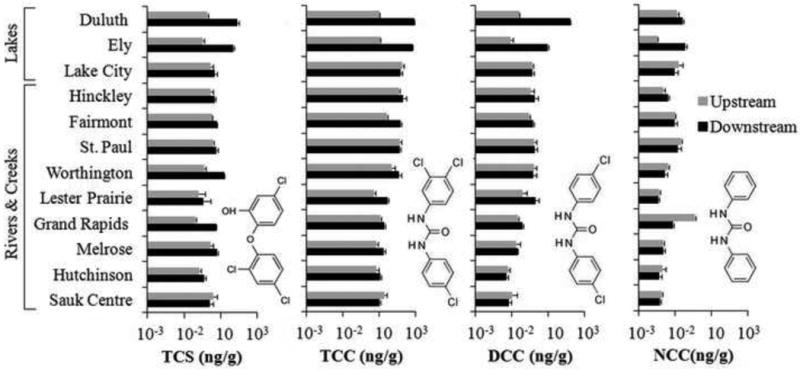
Concentrations of triclosan (TCS), triclocarban (TCC), dichlorocarbanilide (DCC), and carbanilide (NCC) in bed sediments located up- and downstream of WWTP discharge locations. Error bars represent the standard deviation of analyte concentrations determined in triplicate independent extractions. MDLs for TCC, TCS, DCC, and NCC by LC-ESI-MS/MS were 0.0003, 0.22, 0.01, and 0.003 μg/kg-dw, respectively.
3.2. Comparison of antimicrobial contamination upstream and downstream of WWTPs
A two tailed t-test was performed to determine whether statistically significant differences existed between antimicrobial concentrations measured in sediment upstream and downstream of WWTP discharge locations. The downstream TCS and TCC sediment concentration was significantly greater than upstream concentration (p<0.05) for 58% (7 out of 12) and 42% (5 out of 12) of the sites, respectively (Figure 2). DCC showed a similar trend with downstream sediment concentration greater in 42% of the sites, in the same locations as TCC (Grand Rapids, Fairmont, Lester Prairie, Ely, and Duluth). NCC concentration in downstream sites was significantly higher in 2 sites (Hinckley and Ely), and interestingly lower in 2 sites (Sauk Centre and Grand Rapids) when compared to upstream locations. The type of surface water system receiving treated wastewater also seemed to dictate contamination levels found in sediments. Biocide concentrations in lake sediments collected in the area of influence of WWTP discharge locations were, on average, about 8 times higher than the concentrations observed in any river or creek sediments downstream of WWTP discharges (Figure 2).
The aqueous phase concentrations of TCS and TCC were provided previously [31] and are summarized in Figure 3. TCS and TCC concentrations were consistently higher in WWTP effluent when compared to upstream and downstream surface water. This suggests WWTPs to be a significant point-source of antimicrobial contamination in downstream surface water and associated sediments. To obtain a perspective on the interrelations in the analytical dataset, PCA was performed in clustering mode based on the correlations between analyte concentrations at different sampling locations [using surface water, WWTP effluent concentration of TCS and TCC, and the sediment concentration (upstream and downstream) of all analytes (TCS, TCC, DCC, and NCC)] (Figure 4A). The first two principal components (PC1 and PC2) explained the highest amount of variance in the dataset and when combined accounted for 57.5% of the total observed variability. The PCA plot reveals that TCS and TCC concentration in downstream sediments are clustered together with WWTP effluent concentration of TCS, thus indicating a correlation between downstream sediment contamination with WWTP discharge. A stronger correlation is seen for TCS when compared to TCC (Figure 4A). Also, downstream sediment concentrations of NCC and DCC nearly superimposed with downstream TCC sediment concentration, indicating little variance and thus suggesting a strong correlation between these analytes. However, significant levels of sediment contamination upstream of some WWTPs, especially for TCC, suggest potential contribution from other point and non-point sources not included in this study. For example, TCC concentrations of more than 100 ng/g dw were detected in sediments upstream of WWTPs in St. Paul, Fairmont, and Lake City. These high levels were comparable with the associated downstream TCC sediment concentration at those sites.
