Abstract
C-reactive protein (CRP), a plasma protein of the innate immune system, is produced by hepatocytes. A critical regulatory region (−42 to −57) on the CRP promoter contains binding site for the IL-6-activated transcription factor C/EBPβ. The IL-1β-activated transcription factor NF-κB binds to a κB site located nearby (−63 to −74). The κB site overlaps an octamer motif (−59 to −66) which is the binding site for the constitutively active transcription factor Oct-1. Oct-1 is known to function both as a transcriptional repressor and as an activator depending upon the promoter context. Also, Oct-1 can regulate gene expression either by binding directly to the promoter or by interacting with other transcription factors bound to the promoter. The aim of this study was to investigate the functions of Oct-1 in regulating CRP expression. In luciferase transactivation assays, overexpressed Oct-1 inhibited (IL-6+IL-1β)-induced CRP expression in Hep3B cells. Deletion of the Oct-1 site from the promoter drastically reduced the cytokine response because the κB site was altered as a consequence of deleting the Oct-1 site. Surprisingly, overexpressed Oct-1 inhibited the residual (IL-6+IL-1β)-induced CRP expression through the promoter lacking the Oct-1 site. Similarly, deletion of the Oct-1 site reduced the induction of CRP expression in response to overexpressed C/EBPβ, and overexpressed Oct-1 inhibited C/EBPβ-induced CRP expression through the promoter lacking the Oct-1 site. We conclude that Oct-1 acts as a transcriptional repressor of CRP expression and it does so by occupying its cognate site on the promoter and also via other transcription factors by an as yet undefined mechanism.
Keywords: C-reactive protein, Oct-1, C/EBPβ, Hep3B cells, κB site
1. Introduction
C-reactive protein (CRP) is defined as an acute phase protein whose serum concentration increases in inflammatory conditions, such as rheumatoid arthritis, and in some non-inflammatory conditions, such as stress and cellular injury (Agrawal et al., 2009; Kushner et al., 2006). CRP is primarily produced by hepatocytes in response to IL-6 and IL-1β and its synthesis is regulated at the transcriptional level (Castell et al., 1990; Eklund, 2005; Goldberger et al., 1987; Toniatti et al., 1990a; Voleti and Agrawal, 2006; Yoshida et al., 2006; Zhang et al., 1995). In human hepatoma Hep3B cells, IL-6 induces CRP expression by activating transcription factors C/EBPβ and STAT3 (Ochrietor et al., 2000; Poli and Cortese, 1989; Ramji et al., 1993; Turkson et al., 1998; Wang et al., 1999; Young et al., 2008; Zhang et al., 1996). IL-1β, which alone does not induce CRP expression in Hep3B cells, synergistically enhances the effects of IL-6 by activating transcription factor NF-κB (Agrawal et al., 2003a; Darlington et al., 1986; Ganapathi et al., 1988; Kramer et al., 2008; Zhang et al., 1995). The proximal 157 bp of the CRP promoter are sufficient for the synergy between IL-6 and IL-1β (Zhang et al., 1995). Transcription factor c-Rel participates in regulating CRP expression, without binding to the promoter, via interactions with C/EBPβ (Agrawal et al., 2003b; Cha-Molstad et al., 2007). Five constitutively active transcription factors (C/EBPζ, RBP-Jκ, HNF-1, HNF-3, and Oct-1) are also involved in regulating CRP expression (Blaschke et al., 2006; Grimm et al., 2011; Nishikawa et al., 2008; Reiner et al., 2008; Toniatti et al., 1990b; Voleti and Agrawal, 2005).
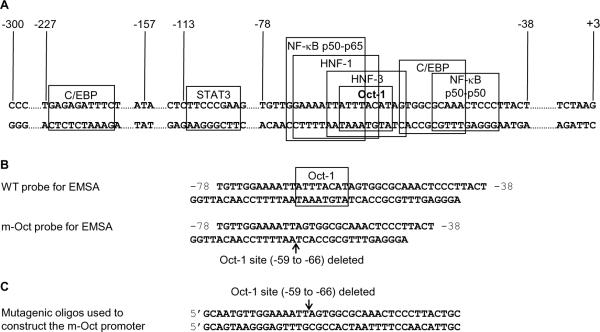
The binding sites of various transcription factors on the CRP promoter are shown in Fig. 1A. C/EBPβ binds to two sites centered at positions −52 and −219 (Li and Goldman, 1996). The STAT3-binding site is located at −108 (Zhang et al., 1996). There are five NF-κB proteins, p50, p52, p65, c-Rel and Rel B, which form homodimers or heterodimers and bind to κB sites on the promoters to regulate gene expression (Ghosh and Hayden, 2012). On the CRP promoter, NF-κB p50-p50 binds to a nonconsensus κB site located at −47, overlapping the proximal C/EBP site, and NF-κB p50-p65 binds to a κB site located at −69 (Agrawal et al., 2001; Cha-Molstad et al., 2000; Voleti and Agrawal, 2005). C/EBPζ binds to the same proximal C/EBP site where C/EBPβ binds (Singh et al., 2007). RBP-Jκ binds to the same site where NF-κB p50-p50 binds (Singh et al., 2007). The binding sites for HNF-1 and HNF-3 are located at positions −67 and −62, respectively (Li and Goldman, 1996). Oct-1 binds to a site centered at position −63 (Arcone et al., 1988; Li and Goldman, 1996).
Fig. 1.
The CRP promoter and the oligos used in this study. (A) The −300 to +3 region of the CRP gene is shown. The binding sites of various transcription factors on the promoter are boxed. (B) Sequences of the oligos derived from the CRP promoter and used as probes in EMSA. (C) Sequences of the mutagenic oligos used for mutagenesis of the CRP promoter to construct the m-Oct promoter.
