Abstract
Background
Basic and clinical studies report that the expression of Fibroblast Growth Factor-2 (FGF2) is decreased in the Prefrontal Cortex (PFC) of depressed subjects or rodents exposed to stress, and increased following antidepressant treatment. Here, we aim to determine if: 1) FGF2/FGF receptor signaling is sufficient and required for mediating an antidepressant response behaviorally and cellularly; and 2) if the antidepressant actions of FGF2 are mediated specifically by the PFC.
Methods
The role of FGF2 signaling in behavioral models of depression and anxiety was tested using chronic unpredictable stress (CUS)/sucrose consumption test (SCT), forced swim test (FST) and novelty suppressed feeding test (NSFT). We also assessed the number of bromodeoxyuridine labeled dividing glial cells in the PFC as a cellular index relevant to depression (i.e. decreased by stress and increased by antidepressant treatment).
Results
Chronic FGF2 infusions (i.c.v.) blocked the deficit in SCT caused by CUS. Moreover, the response to antidepressant treatment in the CUS/SCT, and FST, were abolished upon administration of an inhibitor of FGF receptor activity, SU5402. These results are consistent with the regulation of proliferating cells in the PFC, a portion of which are of oligodendrocyte lineage. Lastly, subchronic infusions of FGF2 into the PFC but not into dorsal striatum produced antidepressant- and anxiolytic-like effects on FST and NSFT respectively.
Conclusions
These findings demonstrate that FGF2-FGFR signaling is sufficient and necessary for the behavioral, as well as gliogenic actions of antidepressants and highlight the PFC as a brain region sensitive to the antidepressant actions of FGF2.
Key works: Fibroblast Growth Factor-2, Depression, Antidepressants, NG2-glia, Prefrontal Cortex, Chronic Unpredictable Stress
Introduction
Depression is a debilitating mental illness that affects nearly 1 in 6 Americans (1). Existing pharmacological agents are not ideal as only a subset of patients display improvement and the therapeutic onset is delayed. Thus, the development of novel, more efficacious antidepressant drugs requires a better understanding of the pathophysiological basis of depression. Brain imaging studies of subjects diagnosed with depression have demonstrated decreased volume of limbic brain regions including prefrontal cortex (PFC) (2, 3) and further histological assessments of the PFC report decreases in the density of glia (oligodendrocytes and astrocytes), and neurons, as well as the size of neurons (4–9). In contrast to the damaging effects of depression and stress, there is evidence that the therapeutic actions of antidepressant are mediated by growth factor signaling molecules that can exert effects directly on neurons or indirectly on glial cells to reverse neuronal damage and atrophy in affected brain regions (10, 11).
Fibroblast Growth Factor-2 (FGF2) is hypothesized to be one such growth factor (12). Levels of FGF2 and its receptors are down regulated in the PFC and other limbic brain regions in depressed subjects (13–15), and antidepressant treatment opposes these effects and up regulates FGF2 in humans (13) and rodents in preclinical models (16, 17). Recent behavioral studies have provided evidence that FGF2 produces antidepressant effects. FGF2 infusion into the brain produces antidepressant-like responses in several different models of acute antidepressant response and/or anxiety (18, 19).
In the current study, we test the role of FGF2 in a Chronic Unpredictable Stress (CUS) model of depression, and determine whether different classes of antidepressants require FGF receptor signaling to produce an antidepressant response. The CUS model, unlike other depression paradigms, has a unique combination of predictive (requires a chronic treatment with “standard” antidepressant drugs), face (produces depressive symptoms) and construct validity (stress is a precipitating factor of depression that produces anhedonia, a core symptom of depression) (20, 21). Furthermore, ketamine, a rapid-acting antidepressant has shown antidepressant efficacy in this model of depression (22). We also assess the effects of FGF2 on the number of proliferating glia in the PFC as a cellular index of antidepressant efficacy (23). FGF2 is a potent gliogenic agent (24) and we predict that altered regulation of FGF2 signaling could underlie the opposing actions of stress and antidepressants on glial proliferation in the PFC. Finally, we determine if FGF2 in the PFC is sufficient to produce an antidepressant response.
Methods and Materials
Animals
Experiments were conducted in male C57Bl/6 mice (8–10 weeks of age; Jackson Laboratories) with the exception of the PFC infusion studies, which were conducted in male Sprague Dawley rats (300–400 g; Charles River) to allow for a larger target area. Animals underwent a 12-hour light dark cycle at constant temperature (20°C) with ad libitum access to water and food. All experimental protocols were consistent with the guidelines issued by the US National Institutes of Health and were approved by the Yale University Institutional Animal Care and Use Committee.
Cannula Implantation and Microinjection
Rodents were anesthetized with a Ketamine (LLOYD laboratories) & Xylazine (Fort Dodge Animal Health) cocktail (100mg/kg and 10mg/kg for mice, 80 mg/kg and 6mg/kg respectively for rats). It is important to consider potential confounding effects of ketamine given reports that this agent produces antidepressant actions in rodent models (25, 26). However, the antidepressant effects are typically observed with subanesthetic doses, are no longer observed at the later time points used for behavioral tests in the current study (2 to 4 weeks after ketamine/surgery), and all animals, control and experimental, undergo the same anesthetic/surgical conditions, making ketamine an acceptable and safe choice in the current study. For central infusions of FGF2, mice were implanted with a mini-osmotic pump (Plastics1; model 2002, Brain infusion Kit3) that infused recombinant mouse FGF2 (R&D systems; 0.5 μl/hour, 14 days; 240 ng/day) or saline (vehicle) into the lateral ventricle (−0.2 mm Anterior/Posterior, ±1 medial/lateral, 2.5mm depth). For central infusions of SU5402 (Calbiochem) or DMSO (100%) (vehicle), mice were implanted with a guide cannula into one of the lateral ventricle (−0.2 mm Anterior/Posterior; ±1 mm medial/lateral; depth 2.25mm). After 1–2 days of recovery, mice were infused with SU5402 (2.5 μg/day; 1μl; 0.25μl/min) or DMSO (vehicle). For infusions of FGF2 into the medial PFC: A guide cannula was implanted bilaterally in the prelimbic cortex of rats (PrL) (Depth −3.5 mm from dura; Anterior/Posterior +3.2mm; Medial/Lateral ±1mm). After a 2-week recovery, cannulated rats were infused with recombinant human FGF2 (R&D systems) (200 ng/μl; 1μl with 0.5 μl/side; 0.1μl/min) or saline (vehicle). For infusions of FGF2 into the dorsal striatum: A guide cannula was implanted bilaterally in the caudate putamen of rats (CPU) (Depth −5 mm from dura; Anterior/Posterior +1.6mm; Medial/Lateral ±1.9mm). After a 10-day recovery, cannulated rats were infused with recombinant human FGF2 (R&D systems) (200 ng/μl; 1μl with 0.5 μl/side; 0.1μl/min) or saline (vehicle).
