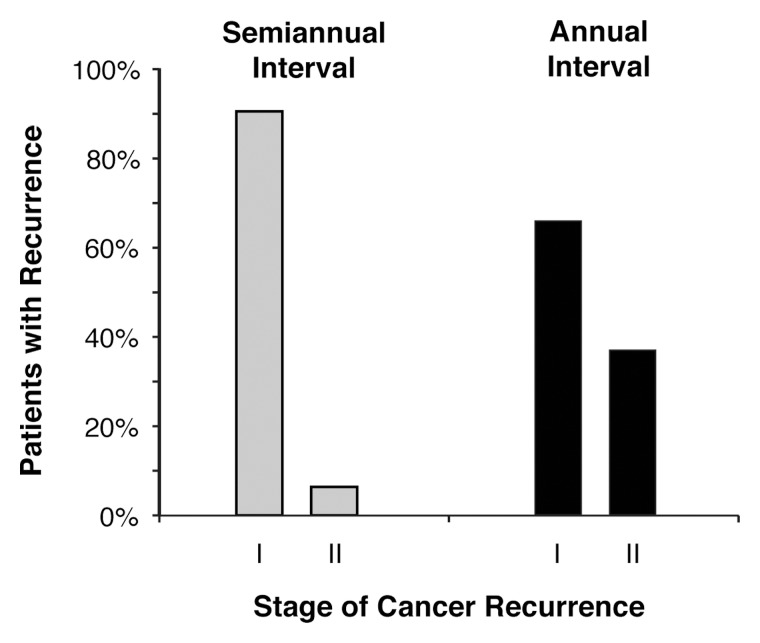
Following breast conservation therapy, asymptomatic recurrences are detected at a significantly earlier stage with semiannual ipsilateral surveillance mammography compared with noncompliant annual mammography.
Abstract
Purpose:
To compare cancer recurrence outcomes on the basis of compliant semiannual versus noncompliant annual ipsilateral mammographic surveillance following breast conservation therapy (BCT).
Materials and Methods:
A HIPAA-compliant retrospective review was performed of post-BCT examinations from 1997 through 2008 by using a deidentified database. The Committee on Human Research did not require institutional review board approval for this study, which was considered quality assurance. Groups were classified according to compliance with institutional post-BCT protocol, which recommends semiannual mammographic examinations of the ipsilateral breast for 5 years. A compliant semiannual examination was defined as an examination with an interval of 0–9 months, although no examination had intervals less than 3 months. A noncompliant annual examination was defined as an examination with an interval of 9–18 months. Cancer recurrence outcomes were compared on the basis of the last examination interval leading to diagnosis.
Results:
Initially, a total of 10 750 post-BCT examinations among 2329 asymptomatic patients were identified. Excluding initial mammographic follow-up, there were 8234 examinations. Of these, 7169 examinations were semiannual with 94 recurrences detected and 1065 examinations were annual with 15 recurrences detected. There were no differences in demographic risk factors or biopsy rates. Recurrences identified at semiannual intervals were significantly less advanced than those identified at annual intervals (stage I vs stage II, P = .04; stage 0 + stage I vs stage II, P = .03). Nonsignificant findings associated with semiannual versus annual intervals included smaller tumor size (mean, 11.7 vs 15.3 mm; P = .15) and node negativity (98% vs 91%, P = .28).
Conclusion:
Results suggest that a semiannual interval is preferable for ipsilateral mammographic surveillance, allowing detection of a significantly higher proportion of cancer recurrences at an earlier stage than noncompliant annual surveillance.
© RSNA, 2012
Introduction
Breast conservation therapy (BCT) is an effective and widely accepted treatment for women with small and early breast cancer (1,2). However, these women continue to be at risk for developing recurrence, with a constant incidence of approximately 1%–2% per year for 10 years (3–6). In comparison, the average woman is at risk for primary breast cancer at an incidence of 0.1% per year at age 40 years to a maximum of 0.5% by age 75 years (7). In prospective trials, researchers who investigated the best overall approach for post-BCT surveillance concluded that only mammography and clinical breast examination are effective for detection of recurrence and maintenance of survival (8,9). Although mammography and clinical breast examination are complementary approaches, mammography has been shown to depict more breast cancers earlier (4,10).