Oct-1 is a ubiquitous and constitutively active transcription factor (Singh et al., 1986; Sturm et al., 1988). Generally, Oct-1 functions as an activator of transcription (Dong et al., 2009; Inamoto et al., 1997; Shakya et al., 2011; Zhou and Yen, 1991). However, Oct-1 also represses transcription of some genes (Bhat et al., 1996; Shakya et al., 2011). Oct-1 binds specifically to an octamer motif (ATGCAAAT) and related sequences on promoters to regulate gene expression (Bhat et al., 1996; Singh et al., 1986). Oct-1 also functions by associating with basal transcription factors and other tissue-specific transcription factors and cofactors (Nakshatri et al., 1995; Préfontaine et al., 1999; Shakya et al., 2011; Ström et al., 1996; Voss et al., 1991; Wysocka and Herr, 2003; Zwilling et al., 1994).
It is not known how Oct-1 acts on the CRP promoter to regulate CRP gene expression. Previously, we reported that Oct-1 competes with NF-κB for binding to its cognate site which overlaps the κB site on the CRP promoter (Voleti and Agrawal, 2005). The aim of this study was to determine whether Oct-1 acts as a repressor or as an activator of CRP expression. Another aim of this study was to determine the requirement of the Oct-1-binding site on the CRP promoter for Oct-1 to regulate CRP expression.
2. Materials and Methods
2.1. Electrophoretic mobility shift assays (EMSA)
Hep3B cells were cultured in 100 mm dish. Cells were subjected to serum starvation overnight and then treated with IL-1β for 15 min, as described previously (Voleti and Agrawal, 2005). The confluency of cells was approximately 60% at the time of IL-1β-treatment. IL-β (R&D) was used at a concentration of 200 U/ml. Nuclear extracts were prepared by using NE-PER nuclear and cytoplasmic kit (Pierce), as described previously (Singh et al., 2007). The sequences of the oligonucleotides (oligo) used in EMSA are shown in Fig. 1B. Oligos were obtained from Integrated DNA Technologies. To prepare the probes, complementary oligos were annealed and labelled with [γ-32P] ATP. The probe-nuclear extract reaction buffer contained 16 mM HEPES (pH 7.9), 40 mM KCl, 1 mM EDTA, 2.5 mM DTT, 0.15% Nonidet P-40, 8% Histopaque, and 1 μ-g of poly dI-dC. In supershift experiments, antibodies (2 μ-g) were added to the reaction mixture and incubated on ice for 15 min before addition of the probe. In oligo competition experiments, 150 ng of unlabelled oligos was added to the reaction mixture before addition of the antibody and probe. The antibodies to p50 (H119), p65 (H286), HNF-1 (H205), HNF-3 (C20) and Oct-1 (C20) were purchased from Santa Cruz Biotechnologies. DNA-protein complexes were resolved in native 5% polyacrylamide gels containing 2.5% glycerol and visualized in a phosphorimager using Image-Quant software (GE Healthcare).
2.2. Construction of CRP promoter-luciferase (Luc) reporter vectors
The construction of wild-type (WT) CRP promoter constructs, Luc-157 WT (−157/+3 of CRP gene) and Luc-300 WT (−300/−1 of CRP promoter), has been reported previously (Agrawal et al., 2001; Kleemann et al., 2003; Voleti and Agrawal, 2005). These two WT constructs were used as templates for mutagenesis (deletion of all 8 bp of the Oct-1 site) of the CRP promoter by using QuickChange site-directed mutagenesis kit (Stratagene). The mutagenic primers are shown in Fig. 1C. Mutations were verified by DNA sequencing. Plasmids were purified using maxiprep plasmid isolation kit (Eppendorf).
2.3. Luciferase transactivation assays (Luc assays)
Hep3B cells were cultured in 6-well plates. Transfections were performed using TransIT-LT1 (Mirus) according to manufacturer's instructions. CRP promoter-Luc reporter constructs were used at 1 μg plasmid per well and the transcription factor expression vectors were used as mentioned in the figure legends. The confluency of cells was approximately 60% at the time of transfection. After transfection, cells were left in serum-free medium. After 16 h of transfection and serum starvation, the transfected cells were either treated with IL-6 and IL-1β for 24 h or left untreated. IL-6 (R&D) was used at a concentration of 1100 U/ml and IL-1β was used at a concentration of 200 U/ml. After 40 h of transfection, Luc assays were performed following the protocol supplied by the manufacturer (Promega). Luc activity was measured in a luminometer (Molecular Devices), as described previously (Singh et al., 2007).
3. Results
3.1. Oct-1 binds to its site on the CRP promoter and competes with the binding of NF-κB to the overlapping κB site
To demonstrate that Oct-1 binds to its cognate site on the CRP promoter, we performed EMSA using a 45 bp oligo (WT oligo; Fig. 1B) which contained the overlapping Oct-1 and κB sites and the adjacent C/EBP site (Fig. 2). We used nuclear extract from IL-1β-treated Hep3B cells as the source of NF-κB. Four specific complexes were formed on the probe (lanes 1 and 2). The fastest migrating complex was NF-κB (p50–p65) because the antibodies to p50 and p65 reduced the intensity of the complex (lanes 3 and 4). By using antibodies to HNF-1 and Oct-1, the top two complexes were found to contain HNF-1 and Oct-1 (lanes 5 and 6). Another specific complex contained HNF-3 (confirmation using antibodies to HNF-3 is shown in Fig. 3). When the Oct-1-containing complex was abolished (and, in part, supershifted) by treatment of the nuclear extract with anti-Oct-1 antibodies, the intensity of the NF-κB complex increased (compare lanes 1 and 6). These results suggest that Oct-1 binds to its site on the CRP promoter and competes with the binding of NF-κB to the overlapping κB site.
Fig. 2.
