Abstract
Objective
To determine if a dose-response relationship exists between percentage body weight changes in persons with symptomatic knee osteoarthritis (OA) and self reported pain and function.
Methods
Data from persons in the Osteoarthritis Initiative (OAI) and the Multicenter Osteoarthritis (MOST) datasets (n=1,410) with symptomatic function limiting knee OA were studied. For the OAI, we used baseline and 3-year follow-up data while for the MOST, baseline and 30-month data were used. Key outcome variables were WOMAC Physical Function and Pain change scores. In addition to covariates, the predictor variable of interest was the extent of weight change over the study period and divided into 5 categories representing different percentages of body weight change.
Results
A significant dose-response relationship (p< 0.003) was found between the extent of percentage change in body weight and the extent of change in WOMAC Physical Function and WOMAC Pain. For example, persons who gained ≥10% of body weight had WOMAC Physical Function score changes of −5.4 (95%CI, −8.7, −2.00) points indicating worsening relative to the reference group of persons with weight changes of between <5% weight gain and <5% weight reduction.
Conclusion
Our data suggest a dose-response relationship exists between changes in body weight and corresponding changes in pain and function. The threshold for this response gradient appears to be ≥10% body weight shifts. Weight changes of ≥10% have potential to lead to important changes in pain and function for patient groups as well as individual patients.
Osteoarthritis (OA) of the knee has multiple causes but one of the more powerful risk factors for OA onset and progression is excessive body weight1,2. The Framingham study, for example, reported that women who lost at least 5kg had a 50% reduction in the odds of developing symptomatic knee OA3. Given the high costs and high prevalence of knee OA, many researchers have focused on attempts to identify interventions that reduce body weight of persons with OA who are overweight or obese4-10.
A meta analysis that examined the effects of various approaches to weight reduction with or without co-interventions for persons with symptomatic knee OA found that a weight reduction on the order of a 5% reduction of body weight was associated with insignificant reductions in knee pain but significant though small improvements in self reported functional status.6 Christensen and colleagues also reported a dose-response effect such that the extent of weight reduction was proportional to the extent of functional improvement. In recently published trials, weight reduction strategies leading to losses approximating 10% or more of body weight have resulted in more substantial reductions in pain and improved function4,7,10. In a recently published cohort study of 44 persons undergoing gastric surgery for severe obesity, the average reduction in body weight from baseline to 6-months following surgery was 20.2%11. Average WOMAC Pain and Physical Function scores were reduced by 50% or more. These data, in combination, suggest that weight reduction and improvements in pain and functional status may be proportional and respond in a dose-response manner.
Several trials have examined the influences of weight loss on pain and function and we found one cohort study that examined effects of body weight gain on pain or functional status12. If a dose-response relationship exists between body weight changes and corresponding changes in pain and function for persons with symptomatic knee OA, one would expect that body weight gains may also be associated with proportional increases in pain and worsening functional status.
We found no studies that determined if a dose-response relationship existed between body weight changes, both gains and losses, and changes in knee related pain and functional status in a large sample of persons with symptomatic knee osteoarthritis. Trial evidence suggests that weight reduction of at least 5% of body weight would lead to improved function and that weight reductions of 10% or more would lead to greater reductions in pain and substantially improved function. Recommendations based on trial findings on persons with knee OA are similar to federal government-based recommendations for weight reduction to optimize health.13,14
Participants in weight loss trials receive extensive attention and training during the trial and it is unclear whether persons in the community who are not part of a weight loss trial and who undergo similar amounts of weight reduction also experience similar changes in pain and function. It also is unknown whether persons who gain weight actually experience worse pain and function and whether this pain and functional loss is proportional to the amount of weight gained. Knowing whether persons in the community report proportionate reductions (or increases) in pain and function following changes in body weight would equip clinicians with additional evidence-based information to aid in managing patients with knee OA. The purpose of our longitudinal cohort study was to determine if a dose-response relationship exists between extent of weight changes (including both weight reduction and weight gain) and extent of changes in self-reported function-related pain and functional status.
Patients and Methods
We analyzed data from two public use datasets. The Osteoarthritis Initiative (OAI) is a publicly and privately funded prospective longitudinal cohort study with a 4 year follow-up. A primary objective of the OAI study was to develop diverse cohorts of persons for the study of the natural history, risk factors, onset and progression of knee tibiofemoral OA. The Multicenter Osteoarthritis study (MOST) also is a publically funded prospective longitudinal cohort study. The overall aims of MOST were to identify novel and modifiable biomechanical factors, bone and joint structural factors and nutritional factors that affect the occurrence and progression of knee OA. All centers in both studies required all subjects to read and sign IRB approved consent forms prior to participation.