Figure 3.
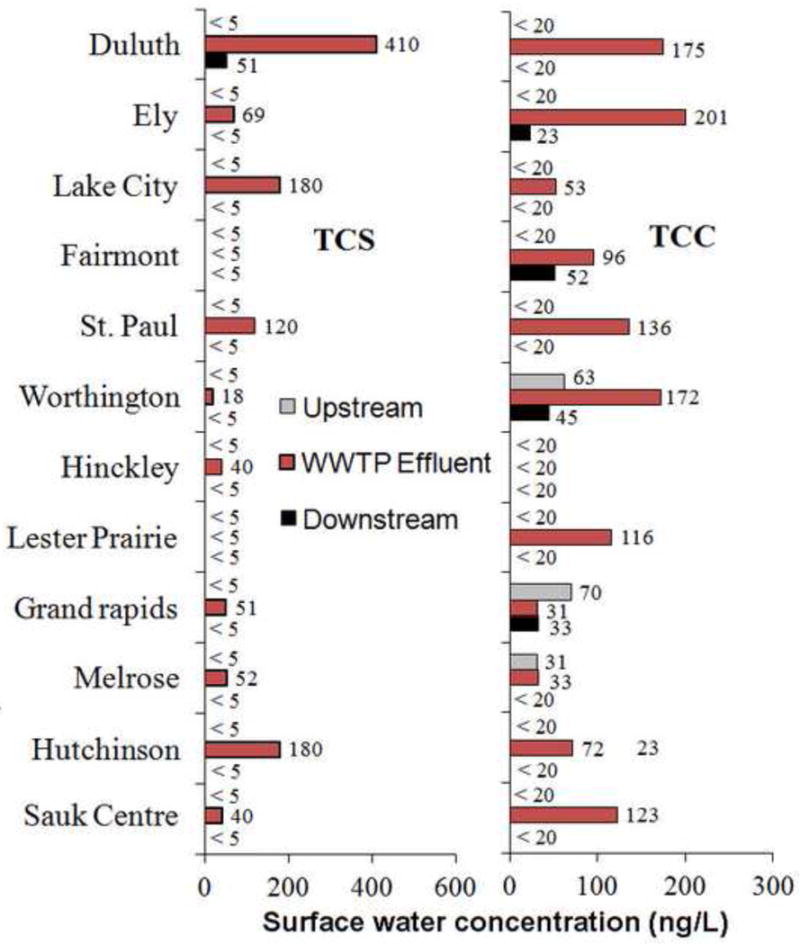
Concentrations of triclocarban (TCC) and triclosan (TCS) in Minnesota surface waters and WWTP effluents at locations where bed sediments were collected [31]. Absence of bars represents non-detect values (below the respective MDL stated).
Figure 4.
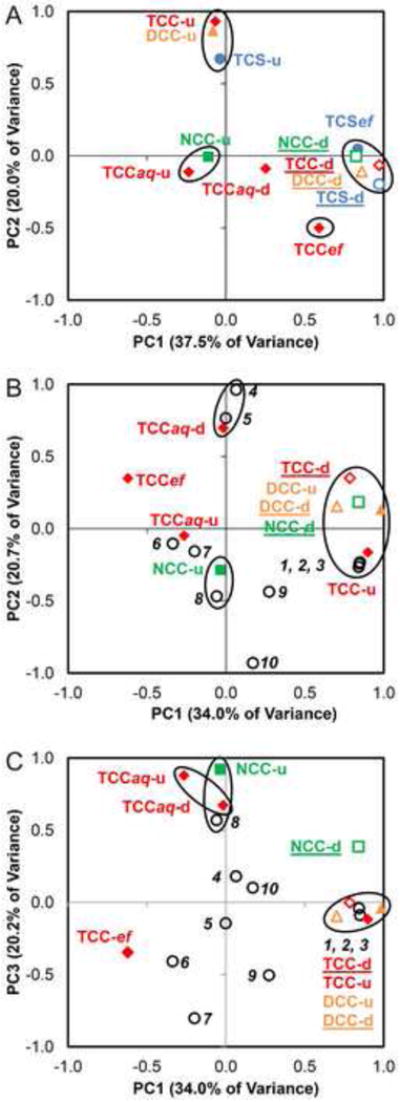
Principal component analysis of the contaminant levels (TCC: diamond, DCC: triangle, NCC: square, and TCS: circle) that were detected in sediment (upstream – u and downstream – d), surface water (upstream- aq-u and downstream- aq-d), and in wastewater treatment plant effluent (ef) of the sampling sites. Plot A represents the variance (57.5%) in sediment and aqueous phase contamination of biocides. Plots B and C represent the variance (54.7% and 54.2%, respectively) in contaminants levels of TCC, DCC, and NCC and various physicochemical properties (empty circles). (1) Population; (2) surface water flow; (3) WWTP discharge; (4) contribution (%) of WWTP discharge to surface water flow; (5) conductance; (6) sediment organic carbon fraction; (7) turbidity of surface water; (8) dissolved oxygen concentration; (9) distance of sampling location from WWTP; and (10) pH of surface water. The highlighted circles represent the cluster of parameters that correlate with each other.
