Abstract
Themis1, a recently identified T cell protein, has a critical function in the generation of mature CD4+CD8− and CD4−CD8+ (CD4- and CD8- single positive; SP) thymocytes and T cells. Although Themis1 has been shown to bind to the adapter proteins LAT and Grb2, previous studies have yielded conflicting results regarding whether or not thymocytes from Themis−/− mice exhibit TCR-mediated signaling defects. Here, we demonstrate that, in the absence of Themis1, TCR-mediated signaling is selectively impaired in CD4 SP and CD8 SP thymocytes but is not affected in CD4+CD8+ Double Positive (DP) thymocytes despite high expression of Themis1 in DP thymocytes. Like Themis1, Themis2, a related member of the Themis family, which is expressed in B cells and macrophages, contains two conserved cysteine-based domains, a proline rich region (PRR) and a nuclear localization signal (NLS). To determine if Themis1 and Themis2 can perform similar functions in vivo, we analyzed T cell development and TCR-mediated signaling in Themis1−/− mice reconstituted with either Themis1 or Themis2 transgenes. Notably, Themis1 and Themis2 exhibited the same potential to restore T cell development and TCR-mediated signaling in Themis1−/− mice. Both proteins were tyrosine phosphorylated and were recruited within Grb2 signaling complexes to LAT following TCR engagement. These results suggest that conserved molecular features of the Themis1 and Themis2 proteins are important for their biological activity and predict that Themis1 and Themis2 may perform similar functions in T and B cells, respectively.
Introduction
Several independent groups recently reported the initial characterization of a new T cell protein, E430004N04Rik, designated Themis1 (Thymocyte expressed molecule important for selection) (1–5). Analysis of Themis1−/− mice, generated either by gene targeting or by induced mutagenesis, identified an important role for Themis1 in T cell development: in the absence of Themis1, positive and negative selection of thymocytes is markedly impaired and numbers of CD4+CD8− and CD4−CD8+ [CD4- or CD8- Single Positive, (SP)] thymocytes and T cells are strongly reduced. Despite the profound developmental defects observed in Themis1−/− mice, the molecular function of Themis1 remains unclear.
Themis1 contains two novel cysteine-based (CABIT) domains (2), a bipartite nuclear localization sequence (NLS) and a proline rich region (PRR). In thymocytes, Themis1 is localized in both the cytoplasm and the nucleus suggesting that it may have functions in both cellular compartments (1). Themis1 has been shown to bind to the ubiquitous cytoplasmic adapter protein Grb2 (1, 5); however, the role of this interaction for Themis1 function has not been elucidated. Following TCR engagement, complexes formed by Grb2 and the exchange factors Vav 1 and/or Sos1 (6, 7) are recruited to the scaffolding adapter LAT (8, 9) and contribute to the activation of the ERK and JNK/P38 signaling pathways (10, 11). One group reported a requirement for Themis1 in T cell antigen receptor (TCR) induced ERK phosphorylation and calcium mobilization in total thymocytes (3). However, we failed to detect any signaling defects in purified CD4+CD8+ (Double Positive, DP) thymocytes from Themis1−/− mice despite the fact that Themis1 is highly expressed in DP cells (1). Thus, no consensus has been reached regarding the role of Themis1 in TCR-mediated signal transduction.
Themis1 is the founding member of a family of related murine proteins that includes BCO13712 (ICB-1), designated Themis2, which is expressed primarily in B cells and macrophages, and 9130404H23Rik, designated Themis3, which is expressed primarily in the intestine (1–3). Mice deficient in Themis2 or Themis3 have not yet been reported and, like Themis1, the function of these proteins has not been elucidated. Themis1 and Themis2 share 29% identity and 65% homology at the amino acid level. Like Themis1, Themis2 contains 2 CABIT domains (2), a putative NLS and a PRR. Although the Themis2 PRR motif (PxxPxK) differs from that of Themis1 (RxPxxP), Themis2 was also shown to bind to Grb2 (12). Additionaly, Themis2 was reported to bind constitutively to Vav1 and Lyn in the Raw264.7 cell line and to regulate LPS-dependent TNFα secretion in primary human macrophages. The NLS of Themis1 and Themis2 are also not completely conserved and the intracellular localization of Themis2 has not been evaluated.
To ascertain if Themis1 and Themis2 perform similar functions in vivo despite their molecular differences, we expressed Themis1 or Themis2 as transgenes under the control of the T cell specific hCD2 promoter/enhancer and crossed both transgenes into the Themis1−/− background. We found that Themis1 and Themis2 exhibit the same potential to restore T cell development in Themis1−/− mice. Moreover, both proteins are detected in the nucleus and the cytoplasm in thymocytes and are recruited within Grb2 signaling complexes to tyrosine phosphorylated LAT following TCR engagement. We identify a selective signaling defect in the phosphorylation of Vav1, ERK and P38 in SP thymocytes but not DP thymocytes in the absence of Themis1 and demonstrate that this defect is corrected by expression of either Themis1 or Themis2 transgenes. These findings favor a model wherein Themis1 functions to transduce signals initiated by the TCR and suggest that Themis2 may perform a similar role in B cells in response to B cell receptor (BCR) engagement.
Materials and Methods
Mice
The human CD2-(hCD2)-Themis1 and hCD2-Themis2 transgenes were generated by substituting murine Themis1 and Themis2 coding sequences for the ζ cDNA sequences in the construct ζ-CT108 (13). Previous studies have shown that the human CD2 promoter/enhancer directs the expression of transgenes in mice to the T lineage (14). AND αβTCR transgenic mice were obtained from Taconic Farms. Animal experiments were approved by the Animal Care and Use Committee of the National Institute of Child Health and Human Development, NIH.