Bromodeoxyuridine injection & Drug administration
Mice were administered (i.p.) saline or fluoxetine hydrochloride (10mg/kg; Lilly) for 22 days or imipramine (20mg/kg; Sigma) for 30 days. To label dividing cells, bromodeoxyuridine (BrdU; 100 mg/kg, i.p.; Sigma) was administered 3 times every 12 hours, 3 days before sacrifice.
Immunohistochemistry & quantification
Studies were conducted as previously described (10, 23) on fixed coronal brain sections (40μm) incubated with mouse anti-BrdU antibody (1:100; overnight; Becton Dickinson), and then biotinylated goat anti-mouse antibody (1:200; Vector laboratories; 1 hour). BrdU-labeled cells were quantified by a blinded investigator in every sixth section throughout the PrL & Infralimbic (IL) cortices using a 0.5 mm2 contour and Stereoinvestigator Software (MicroBrightField).
For the double labeling, sections were incubated with rat anti-BrdU (1:100; Accurate) and oligodendrocyte precursor marker rabbit anti-NG2 (1:100, Millipore) for 3 days at 4°C. Sections were then exposed to Alexa Fluor 488 goat anti-rat and Alexa Fluor 546 goat anti-rabbit (1:200; 1 hour). For triple labeling, sections were incubated with rat anti-BrdU (1:100; Accurate), the oligodendrocyte marker mouse anti-receptor interacting protein (RIP) (1:1000; Developmental Studies Hypridoma Bank) for 4 days at 4°C. Sections were then exposed for 1 hour to Alexa Fluor 488 goat anti-rat, Alexa Fluor 633 goat anti-mouse (1:200; Molecular Probes) and the endothelial cell marker DyLight 594 conjugated Tomato Lectin (LEL,TL) (4:1000, Vector Laboratories). For quantification, sections were analyzed for colocalization in the PrL & IL cortices using a confocal microscope (Fluoview, Olympus). On average, 25 BrdU+ cells per animal were analyzed with Z-plane sectioning (1μm steps) to confirm the colocalization of BrdU with each cell marker.
CUS procedure
We used two different CUS protocols for mice adapted from our earlier studies. The number and type of stressors differed depending on the length of the CUS study and were devised to ensure a depressive-like phenotype. For the FGF2 infusion study, mice were exposed to three stressors per day for fourteen days (27) (details of stressors in Table S2 in the Supplement). For the antidepressant imipramine blockade study, mice were exposed to 2–3 stressors/day for 4 weeks (28) (details in Table S3 in the Supplement). A longer treatment regimen accompanied by a longer stress exposure was needed to elicit the antidepressant effects of imipramine in this model of depression. Since FGF2 is a downstream signaling molecule of antidepressants, a shorter treatment regimen was expected to be sufficient.
Sucrose Consumption Test
Mice were first habituated to 1 % sucrose solution. After overnight fluid deprivation, sucrose intake over a 1-hour period was measured followed by 1-hour water test the day after. The amount of fluid intake was normalized to body weight of the respective animal. All sucrose and water tests were done between 9 and 11 AM. For the CUS/imipramine study, 1-hour sucrose testing was repeated over a period of 1 week in the morning starting on day 20 and the result was expressed as an average of the individual tests. 1% sucrose solution was initially presented to mice during testing and was then increased to 2% to enhance sensitivity to sucrose intake (29). In this study, water intake was measured overnight, given the repeated testing of sucrose.
Forced Swim Test
Mice: FST was performed according to previously published methods from our laboratory (30). Briefly, a 5-min swim test session was videotaped, and the time spent immobile during the swim session was recorded blindly. The first min was excluded from the analysis and scoring was done for the last 4 min of the test period. Rats: were exposed to a pre-swim test for 15 minutes and then tested on the following day. Sessions were videotaped and scored blindly for the duration of the 10 min test. Immobility was defined as the least movement to stay afloat.
Locomotor Activity
Mice: animals were placed in a novel cage (width 17.3 cm, length 28.3 cm, height 12 cm) and distance moved (cm) was recorded for a total of 45 min with a video tracking software (Anymaze software, Stoelting). Rats: Ambulatory locomotor activity was assessed in clear plastic boxes fitted to automated activity meters (Omnitech Electronics) consisting of two parallel rows of photosensors (16 sensors per row, 2.5 cm apart). Locomotor activity was recorded in 5-min bins for a total of 30 min by using Micropro software.
Novelty Suppressed Feeding Test
NSFT was performed as previously described (31). Briefly, food deprived rats were placed in an open field (76.5 cm × 76.5cm × 40cm, Plexiglas) with a small amount of food in the center. The amount of time to take the first bite was recorded as latency to feed. Home cage food intake was measured right after the test as a control value.
Statistical Analysis
Statistical differences for experiments with more than 3 groups were determined by ANOVA and Fisher’s least significant difference post-hoc tests (SPSSInc, PASWStatistics18). For experiments with two groups, Student’s t-test was used. The level of statistical significance was set at p <0.05. Animals that were determined not healthy due to surgery and drug treatment or when the infusion cannula was not in the correct location were removed from the study.