The geographic variability in sediment contamination might in part be attributed to different physical and chemical properties in various sites that include WWTP discharge, population density, turbidity, pH, sediment organic carbon content, etc. The covariance was investigated through PCA analyses based on the complete analytical dataset and the physicochemical site-specific measurements [i.e., TCC concentrations (in surface water, WWTP effluent, and sediments), DCC and NCC sediment concentrations, stream flow and WWTP effluent discharge, and other physical chemical parameters (specific conductance, pH, turbidity, dissolved oxygen, and sediment organic carbon content)] as inputs (see Supplementary Information for the datasets used). The PCA plots reveal that the downstream sediment concentrations of TCC, DCC, and NCC clustered closely together with stream flow (million gallons/day [mgd]), WWTP discharge (mgd), and population density, suggesting a relation between sediment contamination levels and these parameters. The correlation was observed to be stronger for TCC and DCC and comparatively weaker for NCC (Figure 4C). These results suggest that higher levels of TCC and DCC can be expected in sediments at locations with high surface water flow, high WWTP discharge, and high population density. The percent contribution of WWTP effluent to surface water flow also seems to relate with the TCC surface water concentration (Figure 4B), thus further confirming WWTP as a significant point source for downstream contamination of these biocides.
3.3. Evidence for ubiquitous dechlorination of TCC
Use of tandem mass spectrometry enabled the detection of impurities and putative dechlorination products of TCC above LOQ in all samples (Figure 2). DCC is known to be present as impurity (∼0.2% w/w) in technical grade TCC (>99%) [33], which corresponds to a ratio of 495:1. The TCC: DCC ratio in freshwater sediments from the present study ranged widely from 5:1 to 221:1. This deviation in the reported TCC: DCC ratio in the present study from the impurity ratio suggests TCC removal relative to DCC or DCC formation either in the WWTP or the bed sediment via dechlorination of TCC, since their adsorptive characteristics are similar. For almost all sites, DCC levels exceeded those of NCC (sediment collected upstream of the WWTP near Grand Rapids representing the one exception). This finding is similar to data collected in a previous study in the Chesapeake Bay, Maryland and in Jamaica Bay, New York [29]. It was previously hypothesized that biological dechlorination of TCC might have taken place under anoxic conditions, which prevail in deeper sections of the sediments [29]. But because dissolved oxygen levels were not monitored in the sediments of the present study, it is unknown whether the site specific redox conditions allow in situ reductive dechlorination of TCC. Nevertheless, the TCC:DCC ratio can be expected to decrease with sediment depth where anaerobic conditions may prevail (as seen in Chesapeake Bay [29]). It is noted that the presence of dehalorespiring bacteria at the TCC-contaminated Chesapeake Bay sediments was later confirmed by isolating an anaerobic microbial consortium that was capable of dechlorinating trichloroethene (TCE) to ethene [34]. Evidence of microbial TCC anaerobic reductive dechlorination remains unreported to date. Since the sediments analyzed in the present study were obtained from the top 10 cm of the bed surface, they might have been partially oxygenated and hence incomplete dechlorination of TCC could be a possible reason for the observed lower concentrations of DCC and NCC when compared to the previous study at Chesapeak Bay. We would like to emphasize that the dissolved oxygen concentrations reported by USGS (and used in the PCA) were measured in the water column and not in the sampled sediment bed. Reductive dechlorination in anoxic sediments is an important pathway for several halogenated compounds, including polychlorinated biphenyls (PCBs), chlorinated dioxins and dibenzofurans, and TCE [35-37]. Presence of oxygen has shown to inhibit reductive dechlorination of these compounds [38, 39]. However, the consistent detection of dechlorination products of TCC in potentially oxygenated bed sediment in this study might suggest either an alternate pathway for dechlorination, or tolerance of certain dehalorespiring bacteria of low oxygen concentrations, perhaps as a result of a favorable facultative anaerobic microbial community. Alternatively, or in addition, anaerobic microbial reductive dechlorination of TCC could also have taken place in the WWTPs; however, a lack of NCC and DCC data for WWTP effluent at the sampling locations makes it impossible to pinpoint with certainty the environmental locale of this dechlorination route.