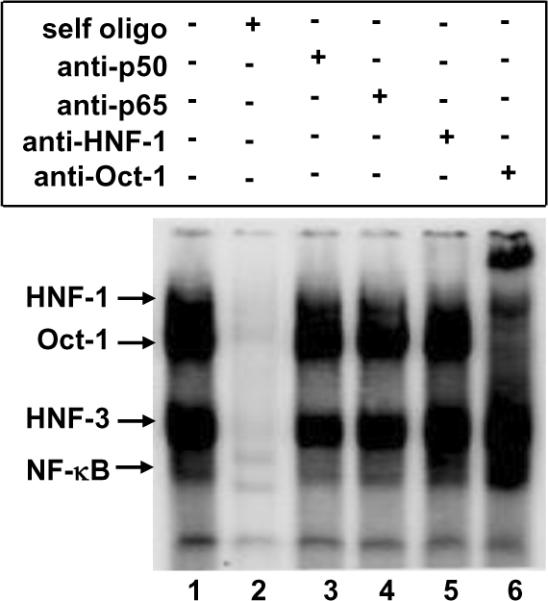
Oct-1 binds to its site on the CRP promoter and competes with the binding of NF-κB to the overlapping κB site. A representative of three EMSA is shown. Radiolabelled WT oligo (−38 to −78) was used as the probe and nuclear extract from IL-β-treated Hep3B cells was used as the source of NF-βB. Self oligo competitor (unlabelled WT oligo) and antibodies were added to nuclear extract before the addition of the probe. DNA probe-protein complexes were visualized by using a phosphorimager. Arrows point to the complexes formed on the probe. The mobility of the free probe is not shown.
Fig. 3.
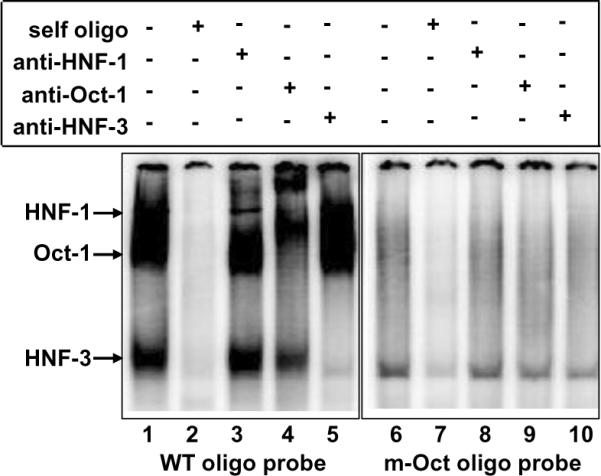
Oct-1 does not bind to CRP promoter in the absence of the Oct-1 site. A representative of two EMSA is shown. Radiolabelled WT oligo (lanes 1–5) and m-Oct oligo (8 bp Oct-1 site deleted, lanes 6–10) were used as probes. Nuclear extract from untreated Hep3B cells was used as the source of constitutively active transcription factors. Self oligo competitors (unlabelled WT and m-Oct oligos) and antibodies were added to nuclear extract before the addition of the probe. DNA probe-protein complexes were visualized by using a phosphorimager. Arrows point to the complexes formed on the WT probe. The mobility of the free probe is not shown.
3.2. Oct-1 does not bind to CRP promoter in the absence of the Oct-1 site
The purpose of the next EMSA was to characterize the m-Oct promoter (promoter with the 8 bp Oct-1 site deleted; Fig. 1B) that we used in the Luc assays. We performed EMSA using WT oligo and m-Oct oligo as probes and nuclear extracts from untreated Hep3B cells (Fig. 3). Three specific complexes containing HNF-1, Oct-1 and HNF-3 were formed on the WT probe (lanes 1–5), whereas deletion of the Oct-1 site abolished the binding of not only Oct-1 but also HNF-1 and HNF-3 to the probe (lanes 6–10). These results suggested that Oct-1 would not bind directly to the m-Oct CRP promoter that we used in the Luc assays.
3.3. Oct-1 inhibits (IL-6+IL-1β)-induced CRP expression
To investigate the role of Oct-1 in regulating CRP expression, we conducted Luc assays using Luc 157-WT and Luc 300-WT promoter constructs and determined the effects of overexpressed Oct-1 on (IL-6+IL-1β)-induced CRP promoter-driven Luc activity. As shown in Fig. 4, Oct-1 inhibited (IL-6+IL-1β)-induced CRP promoter-driven Luc activity in a dose-dependent manner, irrespective of the size of the CRP promoter, that is, irrespective of the presence of one or both of the C/EBP sites (located at positions −52 and −219). From these results we conclude that Oct-1 acts as a transcriptional repressor of CRP expression.
Fig. 4.
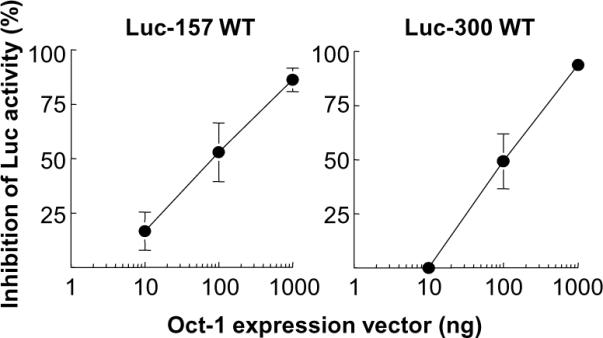
Oct-1 inhibits (IL-6+IL-1β)-induced CRP promoter-driven Luc activity. Luc-157 WT and Luc-300 WT CRP promoter constructs were transfected into Hep3B cells along with increasing amounts of the expression vector encoding Oct-1. After 16 h, cells were treated with IL-6 and IL-1β for 24 h. CRP expression was measured as Luc activity. Percent inhibition of Luc activity is plotted on the y-axis. Average ± S.E.M. of three experiments are shown. For some data points, error bars are not visible because the S.E.M. was low.