Results
FGF2 infusions block the decrease in Sucrose consumption caused by CUS exposure
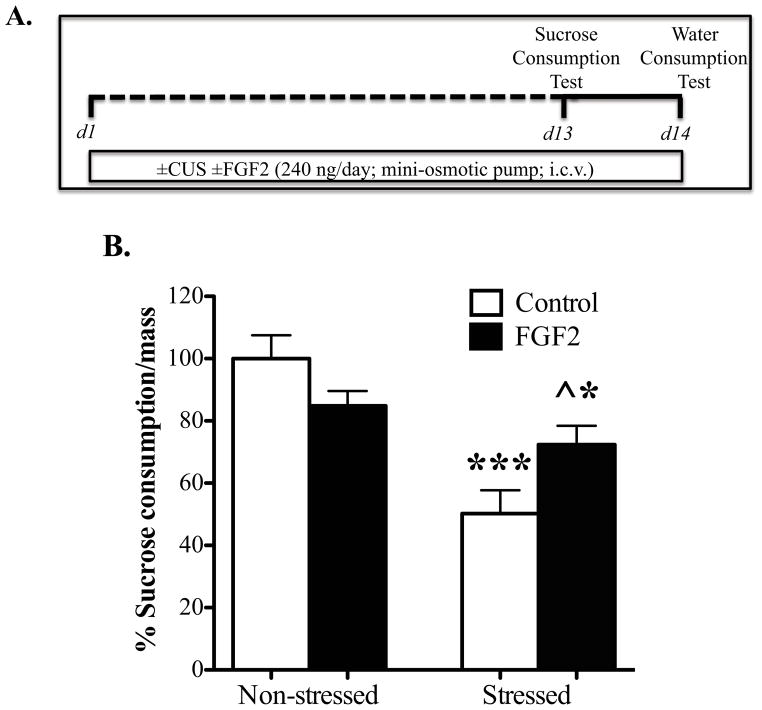
The influence of FGF2 (i.c.v., 14 d) on the deficit in sucrose consumption in mice resulting from CUS exposure was determined according to previously reported procedures (27). Two way ANOVA analysis showed a significant interaction between CUSxFGF2 on sucrose intake (F1,23= 5.73, p=0.025, Fig 1B). Post-hoc analysis indicated that there was a decrease in sucrose intake for the CUS group (approximately 50%, p<0.001) when compared to non-stressed controls. In addition, CUS mice treated with FGF2 consumed more sucrose solution (~22% more, p=0.04) than the CUS vehicle-treated mice. Nevertheless, this blockade was partial, as the amount consumed by the FGF2+CUS group was significantly different from controls (p=0.014). Water intake did not differ significantly between the groups (ctrl: 100% ±21.5%; FGF2: 100% ±18%; CUS: 75%±7.5%; CUS+FGF2: 89%±12%).
Figure 1. Continuous FGF2 infusions (i.c.v.) block the effects of Chronic Unpredictable Stress (CUS) on sucrose intake.
(A) Schematic diagram indicates the timeline for drug treatment, CUS exposure, and behavioral testing. Mice were implanted with a mini-osmotic pump that infuses saline or FGF2 (240 ng/day) intracerebroventricular (i.c.v.) for 14 days while being exposed to CUS or control conditions. On day 13, 1-hour (B) sucrose consumption test was conducted (Control n=8; FGF2, n=4; CUS, n=9; CUS+FGF2, n=6). Amount of sucrose consumed is normalized to the respective body weight (sucrose intake/mass for controls= 0.07 ±0.005). The data are expressed as mean percentage from control group ± s.e.m. * p< 0.05,** p< 0.01, ***p<0.001 compared with control animals; ^ p< 0.05 compared with CUS saline treated-group (analysis of variance and Fisher’s PLSD post hoc test).
FGF receptor antagonist (SU5402) blocks the behavioral effects of two different classes of antidepressants
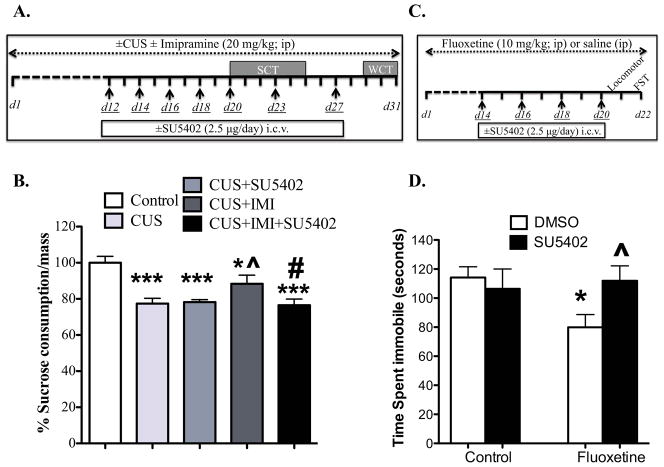
We confirmed the upregulation of FGF2 expression in the rat PFC following antidepressant treatment (see Supplement). Furthermore, we showed that the expression of a high affinity receptor for FGF2, FGFR1 is decreased in the rat PrL following CUS exposure and then restored to control levels after fluoxetine treatment (see Supplement). To further examine the role of FGF2 in mediating the behavioral actions of antidepressant treatment, the influence of an inhibitor of FGF2 receptor signaling, SU5402 on the response to imipramine (IMI, 20 mg/kg) in the CUS model was determined in mice. This dose was chosen based on previous studies demonstrating antidepressant effects in animal models of depression (32), including CUS (29). Post-hoc analysis indicated a significant decrease in sucrose intake with CUS compared to controls (p=0.000, Fig 2B), and SU5402 had no effect on the CUS response (22%) when compared to control (p=0.001). The effect of CUS was significantly blocked by imipramine treatment (p=0.037), although this effect was partial as consumption of the IMI+CUS group was significantly different from control (p=0.032). The antidepressant effect of imipramine was completely blocked by SU5402 (CUS+IMI+SU5402 group was significantly lower than CUS+IMI group, p=0.029). SU5402 alone (in non-stressed conditions) did not influence sucrose consumption (S2) (ctrl: 100% ±11%; SU5402: 100% ±15.7%), and there were no significant effects on overnight water consumption (ctrl: 100% ±5%; CUS: 89.8% ±7%; CUS+SU5402: 87% ±10%; CUS+IMI: 91% ±4.6%; CUS+IMI+SU5402: 86% ±7%).
Figure 2. Fibroblast Growth Factor receptor (FGFR) antagonist (SU5402) infusion blocks the behavioral actions of antidepressant treatment.
(A) Schematic diagrams indicate the timeline for imipramine (IMI) (A) or fluoxetine (C) treatment, days of SU5402 infusions (underlined), CUS exposure (A only) and behavioral testing. CUS and imipramine treatment persisted for 30 days. (B) A 1-hour sucrose consumption test (SCT) was conducted repeatedly between days 20 and 25. Amount of sucrose consumed is averaged across days and normalized to the respective body weight (average sucrose intake/mass for controls= 0.074 ±0.003) and the data are expressed as mean percentage from control group ± s.e.m (Control, n=6; CUS, n=7; CUS+SU5402, n=3; CUS+IMI, n=5; CUS+IMI+SU5402, n=6); * p< 0.05 and *** p<0.001 compared to control group; ^ p<0.05 compared to CUS group; # p<0.05 compared to CUS+IMI group (analysis of variance and Fisher’s PLSD post-hoc test). (C) After two weeks of fluoxetine treatment, FGFR inhibitor (SU5402; 2.5 μg/μl) was infused (i.c.v.) every other day. Mice were tested on (D) forced swim test (FST) (Control, n=11; SU5402, n=9; Flx, n=10; Flx+SU5402, n=10) on day 22. Results are expressed as mean ± s.e.m; * p< 0.05 and ** p< 0.01 compared with control animals; ^ p< 0.05 compared with fluoxetine-treated DMSO-injected group (analysis of variance and Fisher’s PLSD post-hoc test). i.c.v., intracerebroventricular; ip, intraperitoneal; WCT, water consumption test.