The OAI and MOST Study Samples
In the OAI, subjects between the ages of 45 and 79 years with or at high risk for knee OA were recruited from communities in and around four clinical sites: 1) the University of Maryland School of Medicine in Baltimore, Maryland, 2) the Ohio State University in Columbus, Ohio, 3) the University of Pittsburgh in Pittsburgh, Pennsylvania, and 4) Memorial Hospital of Rhode Island, in Pawtucket, Rhode Island. Persons recruited for the MOST study also had or were at high risk for knee OA and were aged 50 to 79 years. Subjects were recruited from communities in and around two clinical sites: 1) University of Iowa in Iowa City, Iowa, 2) University of Alabama, Birmingham in Birmingham, Alabama. Details of study populations from both cohorts have been described in detail elsewhere.15,16
Persons from both the OAI and MOST had to have the following features to be recruited for our study: 1) radiographic tibiofemoral knee OA defined as definite osteophytes (Osteoarthritis Research Society International (OARSI) atlas grades 1 to 317 in the OAI or Kellgren-Lawrence (KL) grades 2 or higher18 in MOST) as measured on a standardized fixed flexion radiograph19,20 , 2) WOMAC Pain Scale scores of 4 or higher, 3) WOMAC Physical Function scores of 9 or higher, and 4) no knee replacement surgery during the follow-up period. We wanted to study a sample of persons who had radiologically confirmed knee OA and who had function limiting pain. Minimal detectable clinical improvement estimates for WOMAC Physical Function scores generally range from 7 to 9 points while for the WOMAC Pain scale, estimates range from 2 to 4 points21-26. We chose the more conservative change score criteria of 9 points for the WOMAC Physical Function scale26 and 4 for the WOMAC Pain scale25 to reduce chances of falsely categorizing a person as changed when in fact they had not changed. Because we were interested in determining effects associated with differing amounts of weight change, we wanted a sample with WOMAC scores that were substantial enough to allow for detection of change at the individual person level to allow for interpretation. We excluded persons who underwent knee arthroplasty because the surgery would have likely resulted in dramatic changes in WOMAC and performance-based measures27,28 and we were interested in weight loss effects in non-surgical cases. The complete protocol for OAI can be viewed at (http://www.oai.ucsf.edu/datarelease/docs/StudyDesignProtocol.pdf). For MOST, details are available at (http://most.ucsf.edu/about.asp).
Baseline Variables
The baseline variables were the knee radiographic data, age, sex, comorbidity status measured on a continuous scale29, body weight (kgs), race (dichotomized to either African American or other), presence of frequent low back pain, depression status using a validated cutscore of 16 or higher indicating likely clinical depression on the Center for Epidemiologic Studies depression scale30,31, a dichotomized variable indicating whether the person was not working, at least in part, for health reasons, educational level, (less than high school degree, high school diploma or at least some college) and current smoking status. We also had a variable that was coded to indicate the presence of unilateral or bilateral knee OA. We used the clinical datasets 0.2.2 and 5.2.1 from the OAI website (http://oai.epi-ucsf.org/datarelease/About.asp). All data for MOST are publically available.
Key Predictor Variable
The primary predictor variable was changes in body weight from baseline to follow-up. We chose to use 5 categories of body weight changes and these are: ≥ 10% body weight reduction, 9.9% to 5% body weight reduction, 4.9% reduction to 4.9% body weight gain, 5% to 9.9% body weight gain and ≥ 10% body weight gain. We chose these categories to reflect weight changes because they capture the categories of weight changes generally described in the literature6,32 and in recommendations from government agencies13,14. In addition, these categories allow for assessment of the effects of proportionally similar changes in body weight on pain and function.
Outcome Variables of Interest
The outcome measures were change scores obtained by subtracting follow-up scores from baseline score. Follow-up measures were obtained during the 3-year visit for OAI and at the 30 month follow-up visit for MOST. The outcome variables were identical for both datasets and were the WOMAC Pain Scale change scores and the WOMAC Physical Function Scale change scores. Both outcome measures have demonstrated high levels of reliability and validity26,33-36. WOMAC Physical Function Likert version 3.1 scores range from 0 to 68 with higher scores indicating worse function. WOMAC Pain scores range from 0 to 20 with higher scores indicating greater function-related pain37.