Cells and Plasmids
Jurkat/TAg and 293T cell lines were maintained in RPMI 1640 medium supplemented with 10% FCS, penicillin, streptomycin and β-mercaptoethanol. pXS-Fyn-myc, pSX-Lck-myc, pSX-Lck Y505F-myc, pSX-Zap70-myc have been described previously (8). PCI-neo plasmid containing the cDNA of Themis1 was used as a template to delete the PRR region of Themis1 (RxPXXP) and the N-terminal (PKR) or the C-terminal (KRRPR) parts of the nuclear localization sequence (NLS) of Themis1. Mutagenesis reactions were performed using the GeneTailer mutagenesis kit from Invitrogen. All mutations were verified by sequencing.
Antibodies and reagents
Sources for antibodies and reagents used in this study include: anti-laminB (M-20), anti-GAPDH (FL-335), recombinant Grb2 N-terminal (1–68) or C-terminal (156–199) SH3 domains, Santa Cruz Biotechnologies; anti-Myc (9E10), anti-Grb2 (81), alexa647-conjugated anti-pERK (pT202 /pY204), pP38 (pT180 /pY182), pLAT (pY171), BD Bioscience; Anti-Vav1 (mouse ascite), anti-SOS1, anti-phospho-tyrosine (4G10), Millipore; anti-pVav1(pY160), Invitrogen. Anti-Themis1 rabbit antibodies were previously described (1). To prepare anti-Themis2 antibodies, peptides corresponding to residues CKISVHKKDRKPNPQTQN of mouse Themis2 were coupled to KLH and injected into New Zealand White rabbits (Covance). Anti-Themis2 antibodies were affinity purified from rabbit serum using a covalently bound antigen column.
Subcellular fractionation
107 thymocytes or 2.106 Jurkat T cells were incubated in 120 µl of hypotonic buffer (Hepes 100 mM, KCl 10 mM, EDTA 1 mM, Na3VO4 2 mM, protease inhibitors tablet (Roche)) for 20 min on ice. After incubation, 1.2 µl of NP40 10% was added. Lysates were mixed vigorously and centrifuged at 3000 r.p.m. for 5 min. Supernatants, which contained plasma membrane and cyotosol, were collected. Pellets were washed with hypotonic buffer and incubated with 50 µl of nuclear lysis buffer (Hepes 100 mM, NaCl 400 mM, EDTA 1 mM, Na3VO4 2 mM, protease inhibitors tablet) for 10 min on ice. Lysates were centrifuged at 14000 r.p.m., for 10 min. Supernatants, which contain nuclear extract, were collected.
Intracytoplasmic staining and Flow cytometry
Thymocytes (1.6×108 /ml) were stimulated for 1 minute with preformed complexes consisting of anti-CD3biotin (145-2C11) + anti-CD4biotin (GK1.5) (60µg/ml) plus avidin (30µg/ml). An excess of Cytofix/Cytoperm buffer (BD Bioscience) was added to each tube to stop stimulations. After centrifugation, cells were resuspended in Permwash buffer (BD Bioscience), incubated 10min at room temperature, centrifuged and resuspended in Permwash buffer containing fluorescent antibodies. Aquisition was performed on a Becton Dickinson Immunocytometry Systems FACScalibur with standard CellQuest software.
In vitro binding experiments and Immunprecipitations
Lysates from unstimulated or stimulated thymocytes were prepared as described previously (1) and incubated with recombinant Grb2 protein for 90 min at 4 °C. For immunprecipitations, anti-Themis1 or anti-Themis2 antibodies were incubated for 2 hours at room temperature with proteinA conjugated agarose gel. Gels were washed three times with lysing buffer and incubate with thymocyte extracts for two hours at 4°C. Gels were washed and resuspended in NuPage LDS sample buffer (Invitrogen). Proteins were resolved by SDS-PAGE and transferred to Immobilon-P membrane (Millipore).
Spreading assay and Confocal analysis
Spreading assays were performed as described previously (15). Briefly, chambered coverslips (LabTek) were coated overnight at 4°C with the stimulatory antibody human anti-CD3ε (HIT3a or UCHT at 10 µg/ml). Cells were plated onto coated coverslips containing imaging buffer (RPMI 1640 without phenol red, 10% fetal calf serum, 20 mM HEPES). Cells were fixed at different time points with 2.4% paraformaldehyde. Immunostaining was performed as described previously (16). Fluorescent images of fixed samples were acquired on a 510 laser-scanning confocal microscope system by using a 63× plan apochromat objective (Carl Zeiss).