A major limitation in implementing mammographic surveillance is identifying the optimal interval for follow-up. To our knowledge, no study directly compares the effect of different mammographic surveillance intervals on the frequency, size, nodal status, and stage of recurrences. In a review (11) of observational studies, each involving a single specific surveillance protocol (ranging from 6- to 48-month interval examinations), investigators found mammography-only detection of recurrence to be variable in the ipsilateral (8%–50%) and contralateral (18%–80%) breasts. To our knowledge, researchers in prospective trials have not randomized the data for surveillance frequency; thus, no data exist to validate a specific interval for follow-up (12).
An annual surveillance interval following BCT has been suggested by several organizations, including the American Society for Clinical Oncology (13,14), the National Comprehensive Cancer Network (15), and the Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer for Health Canada (16). Because of the paucity of supporting data, these groups have based their guidelines for recommending annual surveillance on expert opinion. These recommendations are conservative, because they are equivalent to screening recommendations for the average-risk general population.
It is unknown whether a shorter than annual interval is preferable for follow-up of patients who have undergone BCT, because they are at higher than average risk for subsequent cancer. Our institution (University of California, San Francisco) has had a semiannual post-BCT surveillance protocol in place for many years. Our purpose was to compare recurrence outcomes on the basis of compliant semiannual versus noncompliant annual ipsilateral mammographic surveillance following BCT.
Materials and Methods
Study Design and Data Collection
This study was compliant with the Health Insurance Portability and Accountability Act and did not require institutional review board approval because it was classified as quality assurance and was approved by our institutional review board as exempt from the requirement for informed consent. Only deidentified data were used. A retrospective review was performed to identify post-BCT surveillance mammographic examinations performed from 1997 through 2008, by using our electronic mammography database in which the indication for examination (including routine surveillance following BCT) was prospectively stored. The post-BCT protocol at our institution recommends surveillance mammography and clinical breast examination of the ipsilateral breast every 6 months for 5 years. Surveillance mammographic examinations represent examinations only in asymptomatic patients who were assessed for recurrence for a maximum of 5 years (a historical milestone at the time this protocol was initiated), after which the protocol was converted to a regular screening mammography protocol.
To compare recurrence outcomes between semiannual and annual examinations, the last mammographic examination interval leading to detection of recurrence was used as the primary predictor of recurrence outcomes. Patients who had recurrences that were detected with surveillance mammography were categorized into either the semiannual or annual groups on the basis of compliance with our semiannual protocol. Examination intervals were determined by the number of days from the prior to the current surveillance examination, and intervals could vary with each visit. Semiannual (compliant) examinations were defined as those with intervals of 0–274 days (representing 0–9 months), although no intervals were shorter than 91 days (3 months). Noncompliant annual examinations were defined as those with intervals of 275–548 days (representing 9–18 months). With this scheme, a patient could be compliant for some visits and not compliant for others. Therefore, analysis was performed by using the last examination interval leading to cancer recurrence detection. Examination intervals in which cancer recurrence was not detected were not used in analysis. It was not feasible to calculate time to recurrence on the basis of the design of the database. This type of analysis is supported by two observations. First, the risk of recurrence is approximately constant and equal during every visit interval for 5 years and is not conditional on the basis of the time to recurrence (3–6). Second, detection of recurrence depends on the interval from the most recent negative mammographic examination result because the number of prior examinations likely does not improve detection in a nondiagnostic setting (17,18).
Examinations with intervals beyond 548 days (18 months) were excluded from the study because there were very few examinations (n = 187) that could be used for analysis. The first surveillance examinations were also excluded because there was no preceding surveillance examination from which an interval could be calculated. The study end point was mammographically identified ipsilateral breast cancer, for which we used the term recurrence. Contralateral cancer detection was not included in our analysis, because the contralateral breast was examined at similar intervals in both compliant and noncompliant cohorts.