Data Analysis
We used Chi Square tests for categorical baseline variables and t tests for continuous variables to compare persons with follow-up weight data and those whose follow-up weight data were missing. The subsequent analyses were performed for the dependent variables of changes in WOMAC function and changes in WOMAC pain. We investigated our primary question by first performing two regression analyses that included WOMAC function and WOMAC pain as dependent variables, and weight changes categorized into five groups (≥10% reduction, 5% to 9.9% reduction, 4.9% reduction to 4.9% gain, 5% to 9.9% gain, ≥10% gain) as the independent variable. These analyses tested whether function or pain differed among the weight reduction or gain categories compared to the reference category (4.9% reduction to 4.9% gain). Next we repeated these analyses adjusting for the following covariates: baseline scores for the dependent variable of interest (i.e., either WOMAC function or pain scores), gender (2-levels: female, male)38, depression (2-levels: depressed, not depressed)39, and number of comorbidities40. Our regression model was as follows:
Changes in WOMAC function or pain = constant + b1(≥10% wt. reduction) + b2(5 to 10% wt. reduction) + b3(5 to 10% wt. gain) + b4(≥10% wt. gain) + b5(baseline function or pain) + b6(number of comorbidities) + b7(gender) + b8(depression)
We applied the following dummy variable coding scheme: 1 if weight category applies, 0 if otherwise; 1 if female, 0 if male; 1 if depressed, 0 if not depressed. Applying this coding scheme, the reference weight category was the 4.9% wt. reduction to 4.9% wt. gain category (i.e., b1=b2=b3=b4=0). We performed a trend analysis to assess whether the results were consistent with a dose-response. Specifically, we examined the extent to which linear, quadratic, cubic, and quartic trends were evident.
Prior to initiating the analyses we examined the distributional properties of the variables and checked for heterogeneity of dependent variable variances among the five weight change categories. For all analyses we applied 2-tailed tests and an effect was considered statistically significant if p < 0.05. Analyses were conducted using STATA version 10.1.
RESULTS
The characteristics of the sample are reported in Table 1. There were some demographic differences between the two datasets. For example, persons in the OAI were generally younger, had more education and weighed less than the persons in MOST. In addition, there was evidence of selective loss of follow-up when comparing persons with follow-up weight data (n=1,410) to persons whose follow-up weight data were missing (n=375). For example, persons with missing follow-up weight measures had less education, were more frequently African American, and had higher levels of pain and worse function. The distribution of persons in each of the 5 weight change categories were as follows: ≥10% weight reduction, n=82, 5% to 9.9% weight reduction, n= 176, 4.9% reduction to 4.9% gain, n= 953, 5% to 9.9% weight gain, n= 148, and >10% weight gain, n=51.
Table 1.
Characteristics of the Samples at Baseline and those with Complete versus Missing Data*
| OAI Baseline (n=976) |
MOST Baseline (n=809) |
Combined Baseline (n=1785) |
Complete Weight Data at Baseline and Follow-up (n=1410) |
Missing Weight Data at Follow-up (n=375) |
Chi Square or t test p-values Comparing Complete and Missing Data |
|
|---|---|---|---|---|---|---|
| Age | 61.72 (9.25) | 63.74 (8.09) | 62.63 (8.79) | 62.73 (8.62) | 62.26 (9.38) | 0.94 (0.35) |
| Female (%) | 62.2 | 64.5 | 63.2 | 62.5 | 66.1 | 1.70 (0.19) |
| Race (% African American) | 33.5 | 22.9 | 28.7 | 25.5 | 40.8 | 34.07 (< 0.001) |
| Marital Status (%) | ||||||
| Married | 58.7 | 68.8 | 62.6 | 65.5 | 54.9 | 16.4 (0.002) |
| Widowed | 10.7 | 12.9 | 11.5 | 10.7 | 15.1 | |
| Divorced | 17.2 | 13.0 | 15.1 | 14.9 | 16.8 | |
| Separated | 3.1 | 0.9 | 2.1 | 1.9 | 3.0 | |
| Never Married | 10.4 | 4.5 | 7.6 | 7.0 | 10.3 | |
| Education (%) | ||||||
| <high school diploma | 6.7 | 7.8 | 7.2 | 6.2 | 11.1 | 15.0 (0.001) |
| High school diploma | 18.9 | 30.2 | 24.0 | 23.2 | 23.7 | |
| At least some college | 74.4 | 62.1 | 68.8 | 70.6 | 61.7 | |
| Comorbidity | 0.55 (0.95) | 0.64 (1.01) | 0.59 (0.98) | 0.57 (0.95) | 0.67 (1.07) | 1.80 (0.07) |
| Weight (kg) | 86.63 (16.50) | 93.55 (20.98) | 89.80 (19.98) | 89.62 (18.6) | 90.32 (20.25) | 0.63 (0.53) |
| Current Smoker (%) | 8.6 | 13.1 | 9.8 | 9.0 | 12.6 | 3.58 (0.06) |
| CESD (% depressed) | 17.9 | 19.2 | 18.5 | 17.7 | 21.6 | 3.03 (0.08) |
| WOMAC Pain | 7.96 (3.20) | 8.15 (3.11) | 8.05 (3.17 | 7.83 (3.06) | 8.85 (3.42) | 5.57 (<0.001) |
| WOMAC Physical Function | 25.23 (10.76) | 26.73 (10.29) | 25.91 (10.57) | 25.12 (10.14) | 28.88 (11.63) | 6.17 (<0.001) |
Values are mean (SD) unless otherwise indicated. OAI is defined as the Osteoarthritis Initiative; MOST is defined as Multicenter Osteoarthritis Study; CESD is defined as Center for Epidemiologic Studies Depression Scale.