Results
Themis1 and Themis2 transgenes can rescue T cell development in Themis1−/− mice
To determine if Themis1 and Themis2 have redundant functions in vivo, we examined if both proteins could independently rescue T cell development when transgenically expressed in Themis1−/− mice. Western blotting of total thymocytes from Themis1−/− mice expressing Themis1 or Themis2 transgenes (hereafter designated Themis1−/−; Themis1 tg and Themis1−/−; Themis2 tg, respectively) demonstrated that the Themis1 and Themis2 transgene encoded proteins were expressed in thymocytes (Fig 1A). Surface staining for CD4 and CD8 revealed similar profiles on thymocytes and peripheral lymphoid T cells in Themis1+/+, Themis1−/−; Themis1 tg and Themis1−/−; Themis2 tg mice. Significantly, whereas CD4 SP and CD8 SP thymocyte and T cell numbers were strongly reduced in Themis1−/− mice, they were restored to normal levels (i.e., equivalent to those in Themis1+/+ mice) in Themis1−/−; Themis1 tg and Themis1−/−; Themis2 tg mice (Fig1B). In addition, differentiation of CD4 SP and CD8 SP from the immature CD24hi to the mature CD24lo stage thymocytes was fully restored when either Themis1 or Themis2 transgenes were expressed in Themis1−/− mice (FigS1A). We previously reported that surface expression of CD5, which parallels and is directly regulated by TCR signal intensity (17) is reduced on CD4 SP thymocytes in Themis1−/− mice. Notably, CD5 surface levels were restored to normal in Themis1−/−; Themis1tg and Themis1−/−; Themis2tg mice (FigS1B and data not shown). Finally, the percentage of memory-like (CD44hiCD62Llo) T cells, which is increased in Themis1−/− mice, presumably due to lymphopenia, was comparable in Themis1+/+, Themis1−/−; Themis1 tg and Themis1−/−; Themis2 tg mice (data not shown).
Figure 1.
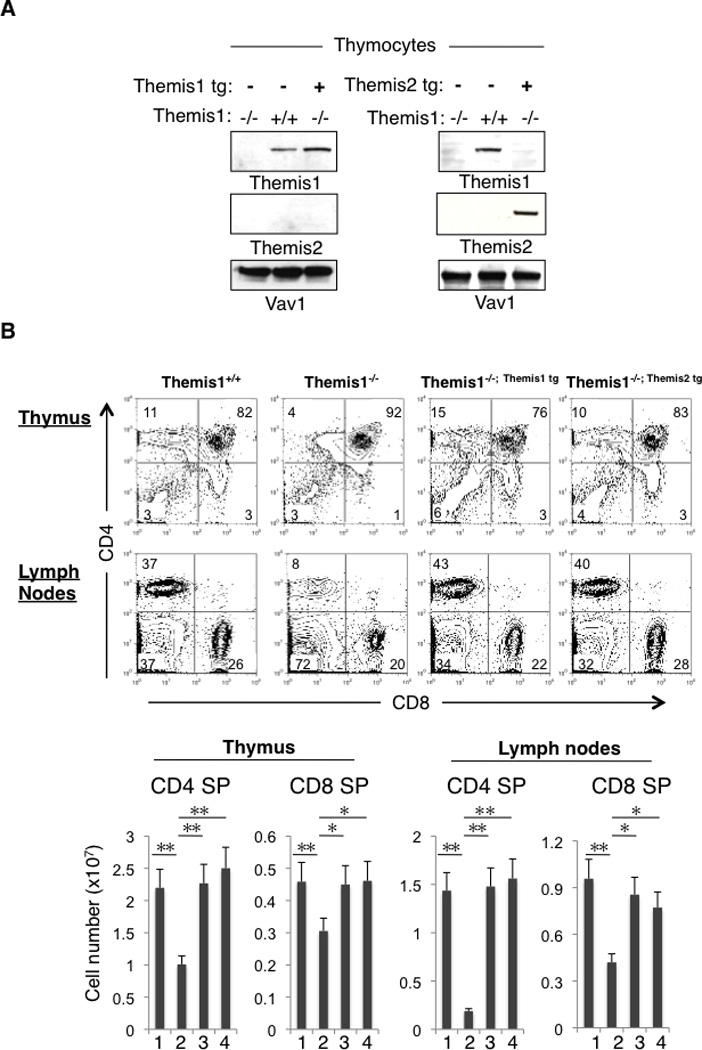
Themis1 or Themis2 transgenic expression restores T cell development in Themis1−/− mice. A, Analysis of Themis1 and Themis2 expression by western blot in Themis1−/−, Themis1−/−; Themis1tg, Themis1−/−; Themis2tg thymocytes. B, Analysis of Themis1+/+, Themis1−/−, Themis1−/−; Themis1tg, or Themis1−/−; Themis2tg thymocytes and lymph nodes T cells by flow cytometry. Two parameter dot plots show CD4 versus CD8 surface staining. Numbers indicate percentage of cells in the indicated quadrant. Bar graphs represent average cell numbers of the indicated thymocyte subsets calculated from 6 mice per group. (1) Themis1+/+, (2) Themis1−/−, (3) Themis1−/−; Themis1tg, (4) Themis1−/−; Themis2tg. (* P<0.05, ** P<0.01). Only significant differences are noted in the graphs.
Although both the Themis1 and Themis2 transgenes restored T cell development in a polyclonal TCR repertoire, it was possible that they might exhibit different potentials to restore positive selection if the specificity of the TCR was fixed. We previously found that positive selection is dramatically impaired in Themis1−/− mice expressing the class II restricted αβTCR transgene (AND) specific for pigeon cytochrome c (1). When either the Themis1 or the Themis2 transgene was expressed in AND TCR transgenic Themis1−/− mice, positive selection was fully restored (Fig2). Collectively, these results demonstrate that Themis2 is capable of substituting for Themis1 during T cell development, indicating that Themis1 and Themis2 have redundant molecular functions in thymocytes.
Figure 2.