3.4. Aquatic toxicity of TCC
The high TCC levels detected in freshwater sediments, especially from downstream site at Shagawa Lake (Ely) and St. Louis Bay in Lake Superior (Duluth) may be of concern due to toxicity effects on aquatic organisms. Lake Superior is the largest freshwater lake in the world. The studied region (St. Louis Bay) is a fresh-water estuary of Lake Superior and is addressed as one of the Areas of Concern in the Great Lakes region by the U.S. Environmental Protection Agency's St. Louis River System Remedial Action Plan [40]. Researchers have reported impairment of beneficial uses of the lake due to contamination, resulting in restrictions on fish and wildlife consumption [40]. The detected level of TCC (822 ng/g) in St. Louis Bay bed sediments adds to the existing concerns in this Area of Concern and throughout the Great lakes region. The St. Louis Bay of Lake Superior has more than 80 species of fish and other aquatic organisms [41]. These include invertebrates (zooplankton, midge larvae, and mysid shrimp), and vertebrates (rainbow trout) which are known to have a pronounced sensitivity to acute toxicity from TCC exposure [42]. Though the surface water TCC concentrations are well below the threshold values for most of these aquatic species in the studied region, very little is known about this compound's biota-sediment accumulation factor. Moreover, the effects of TCC in mixtures of other chemicals present in the Area of Concern are unknown. Based on the detected levels of TCC (Csed), the fraction of organic carbon in lake sediments (fOC), and the organic carbon partition coefficient of TCC (KOC), the pore water concentration of TCC (Cpore) were estimated to be 0.2 and 1.7 μg/L for the downstream site at St. Louis Bay and Shagawa Lake, respectively using the following equilibrium expression.
The estimated pore water concentration at Shagawa Lake exceeds the no-observed effective concentration of 1.46 μg/L for the zooplankton Ceriodaphnia sp. and concentration in both lakes exceeds the half maximal effective concentration (EC50) of 0.2 μg/L for reproduction in the mysid shrimp Mysidopsis bahia [42]. These invertebrates are commonly found in both lakes. This discussion excludes the possibility of biomagnification, which would further lower the threshold level for concern of TCC exposure for inducing chronic and acute toxicity in aquatic organisms.