The distributions of the dependent variables approximated a normal distribution and for each dependent variable the variances among the five weight categories did not differ statistically. Table 2 provides a descriptive summary of the WOMAC Physical Function and Pain change scores for the five weight change categories as well as the percentage of patients whose change scores met or exceeded the minimal clinically important thresholds of 9 WOMAC Physical Function points26 or 4 WOMAC Pain points25. Unadjusted analyses revealed statistically significant differences among the weight change categories for both WOMAC function (F4, 1381 = 4.72, p = 0.001) and pain (F4, 1401 = 2.50, p = 0.041). Table 3 displays the difference between the weight change categories and the reference category (4.9% reduction to 4.9% gain) in WOMAC function and pain points. Unadjusted and adjusted coefficients are reported in this table. The results show that for both the unadjusted and adjusted analyses only the weight change categories ≥10% reduction and ≥10% gain differ from the reference category. The dose-response relationship for weight changes and WOMAC Physical Function scale is illustrated in Figure 1 while Figure 2 illustrates the dose-response relationship for the WOMAC Pain scale. The trend analyses identified statistically significant linear and cubic trends for both the WOMAC Physical Function (linear: F1,1362= 16.47, P < 0.001; cubic: F1,1362 = 12.11, P < 0.001) and Pain (linear: F1,1381 = 8.77, P < 0.003; cubic: F1,1381 = 13.79, P < 0.001) measures. The linear trend comments on the extent to which there is a steady decline in outcome measures’ scores across weight change categories, while the cubic trend acknowledges there is a "flattening" or similarity in outcome measures’ scores for the central three weight change categories.
Table 2.
Changes in WOMAC Physical Function and Pain and the Percentage of Persons with Changes of at least 9 WOMAC Physical Function Points or 4 WOMAC Pain Points
| Weight Change Group | WOMAC Physical Function Change Mean (SD) |
Percentage with WOMAC Physical Function change scores of 9 or more points |
WOMAC Pain Change Mean (SD) |
Percentage with WOMAC Pain change scores of 4 or more points |
|---|---|---|---|---|
| ≥10% reduction | 7.50 (13.24) | 37/82 (45.1%) | 2.05 (4.60) | 31/82 (37.8%) |
| 5% to 9.9% reduction | 3.34 (12.62) | 50/171 (29.2%) | 0.99 (4.34) | 33/171 (19.3%) |
| 4.9% reduction to 4.9% gain | 2.78 (11.82) | 269/940 (28.6%) | 1.09 (3.86) | 233/951 (24.5%) |
| 5% to 9.9% gain | 3.23 (12.34) | 41/145 (28.3) | 1.40 (3.99) | 42/148 (28.4%) |
| ≥10% gain | −1.67 (13.6) | 10/48 (20.8%) | −0.06 (3.93) | 8/51 (15.7%) |
Table 3.
Comparison of Weight Change Groups with the 4.9% Reduction to 4.9% Gain Reference Group
| Unadjusted Analysis | Adjusted Analysis | |||
|---|---|---|---|---|
| Variable | WOMAC Function Regression coefficient (95% CI), p |
WOMAC Pain Regression coefficient (95% CI), p |
WOMAC Function Regression coefficient (95% CI), p |
WOMAC Pain Regression coefficient (95% CI), p |
| Weight Change Category* | ||||
| ≥10% reduction | 4.71 (1.97, 7.45), 0.001 | 0.96 (0.06, 1.86), 0.036 | 4.07 (1.49, 6.65), 0.002 | 0.90 (0.06, 1.74), 0.035 |
| 5% to 9.9% reduction | 0.55 (−1.42, 2.53), 0.582 | −0.10 (−0.74, 0.54), 0.760 | 0.01 (−1.87, 1.89), 0.991 | −0.26 (−0.87, 0.35), 0.402 |
| 4.9% reduction to 4.9% gain | reference | reference | reference | reference |
| 5% to 9.9% gain | 0.44 (−1.68, 2.57), 0.682 | 0.31 (−0.37, 1.00), 0.371 | 1.08 (−0.91, 3.07), 0.288 | 0.50 (−0.14, 1.15), 0.128 |
| ≥10% gain | −4.45 (−7.98, −0.93), 0.013 | −1.15 (−2.27, −0.03), 0.045 | −5.36 (−8.74, −2.00), 0.002 | −1.56 (−2.62, −0.49), 0.004 |
| Baseline dependent | NA | NA | 0.43 (0.36, 0.49), <0.001 | 0.49 (0.42, 0.56), <0.001 |
| Female | NA | NA | −1.60 (−2.85, −0.35), 0.012 | −0.39 (−0.80, 0.01), 0.058 |
| Comorbidity (number) | NA | NA | −1.26 (−1.91, −0.61), <0.001 | −0.33 (−0.55, −0.12), 0.002 |
| Depressed (yes) | NA | NA | −2.02 (−3.73, −0.31), 0.020 | −0.96 (−1.50, −0.42), 0.001 |
| Constant | 2.78 (2.01, 3.56), <0.001 | 1.08 (0.83, 1.34), <0.001 | −4.2 (−6.73, −1.68), 0.001 | −1.74 (−2.58, −0.92), <0.001 |
Reference category was weight change 4.9% reduction to 4.9% gain
Figure 1.