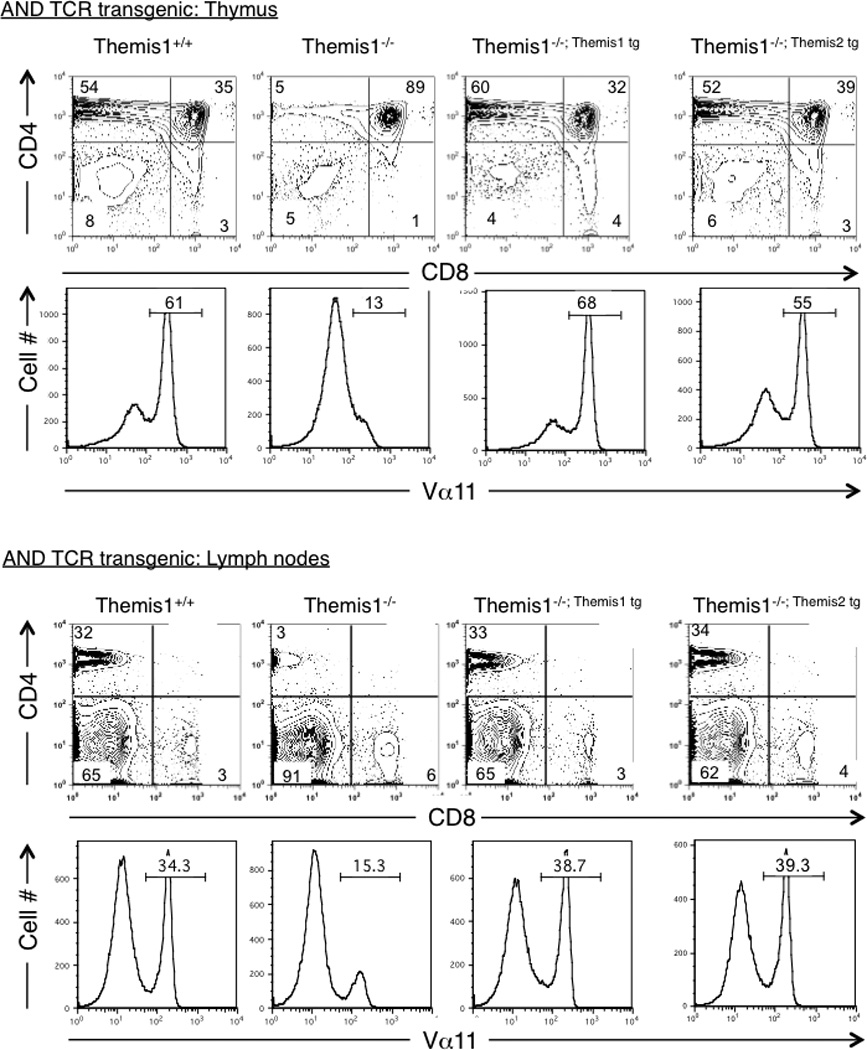
Themis1 or Themis2 transgene expression restores positive selection in AND TCR transgenic Themis1−/− mice. Analysis by flow cytometry of thymocytes and lymph nodes T cells in Themis1+/+, Themis1−/−, Themis1−/−; Themis1tg, Themis1−/−; Themis2tg mice expressing MHC class II-restricted (AND) TCR. Representative two parameter plots (n=4) show CD4 versus CD8 staining on total thymocytes or lymph node cells. Numbers indicate percentage of cells in the indicated quadrant. Single parameter histogram plots show AND TCR staining (Vα11) on total thymocytes or peripheral lymph nodes cells.
Themis1 and Themis2 are localized in both the nucleus and the cytosol in thymocytes and are tyrosine phosphorylated in response to TCR engagement
We reasoned that since Themis2 is capable of restoring T cell development in Themis1−/− mice, then conserved motifs/domains between Themis1 and Themis2 would likely be functionally important. Using sub-cellular fractionation, we previously reported that Themis1 constitutively localizes to both the nucleus and the cytosol in thymocytes (1). To confirm that the nuclear localization of Themis1 was dependent upon the bipartite NLS, we deleted either the first or the second NLS domain by mutagenesis and analyzed Themis1 cellular distribution in Jurkat T cells. Themis1 proteins lacking either domain of the bipartite NLS were undetectable in the nucleus demonstrating a requirement for this sequence for nuclear localization (Fig 3A). Moreover, we found that the nuclear/cytoplasmic distribution of Themis1 remained unchanged in thymocytes following stimulation with PMA + ionomycin or following stimulation with anti-TCR antibodies (data not shown). The NLS of Themis1 and Themis2 are not fully conserved: PKR(X)12KRRPR for Themis1, VPR(X)12KRRPR for Themis2. Nevertheless, like Themis1, transgenic Themis2 was also detected in the nucleus as well as the cytoplasm in unstimulated thymocytes (Fig 3B).
Figure 3.