Database Design
Breast Imaging Reporting and Data System assessment data and biopsy data were acquired contemporaneously as part of an ongoing quality assurance program. For both assessments and outcomes, the database maintains cumulative deidentified data rather than examination-by-examination data. A subset of this database contains examination-by-examination data for patients following the BCT protocol, in which the data stored are limited to the number of days since the last examination, the number of examinations per patient, the sequence number of examinations, demographic information, and cancer recurrence data for a given deidentified patient.
Demographic Information
Cancer risk profile based on deidentified demographic information and a self-reported breast health history were prospectively collected at each mammographic examination and were compared to assess group differences at the time of recurrence. Patient age was recorded as that on the day of the mammographic examination. A patient with a strong family history was defined as a patient with one first-degree relative who had a postmenopausal diagnosis of unilateral breast cancer. A patient with a very strong family history of breast cancer was defined as a patient with two or more first-degree relatives with a breast cancer history, a first-degree relative with premenopausal breast cancer, or a first-degree relative with ovarian cancer. Dates of primary cancer diagnosis and BCT were not entered prospectively into our database and were not available for many study subjects who had previous breast care at an outside institution.
Cancer Recurrence Assessment
Cancer recurrence was defined as a diagnosis of ductal carcinoma in situ or invasive carcinoma within 6 months of a surveillance mammographic examination (to match the semiannual interval of our imaging protocol), as determined through standard mammography auditing and by linkage with our regional Surveillance Epidemiology and End Results program and the California Cancer Registry. Per cancer registry protocol, co-occurring invasive cancer and ductal carcinoma in situ were recorded as invasive cancer only. Previous research showed cancer ascertainment to be at least 95% complete (19). Linkage was conducted according to protocols for human subjects that maintain patient confidentiality. Breast cancer recurrences were staged by using rTNM criteria, defined by the American Joint Committee on Cancer, which have equivalent classifications as primary cancer staging (20). Recurrence tumor size and node status were obtained contemporaneously from pathology reports as part of our routine mammography auditing practice. Size was defined by using the longest diameter at pathologic analysis and was recorded only for invasive lesions.
Statistical Analysis
Outcomes between the compliant semiannual and noncompliant annual examination intervals were analyzed for differences in proportions of cancer recurrence by using the Fisher exact test. Continuous data were analyzed by using a two-sample t test, assuming unequal variance. Data were analyzed by using statistical software (Matlab 2007a; MathWorks, Natick, Mass).
Results
A total of 10 750 mammographic examinations among 2329 patients (mean and range of examinations per patient, 4.6 and 1–13, respectively) were initially identified as post-BCT surveillance examinations. The distribution of the number of examinations per patient is shown in Table 1. Among the 8421 examinations with reliably determined examination intervals (2329 first surveillance examinations were excluded), 7169 (85.1%) were compliant semiannual examination and 1065 were noncompliant annual examinations. A total of 187 examinations had intervals longer than 18 months and were not included in the analysis. The mean number of days between examinations was 190 days for the compliant semiannual group and 369 days for the noncompliant annual group. There were 158 examinations with abnormal interpretations, 158 biopsies performed, and 114 cancers diagnosed, resulting in a positive biopsy rate of 72%. Of the 114 total cancers, three cancers were detected at the first surveillance examination, and two cancers were detected at intervals longer than 18 months, leaving 109 cancers that were detected at either compliant semiannual or noncompliant annual examinations.
Table 1.
Distribution of Mammographic Surveillance Mammographic Examinations

Note.—The protocol at our institution recommends semiannual surveillance.
Total number of examinations include the first surveillance examination and examinations with intervals greater than 547 days (not shown).
Mean number of days for compliant semiannual and noncompliant annual examinations were 190 days and 369 days, respectively. Subsequent examinations according to interval do not include the first surveillance examinations because there was no preceding surveillance examination from which an interval could be calculated.
Of the 8234 examinations in 1841 women included for analysis, 155 biopsies were performed, yielding a total of 109 ipsilateral cancer recurrences identified in 109 women (Table 2). The 7169 compliant semiannual examinations resulted in 133 biopsies, by means of which 94 recurrences were detected in 94 women (positive biopsy rate, 71%). The 1065 noncompliant annual examinations resulted in 22 biopsies, by means of which 15 recurrences were identified in 15 women (positive biopsy rate, 68%). In either group, only one cancer recurrence was detected; no women had multiple recurrences. At the time of recurrence, cohort demographics were approximately equivalent between compliant and noncompliant groups, with similarities in age (60.4 years vs 60.7 years), strong family history (13.8% vs 13.3%), and very strong family history (7.4% vs 6.7%).