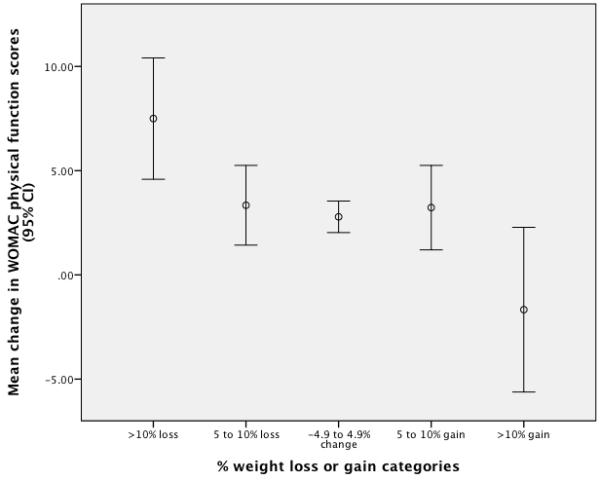
Dose-response relationship for the WOMAC Physical Function Scale. Point estimates and 95% CI bars were derived from unadjusted estimates based on the ANCOVA.
Figure 2.
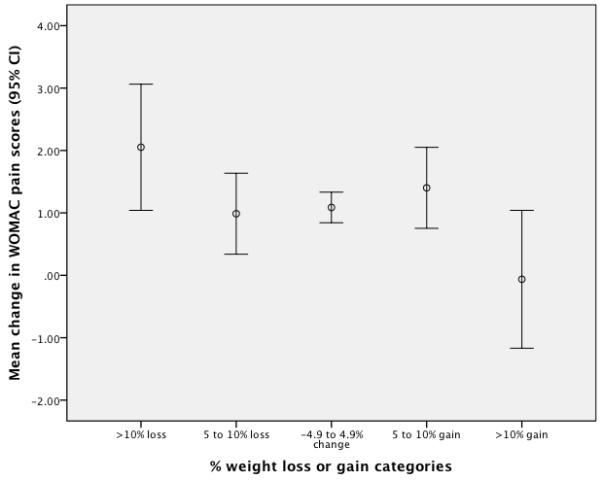
Dose-response relationship for the WOMAC Pain Scale. Point estimates and 95% CI bars were derived from unadjusted estimates based on the ANCOVA.
DISCUSSION
Our study demonstrated a dose-response relationship between body weight changes and changes in self-reported pain and functional status. Such a study would, at minimum, require: 1) a large sample of persons followed over an extended period of time, 2) well defined methods for quantifying arthritis status, pain and functional status, 3) a high rate of follow-up, and 4) a sufficient number of persons who either lost or gained weight during the follow-up period.
We found that persons who lose ≥10% of their body weight over an approximate 3-year period report a significantly lower function related pain and improved functional status relative to the reference category. In contrast, persons who gain ≥10% of their baseline body weight have significantly worse function-related pain and function than persons in the reference category. The dose-response relationship between weight changes and pain and functional status changes was highly significant (p< 0.003) both for linear and cubic trends.