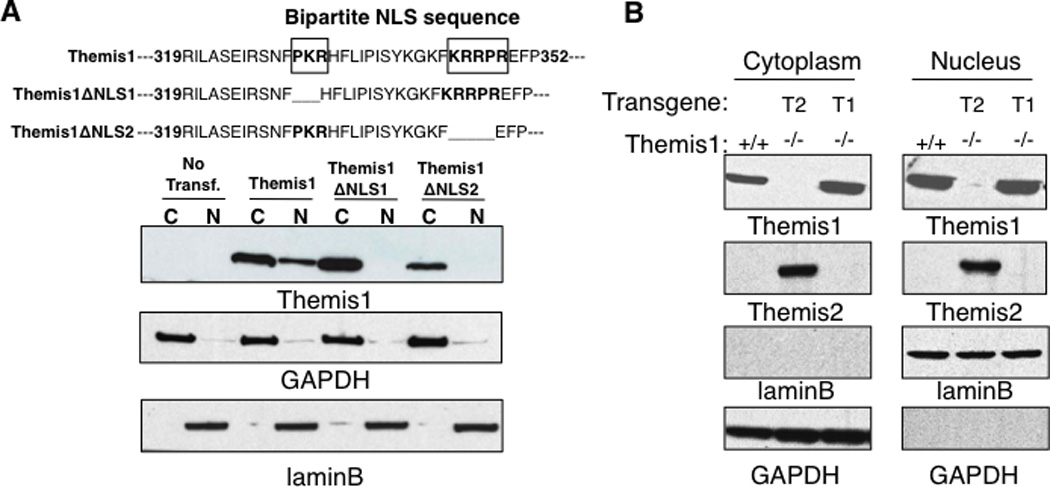
Themis1 and Themis2 are localized in the nucleus and in the cytoplasm in thymocytes. A, Deletions of the bi-partite NLS prevent Themis1 translocation/retention in the nucleus. Jurkat cells were transfected with plasmid encoding Wt or mutated isoform of Themis1 deleted of the N-ter (ΔNLS1) or the C-ter (ΔNLS2) parts of the NLS sequence. The expression of Themis1 was analyzed by western blot (four independent experiments) in cytoplasmic and nuclear extracts. B, Expression of Themis1 and Themis2 in cytoplasmic and nuclear fractions. Thymocytes from Themis1+/+, Themis1−/−; Themis1tg (T1−/−) and Themis1−/−; Themis2tg (T2−/−) mice were lysed. Extracts were fractionated to separate the cytosol and the nuclei (three independent experiments). GAPDH and LaminB proteins were used as loading control in the cytoplasmic and nuclear fraction respectively.
Themis1 is tyrosine phosphorylated following TCR engagement (3, 18). We found that transgenic Themis2 is also rapidly tyrosine phosphorylated in thymocytes following stimulation with anti-CD3+anti-CD4 (Fig.4A). In addition, Themis2 was tyrosine phosphorylated in B cells following B cell receptor stimulation with F(ab’)2 anti-IgM antibodies (Fig4A). Previous reports indicate that the phosphorylation of Themis1 and Themis2 is controlled by Src family kinases. Lck silencing in Jurkat T cells was shown to impair Themis1 phosphorylation (18) and Lyn was reported to bind constitutively to Themis2 in the Raw264.7 macrophages cell line (12). Co-transfection experiments in 293T cells demonstrated that Src kinases (Lck or Fyn) but not the ZAP-70 kinase can phosphorylate both Themis1 and Themis2 (Fig 4B). Moreover, Themis1 was phosphorylated following pervanadate treatment in Jurkat T cells deficient for ZAP70 (P116) but not in Jurkat T cells deficient for Lck (JCam1.6) (Fig 4C).
Figure 4.
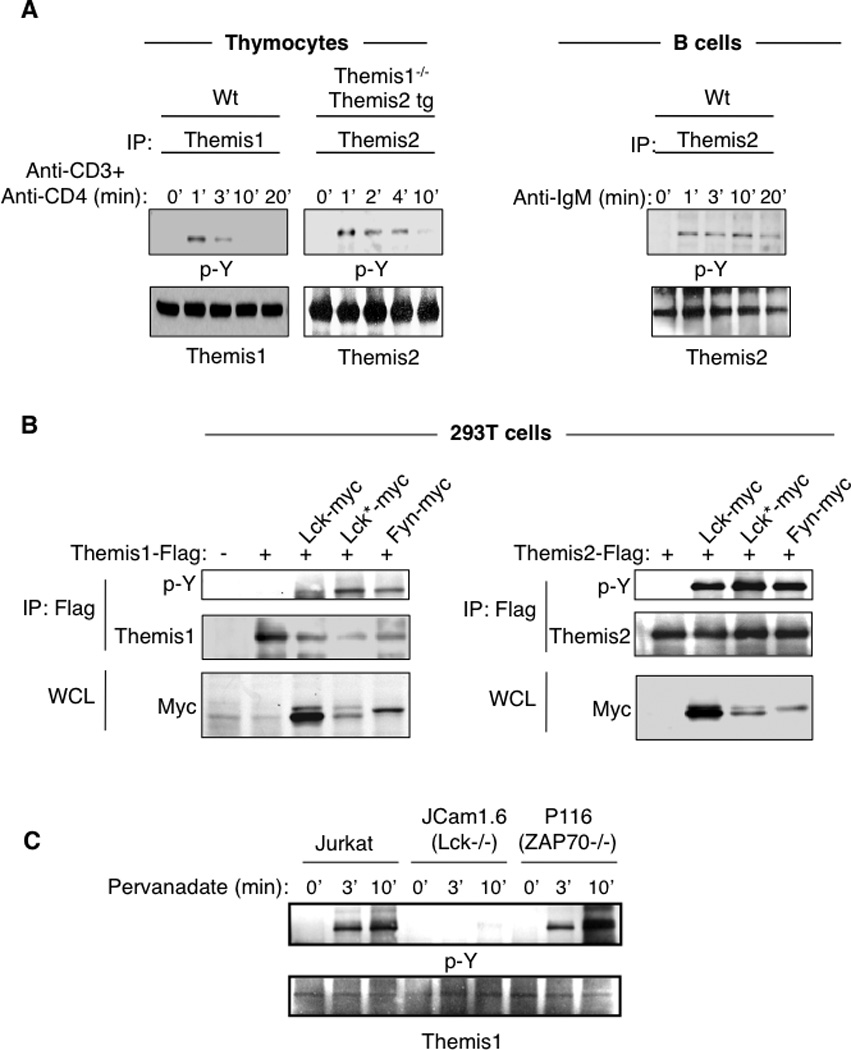
Themis1 and Themis2 are tyrosine phosphorylated by src kinases following TCR or BCR engagement. A, Analysis of Themis1 and Themis2 phosphorylation in thymocytes following stimulation with anti-CD3+anti-CD4 antibodies or of Themis2 phosphorylation in B cells following stimulation with F(ab’)2 anti-IgM antibodies. B, Analysis of Themis1 and Themis2 phosphorylation by Src or ZAP70 kinases in 293T cells. 293T cells were transfected with expression plasmids encoding Flag-tagged Themis1 or Themis2 and myc-tagged tyrosine kinases [wild-type Lck, active-Lck* (Lck Y505F), Fyn, Zap70]. Themis1 and Themis2 were immunoprecipitated with anti-Flag resin and immunoblotted with anti-phosphotyrosine. Whole cell lysates (WCL) were also analyzed by immunoblotting with anti-myc antibodies. C, Jurkat T cells deficient or not for Lck (JCam1.6) or ZAP70 (P116) were stimulated with pervanadate for the indicated periods of time. Themis1 was immunoprecipitated and immunoblotted with anti-phosphotyrosine. Results shown are representative of three experiments.