4. Conclusion
Analytical data on the occurrence of TCS and TCC in surface waters, WWTP effluents, and bed sediments in Minnesota suggest that WWTPs are an important point source of freshwater sediment contamination. In decreasing order, the relative abundances of the studied antimicrobial compounds in sediments are TCC>TCS>DCC>NCC. Principal component analysis revealed that freshwater sediment contamination strongly correlated with WWTP discharge, stream flow, and population density. Elevated concentrations in lake sediments may potentially exert toxicity to aquatic organisms and should be the subject of further study. Consistent detection of dechlorination products of TCC (specifically, DCC and NCC) in all sampled locations in the current study area strongly suggests that dechlorination of TCC is a ubiquitous and important attenuation process in freshwater environments. Though the known reductive dechlorination mechanisms are strictly anaerobic, detection of these transformation products in potentially partially-oxygenated sediments suggest an alternate dechlorination pathway for TCC, the existence of certain dehalorespiring bacteria that can tolerate low concentrations of oxygen, the presence of dehalorespiring bacteria as an integral part of facultative anaerobic microbial communities (whether they be located in the WWTP or the bed sediment) or alternative abiotic processes. Further studies are required on biocide occurrence in sediments in other parts of the U.S. and on the aquatic toxicity exerted by the parental biocides TCS, TCC and their transformation products to bridge existing knowledge gaps in the environmental fate and transport of these widely used antimicrobials.
Supplementary Material
Highlights.
First statewide U.S. survey finds antimicrobial compounds in Minnesota freshwater sediments.
Triclosan, triclocarban (TCC) and its lesser chlorinated analogues were detected in all the samples.
Antimicrobials occur both upstream and downstream of wastewater discharge locations.
TCC is more abundant than its dechlorinated transformation products and triclosan.
Detected degradates suggest dechlorination of TCC in freshwater environments is ubiquitous.
Acknowledgments
This project was supported in part by Award Number R01ES015445 from the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health (NIH). Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halden RU, Paull DH. Analysis of triclocarban in aquatic samples by liquid chromatography electrospray ionization mass spectrometry. Environ Sci Technol. 2004;38:4849–4855. doi: 10.1021/es049524f. [DOI] [PubMed] [Google Scholar]
- 2.Sabaliunas D, Webb SF, Hauk A, Jacob M, Eckhoff WS. Environmental fate of triclosan in the River Aire Basin, UK. Water Res. 2003;37:3145–3154. doi: 10.1016/S0043-1354(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 3.Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health, Part A. 2006;69:1861–1873. doi: 10.1080/15287390600631706. [DOI] [PubMed] [Google Scholar]
- 4.Howes D, Black J. Percutaneous absorption of triclocarban in rat and man. Toxicol. 1976;6:67–76. doi: 10.1016/0300-483x(76)90008-1. [DOI] [PubMed] [Google Scholar]
- 5.Allmyr M, Adolfsson-Erici M, McLachlan MS, Sandborgh-Englund G. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci Total Environ. 2006;372:87–93. doi: 10.1016/j.scitotenv.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Ye X, Zhou X, Furr J, Ahn KC, Hammock BD, Gray EL, Calafat AM. Biomarkers of exposure to triclocarban in urine and serum. Toxicol. 2011;286:69–74. doi: 10.1016/j.tox.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanioka N, Omae E, Nishimura T, Jinno H, Onodera S, Yoda R, Ando M. Interaction of 2, 4, 4′-trichloro-2′-hydroxydiphenyl ether with microsomal cytochrome P450-dependent monooxygenases in rat liver. Chemosphere. 1996;33:265–276. doi: 10.1016/0045-6535(96)00169-5. [DOI] [PubMed] [Google Scholar]