Table 2.
Results of 109 Asymptomatic Cancer Recurrences Detected through 5 Years of Mammographic Surveillance Following BCT

Numbers in parentheses are percentages.
Stage I versus stage II.
Stage 0 + stage I versus stage II.
Mean invasive size excludes stage 0. Numbers in parentheses are standard deviations.
No cancer recurrences beyond stage IIA were detected (Table 2). When analyzing surveillance cohorts for recurrence by using stage I versus stage II (Figure), a significantly higher proportion of stage I recurrences were detected with compliant semiannual examinations than were detected with noncompliant annual examinations (90% vs 64%, P = .04). Repeating this analysis by including stage 0 recurrences, a higher proportion of stage 0 + stage I recurrences (94% vs 73%, P = .03) continued to be detected with semiannual examinations. When invasive cancer recurrences were stratified according to size only, a higher proportion of lesions of 1 cm or smaller were detected with compliant semiannual examinations, approaching significance (67% vs 36%, P = .09). Additional nonsignificant findings for invasive recurrences detected with semiannual versus annual examinations included smaller tumor size (mean diameter, 11.7 mm ± 7.4 [standard deviation] vs 15.3 mm ± 8.8, P = .15) and more frequent node negativity (98% vs 91%, P = .28).
Proportions of patients with asymptomatic invasive cancer recurrence according to stage of cancer recurrence and surveillance interval. Routine surveillance mammograms were acquired every 6 months for 5 years following BCT. Cohort assignments were based on compliance with semiannual examinations compared with noncompliance with annual examinations. Semiannual-interval surveillance mammography depicted a significantly higher proportion of asymptomatic stage I cancer recurrences than annual-interval surveillance mammography (P = .04). When including noninvasive recurrences (not shown), semiannual-interval surveillance mammography continued to depict more stage 0 + stage I recurrences than stage ‖ recurrences (P = .03).
Discussion
The major finding of this study was that compliant ipsilateral semiannual mammographic surveillance aided detection of nonpalpable breast cancer recurrences at an earlier stage. Cancer recurrences detected during routine semiannual surveillance mammography following BCT had a significantly higher probability of being stage I or earlier when compared with recurrences detected during noncompliant annual surveillance examinations. Stated in another way, noncompliant annual mammographic surveillance allowed more lesions to progress to stage IIA prior to detection, diagnosis, and treatment. Recurrences detected at semiannual examinations also demonstrated a nonsignificant trend toward being smaller and node negative.
A similar significant reduction in overall cancer stage was observed for our semiannual surveillance cohort, as was observed when analysis was limited to invasive cancers. These results suggest that the earlier detection provided by semiannual surveillance did not substantially involve overdiagnosis of recurrences.
Compliance with our recommended semiannual surveillance protocol was high (85%). On the basis of this high rate of compliance, we believe that a large percentage of patients who have undergone BCT, when offered ipsilateral semiannual surveillance, are likely to take advantage of its benefits.
Our results are relevant because breast cancer recurrence following BCT substantially increases mortality and because early detection during surveillance may decrease mortality, similar to the benefit seen with the screening of asymptomatic women. In the National Surgical Adjuvant Breast and Bowel Project randomized controlled trial, patients who developed recurrences following BCT had up to three times higher mortality than patients who did not develop recurrences at the 20-year follow-up (2). Moreover, detection of recurrence by using surveillance mammography has been associated with a higher rate of survival in retrospective studies (21–23). Early detection (mammographically detected recurrence in asymptomatic patients) was associated with 47% improved survival relative to symptomatic patients, or 27% improved survival when adjusting for both lead-time and length biases (22). Finally, an association is also reported between mortality and recurrence tumor size of 1 cm or smaller, suggesting that mammographic detection of smaller recurrences is predictive of improved survival (24–26). The mortality difference between patients with stage I and patients with stage II lesions among recurrences has not been analyzed in prior studies, but if similar to mortality for primary breast cancer, one can infer an approximately 10%–25% relative difference in mortality (27). In comparison, the mortality difference between patients with stage 0 and stage I cancer recurrence may be inferred to be an approximately 5% difference (27). This finding implies that earlier detection of recurrence by semiannual surveillance mammography may improve survival substantially.