Themis1 and Themis2 are recruited within Grb2 signaling complexes to tyrosine phosphorylated LAT following TCR engagement
The rapid TCR- or BCR-dependent tyrosine phosphorylation of Themis1 and Themis2 suggested that they may be recruited to immunoreceptor signaling complexes following T cell or B activation. Supporting this, we found that spreading of Jurkat T cells (transfected with Themis1-GFP plasmid) onto anti-CD3 coated coverslips, induced the redistribution of Themis1 into clusters that co-localize with the TCR, ZAP-70 kinase and the transmembrane adaptor protein LAT (Fig 5A). In Themis1+/+ or Themis1−/−; Themis2 tg thymocytes, LAT co-precipitated with both Themis1 and Themis2 following anti-CD3+anti-CD4 stimulation. (Fig5B). LAT is tyrosine phosphorylated after TCR engagement and recruits molecular complexes formed by the adaptor protein Grb2 and the exchange factors Vav1 and Sos1 (8, 9). We found that Grb2 co-precipitated with both Themis1 and transgenic Themis2 in unstimulated or stimulated thymocytes (Fig. 5B). Grb2 association with Themis2 was also demonstrated in both unstimulated and stimulated B cells (Fig.5B). Interestingly, Vav1 but not Sos1 co-precipitated with Themis1 and with Themis2 in unstimulated thymocytes and with Themis2 in B cells (Fig5B). Grb2 binds constitutively to the proline rich regions of Sos1 and Vav1 through its N-terminal and C-terminal SH3 domains, respectively. Deletion of the Themis1 PRR prevented its association with Grb2 in Jurkat T cells indicating that this domain is essential for Themis1/Grb2 binding (Fig 5C). A previous report showed that mutation of the Grb2 N-terminal SH3 domain, but not the C-terminal SH3 domain, prevents the binding of Themis1 to Grb2 (5). To confirm this observation, we analyzed the interaction of GST fusion proteins containing either the N-terminal or the C-terminal SH3 domain of Grb2 to Themis1 or Themis2 in whole cell thymocyte extracts. We found that Themis1 and Themis2 could interact with both the N-terminal and the C-terminal SH3 domains of Grb2 (Fig. 5D). Under these experimental conditions, Themis1 bound preferentially to the N-terminal domain of Grb2 (Fig. 5D) whereas Themis2 appeared to bind equally to the N-terminal and the C-terminal SH3 domain of Grb2. Taken together, these results suggest that Themis1 and Themis2 may form a tri-molecular complex with Grb2 and Vav1 (but not Sos1) in which Themis1 or Themis2 binds to the Grb2 N-terminal SH3 domain and Vav1 binds to Grb2 C-terminal SH3 domain and that this complex is recruited to LAT following TCR-induced LAT phosphorylation.
Figure 5.