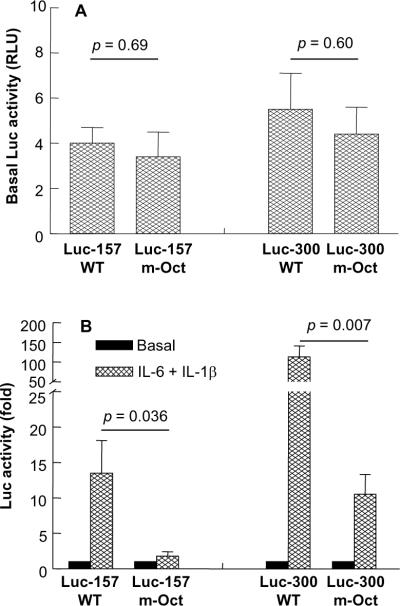
3.4. Effects of deleting the Oct-1 site on basal and (IL-6+IL-1β)-induced CRP expression
To explore the mechanism of Oct-1-mediated repression of CRP expression, our approach was to delete the Oct-1-site (−59 to −66) from the promoter and use the m-Oct promoter construct, which does not bind Oct-1, in the Luc assays. We determined the contribution of the Oct-1 site, which overlaps the HNF-1, HNF-3 and κB sites, to the basal and (IL-6+IL-1β)-induced CRP expression. Deletion of the Oct-1 site from the promoter did not affect basal CRP expression (Fig. 5A). However, (IL-6+IL-1β)-induced CRP expression was drastically reduced when the Oct-1 site was deleted, irrespective of the size of the CRP promoter used in the assay (Fig. 5B). CRP expression was reduced by about 87% (from 14-fold to 2-fold) when Luc-157 m-Oct promoter was used and by about 91% (from 113-fold to 11-fold) when Luc-300 m-Oct promoter was used, compared to their respective WT constructs. These results suggest that the −59 to −66 region of the CRP promoter is required for full (IL-6+IL-1β)-induced CRP expression.
Fig. 5.
Effects of deleting the Oct-1 site on basal and (IL-6+IL-1β)-induced CRP expression. (A) Hep3B cells were transfected with Luc-157 WT, Luc-157 m-Oct, Luc-300 WT and Luc-300 m-Oct CRP promoter constructs. After 40 h, CRP transcription was measured as Luc activity and plotted on the y-axis. Average ± S.E.M. of four experiments are shown. Unpaired two-tailed Students t-test was used to calculate p values. (B) Hep3B cells were transfected with Luc-157 WT, Luc-157 m-Oct, Luc-300 WT and Luc-300 m-Oct CRP promoter constructs. After 16 h, cells were either treated with IL-6 and IL-1β or left untreated for 24 h. CRP expression was measured as Luc activity. Basal Luc activity was taken as 1 and (IL-6+IL-1β)-induced Luc activity was plotted on the y-axis as fold over basal Luc activity. Average ± S.E.M. of five experiments are shown. Unpaired two-tailed Students t-test was used to calculate p values.
3.5. Oct-1 inhibits (IL-6+IL-1β)-induced CRP expression even if the Oct-1 site is deleted from the promoter
Because some residual (IL-6+IL-1β)-induced CRP transcription activity was present even if the Oct-1 site was deleted, we next determined whether overexpressed Oct-1 would have an effect on the residual (IL-6+IL-1β)-induced CRP expression. Surprisingly, overexpressed Oct-1 inhibited the (IL-6+IL-1β)-induced CRP expression through the m-Oct promoters of both size; the inhibition was about 55%, 71%, 83% and 64% on Luc-157 WT, Luc-157 m-Oct, Luc-300 WT and Luc-300 m-Oct promoters, respectively (Fig. 6). The Oct-1-mediated inhibition of CRP expression on the m-Oct promoters was not significantly different from that on the WT promoters. These results suggest that the inhibitory effect of Oct-1 on CRP expression is not mediated solely through the Oct-1 site and that Oct-1 represses CRP expression even when Oct-1 is not bound directly to the promoter.
Fig. 6.
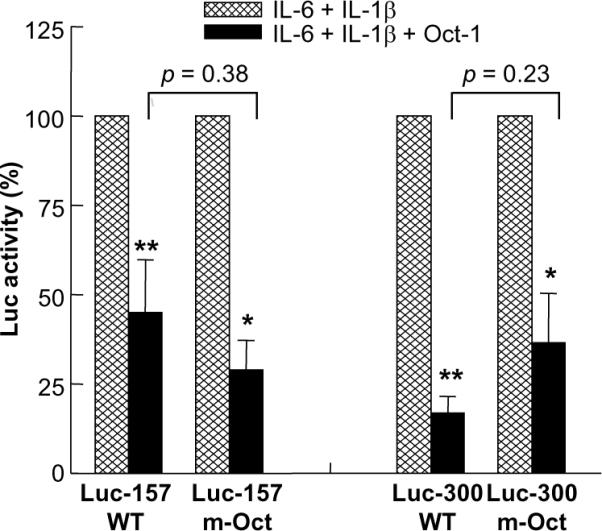
Oct-1 inhibits (IL-6+IL-1β)-induced CRP expression even if the Oct-1 site is deleted from the promoter. Hep3B cells were transfected with Luc-157 WT, Luc-157 m-Oct, Luc-300 WT and Luc-300 m-Oct CRP promoter constructs. One set of cells were co-transfected with the expression vector encoding Oct-1 (1 μg). After 16 h, cells were either treated with IL-6 and IL-1β or left untreated for 24 h. CRP expression was measured as Luc activity. Luc activity in the absence of Oct-1 was taken as 100%. Average ± S.E.M. of four experiments are shown. The p values for the difference between (IL-6+IL-1β) and (IL-6+IL-1β+Oct-1) groups were calculated by using paired two-tailed Students t-test (*p < 0.05, **p < 0.005). The p values for the difference between WT and corresponding m-Oct groups were calculated by using unpaired two-tailed Students t-test.