We also determined in mice the influence of SU5402 on the FST response to fluoxetine to examine another model of antidepressant treatment and a different class of antidepressant. A dose of 10 mg/kg was used based on previous reports of efficacy in this model (33). A two way ANOVA analysis revealed a marginally significant interaction between fluoxetine × SU5402 on time spent immobile (F(1,36)=3.96; p= 0.054, Fig 2D), which was significantly reduced by fluoxetine (p=0.018) and abolished by SU5402 (p=0.029). Fluoxetine-treated groups did not show enhanced locomotor activity (control: 1378 cm ±58; SU5402: 1308 cm ±136; FLX: 1048 cm ±113; FLX+SU5402: 1025 cm ±67) indicating that the decrease in time spent immobile (i.e., increased swimming) reflects an antidepressant-like behavior, rather than an inhibitory effect on general ambulatory activity.
FGF2 infusions block CUS-inhibition of glial proliferation in the PFC
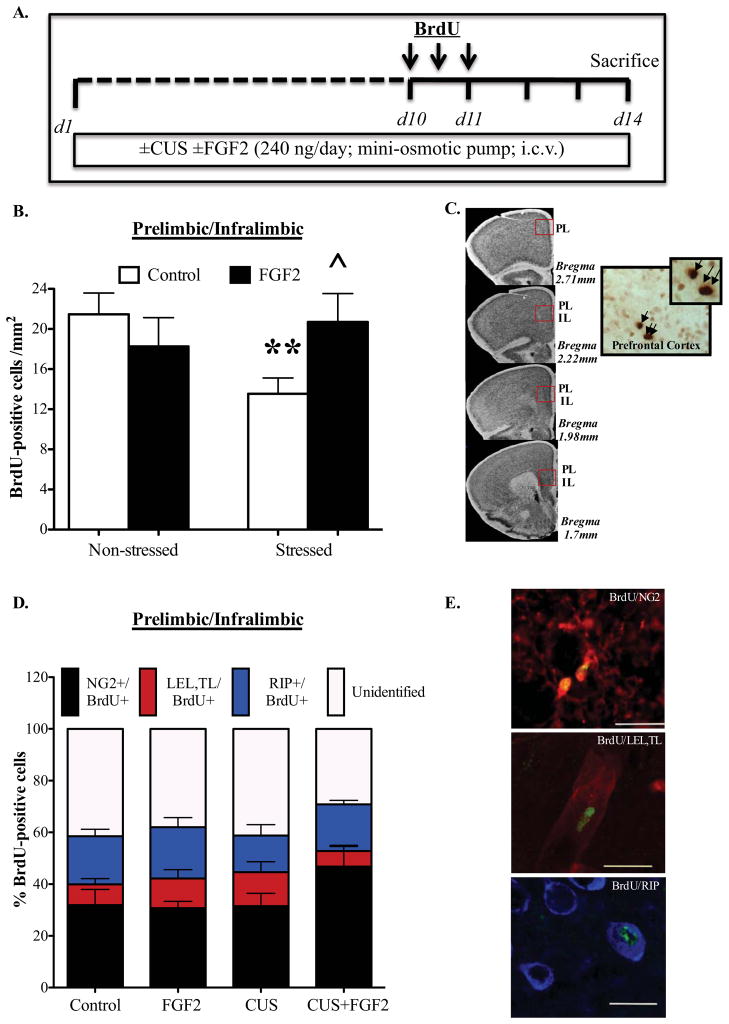
Because CUS decreases glial proliferation in the PFC and reduced glial number is sufficient to cause depressive-like behavior in rodents (23, 34, 35), we next set out to examine the effects of chronic FGF2 infusion (i.c.v.) on PFC cell proliferation in mice (Fig 3A–D). Two-way ANOVA analysis revealed significant interaction effects for CUSxFGF2 on the number of BrdU+ cells (F1,30= 4.953, p=0.034) (Fig 3B). Post-hoc analysis demonstrated that CUS significantly reduced the number of BrdU+ cells (p=0.01) and that chronic FGF2 infusion blocked this decrease (p=0.044) (Fig 3B). FGF2 infusions did not influence the number of BrdU-labeled cells in non-stressed mice (Fig 3B).
Figure 3. Continuous FGF2 infusions block Chronic Unpredictable Stress (CUS)-inhibition of glial proliferation in the Prelimbic/Infralimbic Cortex.
(A) Schematic diagram indicates the timeline for drug treatment, CUS exposure and Bromodeoxyuridine (BrdU) injections. Mice were implanted with a mini-osmotic pump that infuses saline or FGF2 (240 ng/day) i.c.v. for 14 days while being exposed to CUS or control conditions. Between days 10 and 11, mice were injected with BrdU 3x every 12 hours and were sacrificed three days later following the last injection. Mice were then perfused and immunostaining against BrdU was done on PFC sections. (B) BrdU+ cells/mm2 were quantified in the Prelimbic & Infralimbic cortices (Control, n=11; FGF2, n=7; CUS, n=10; CUS+FGF2, n=6). (C) Representative micrographs are shown from various anterior-posterior levels analyzed, with corresponding plates adapted from BML Mouse brain Atlas (65). The number of BrdU+ cells was quantified using a 0.5 mm2 rectangle on the region of interest. Shown on the right are images of BrdU+ cells (40x magnification, indicated by arrows) in the PFC. (D) Colocalization of BrdU with NG2, a specific cell marker for oligodendrocyte progenitor cells, RIP, a marker for oligodendrocyte cells and lastly LEL,TL a marker for endothelial cells. The percent of colabeled cells was not altered by CUS or by FGF2 (NG2: ctrl, 31.9% ±6.1; FGF2 alone, 30.7%± 2.6; CUS, 31.56% ±4.9; CUS+FGF2, 46.8%±8.18; LEL,TL: ctrl, 8% ±2.2; FGF2 alone, 11.5%± 3.4; CUS, 13.1% ±4; CUS+FGF2, 6%±1.8; RIP: ctrl, 18.6% ±2.74; FGF2 alone, 19.8%± 3.7; CUS, 14.2% ±4.2; CUS+FGF2, 18%±1.57). (E) Representative figures showing BrdU-labeled cells (green) in Prelimbic & Infralimbic cortices that are colabeled with NG2-labeled cells (red), endothelial cells (red) or RIP-labeled cells (blue). Results are expressed as mean ± s.e.m; * p< 0.05,** p< 0.01, ***p<0.001 compared with control animals; ^ p< 0.05 compared with CUS saline treated-group (analysis of variance and Fisher’s PLSD post-hoc test). i.c.v., intracerebroventricular.