We found no other evidence that quantified the potential impact of body weight gain on subsequent pain and functional status. Our study suggests that body weight gains on the order of ≥10% body weight has significant effects particularly on self-reported function but also on pain. After adjusting for covariates, average WOMAC Physical Function scores worsened (increased) by 5.4 (95% CI, 2,0, 8.7) points while WOMAC Pain scores worsened (increased) by 1.6 (95% CI, 0.5, 2.6) points relative to that seen in the reference group. While these differences are approximately half that required to infer change at the level of the individual patient21-26, for group level changes, these estimates approximate the magnitude of changes reported in successful weight loss6-8 and knee exercise41 trials. When interpreting the meaningfulness of these group level changes it is necessary to distinguish between an important within patient change and an important between group difference. Goldsmith and colleagues have shown that an important within patient change is substantially greater than an important between group difference42. For example, in randomized trials of rheumatoid arthritis patients they found an important within patient change in pain to be 36% of baseline scores compared to 20% of baseline scores for a between group difference. Similarly, Goldsmith et al. identified an important within patient change in disability to be 49% of baseline compare to 16% of baseline for a between group difference42. Applying Goldsmith et al’s. percentage difference estimates to the 9 point WOMAC Physical Function26 and 4 point WOMAC Pain25 minimal detectable change estimates used in our study yields important between group differences of approximately 2.2 and 2.9 WOMAC pain and function points respectively. Referring to Table 3 we see that the ≥10% reduction and gain groups’ mean disability change scores differ significantly from the reference group. For pain, all between group comparisons with the reference group are less than 2.2 points.
Table 2 delineates the percentages of patients who met or exceeded the minimal clinically important thresholds for important within person change. For example, 45.1% of patients who lost ≥ 10% of bodyweight reported changes that met or exceeded the criterion of 9 WOMAC Physical Function points while 29.2% of persons who lost between 5% and 9.9% of body weight met the WOMAC Physical Function change threshold. Similar estimates were reported for the WOMAC Pain scale. These data suggest that weight changes of ≥ 10% appear to be important thresholds for individual patient pain and function changes.
Most of the work examining the effects of body weight changes on pain and function has been directed toward weight loss. Trials have consistently shown that a 5% or greater weight loss resulted in significantly reduced pain or improved function. For example, Messier and colleagues examined the efficacy of a diet and exercise intervention on obese persons with symptomatic knee OA.8 The authors found that an average 5.7% weight loss was associated with significant reductions of 5.7 WOMAC Physical Function points and 2.2 WOMAC Pain points after 18 months. These WOMAC changes are somewhat larger than our estimates for persons with ≥10 loss in body weight (point estimate of 4.1 WOMAC Physical Function points and 0.9 WOMAC Pain points). In another trial in which loss in body weight approached 9% in the experimental group, reductions particularly in WOMAC Physical Function scores were even greater with an average 8.4 point reduction in 6 months9. Bliddal and colleagues report a similar magnitude of changes in both WOMAC Physical Function and Pain scores for persons in their 1-year trial who lost an average of 11% of body weight4. Baseline WOMAC Pain and Physical Function scores in these previous trials were very similar to our sample suggesting our sample was similar though with a lower BMI than these previous trials.
For persons in our study with comparable weight reduction (between a 5% and a 9.9% weight loss) to that reported in two successful trials8,9 and a systematic review6, changes in WOMAC Pain and Physical Function were essentially non-existent. We suspect this may be related to the fact that persons in our study were not enrolled in a weight loss study and did not receive the additional training and attention as compared to persons in a weight loss trial. The effects of weight loss on pain in function, when assessed in multiple sites in the context of a cohort study like ours with no focus on weight changes per se may dilute the effects as compared to that seen in trials. It is also possible that the longer follow-up period in our study influenced the effect of weight loss on pain and function. Even with these differences in study design and length, weight loss when appreciable (≥10% of body weight) was shown to have therapeutic effects.
The National Institutes of Health published Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults.14 The recommendation for extent of weight loss was the following: "The initial goal of weight loss therapy should be to reduce body weight by approximately 10 percent from baseline." While the NIH guidelines were not designed specifically for persons with knee OA, our findings support the NIH recommendations for weight loss when applied to OA related pain reduction and functional improvement.
Our study has some notable strengths but also some important limitations. First, in spite of our large sample size, the loss to follow-up was substantial in that 21% of the sample did not have follow-up weight data. Those lost to follow-up were generally more symptomatic, tended to be African American, and were less likely to report being married. A selective loss to follow-up, while not uncommon in large cohort studies43, likely impacted our findings. We suspect that this loss likely diluted the effects of weight changes given that those lost to follow-up were more symptomatic. Future research should focus on enhanced methods of recruitment and retention for these at-risk populations. In addition, persons in the OAI and MOST datasets met all inclusion criteria but also differed in several ways. We chose not to adjust for these differences. In total, the combined dataset is more heterogeneous than either the MOST or OAI in isolation and therefore better reflects variation in the types of patients with knee OA seen in clinical practice. The sample sizes for the ≥10% gain and ≥10% loss groups were fairly small relative to the other weight change categories, 51 and 82 persons respectively. In spite of these relatively small samples our findings were consistent and statistically robust. Finally, our study design is descriptive in nature and we cannot determine whether the weight loss (or gain) causes predictable changes in pain and functional status or vice versa.