- 8.Wang LQ, Falany CN, James MO. Triclosan as a substrate and inhibitor of 3′-phosphoadenosine 5′-phosphosulfate-sulfotransferase and UDP-glucuronosyl transferase in human liver fractions. Drug Metab Disposition. 2004;32:1162–1169. doi: 10.1124/dmd.104.000273. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs MN, Nolan GT, Hood SR. Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR) Toxicol Appl Pharmacol. 2005;209:123–133. doi: 10.1016/j.taap.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, Stoker TE. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicolog Sci. 2009;107:56. doi: 10.1093/toxsci/kfn225. [DOI] [PubMed] [Google Scholar]
- 11.Murray KE, Thomas SM, Bodour AA. Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environ Poll. 2010;158:3462–3471. doi: 10.1016/j.envpol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Nolen G, Dierckman T. Reproduction and teratogenic studies of a 2: 1 mixture of 3, 4, 4′-trichlorocarbanilide and 3-trifluoromethyl-4, 4′-dichlorocarbanilide in rats and rabbits. Toxicol Appl Pharmacol. 1979;51:417–425. doi: 10.1016/0041-008x(79)90366-1. [DOI] [PubMed] [Google Scholar]
- 13.Ponte C, Richard J, Bonte C, Lequien P, Lacombe A. Methemoglobinemia in Newborn-Discussion of Etiological Role of Trichlorocarbanilide. Semaine Des Hopitaux. 1974;50:359–365. [Google Scholar]
- 14.Johnson RR, Navone R, Larson EL. An unusual epidemic of methemoglobinemia. Pediatrics. 1963;31:222. [PubMed] [Google Scholar]
- 15.Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, Denison MS, Lasley B, Pessah IN, Kültz D, Chang DPY. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: receptor-based bioassay screens. Environ Health Perspect. 2008;116:1203. doi: 10.1289/ehp.11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adolfsson-Erici M, Pettersson M, Parkkonen J, Sturve J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere. 2002;46:1485–1489. doi: 10.1016/s0045-6535(01)00255-7. [DOI] [PubMed] [Google Scholar]
- 17.Coogan MA, Edziyie RE, La Point TW, Venables BJ. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere. 2007;67:1911–1918. doi: 10.1016/j.chemosphere.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 18.Kim JW, Ramaswamy BR, Chang KH, Isobe T, Tanabe S. Multiresidue analytical method for the determination of antimicrobials, preservatives, benzotriazole UV stabilizers, flame retardants and plasticizers in fish using ultra high performance liquid chromatography coupled with tandem mass spectrometry. J Chrom A. 2011 doi: 10.1016/j.chroma.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Houtman CJ, van Oostveen AM, Brouwer A, Lamoree MH, Legler J. Identification of estrogenic compounds in fish bile using bioassay-directed fractionation. Environ Sci Technol. 2004;38:6415–6423. doi: 10.1021/es049750p. [DOI] [PubMed] [Google Scholar]
- 20.Ishibashi H, Matsumura N, Hirano M, Matsuoka M, Shiratsuchi H, Ishibashi Y, Takao Y, Arizono K. Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat Toxicol. 2004;67:167–179. doi: 10.1016/j.aquatox.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Matsumura N, Ishibashi H, Hirano M, Nagao Y, Watanabe N, Shiratsuchi H, Kai T, Nishimura T, Kashiwagi A, Arizono K. Effects of nonylphenol and triclosan on production of plasma vitellogenin and testosterone in male South African clawed frogs (Xenopus laevis) Biol Pharm Bull. 2005;28:1748–1751. doi: 10.1248/bpb.28.1748. [DOI] [PubMed] [Google Scholar]
- 22.Veldhoen N, Skirrow RC, Osachoff H, Wigmore H, Clapson DJ, Gunderson MP, Van Aggelen G, Helbing CC. The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development. Aquat Toxicol. 2006;80:217–227. doi: 10.1016/j.aquatox.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Gee R, Charles A, Taylor N, Darbre P. Oestrogenic and androgenic activity of triclosan in breast cancer cells. J Appl Toxicol. 2008;28:78–91. doi: 10.1002/jat.1316. [DOI] [PubMed] [Google Scholar]
- 24.Halden RU, Paull DH. Co-occurrence of triclocarban and triclosan in U. S. water resources. Environ Sci Technol. 2005;39:1420–1426. doi: 10.1021/es049071e. [DOI] [PubMed] [Google Scholar]