Phenotype analysis demonstrated that the majority of BrdU+ cells (32–47%) co-express NG2, a marker for oligodendrocyte precursor cells (OPCs) (Fig 3D). The remaining BrdU+ cells expressed markers for mature oligodendrocytes (RIP) (14–20%) or endothelial cells (LEL/TL) (6–13%). There was no significant effect of CUS or FGF2 on the percentage of NG2, RIP or endothelial cells, although there was a trend for an increase in NG2+ cells in the CUS+FGF2 group (p=0.085). Approximately 40% of BrdU+ cells were unidentified, consistent with previous reports (23). These results indicate that: 1) CUS decreases the total number of proliferating glial and endothelial cells, and 2) that FGF2 blocks this decrease.
FGF receptor antagonist blocks fluoxetine-induction of proliferating cells in PFC
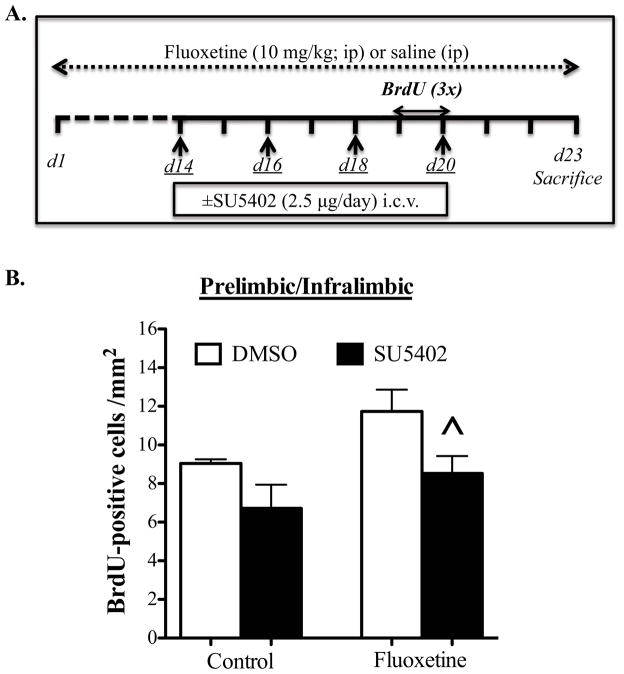
Because FGFR1 is expressed by glial cells (36), we examined if the FGFR inhibitor blocks fluoxetine-induction of cell proliferation in the mouse PFC. Mice were administered fluoxetine for 23 d and infused with SU5402 (i.c.v., 2.5μg) every other day for the last 9 d (Fig 4A). This paradigm was used because of the time delay for up-regulating FGF2 expression following antidepressant treatment (16, 17, 37) and to minimize the number of infusions of SU5402/DMSO and still provide sufficient blockade of FGFR signaling. Two-way ANOVA demonstrated a significant effect of fluoxetine (F1,24= 5.253, p=0.03, Fig 4B) and SU5402 (F1,24= 7.915, p=0.01, Fig 4B) on the number of BrdU+ cells in the PFC. There was a trend for increased BrdU+ cells (+30%) following fluoxetine treatment when compared to control group (p=0.085), consistent with previously reported effects of fluoxetine (38). This increase was blocked by SU5402 (p=0.038). There was no significant effect of SU5402 alone on BrdU+ cells in vehicle treated animals (Fig 4B). Note that there is a baseline (i.e., control) difference in the number of BrdU+ cells (Fig 4 compared to Fig 3), which could be due to the stress of daily injections in the experiment shown in Fig 4.
Figure 4. Fibroblast Growth Factor receptor (FGFR) antagonist (SU5402) blocks fluoxetine-induction of Bromodeoxyuridine (BrdU) labeled cells.
(A) Schematic diagram indicates the timeline for fluoxetine treatment, days of infusions of SU5402 (underlined) and Bromodeoxyuridine (BrdU) injections. BrdU was injected three times between days 19 and 20. Three days following BrdU injection, mice were perfused and immunostaining for BrdU was conducted. (B) BrdU+ cells/mm2 were counted in the Prelimbic & Infralimbic cortices (Control, n=7; SU5402, n=8; Flx, n=5; Flx+SU5402, n=8). Results are expressed as mean ± s.e.m; * p< 0.05 and ** p< 0.01 compared with control animals; ^ p< 0.05 compared with fluoxetine-treated DMSO-injected group (analysis of variance and Fisher’s PLSD post-hoc test). i.c.v., intracerebroventricular; ip, intraperitoneal.
FGF2 infusions specifically into the medial PFC produce antidepressant behavioral responses
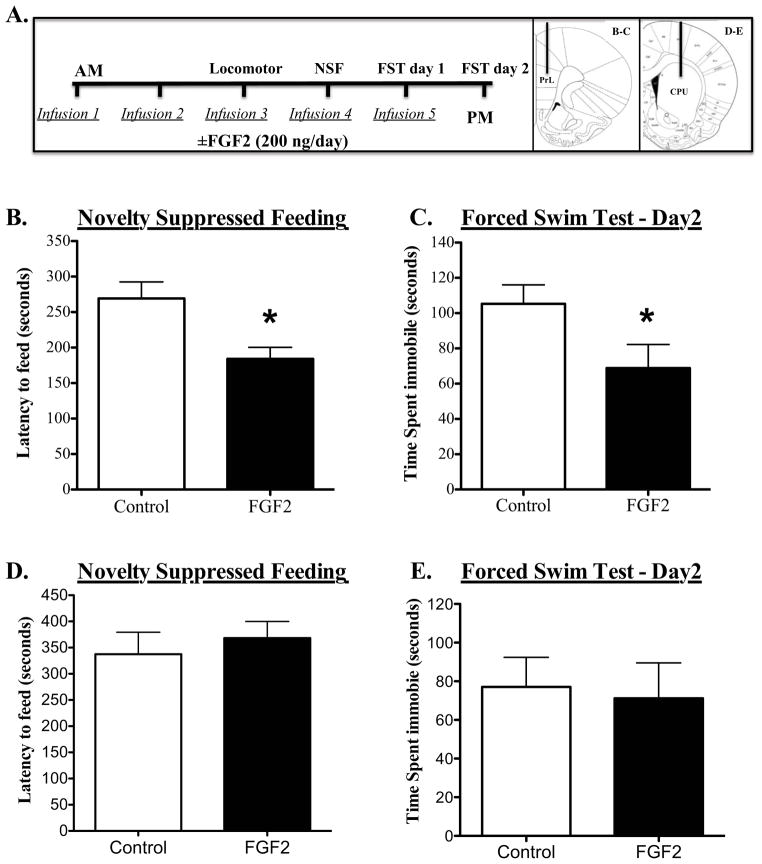
To directly test the role of PFC, FGF2 was infused over 5 days into medial PFC of rats, which were used here because of the larger target region. FGF2 produced significant anxiolytic and antidepressant effects in the NSFT (decreased latency to feed) (p=0.012; Fig 5B) and FST (decreased immobility) (p=0.053; Fig 5C) respectively. There were no significant effects on home cage feeding (control: 2.95 g ± 0.29; FGF2: 2.7 g ± 0.49; p= 0.67) or locomotor activity expressed as number of beam breaks (control: 2927 ± 343; FGF2: 3820 ± 1150; p= 0.12).