Mortality could not be analyzed in our study because there has not been sufficient follow-up time. As with screening, the effect of early detection of recurrence is likely affected by lead-time and length biases. The best approach to establish true clinical effectiveness would involve a prospective controlled trial, with randomization of women by invitation to either semiannual-interval or annual-interval mammography following BCT. However, as has been discussed by others, such a trial would require large numbers of subjects and a very long period of observation after completion of 5-year post-BCT surveillance (22). Houssami et al (22) proposed an alternative experimental design to study effectiveness, making use of surrogate measures for survival and methods that account for the effects of lead-time–bias and length-bias sampling.
Several organizations currently endorse annual surveillance intervals (14–16) or offer general guidance (28) for the frequency of post-BCT mammography but admit that these recommendations are based on expert opinion and that direct evidence is needed (12). To our knowledge, ours is the first study to provide such direct evidence, by comparing the effect of surveillance intervals on recurrence outcomes in the same study population.
As a result of the retrospective design of our study, selection bias may have contributed to some of the differences we observed. We performed our analyses on the basis of patient compliance; therefore, our results indicate the outcomes found when patients choose to undergo examination at semiannual versus annual intervals. The potential contribution of selection bias to our observed results as a consequence of patient compliance may not be substantial, as we found no demographic-based differences in cancer risk factors between our semiannual and annual surveillance cohorts.
Patients who develop symptomatic recurrences would have only their asymptomatic surveillance examinations included in our database. However, this study attempts to report only outcomes of screening asymptomatic women following BCT, and not on the outcomes of diagnostic mammography in symptomatic women following BCT.
Finally, our database does not contain information on the primary tumor, BCT regimen (including radiation therapy), or time to recurrence in our patients. We also could not distinguish between recurrence and second primary cancer because of the clinical difficulty in making the distinction (17), because both types of malignancy can be identified with mammographic surveillance and because we did not have access to further imaging characteristics (eg, breast quadrant). Our database stored deidentified information that was not linked to patient data. Attempting to gather these additional data retrospectively would be unfeasible and almost certainly impossible for most of the patients because of the long interval since the data collection began and because many patients were referrals from outside institutions.
Although our analysis does not preclude our observation that earlier recurrences were detected with semiannual surveillance, it does limit our ability to comment on what interval may be most effective for semiannual examinations during the course of surveillance. While a previous study has shown that semiannual mammography is associated with a low yield of detection for invasive cancer recurrence at the initial 6-month surveillance examination (29), we recommend continuous semiannual surveillance for 5 years to avoid confusing our patients with a complex protocol that appears to include some and skip other 6-month examinations. Confusion concerning mammography protocols has been shown to lead to decreased compliance (30). We also were unable to analyze the effect of biannual-interval examinations because very few patients had such intervals, as compliance with the semiannual protocol was high.
In conclusion, our results suggest that, following BCT, asymptomatic recurrences are detected at a significantly earlier stage with semiannual ipsilateral surveillance mammography compared with noncompliant annual mammography. Indirect evidence suggests that patients who accept semiannual surveillance following BCT may derive a survival benefit compared with patients who undergo annual-interval surveillance instead. Reliance on annual surveillance allows more second cancers to progress from stage I to stage IIA prior to detection, diagnosis, and treatment. Although demonstration of true clinical effectiveness may require a randomized controlled trial, our results are the first evidence, to our knowledge, to support more frequent surveillance following BCT than is recommended by current expert-opinion–based guidelines.
Advance in Knowledge.
• Breast cancer recurrences were significantly less advanced in women who were compliant with a semiannual ipsilateral surveillance protocol than in women who were noncompliant and followed annual surveillance; the difference in proportion of stage I recurrence between semiannual- and annual-surveillance groups was 90% and 64%, respectively (P = .04).