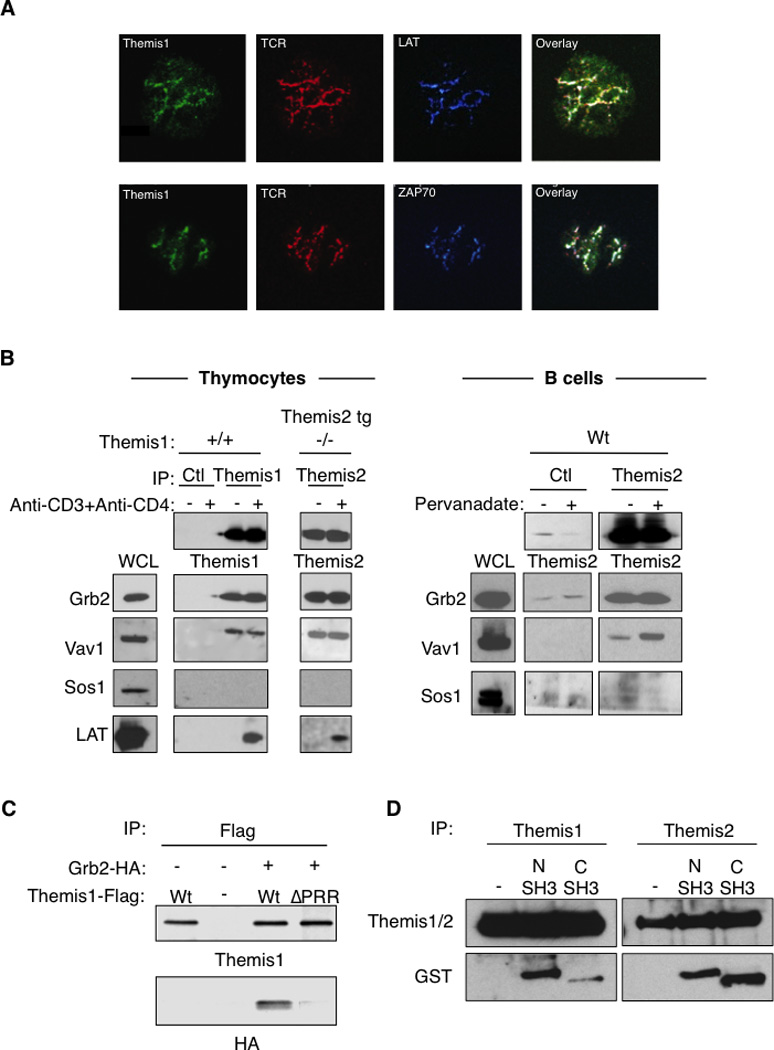
Themis1 and Themis2 are recruited within Grb2/Vav1 signaling complexes to LAT following TCR engagement. A, Formation of TCR activation clusters in Jurkat T cells expressing Themis1-GFP protein plated onto anti-CD3 coated coverslips. The colocalization of Themis1-GFP (green) with the TCR (red), ZAP70 (blue) and LAT (blue) were analyzed by confocal microscopy. B, Analysis of LAT, Vav1, Sos1 and Grb2 co-immunoprecipitation with Themis1 and Themis2 in thymocytes and B cells. Thymocytes (Themis1+/+ or Themis1−/−; Themis2tg) or B cells were stimulated with anti-CD3+anti-CD4 antibodies or pervanadate respectively. Anti-Themis1 (thymocytes) or anti-Themis2 (thymocytes and B cells) immunoprecipitates were analyzed by western blot with the indicated antibodies. C, The proline rich region of Themis1 mediates its association with Grb2. 293T cells were transfected with expression plasmids encoding Themis1-Flag, Grb2-HA, or Themis1-ΔPRR (deleted of the RxPXXP motif) in the combinations shown. Anti-Themis1 immunoprecipitates were blotted with anti-HA and anti-Themis1 antibodies. D, Themis1 and Themis2 interact with the N-terminal and the C-terminal SH3 domains of Grb2. Thymocyte lysates from Wt or Themis2 transgenic mice were incubated with GST fusion protein expressing the N-terminal SH3 domain (1–68) or the C-terminal SH3 domain (156–199) of Grb2. Themis1 or Themis2 were immunoprecipitated and co-precipitation of GST-fusion proteins were analyzed by immunoblot with the indicated antibodies. Results shown are representative of three experiments.
Themis1 or Themis2 can rescue the TCR-dependent signaling defect in Themis1−/− thymocytes
Using total thymocyte extracts, Fu et al reported that Themis1 is required for phosphorylation of ERK following TCR engagement (3). However, we failed to detect any signaling defects in DP thymocytes isolated from Themis1−/− mice (1). To resolve this discrepancy, we performed an analysis of ERK phosphorylation following TCR+CD4 engagement by intracellular staining and flow cytometry which enabled us to examine specific thymocyte subpopulations. We again found that phosphorylation of ERK1/2 (pY202/pY204) was comparable in Themis1+/+ and Themis1−/− DP thymocytes after stimulation with preformed anti-CD3+anti-CD4 immune complexes (Fig.6A). However, phosphorylation of ERK1/2 was significantly reduced in Themis1−/− CD4 SP thymocytes (Fig.6A,B). We next examined TCR induced phosphorylation of Vav1 (pY160), P38 (pT180/pY182) and LAT (pY171) by intracellular staining. Similar to ERK1/2, phosphorylation of Vav1, P38 and LAT was comparable in Themis1+/+ and Themis1−/− DP thymocytes (Fig.6A). However, phosphorylation of Vav1 and P38 was significantly reduced in Themis1−/− CD4 SP thymocytes (Fig.6A,B). Phosphorylation of Vav1 and P38 was also reduced in CD8 SP thymocytes (data not shown). In contrast, phosphorylation of LAT was unaffected by the absence of Themis1 in both DP and CD4 SP thymocytes indicating that the signaling defect was upstream of LAT (Fig.6A,B). Importantly, when either the Themis1 or the Themis2 transgene was expressed in Themis1−/− thymocytes, phosphorylation of Vav1, ERK and P38 was restored to levels comparable to Wt (Themis1+/+) CD4 SP (Fig.6A,B). No significant changes were observed for LAT, Vav1, ERK and P38 phosphorylation when Themis1−/−; Themis1 tg and Themis1−/−; Themis2 tg were compared indicating that Themis2 is capable of performing the same signaling functions as Themis1 in thymocytes (Fig6A,B).
Figure 6.