Figure 5. Subchronic FGF2 infusions into the Prefrontal cortex (PFC) but not into the dorsal striatum produce behavioral changes in novelty-suppressed feeding (NSF) and forced swim tests (FST).
(A) Schematic diagram shows the timeline for FGF2 infusions and the sequence of behavioral testing. Images on the right are representative micrographs shown at different anterior-posterior levels adapted from Paxinos and Watson (66). Rats received saline or FGF2 (200 ng/day) infusions into the PFC for 5 days and were then tested in (B) NSF on day 3 or (C) FST on day 6. Day 2 of FST was recorded and time spent immobile was analyzed. Values represent mean ± s.e.m (n=6–8 per group). Another subset of rats received saline or FGF2 (200 ng/day) infusions into the dorsal striatum (Caudate Putamen) for 5 days. The same paradigm used for the FGF2 infusion into the PFC was followed. Rats were tested in (D) NSF on day 3 or (E) FST on day 6. Day 2 of FST was recorded and time spent immobile was analyzed. Values represent mean ± s.e.m (n=10 per group). * p <0.05 compared to control group (Student’s t test). PrL, Prelimbic; CPU, Caudate Putamen.
We next examined the regional selectivity of the antidepressant actions of FGF2. Dorsal striatum (caudate putamen) is not implicated in the pathophysiology of mood disorders but does express FGF2 and FGFR1 (39), and as such was used as a control brain region. The same paradigm used for the FGF2 infusion into the PFC was followed (Fig 5A). Infusion of FGF2 into the caudate putamen for 5 days did not produce behavioral changes in the NSFT (Fig 5D) nor in FST (Fig 5E). There were no significant effects on home cage feeding (control: 3.5 g± 0.28; FGF2: 3.7g± 0.42) or locomotor activity (control: 2348± 241; FGF2: 2518±290 number of beam breaks).
Discussion
The results demonstrate that FGF2 treatment is sufficient to block anhedonia-like behavior caused by CUS, and that both imipramine and fluoxetine require FGFR signaling to abolish anhedonia and despair behaviors, respectively. The results also demonstrate that FGF2 blocks CUS-induced inhibition of proliferating glia in the PFC, and that fluoxetine-induction of proliferating cells requires FGFR signaling. Lastly, FGF2 infusions into the PFC but not into dorsal striatum are sufficient to produce antidepressant actions in the NSFT and FST. Together, these studies implicate FGF2 signaling in the PFC in the behavioral and cellular responses to antidepressant treatment.
FGF2-FGFR signaling is sufficient and necessary for antidepressant-induced behaviors
Previous studies have demonstrated that FGF2 has antidepressant-like effects in behavioral models responsive to acute antidepressant treatment such as FST or have limited construct validity (e.g. olfactory bulbectomy model) (18, 19). Our work extends these studies in an important and novel way to reveal that FGF2 treatment can exert antidepressant effects in a model of depression with predictive, face and construct validity (e.g CUS). FGF2 did not completely block the effects of CUS, suggesting that other factors may also be required. The results also demonstrate that the behavioral responses to two different classes of antidepressants (the tricyclic imipramine and 5-HT selective reuptake inhibitor fluoxetine) require FGF2-FGFR signaling. The inhibitor used for these studies, SU5402, blocks the tyrosine kinase domain of FGFR1, but does not inhibit insulin receptor-1, epidermal growth factor, or platelet derived growth factor receptor subtypes (40–42). This combined with expression/cell signaling studies demonstrating that FGFR1 is regulated by stress (43) and antidepressant treatment (44) suggest that this receptor subtype underlies the behavioral as well as cellular antidepressant responses. However, FGF2 receptor subtypes, R1–4, share a high degree of amino acid homology (24), and there is evidence that SU5402 also blocks these other receptor subtypes (41). There is also a report that SU5402 can block VEGF receptor signaling (45). Thus, it is important to note that the effects of SU5402 treatment may not be exclusive to FGFR1 and FGFRs in general, and studies are currently underway to develop shRNA approaches for selective knockdown of FGFR1 to further examine this issue.
Antidepressant-induction of FGF2 (16, 17), FGFR1 (see Supplement), and cell proliferation in the PFC (38) raises the possibility that this brain region underlies the behavioral actions of FGF2. The results demonstrate that FGF2 infusion into PFC has antidepressant properties in two behavioral paradigms, the FST and NSFT, and that the response in one of these tests (NSFT) occurs within a much shorter period of time (3 days) than conventional antidepressants (21 days) (32). These behavioral responses were specific to the PFC, as FGF2 infusions into the dorsal striatum did not produce similar effects. Additional studies of other paradigms, including the CUS/anhedonia model will provide a more rigorous test of the antidepressant actions of FGF2 in the PFC, although long-term local infusions into the PFC could cause damage.
FGF2-FGFR signaling is sufficient and necessary for antidepressant induction of gliogenesis in the PFC
FGF2 also blocked the down-regulation of cell proliferation in the PFC resulting from CUS exposure, similar to the actions of antidepressants (23, 34). FGF2 had no effect in non-stressed mice, suggesting that endogenous levels are sufficient to sustain cell proliferation in the absence of stress. Because BrdU was injected 3 days before sacrifice, it is possible that FGF2 influences the survival, as well as proliferation of dividing cells, which would be consistent with previous in vitro (36, 46, 47) and in vivo studies (48–52). Phenotype analysis demonstrates that 32–47% of BrdU+ cells in the PFC are NG2+, as previously reported (23, 53), representing the largest pool of proliferating cells in the adult cortex (53). NG2+ cells differentiate into oligodendrocytes (54, 55), and either remain NG2+ glial cells with unique characteristics (23, 53) or differentiate into neurons (35, 55), although the latter is controversial (56, 57). BrdU+ cells were also colabeled with RIP (14–20%), a marker for immature and mature oligodendrocytes (58), or a marker of endothelial cells (LEL/TL) (6–13%) as previously reported (23, 34, 53). The percent of each of the different cell types was not significantly influenced by FGF2 or CUS indicating that cell fate was not altered, although there was a trend for an increase of NG2+ cells.