Implication for Patient Care.
• Women who have undergone breast conservation therapy for breast cancer are at increased risk for recurrence compared with the general population and may benefit from more frequent mammographic surveillance.
Disclosures of Potential Conflicts of Interest: V.A.A. No potential conflicts of interest to disclose. B.N.J. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: received royalties for authorship of chapters from UpToDate. Other relationships: none to disclose. N.M.L. No potential conflicts of interest to disclose. J.W.T.L. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: received payment for medicolegal consultation, and Hologic paid for lectures including service on speakers bureaus. Other relationships: none to disclose. R.J.B. No potential conflicts of interest to disclose. C.I.F. No potential conflicts of interest to disclose. D.H.M. No potential conflicts of interest to disclose. E.A.S. No potential conflicts of interest to disclose.
Acknowledgments
This research was supported by NIH TL1 RR024129 and by National Cancer Institute–funded Breast Cancer Surveillance Consortium cooperative agreement U01CA63740. The collection of cancer incidence data used in this study was supported, in part, by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; by the Surveillance, Epidemiology and End Results Program of the National Cancer Institute under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the National Program of Cancer Registries of the Centers for Disease Control and Prevention under agreement no. U55/CCR921930-02 awarded to the Public Health Institute. We thank Eoin Galvin for his editing assistance.
Received July 12, 2011; revision requested August 25; final revision received January 26, 2012; accepted February 9; final version accepted March 6.
Funding: This research was supported by the National Institutes of Health (grants NIH TL1 RR024129, U01CA63740, N01-PC-35136, N01-PC-35139, N02-PC-15105, and U55/CCR921930-02).
The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Abbreviations:
- BCT
- breast conservation therapy
References
- 1.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med 1985;312(11):665–673 [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347(16):1233–1241 [DOI] [PubMed] [Google Scholar]
- 3.Recht A, Silen W, Schnitt SJ, et al. Time-course of local recurrence following conservative surgery and radiotherapy for early stage breast cancer. Int J Radiat Oncol Biol Phys 1988;15(2):255–261 [DOI] [PubMed] [Google Scholar]
- 4.Orel SG, Troupin RH, Patterson EA, Fowble BL. Breast cancer recurrence after lumpectomy and irradiation: role of mammography in detection. Radiology 1992;183(1):201–206 [DOI] [PubMed] [Google Scholar]
- 5.Touboul E, Buffat L, Belkacémi Y, et al. Local recurrences and distant metastases after breast-conserving surgery and radiation therapy for early breast cancer. Int J Radiat Oncol Biol Phys 1999;43(1):25–38 [DOI] [PubMed] [Google Scholar]
- 6.Montgomery DA, Krupa K, Jack WJ, et al. Changing pattern of the detection of locoregional relapse in breast cancer: the Edinburgh experience. Br J Cancer 2007;96(12):1802–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Copeland G, Lake A, Firth R. Cancer in North America, 2002-2006. Volume One: Combined cancer incidence for the United States and Canada. Springfield, Ill: North American Association of Central Cancer Registries, 2009 [Google Scholar]
- 8.Rosselli Del Turco M, Palli D, Cariddi A, Ciatto S, Pacini P, Distante V. Intensive diagnostic follow-up after treatment of primary breast cancer: a randomized trial. National Research Council Project on Breast Cancer follow-up. JAMA 1994;271(20):1593–1597 [DOI] [PubMed] [Google Scholar]
- 9.Palli D, Russo A, Saieva C, et al. Intensive vs clinical follow-up after treatment of primary breast cancer: 10-year update of a randomized trial. National Research Council Project on Breast Cancer Follow-up. JAMA 1999;281(17):1586. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery DA, Krupa K, Cooke TG. Follow-up in breast cancer: does routine clinical examination improve outcome? A systematic review of the literature. Br J Cancer 2007;97(12):1632–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunfeld E, Noorani H, McGahan L, et al. Surveillance mammography after treatment of primary breast cancer: a systematic review. Breast 2002;11(3):228–235 [DOI] [PubMed] [Google Scholar]