The results of our study have potential to impact clinical practice. Clinicians should encourage patients who are overweight to lose weight and the target magnitude of weight loss, based on our study, and NIH recommendations14 should be 10% or more of body weight. Guidance also can be provided regarding weight gain and the potential impact of future weight gain on subsequent pain and functional status. Patients can benefit by knowing that the dosage of changes in body weight is important and is related to pain and functional status.
Significance and Innovations.
A dose-response relationship was found between changes in body weight over an approximate 3-year period and subsequent self-reported pain and functional status.
The threshold for statistically significant changes in pain and function appears to be a ≥10% weight gain or weight reduction.
The study indicates that body weight changes are associated with changes in pain and functional status in a dose-response fashion.
Footnotes
Financial Support Statement: The authors disclose no financial support or other benefits from commercial sources for the work reported on in the manuscript, or any other financial interests which could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
Reference List
- (1).Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- (2).Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2008;34:515–529. doi: 10.1016/j.rdc.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Felson DT, Zhang Y, Anthony JM, Naimark A, Anderson JJ. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann Intern Med. 1992;116:535–539. doi: 10.7326/0003-4819-116-7-535. [DOI] [PubMed] [Google Scholar]
- (4).Bliddal H, Leeds AR, Stigsgaard L, Astrup A, Christensen R. Weight loss as treatment for knee osteoarthritis symptoms in obese patients: 1-year results from a randomised controlled trial. Ann Rheum Dis. 2011;70:1798–1803. doi: 10.1136/ard.2010.142018. [DOI] [PubMed] [Google Scholar]
- (5).Christensen R, Astrup A, Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthritis Cartilage. 2005;13:20–27. doi: 10.1016/j.joca.2004.10.008. [DOI] [PubMed] [Google Scholar]
- (6).Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2007;66:433–439. doi: 10.1136/ard.2006.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Gudbergsen H, Boesen M, Christensen R, Astrup A, Bliddal H. Radiographs and low field MRI (0.2T) as predictors of efficacy in a weight loss trial in obese women with knee osteoarthritis. BMC Musculoskelet Disord. 2011;12:56. doi: 10.1186/1471-2474-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- (9).Miller GD, Nicklas BJ, Davis C, Loeser RF, Lenchik L, Messier SP. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity (Silver Spring) 2006;14:1219–1230. doi: 10.1038/oby.2006.139. [DOI] [PubMed] [Google Scholar]
- (10).Riecke BF, Christensen R, Christensen P, Leeds AR, Boesen M, Lohmander LS, et al. Comparing two low-energy diets for the treatment of knee osteoarthritis symptoms in obese patients: a pragmatic randomized clinical trial. Osteoarthritis Cartilage. 2010;18:746–754. doi: 10.1016/j.joca.2010.02.012. [DOI] [PubMed] [Google Scholar]
- (11).Richette P, Poitou C, Garnero P, Vicaut E, Bouillot JL, Lacorte JM, et al. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis. 2011;70:139–144. doi: 10.1136/ard.2010.134015. [DOI] [PubMed] [Google Scholar]
- (12).White-O’Connor B, Sobal J, Muncie HL., Jr. Dietary habits, weight history, and vitamin supplement use in elderly osteoarthritis patients. J Am Diet Assoc. 1989;89:378–382. [PubMed] [Google Scholar]
- (13).Center for Drug Evaluation and Research. United States Food and Frug Administration . Guidance for Industry: developing products for weight management. FDA; Rockville, MD: 2007. Guidance for Industry: developing products for weight management. Ref Type: Conference Proceeding
- (14).Evaluation, and Treatment of Overweight and Obesity in Adults. National Institutes of Health, National Heart Lung and Blood Institute; 1998. Clinical Guidelines on the Identification; pp. 98–483. Ref Type: Conference Proceeding
- (15).Thompson LR, Boudreau R, Newman AB, Hannon MJ, Chu CR, Nevitt MC, et al. The association of osteoarthritis risk factors with localized, regional and diffuse knee pain. Osteoarthritis Cartilage. 2010;18:1244–1249. doi: 10.1016/j.joca.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–2992. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- (17).Altman RD, Hochberg M, Murphy WA, Jr., Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3(Suppl A):3–70. [PubMed] [Google Scholar]
- (18).Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128–132. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- (20).Kothari M, Guermazi A, von IG, Miaux Y, Sieffert M, Block JE, et al. Fixed-flexion radiography of the knee provides reproducible joint space width measurements in osteoarthritis. Eur Radiol. 2004;14:1568–1573. doi: 10.1007/s00330-004-2312-6. [DOI] [PubMed] [Google Scholar]