The results demonstrate that FGFR signaling is required for the proliferative/survival effects of fluoxetine treatment in the PFC. Given that FGF2/FGFR1 signaling promotes proliferation of oligodendrocyte progenitors in vitro (36) and that FGF2 and FGFR1 are up regulated in the PFC following chronic antidepressant treatment (see Supplement), it is possible that the fluoxetine-induced increase in number of proliferating cells in the PFC is mediated via FGFR1. However, since SU5402 may not be selective for FGFR1, further studies to selectively block or knockdown FGFR1 are required to test this hypothesis. The antidepressant actions of imipramine, which like fluoxetine increases FGF2 (16), may also require FGF2/FGFR-induction of cell proliferation, although further studies will be required to directly test this possibility.
The consequences of FGF2 and increased gliogenesis could influence neuronal function in a number of ways, including modulation of glutamate transmission and/or effects on neuroprotection and synaptic plasticity. For example, NG2-glia express glutamate receptors and transporters indicative of involvement in glutamate transmission and reuptake (59, 60). These cells can also release brain derived neurotrophic factor (BDNF) (61), another growth factor implicated in both the etiology and treatment of depression (27). Furthermore, FGF2 has been shown to increase functional excitatory synapses in neuronal cultures (62). Since both glial and neuronal cells express FGF2 receptors (36, 63, 64), modulation of neuronal function could thus occur via either direct actions on neurons and/or indirect actions on glial cells. Regardless of the mechanisms, the results of the current study provide new evidence for the involvement of FGF2 in the behavioral and cellular actions of antidepressants.
Supplementary Material
Acknowledgments
We thank Drs. Jane Taylor and Angus Nairn for the use of their locomotor activity apparatus and confocal microscope setup. We also would like to thank Drs. Regina Armstrong, Natalina Salmaso and Mila Komitova for their helpful discussions. This study was supported by R37 (MH45481) and by the Connecticut Mental Health Center. ME was partly supported by George Robert Pfeiffer fellowship. NMF was supported from a Brown-Coxe fellowship and funding provided by the Natural Sciences and Engineering council of Canada. This data has been published as an abstract and poster for Society for Neuroscience.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of general psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Schweizer E, Jin Z, Miller D, Bilker W, Swan LL, et al. Neuroanatomical substrates of late-life minor depression. A quantitative magnetic resonance imaging study. Archives of neurology. 1997;54:613–617. doi: 10.1001/archneur.1997.00550170085018. [DOI] [PubMed] [Google Scholar]
- 3.Chang CC, Yu SC, McQuoid DR, Messer DF, Taylor WD, Singh K, et al. Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry research. 2011;193:1–6. doi: 10.1016/j.pscychresns.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cerebral cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 5.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 6.Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophrenia research. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 7.Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, et al. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biological psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- 9.Si X, Miguel-Hidalgo JJ, O’Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2004;29:2088–2096. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS & neurological disorders drug targets. 2007;6:311–320. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behavioural pharmacology. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 12.Akil H, Evans SJ, Turner CA, Perez J, Myers RM, Bunney WE, et al. The fibroblast growth factor family and mood disorders. Novartis Foundation symposium. 2008;289:94–96. doi: 10.1002/9780470751251.ch8. discussion 97–100, 193-105. [DOI] [PubMed] [Google Scholar]
- 13.Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, et al. Dysregulation of the fibroblast growth factor system in major depression. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang HJ, Adams DH, Simen A, Simen BB, Rajkowska G, Stockmeier CA, et al. Gene expression profiling in postmortem prefrontal cortex of major depressive disorder. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:13329–13340. doi: 10.1523/JNEUROSCI.4083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain research bulletin. 2006;70:221–227. doi: 10.1016/j.brainresbull.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Bachis A, Mallei A, Cruz MI, Wellstein A, Mocchetti I. Chronic antidepressant treatments increase basic fibroblast growth factor and fibroblast growth factor-binding protein in neurons. Neuropharmacology. 2008;55:1114–1120. doi: 10.1016/j.neuropharm.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallei A, Shi B, Mocchetti I. Antidepressant treatments induce the expression of basic fibroblast growth factor in cortical and hippocampal neurons. Molecular pharmacology. 2002;61:1017–1024. doi: 10.1124/mol.61.5.1017. [DOI] [PubMed] [Google Scholar]
- 18.Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H. Antidepressant-like effects of intracerebroventricular FGF2 in rats. Brain research. 2008;1224:63–68. doi: 10.1016/j.brainres.2008.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarosik J, Legutko B, Werner S, Unsicker K, von Bohlen Und Halbach O. Roles of exogenous and endogenous FGF-2 in animal models of depression. Restorative neurology and neuroscience. 2011;29:153–165. doi: 10.3233/RNN-2011-0588. [DOI] [PubMed] [Google Scholar]
- 20.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 21.Cox BM, Alsawah F, McNeill PC, Galloway MP, Perrine SA. Neurochemical, hormonal, and behavioral effects of chronic unpredictable stress in the rat. Behavioural brain research. 2011;220:106–111. doi: 10.1016/j.bbr.2011.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biological psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biological psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Reuss B, von Bohlen und Halbach O. Fibroblast growth factors and their receptors in the central nervous system. Cell and tissue research. 2003;313:139–157. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monleon S, D’Aquila P, Parra A, Simon VM, Brain PF, Willner P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology. 1995;117:453–457. doi: 10.1007/BF02246218. [DOI] [PubMed] [Google Scholar]