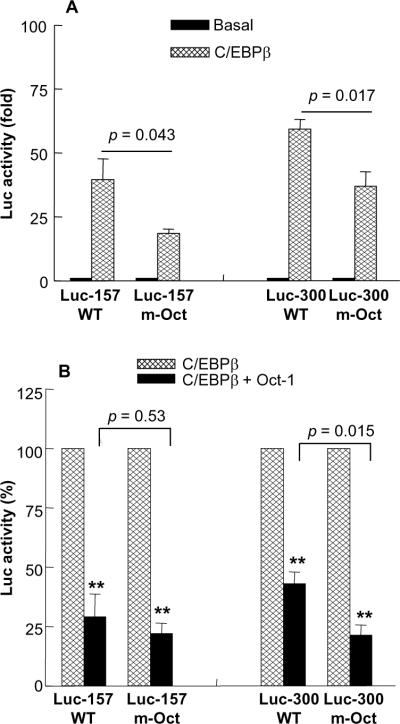
3.6. Oct-1 inhibits C/EBPβ-induced CRP expression regardless of the Oct-1 site
To further explore the mechanism of repressive action of Oct-1 on the CRP promoter, we investigated the effects of overexpressed Oct-1 on overexpressed C/EBPβ-induced CRP expression, in the presence and absence of the Oct-1 site. We first determined the effects of deleting the Oct-1 site on C/EBPβ-induced CRP expression. C/EBPβ-induced CRP expression was reduced when the Oct-1 site was deleted, irrespective of the size of the CRP promoter used in the assay (Fig. 7A). CRP expression was reduced by about 53% (from 40-fold to 19-fold) when Luc-157 m-Oct promoter was used and by about 38% (from 59-fold to 37-fold) when Luc-300 m-Oct promoter was used, compared to their respective WT constructs. These results suggest that the −59 to −66 region of the CRP promoter is also required for full C/EBPβ-induced CRP expression. We next investigated whether overexpressed Oct-1 would have an effect on C/EBPβ-induced CRP expression. Surprisingly, overexpressed Oct-1 inhibited C/EBPβ-induced CRP expression through the m-Oct promoters of both size; the inhibition was about 71%, 78%, 57% and 79% on Luc-157 WT, Luc-157 m-Oct, Luc-300 WT and Luc-300 m-Oct promoters, respectively (Fig. 7B). These results further suggest that the inhibitory effect of Oct-1 on CRP expression is also mediated via interactions with other transcription factors bound to the CRP promoter.
Fig. 7.
Oct-1 inhibits C/EBPβ-induced CRP expression regardless of the Oct-1 site. (A) Hep3B cells were transfected with Luc-157 WT, Luc-157 m-Oct, Luc-300 WT and Luc-300 m-Oct CRP promoter constructs. One set of cells were co-transfected with the expression vector encoding C/EBPβ (50 ng). After 40 h, CRP expression was measured as luciferase activity. Basal Luc activity was taken as 1 and C/EBPβ-induced Luc activity was plotted on the y-axis as fold over basal Luc activity. Average ± S.E.M. of four experiments are shown. Unpaired two-tailed Students t-test was used to calculate p values. (B) Hep3B cells were transfected with Luc-157 WT, Luc-157 m-Oct, Luc-300 WT and Luc-300 m-Oct CRP promoter constructs. One set of cells were co-transfected with the expression vector encoding C/EBPβ (50 ng). Another set of cells were co-transfected with the expression vectors encoding C/EBPβ (50 ng) and Oct-1 (1 μg). After 40 h, CRP expression was measured as Luc activity. Luc activity in the absence of Oct-1 was taken as 100%. Average ± S.E.M. of four experiments are shown. The p values for the difference between C/EBPβ and (C/EBPβ + Oct-1) groups were calculated by using paired two-tailed Students t-test (**p < 0.005). The p values for the difference between WT and corresponding m-Oct groups were calculated by using unpaired two-tailed Students t-test.
4. Discussion
The aim of this study was to determine whether Oct-1 acts as a transcriptional repressor or as an activator of CRP expression. Our approach included overexpressing Oct-1 in (IL-6+IL-1β)-treated Hep3B cells transfected with CRP promoter linked to Luc reporter. We used a 157 bp promoter which contained only one C/EBP-site and a 300 bp promoter which contained both C/EBP-sites. Our major findings were: 1. Overexpressed Oct-1 inhibited (IL-6+IL-1β)-induced CRP promoter-driven Luc expression. 2. The inhibition of 157 bp CRP promoter-driven Luc expression was not different from that of 300 bp CRP promoter-driven Luc expression. These findings suggest that Oct-1 acts as a transcriptional repressor of CRP expression and that the proximal 157 bp of the CRP promoter is sufficient for the repressive action of Oct-1.
Because Oct-1 is also known to regulate expression of certain genes by associating with other transcription factors and cofactors (Nakshatri et al., 1995; Préfontaine et al., 1999; Shakya et al., 2011; Ström et al., 1996; Voss et al., 1991; Wysocka and Herr, 2003; Zwilling et al., 1994), we determined whether Oct-1 can regulate CRP expression without binding to its cognate site on the CRP promoter. Our approach included overexpressing Oct-1 in (IL-6+IL-1β)-treated Hep3B cells transfected with either WT CRP promoter or m-Oct CRP promoter linked to Luc reporter. Another approach was to investigate the effects of overexpressed Oct-1 on overexpressed C/EBPβ-induced CRP expression in Hep3B cells transfected with either WT CRP promoter or m-Oct CRP promoter linked to Luc reporter. Our major findings were: 1. (IL-6+IL-1β)-induced CRP expression was drastically reduced when the Oct-1 site was deleted, indicating that the −59 to −66 region of the CRP promoter was required for full (IL-6+IL-1β)-induced CRP expression. This finding was expected because the −59 to −66 region overlaps the binding site for the IL-1β-activated transcription factor NF-κB. 2. Overexpressed Oct-1 inhibited the residual (IL-6+IL-1β)-induced CRP expression through the m-Oct promoter, indicating that the repressive effect of Oct-1 on CRP expression was not mediated solely via the binding of Oct-1 to the Oct-1 site. 3. C/EBPβ-induced CRP expression was reduced when the Oct-1 site was deleted, indicating that the −59 to −66 region of the CRP promoter was also required for full C/EBPβ-induced CRP expression. This finding was unexpected because the −59 to −66 region does not overlap the C/EBP-site and, therefore, the deletion of this region should not have affected C/EBPβ-induced CRP expression. This finding therefore suggests that C/EBPβ cooperates with one or more transcription factors bound to the −59 to −66 region. 4. Overexpressed Oct-1 inhibited the residual C/EBPβ-induced CRP expression through the m-Oct promoter, further indicating that the repressive effect of Oct-1 on CRP expression was not mediated solely via the binding of Oct-1 to the Oct-1 site.