- (21).Angst F, Aeschlimann A, Steiner W, Stucki G. Responsiveness of the WOMAC osteoarthritis index as compared with the SF-36 in patients with osteoarthritis of the legs undergoing a comprehensive rehabilitation intervention. Ann Rheum Dis. 2001;60:834–840. [PMC free article] [PubMed] [Google Scholar]
- (22).Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384–391. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- (23).Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol. 2002;29:131–138. [PubMed] [Google Scholar]
- (24).Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635–2641. [PubMed] [Google Scholar]
- (25).Halket A, Stratford PW, Kennedy DM, Woodhouse LJ, Spadoni G. Measurement properties of performance-specific pain ratings of patients awaiting total joint arthroplasty as a consequence of osteoarthritis. Physiother Can. 2008;60:255–263. doi: 10.3138/physio.60.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Pua YH, Cowan SM, Wrigley TV, Bennell KL. The Lower Extremity Functional Scale could be an alternative to the Western Ontario and McMaster Universities Osteoarthritis Index physical function scale. J Clin Epidemiol. 2009;62:1103–1111. doi: 10.1016/j.jclinepi.2008.11.011. [DOI] [PubMed] [Google Scholar]
- (27).Kennedy DM, Stratford PW, Riddle DL, Hanna SE, Gollish JD. Assessing Recovery and Establishing Prognosis Following Total Knee Arthroplasty. Phys Ther. 2007 doi: 10.2522/ptj.20070051. [DOI] [PubMed] [Google Scholar]
- (28).Lingard EA, Katz JN, Wright EA, Sledge CB. Predicting the outcome of total knee arthroplasty. J Bone Joint Surg Am. 2004;86-A:2179–2186. doi: 10.2106/00004623-200410000-00008. [DOI] [PubMed] [Google Scholar]
- (29).Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- (30).Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- (31).Blalock SJ, DeVellis RF, Brown GK, Wallston KA. Validity of the Center for Epidemiological Studies Depression Scale in arthritis populations. Arthritis Rheum. 1989;32:991–997. doi: 10.1002/anr.1780320808. [DOI] [PubMed] [Google Scholar]
- (32).Messier SP. Diet and exercise for obese adults with knee osteoarthritis. Clin Geriatr Med. 2010;26:461–477. doi: 10.1016/j.cger.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Bellamy N, Buchanan WW. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol. 1986;5:231–241. doi: 10.1007/BF02032362. [DOI] [PubMed] [Google Scholar]
- (34).Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- (35).Bellamy N. The WOMAC Knee and Hip Osteoarthritis Indices: development, validation, globalization and influence on the development of the AUSCAN Hand Osteoarthritis Indices. Clin Exp Rheumatol. 2005;23:S148–S153. [PubMed] [Google Scholar]
- (36).Stratford PW, Kennedy DM, Hanna SE. Condition-specific Western Ontario McMaster Osteoarthritis Index was not superior to region-specific Lower Extremity Functional Scale at detecting change. J Clin Epidemiol. 2004;57:1025–1032. doi: 10.1016/j.jclinepi.2004.03.008. [DOI] [PubMed] [Google Scholar]
- (37).Baron G, Tubach F, Ravaud P, Logeart I, Dougados M. Validation of a short form of the Western Ontario and McMaster Universities Osteoarthritis Index function subscale in hip and knee osteoarthritis. Arthritis Rheum. 2007;57:633–638. doi: 10.1002/art.22685. [DOI] [PubMed] [Google Scholar]
- (38).Martinson ML, Teitler JO, Reichman NE. Health across the life span in the United States and England. Am J Epidemiol. 2011;173:858–865. doi: 10.1093/aje/kwq325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Zhao G, Ford ES, Li C, Tsai J, Dhingra S, Balluz LS. Waist circumference, abdominal obesity, and depression among overweight and obese U.S. adults: national health and nutrition examination survey 2005-2006. BMC Psychiatry. 2011;11:130. doi: 10.1186/1471-244X-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med. 2011;154:217–226. doi: 10.1059/0003-4819-154-4-201102150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Jenkinson CM, Doherty M, Avery AJ, Read A, Taylor MA, Sach TH, et al. Effects of dietary intervention and quadriceps strengthening exercises on pain and function in overweight people with knee pain: randomised controlled trial. BMJ. 2009;339:b3170. doi: 10.1136/bmj.b3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Goldsmith CH, Boers M, Bombardier C, Tugwell P, OMERACT Committee Criteria for clinically important changes in outcomes: development, scoring and evaluation of rheumatoid arthritis patient and trial profiles. J Rheumatol. 1993;20:561–565. [PubMed] [Google Scholar]
- (43).Hunt JR, White E. Retaining and tracking cohort study members. Epidemiol Rev. 1998;20:57–70. doi: 10.1093/oxfordjournals.epirev.a017972. [DOI] [PubMed] [Google Scholar]