- 12.Rojas MP, Telaro E, Russo A, et al. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev 2005 Jan 25;(1):CD001768 [DOI] [PubMed] [Google Scholar]
- 13.Recommended breast cancer surveillance guidelines American Society of Clinical Oncology. J Clin Oncol 1997;15(5):2149–2156 [DOI] [PubMed] [Google Scholar]
- 14.Khatcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol 2006;24(31):5091–5097 [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network Clinical Practice Guidelines in Breast Cancer 2008. http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf. Published September 8, 2009. Accessed September 5, 2009
- 16.Grunfeld E, Dhesy-Thind S, Levine M; Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Clinical practice guidelines for the care and treatment of breast cancer: follow-up after treatment for breast cancer (summary of the 2005 update). CMAJ 2005;172(10):1319–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buist DS, Abraham LA, Barlow WE, et al. Diagnosis of second breast cancer events after initial diagnosis of early stage breast cancer. Breast Cancer Res Treat 2010;124(3):863–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roelofs AA, Karssemeijer N, Wedekind N, et al. Importance of comparison of current and prior mammograms in breast cancer screening. Radiology 2007;242(1):70–77 [DOI] [PubMed] [Google Scholar]
- 19.Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst 2002;94(20):1546–1554 [DOI] [PubMed] [Google Scholar]
- 20.Greene FL; American Joint Committee on Cancer, American Cancer Society. AJCC cancer staging manual. 6th ed. New York, NY: Springer-Verlag, 2002 [Google Scholar]
- 21.Lash TL, Fox MP, Buist DS, et al. Mammography surveillance and mortality in older breast cancer survivors. J Clin Oncol 2007;25(21):3001–3006 [DOI] [PubMed] [Google Scholar]
- 22.Houssami N, Ciatto S, Martinelli F, Bonardi R, Duffy SW. Early detection of second breast cancers improves prognosis in breast cancer survivors. Ann Oncol 2009;20(9):1505–1510 [DOI] [PubMed] [Google Scholar]
- 23.Paszat L, Sutradhar R, Grunfeld E, et al. Outcomes of surveillance mammography after treatment of primary breast cancer: a population-based case series. Breast Cancer Res Treat 2009;114(1):169–178 [DOI] [PubMed] [Google Scholar]
- 24.Kurtz JM, Amalric R, Brandone H, et al. Local recurrence after breast-conserving surgery and radiotherapy. Frequency, time course, and prognosis. Cancer 1989;63(10):1912–1917 [DOI] [PubMed] [Google Scholar]
- 25.Doyle T, Schultz DJ, Peters C, Harris E, Solin LJ. Long-term results of local recurrence after breast conservation treatment for invasive breast cancer. Int J Radiat Oncol Biol Phys 2001;51(1):74–80 [DOI] [PubMed] [Google Scholar]
- 26.Voogd AC, van Oost FJ, Rutgers EJ, et al. Long-term prognosis of patients with local recurrence after conservative surgery and radiotherapy for early breast cancer. Eur J Cancer 2005;41(17):2637–2644 [DOI] [PubMed] [Google Scholar]
- 27.Singletary SE, Connolly JL. Breast cancer staging: working with the sixth edition of the AJCC Cancer Staging Manual. CA Cancer J Clin 2006;56(1):37–47; quiz 50–51 [DOI] [PubMed] [Google Scholar]
- 28.Morrow M, Strom EA, Bassett LW, et al. Standard for breast conservation therapy in the management of invasive breast carcinoma. CA Cancer J Clin 2002;52(5):277–300 [DOI] [PubMed] [Google Scholar]
- 29.Lin K, Eradat J, Mehta NH, et al. Is a short-interval postradiation mammogram necessary after conservative surgery and radiation in breast cancer? Int J Radiat Oncol Biol Phys 2008;72(4):1041–1047 [DOI] [PubMed] [Google Scholar]
- 30.Han PK, Kobrin SC, Klein WM, Davis WW, Stefanek M, Taplin SH. Perceived ambiguity about screening mammography recommendations: association with future mammography uptake and perceptions. Cancer Epidemiol Biomarkers Prev. 2007. 16(3):458–466 [DOI] [PubMed] [Google Scholar]