We have previously shown that Oct-1 binds to a 25 bp oligo containing the overlapping Oct-1 and κB sites derived from the CRP promoter and that Oct-1 and NF-κB compete with each other for binding to their overlapping binding sites (Voleti and Agrawal, 2005). In this study, we found similar results using the 45 bp oligo derived from the CRP promoter which contained the overlapping Oct-1 and κB sites and the adjacent C/EBP-site. Deleting the Oct-1 site from the oligo abolished the binding of not only Oct-1 to the oligo but also abolished the binding of HNF-1 and HNF-3 and perhaps also of NF-κB. This result was expected because the binding sites for these four transcription factors overlap. Our attempts to specifically abolish the binding of only Oct-1 to the CRP promoter, without disrupting the binding of HNF-1, HNF-3 and NF-κB to their binding sites on the promoter, have been unsuccessful; mutating either the last 2 bp or 4 bp of the Oct-1 site did not abolish the binding of Oct-1 to its site (data not shown).
It has been reported previously that the −42 to −57 region of the CRP promoter, where NF-κB p50-p50, C/EBPβ, C/EBPξ, RBP-Jκ, and c-Rel form complexes with the promoter, is a critical regulatory region participating in CRP expression (Agrawal et al., 2001, 2003a, 2003b; Cha-Molstad et al., 2000, 2007; Singh et al., 2007; Voleti and Agrawal, 2005; Young et al., 2008). Our current data suggest that the −57 to −74 region, where Oct-1, HNF-1, HNF-3, and NF-κB form complexes with the promoter, is also a critical regulatory region on the CRP promoter, that Oct-1 works through both regulatory regions, and that the two regulatory regions on the CRP promoter regulate CRP expression cooperatively.
Combined data suggest that Oct-1 acts as a transcriptional repressor of CRP gene expression and that it does so through two mechanisms. The first mechanism involves the binding of Oct-1 to its site; the competition between Oct-1 and NF-κB for binding to their overlapping sites contributes to the regulation of CRP expression, as we have proposed previously (Voleti and Agrawal, 2005). Competition between Oct-1 and NF-κB, between Oct-1 and C/EBPβ, and between Oct-1 and NF-Y, and subsequent repression of the promoter activity, has been shown for several other genes (dela Paz et al., 2007; Osborne et al., 2004; Wu et al., 1997). It is possible that when Oct-1 binds to an overlapping site of a transcriptional activator, then Oct-1 usually acts as a transcriptional repressor. The second mechanism of Oct-1-mediated repression of CRP expression, as yet undefined, involves possible interactions with other transcription factors bound to the CRP promoter.
Highlights
On the CRP promoter, Oct-1 acts as a transcriptional repressor.
To repress CRP expression, Oct-1 uses two mechanisms.
One mechanism involves binding of Oct-1 to the CRP promoter.
The other mechanism involves interactions of Oct-1 with other transcription factors bound to the CRP promoter.
Acknowledgements
We are grateful to Dr. Peter F. Johnson for the gift of expression vector encoding C/EBPβ and Dr. Winship Herr for the gift of expression vector encoding Oct-1.
This work was supported in part by the National Institutes of Health (R01HL071233; A.A.)
Abbreviations
- CRP
C-reactive protein
- EMSA
electrophoretic mobility shift assay
- Luc
luciferase
- m-OCT
promoter and oligos with the Oct-1 site deleted
- Oligo
oligonucleotide
- RLU
relative luciferase units
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal A, Cha-Molstad H, Samols D, Kushner I. Transactivation of C-reactive protein by IL-6 requires synergistic interaction of CCAAT/enhancer binding protein β (C/EBPβ) and Rel p50. J. Immunol. 2001;166:2378–2384. doi: 10.4049/jimmunol.166.4.2378. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Cha-Molstad H, Samols D, Kushner I. Overexpressed nuclear factor-κB can participate in endogenous C-reactive protein induction, and enhances the effects of C/EBPβ and signal transducer and activator of transcription-3. Immunology. 2003a;108:539–547. doi: 10.1046/j.1365-2567.2003.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Samols D, Kushner I. Transcription factor c-Rel enhances C-reactive protein expression by facilitating the binding of C/EBPβ to the promoter. Mol. Immunol. 2003b;40:373–380. doi: 10.1016/s0161-5890(03)00148-2. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Singh PP, Bottazzi B, Garlanda C, Mantovani A. Pattern recognition by pentraxins. Adv. Exp. Med. Biol. 2009;653:98–116. doi: 10.1007/978-1-4419-0901-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcone R, Gualandi G, Ciliberto G. Identification of sequences responsible for acute-phase induction of human C-reactive protein. Nucl. Acids Res. 1988;16:3195–3207. doi: 10.1093/nar/16.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Weaver JA, Sterling KM, Bresnick E. Nuclear transcription factor Oct-1 binds to the 5'-upstream region of CYP1A1 and negatively regulates its expression. Int. J. Biochem. Cell Biol. 1996;28:217–227. doi: 10.1016/1357-2725(95)00122-0. [DOI] [PubMed] [Google Scholar]
- Blaschke F, Takata Y, Caglayan E, Collins A, Tontonoz P, Hsueh WA, Tangirala RK. A nuclear receptor corepressor-dependent pathway mediates suppression of cytokine-induced C-reactive protein gene expression by liver X receptor. Circ. Res. 2006;99:e88–e99. doi: 10.1161/01.RES.0000252878.34269.06. [DOI] [PubMed] [Google Scholar]