- 30.Duman CH, Schlesinger L, Terwilliger R, Russell DS, Newton SS, Duman RS. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav Brain Res. 2009;198:366–371. doi: 10.1016/j.bbr.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 33.Mason SS, Baker KB, Davis KW, Pogorelov VM, Malbari MM, Ritter R, et al. Differential sensitivity to SSRI and tricyclic antidepressants in juvenile and adult mice of three strains. European journal of pharmacology. 2009;602:306–315. doi: 10.1016/j.ejphar.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- 35.Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biological psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fortin D, Rom E, Sun H, Yayon A, Bansal R. Distinct fibroblast growth factor (FGF)/FGF receptor signaling pairs initiate diverse cellular responses in the oligodendrocyte lineage. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:7470–7479. doi: 10.1523/JNEUROSCI.2120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maragnoli ME, Fumagalli F, Gennarelli M, Racagni G, Riva MA. Fluoxetine and olanzapine have synergistic effects in the modulation of fibroblast growth factor 2 expression within the rat brain. Biological psychiatry. 2004;55:1095–1102. doi: 10.1016/j.biopsych.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Kodama M, Fujioka T, Duman RS. Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biological psychiatry. 2004;56:570–580. doi: 10.1016/j.biopsych.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain research. 1995;701:201–226. doi: 10.1016/0006-8993(95)01002-x. [DOI] [PubMed] [Google Scholar]
- 40.Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, et al. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- 41.Widberg CH, Newell FS, Bachmann AW, Ramnoruth SN, Spelta MC, Whitehead JP, et al. Fibroblast growth factor receptor 1 is a key regulator of early adipogenic events in human preadipocytes. American journal of physiology Endocrinology and metabolism. 2009;296:E121–131. doi: 10.1152/ajpendo.90602.2008. [DOI] [PubMed] [Google Scholar]
- 42.Zhen Y, Sorensen V, Jin Y, Suo Z, Wiedlocha A. Indirubin-3′-monoxime inhibits autophosphorylation of FGFR1 and stimulates ERK1/2 activity via p38 MAPK. Oncogene. 2007;26:6372–6385. doi: 10.1038/sj.onc.1210473. [DOI] [PubMed] [Google Scholar]
- 43.Turner CA, Calvo N, Frost DO, Akil H, Watson SJ. The fibroblast growth factor system is downregulated following social defeat. Neuroscience letters. 2008;430:147–150. doi: 10.1016/j.neulet.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hisaoka K, Tsuchioka M, Yano R, Maeda N, Kajitani N, Morioka N, et al. Tricyclic antidepressant amitriptyline activates fibroblast growth factor receptor signaling in glial cells: involvement in glial cell line-derived neurotrophic factor production. The Journal of biological chemistry. 2011;286:21118–21128. doi: 10.1074/jbc.M111.224683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun L, Tran N, Liang C, Tang F, Rice A, Schreck R, et al. Design, synthesis, and evaluations of substituted 3-[(3- or 4-carboxyethylpyrrol-2-yl)methylidenyl]indolin-2-ones as inhibitors of VEGF, FGF, and PDGF receptor tyrosine kinases. Journal of medicinal chemistry. 1999;42:5120–5130. doi: 10.1021/jm9904295. [DOI] [PubMed] [Google Scholar]
- 46.Baron W, Metz B, Bansal R, Hoekstra D, de Vries H. PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Molecular and cellular neurosciences. 2000;15:314–329. doi: 10.1006/mcne.1999.0827. [DOI] [PubMed] [Google Scholar]
- 47.Walicke PA. Basic and acidic fibroblast growth factors have trophic effects on neurons from multiple CNS regions. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1988;8:2618–2627. doi: 10.1523/JNEUROSCI.08-07-02618.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rai KS, Hattiangady B, Shetty AK. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. The European journal of neuroscience. 2007;26:1765–1779. doi: 10.1111/j.1460-9568.2007.05820.x. [DOI] [PubMed] [Google Scholar]
- 49.Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:5012–5023. doi: 10.1523/JNEUROSCI.20-13-05012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaccarino FM, Schwartz ML, Raballo R, Nilsen J, Rhee J, Zhou M, et al. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nature neuroscience. 1999;2:246–253. doi: 10.1038/6350. [DOI] [PubMed] [Google Scholar]
- 51.Ruffini F, Furlan R, Poliani PL, Brambilla E, Marconi PC, Bergami A, et al. Fibroblast growth factor-II gene therapy reverts the clinical course and the pathological signs of chronic experimental autoimmune encephalomyelitis in C57BL/6 mice. Gene therapy. 2001;8:1207–1213. doi: 10.1038/sj.gt.3301523. [DOI] [PubMed] [Google Scholar]
- 52.Lachapelle F, Avellana-Adalid V, Nait-Oumesmar B, Baron-Van Evercooren A. Fibroblast growth factor-2 (FGF-2) and platelet-derived growth factor AB (PDGF AB) promote adult SVZ-derived oligodendrogenesis in vivo. Molecular and cellular neurosciences. 2002;20:390–403. doi: 10.1006/mcne.2002.1124. [DOI] [PubMed] [Google Scholar]
- 53.Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Molecular and cellular neurosciences. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 54.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 55.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nature neuroscience. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiological reviews. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 59.Haberlandt C, Derouiche A, Wyczynski A, Haseleu J, Pohle J, Karram K, et al. Gray matter NG2 cells display multiple Ca2+-signaling pathways and highly motile processes. PloS one. 2011;6:e17575. doi: 10.1371/journal.pone.0017575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frohlich N, Nagy B, Hovhannisyan A, Kukley M. Fate of neuron-glia synapses during proliferation and differentiation of NG2 cells. Journal of anatomy. 2011;219:18–32. doi: 10.1111/j.1469-7580.2011.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanaka Y, Tozuka Y, Takata T, Shimazu N, Matsumura N, Ohta A, et al. Excitatory GABAergic activation of cortical dividing glial cells. Cerebral cortex. 2009;19:2181–2195. doi: 10.1093/cercor/bhn238. [DOI] [PubMed] [Google Scholar]
- 62.Li AJ, Suzuki S, Suzuki M, Mizukoshi E, Imamura T. Fibroblast growth factor-2 increases functional excitatory synapses on hippocampal neurons. The European journal of neuroscience. 2002;16:1313–1324. doi: 10.1046/j.1460-9568.2002.02193.x. [DOI] [PubMed] [Google Scholar]
- 63.Miyake A, Hattori Y, Ohta M, Itoh N. Rat oligodendrocytes and astrocytes preferentially express fibroblast growth factor receptor-2 and -3 mRNAs. Journal of neuroscience research. 1996;45:534–541. doi: 10.1002/(SICI)1097-4547(19960901)45:5<534::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 64.Takami K, Matsuo A, Terai K, Walker DG, McGeer EG, McGeer PL. Fibroblast growth factor receptor-1 expression in the cortex and hippocampus in Alzheimer’s disease. Brain research. 1998;802:89–97. doi: 10.1016/s0006-8993(98)00552-6. [DOI] [PubMed] [Google Scholar]
- 65.Rosen GDWA, Capra JA, Connolly MT, Cruz B, Lu L, Airey DC, Kulkarni K, Williams RW. The Mouse Brain Library @. 2000 http://www.mbl.org Int Mouse Genome Conference 14.
- 66.Paxinos G, Charles W. The Rat Brain in Stereotaxic Coordinates. 5. Burlington, MA: Elsevier Academic Press; 2005. Copyright © 2005 by George Paxinos and Charles Watson. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.