- 25.Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999-2000: a national reconnaissance. Environ Sci Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- 26.Heidler J, Sapkota A, Halden RU. Partitioning, persistence, and accumulation in digested sludge of the topical antiseptic triclocarban during wastewater treatment. Environ Sci Technol. 2006;40:3634–3639. doi: 10.1021/es052245n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heidler J, Halden RU. Mass balance assessment of triclosan removal during conventional sewage treatment. Chemosphere. 2007;66:362–369. doi: 10.1016/j.chemosphere.2006.04.066. [DOI] [PubMed] [Google Scholar]
- 28.Heidler J, Halden RU. Meta-analysis of mass balances examining chemical fate during wastewater treatment. Environ Sci Technol. 2008;42:6324–6332. doi: 10.1021/es703008y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller TR, Heidler J, Chillrud SN, DeLaquil A, Ritchie JC, Mihalic JN, Bopp R, Halden RU. Fate of triclosan and evidence for reductive dechlorination of triclocarban in estuarine sediments. Environ Sci Technol. 2008;42:4570–4576. doi: 10.1021/es702882g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singer H, Müller S, Tixier C, Pillonel L. Triclosan: occurrence and fate of a widely used biocide in the aquatic environment: field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ Sci Technol. 2002;36:4998–5004. doi: 10.1021/es025750i. [DOI] [PubMed] [Google Scholar]
- 31.Lee KE, Langer SK, Barber LB, Writer JH, Ferrey ML, Schoenfuss HL, Furlong ET, Foreman WT, Gray JL, ReVello RC, Martinovic D, Woodruff OP, Keefe SH, Brown GK, Taylor HE, Ferrer I, Thurman EM. Endocrine active chemicals, pharmaceuticals, and other chemicals of concern in surface water, wastewater-treatment plant effluent, and bed sediment, and biological characteristics in selected streams, Minnesota— design, methods, and data. 2009: US Geol Survey Data Series. 2011;575:54. [Google Scholar]
- 32.USEPA, Appendix B to Part 136—Definition and Procedure for the Determination of the Method Detection Limit—Revision 1.11 (October 26, 1984)
- 33.Sapkota A, Heidler J, Halden RU. Detection of triclocarban and two co-contaminating chlorocarbanilides in US aquatic environments using isotope dilution liquid chromatography tandem mass spectrometry. Environ Res. 2007;103:21–29. doi: 10.1016/j.envres.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Ziv-El M, Delgado AG, Yao Y, Kang D, Nelson KG, Halden RU, Krajmalnik-Brown R. Development and characterization of DehaloRˆ2, a novel anaerobic microbial consortium performing rapid dechlorination of TCE to ethane. Appl Microbiol Biotechnol. 2011;92:1063–1071. doi: 10.1007/s00253-011-3388-y. [DOI] [PubMed] [Google Scholar]
- 35.Häggblom M, Knight V, Kerkhof L. Anaerobic decomposition of halogenated aromatic compounds. Environ Poll. 2000;107:199–207. doi: 10.1016/s0269-7491(99)00138-4. [DOI] [PubMed] [Google Scholar]
- 36.Häggblom MM, Ahn YB, Fennell DE, Kerkhof LJ, Rhee SK. Anaerobic dehalogenation of organohalide contaminants in the marine environment. Adv Appl Microbiol. 2003;53:61–84. doi: 10.1016/s0065-2164(03)53002-7. [DOI] [PubMed] [Google Scholar]
- 37.Holliger C, Regeard C, Diekert G. Dehalogenation by anaerobic bacteria. Dehalogenation. 2004:115–157. [Google Scholar]
- 38.Amos BK, Ritalahti KM, Cruz-Garcia C, Padilla-Crespo E, Löffler FE. Oxygen effect on Dehalococcoides viability and biomarker quantification. Environ Sci Technol. 2008;42:5718–5726. doi: 10.1021/es703227g. [DOI] [PubMed] [Google Scholar]
- 39.Natarajan M, Wang H, Hickey R, Bhatnagar L. Effect of oxygen and storage conditions on the metabolic activities of polychlorinated biphenyls dechlorinating microbial granules. Appl Microbiol Biotechnol. 1995;43:733–738. doi: 10.1007/BF00164781. [DOI] [PubMed] [Google Scholar]
- 40.U.S. Environmental Protection Agency, St. Louis River Area of Concern. 2011. http://www.epa.gov/greatlakes/aoc/stlouis.html.
- 41.Minnesota Sea Grant, 2012. http://www.seagrant.umn.edu/fisheries/superior_fish_species.php#all.
- 42.TCC Consortium. High Production Volume (HPV) Chemical Challenge Program Data Availability and Screening Level Assessment for Triclocarban CAS#: 101–20–2. US Environmental Protection Agency; Washington DC: 2002. pp. 1–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