- Castell JV, Gomez-Lechon MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: Regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12:1179–1186. doi: 10.1002/hep.1840120517. [DOI] [PubMed] [Google Scholar]
- Cha-Molstad H, Agrawal A, Zhang D, Samols D, Kushner I. The Rel family member p50 mediates cytokine-induced C-reactive protein expression by a novel mechanism. J. Immunol. 2000;165:4592–4597. doi: 10.4049/jimmunol.165.8.4592. [DOI] [PubMed] [Google Scholar]
- Cha-Molstad H, Young DP, Kushner I, Samols D. The interaction of c-Rel with C/EBPbeta enhances C/EBPbeta binding to the C-reactive protein gene promoter. Mol. Immunol. 2007;44:2933–2942. doi: 10.1016/j.molimm.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Darlington GJ, Wilson DR, Lachman LB. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J. Cell Biol. 1986;103:787–93. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Paz NG, Simeonidis S, Leo C, Rose DW, Collins T. Regulation of NF-κB-dependent gene expression by the POU domain transcription factor Oct-1. J. Biol. Chem. 2007;282:8424–8434. doi: 10.1074/jbc.M606923200. [DOI] [PubMed] [Google Scholar]
- Dong B, Huang C, Li D, Zhao FQ. Oct-1 functions as a transactivator in the hormonal induction of beta-casein gene expression. Mol. Cell. Biochem. 2009;328:93–99. doi: 10.1007/s11010-009-0078-5. [DOI] [PubMed] [Google Scholar]
- Eklund CM. Proinflammatory cytokines in CRP baseline regulation. Adv. Clin. Chem. 2009;48:111–136. doi: 10.1016/s0065-2423(09)48005-3. [DOI] [PubMed] [Google Scholar]
- Ganapathi MK, Schultz D, Mackiewicz A, Samols D, Hu S-I, Brabenec A, Macintyre SS, Kushner I. Heterogeneous nature of the acute phase response: Differential regulation of human serum amyloid A, C-reactive protein, and other acute phase proteins by cytokines in Hep3B cells. J. Immunol. 1988;141:564–569. [PubMed] [Google Scholar]
- Ghosh S, Hayden MS. Celebrating 25 years of NF-κB research. Immunol. Rev. 2012;246:5–13. doi: 10.1111/j.1600-065X.2012.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger G, Bing DH, Sipe JD, Rits M, Colten HR. Transcriptional regulation of genes encoding the acute-phase proteins CRP, SAA, and C3. J. Immunol. 1987;138:3967–3971. [PubMed] [Google Scholar]
- Grimm AA, Brace CS, Wang T, Stormo GD, Imai S-I. A nutrient-sensitive interaction between Sirt1 and HNF-1α regulates Crp expression. Aging Cell. 2011;10:305–317. doi: 10.1111/j.1474-9726.2010.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamoto S, Segil N, Pan Z-Q, Kimura M, Roeder RG. The cyclin-dependent kinase-activating kinase (CAK) assembly factor, MAT1, targets and enhances CAK activity on the POU domains of octamer transcription factors. J. Biol. Chem. 1997;272:29852–29858. doi: 10.1074/jbc.272.47.29852. [DOI] [PubMed] [Google Scholar]
- Kleemann R, Gervois PP, Verschuren L, Staels B, Princen HMG, Kooistra T. Fibrates down-regulate IL-1-stimulated C-reactive protein gene expression in hepatocytes by reducing nuclear p50-NFκB-C/EBP-β complex formation. Blood. 2003;101:545–551. doi: 10.1182/blood-2002-06-1762. [DOI] [PubMed] [Google Scholar]
- Kramer F, Torzewski J, Kamenz J, Veit K, Hombach V, Dedio J, Ivashchenko Y. Interleukin-1β stimulates acute phase response and C-reactive protein synthesis by inducing an NFκB- and C/EBPβ-dependent autocrine interleukin-6 loop. Mol. Immunol. 2008;45:2678–2689. doi: 10.1016/j.molimm.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am. J. Med. 2006;119:166.e17–166.e28. doi: 10.1016/j.amjmed.2005.06.057. [DOI] [PubMed] [Google Scholar]
- Li S-P, Goldman ND. Regulation of human C-reactive protein gene expression by two synergistic IL-6 responsive elements. Biochemistry. 1996;35:9060–9068. doi: 10.1021/bi953033d. [DOI] [PubMed] [Google Scholar]
- Nakshatri H, Nakshatri P, Currie RA. Interaction of Oct-1 with TFIIB: Implications for a novel response elicited through the proximal octamer site of the lipoprotein lipase promoter. J. Biol. Chem. 1995;270:19613–19623. doi: 10.1074/jbc.270.33.19613. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Hagihara K, Serada S, Isobe T, Matsumura A, Song J, Tanaka T, Kawase I, Naka T, Yoshizaki K. Transcriptional complex formation of c-Fos, STAT3, and hepatocyte NF-1α is essential for cytokine-driven C-reactive protein gene expression. J. Immunol. 2008;180:3492–3501. doi: 10.4049/jimmunol.180.5.3492. [DOI] [PubMed] [Google Scholar]
- Ochrietor JD, Harrison KA, Zahedi K, Mortensen RF. Role of STAT3 and C/EBP in cytokine-dependent expression of the mouse serum amyloid P-component (SAP) and C-reactive protein (CRP) genes. Cytokine. 2000;12:888–899. doi: 10.1006/cyto.2000.0668. [DOI] [PubMed] [Google Scholar]
- Osborne AR, Zhang H, Fejer G, Palubin KM, Niesen MI, Blanck G. Oct-1 maintains an intermediate, stable state of HLA-DRA promoter repression in Rb-defective cells: An Oct-1-containing repressosome that prevents NF-Y binding to the HLA-DRA promoter. J. Biol. Chem. 2004;279:28911–28919. doi: 10.1074/jbc.M403118200. [DOI] [PubMed] [Google Scholar]
- Préfontaine GG, Walther R, Giffin W, Lemieux ME, Pope L, Hache RJG. Selective binding of steroid hormone receptors to octamer transcription factors determines transcriptional synergism at the mouse mammary tumor virus promoter. J. Biol. Chem. 1999;274:26713–26719. doi: 10.1074/jbc.274.38.26713. [DOI] [PubMed] [Google Scholar]
- Poli V, Cortese R. Interleukin 6 induces a liver-specific nuclear protein that binds to the promoter of acute-phase genes. Proc. Natl. Acad. Sci. USA. 1989;86:8202–8206. doi: 10.1073/pnas.86.21.8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramji DP, Vitelli A, Tronche F, Cortese R, Ciliberto G. The two C/EBP isoforms, IL-6DBP/NF-IL6 and C/EBPδ/NF-IL6β, are induced by IL-6 to promote acute phase gene transcription via different mechanisms. Nucl. Acids Res. 1993;21:289–294. doi: 10.1093/nar/21.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner AP, Barber MJ, Guan Y, Ridker PM, Lange LA, Chasman DI, Walston JD, Cooper GM, Jenny NS, Rieder MJ, Durda JP, Smith JD, Novembre J, Tracy RP, Rotter JI, Stephens M, Nickerson DA, Krauss RM. Polymorphisms of the HNF1A gene encoding hepatocyte nuclear factor-1α are associated with C-reactive protein. Am. J. Human Genet. 2008;82:1193–1201. doi: 10.1016/j.ajhg.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya A, Kang J, Chumley J, Williams MA, Tantin D. Oct1 is a switchable, bipotential stabilizer of repressed and inducible transcriptional states. J. Biol. Chem. 2011;286:450–459. doi: 10.1074/jbc.M110.174045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Sen R, Baltimore D, Sharp PA. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986;319:154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Singh PP, Voleti B, Agrawal A. A novel RBP-Jκ-dependent switch from C/EBPβ to C/EBPζ at the C/EBP binding site on the C-reactive protein promoter. J. Immunol. 2007;178:7302–7309. doi: 10.4049/jimmunol.178.11.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ström A-C, Forsberg M, Lillhager P, Westin G. The transcription factors Sp1 and Oct-1 interact physically to regulate human U2 snRNA gene expression. Nucl. Acids Res. 1996;24:1981–1986. doi: 10.1093/nar/24.11.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm RA, Das G, Herr W. The ubiquitous octamer-binding protein Oct-1contains a POU domain with a homeo box subdomain. Genes Dev. 1988;2:1582–1599. doi: 10.1101/gad.2.12a.1582. [DOI] [PubMed] [Google Scholar]
- Toniatti C, Arcone R, Majello B, Ganter U, Arpaia G, Ciliberto G. Regulation of the human C-reactive protein gene, a major marker of inflammation and cancer. Mol. Biol. Med. 1990a;7:199–212. [PubMed] [Google Scholar]
- Toniatti C, Demartis A, Monaci P, Nicosia A, Ciliberto G. Synergistic trans-activation of the human C-reactive promoter by transcription factor HNF-1 binding at two distinct sites. EMBO J. 1990b;9:4467–4475. doi: 10.1002/j.1460-2075.1990.tb07897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol. Cell. Biol. 1998;18:2545–2552. doi: 10.1128/mcb.18.5.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voleti B, Agrawal A. Regulation of basal and induced expression of C-reactive protein through an overlapping element for OCT-1 and NF-κB on the proximal promoter. J. Immunol. 2005;175:3386–3390. doi: 10.4049/jimmunol.175.5.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voleti B, Agrawal A. Statins and nitric oxide reduce C-reactive protein production while inflammatory conditions persist. Mol. Immunol. 2006;43:891–896. doi: 10.1016/j.molimm.2005.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JW, Wilson L, Rosenfeld MG. POU-domain proteins Pit-1 and Oct-1 interact to form a heteromeric complex and can cooperate to induce expression of the prolactin promoter. Genes Dev. 1991;5:1309–1320. doi: 10.1101/gad.5.7.1309. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ripperger J, Fey GH, Samols D, Kordula T, Wetzler M, Van Etten RA, Baumann H. Modulation of hepatic acute phase gene expression by epidermal growth factor and Src protein tyrosine kinases in murine and human hepatic cells. Hepatology. 1999;30:682–697. doi: 10.1002/hep.510300318. [DOI] [PubMed] [Google Scholar]
- Wu GD, Lai EJ, Huang N, Wen X. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter: The role of Oct-1 as a transcriptional repressor. J. Biol. Chem. 1997;272:2396–2403. [PubMed] [Google Scholar]
- Wysocka J, Herr W. The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem. Sci. 2003;28:294–304. doi: 10.1016/S0968-0004(03)00088-4. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Yamagishi S, Nakamura K, Matsui T, Imaizumi T, Inoue H, Ueno T, Sata M. Pigment epithelium-derived factor (PEDF) blocks the interleukin-6 signaling to C-reactive protein expression in Hep3B cells by suppressing Rac-1 activation. Life Sci. 2006;79:1981–1987. doi: 10.1016/j.lfs.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Young DP, Kushner I, Samols D. Binding of C/EBPβ to the C-reactive protein (CRP) promoter in Hep3B cells is associated with transcription of CRP mRNA. J. Immunol. 2008;181:2420–2427. doi: 10.4049/jimmunol.181.4.2420. [DOI] [PubMed] [Google Scholar]
- Zhang D, Jiang S-L, Rzewnicki D, Samols D, Kushner I. The effect of interleukin-1 on C-reactive protein expression in Hep3B cells is exerted at the transcriptional level. Biochem. J. 1995;310:143–148. doi: 10.1042/bj3100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Sun M, Samols D, Kushner I. STAT3 participates in transcriptional activation of the C-reactive protein gene by interleukin-6. J. Biol. Chem. 1996;271:9503–9509. doi: 10.1074/jbc.271.16.9503. [DOI] [PubMed] [Google Scholar]
- Zhou D-X, Yen TSB. The ubiquitous transcription factor Oct-1 and the liver-specific factor HNF-1 are both required to activate transcription of a hepatitis B virus promoter. Mol. Cell. Biol. 1991;11:1353–1359. doi: 10.1128/mcb.11.3.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwilling S, Annweiler A, Wirth T. The POU domains of the Oct1 and Oct2 transcription factors mediate specific interaction with TBP. Nucl. Acids Res. 1994;22:1655–1662. doi: 10.1093/nar/22.9.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]