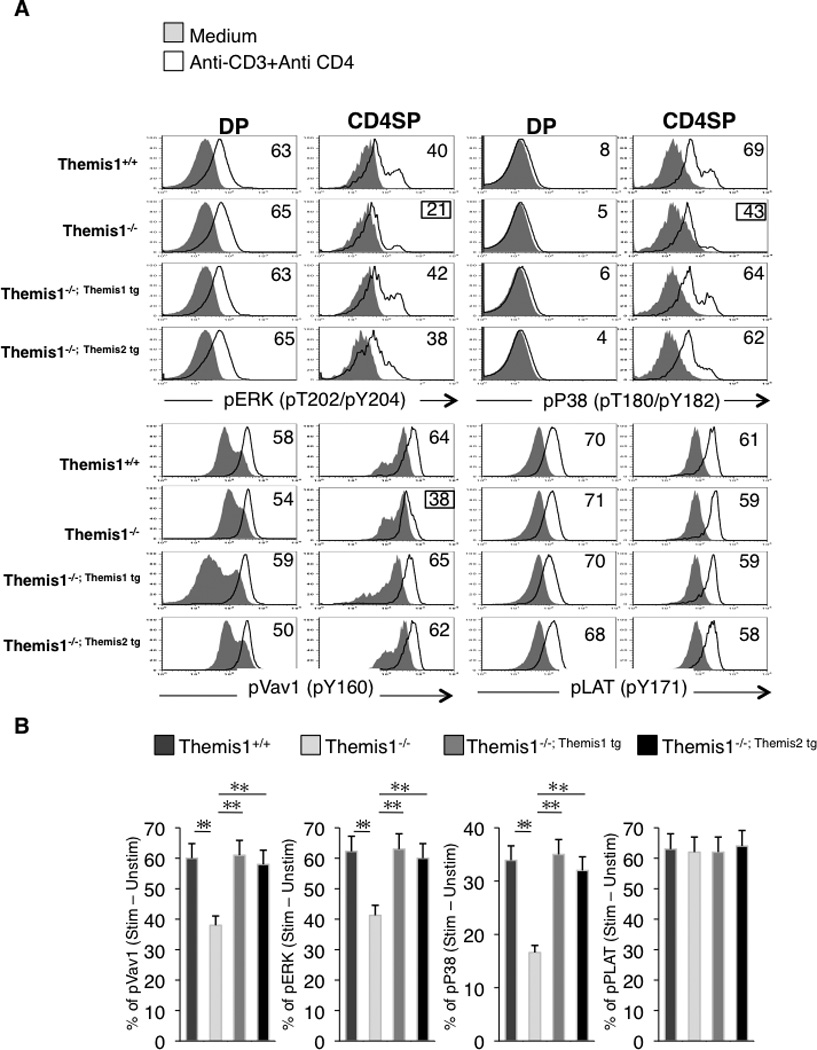
Expression of Themis1 or Themis2 in Themis1−/− thymocytes restores TCR-mediated signaling in CD4 SP thymocytes. A, Representative single parameter histogram plots (n=4) show pERK, pP38, pVav1 and pLAT staining on CD4+CD8+ (DP) or CD4+CD8− (CD4SP) from Themis1+ /+, Themis1−/−, Themis1−/−; Themis1tg and Themis1−/−; Themis2tg thymocytes. Thymocytes were stimulated with anti-CD3+anti-CD4 antibodies, fixed, permeabilized and stain with fluorescent antibodies. Numbers represent percentages of cells that up-regulate the indicated phospho-protein after stimulation (see calculation method below). Histograms are representative of four independent experiments. B, Bar graphs represent percentages of CD4 SP thymocytes that up-regulate the indicated phospho-protein following stimulation (t-test, * P<0.05, ** P<0.01). Percentages were calculated by drawing a gate from the intersection of the two histograms (solid and black line) to the superior edge of the stimulatory histogram (black line). Percentages of cells in this gate for each histogram were calculated and subtracted to each other.
Discussion
To investigate the role of Themis1 in TCR-mediated signaling and to search for common functional domains within the related Themis1 and Themis2 proteins, we analyzed T cell development and TCR-mediated signaling in Themis1−/− mice reconstituted with Themis1 or Themis2 transgenes. We found that Themis1 and Themis2 exhibit the same potential to restore T cell development and TCR-mediated signaling when expressed as transgenes in Themis1−/− mice indicating that conserved domains in these proteins are likely important for their biological activity.
The role of Themis1 in TCR signaling has remained controversial as signaling defects in Themis1−/− thymocytes have been detected in one (3) but not other studies (1, 2, 5). Here we show that, consistent with our previous findings, no signaling defects are evident in DP thymocytes from Themis1−/− mice. Interestingly, however, phosphorylation of ERK, P38, and Vav1 was impaired in CD4 SP thymocytes in the same mice. We also demonstrate that LAT phosphorylation is not impaired in either DP or CD4 SP thymocyes from Themis1−/− mice identifying a stage and pathway specific signaling defect in the absence of Themis1. Since Themis1 is recruited to LAT following TCR engagement, these results indicate that Themis1 regulates signaling effectors assembling on LAT complexes.
The absence of any discernable signaling defects in DP thymocytes is surprising as Themis1 expression is highest in this population. One possible explanation for this phenotype is that Themis1 is functionally redundant with other signaling molecules in DP thymocytes that can, independently of Themis1, activate the same pathways. A candidate for such redundancy could be the exchange factor Ras-GRP1, which is critical for ERK activation in DP thymocytes and which functions independently of Grb2-Sos1 complexes (19). It is also possible that other protein(s) that are required for Themis1 function are poorly expressed in DP thymocytes but are up-regulated in SP thymocytes after positive selection. Another explanation, which we favor, is that Themis1 does not participate in the initiation of TCR-mediated signaling in DP thymocytes, but instead functions to sustain signals initiated during positive selection as thymocytes transition from the DP to the SP stage. A widely accepted model of CD4/CD8 lineage choice contends that this event is dictated by the intensity or duration of TCR signals, with stronger or more persistent signaling initiated by MHC Class-II restricted TCRs plus CD4 promoting CD4 lineage commitment and attenuated or interrupted signaling by MHC Class I restricted TCRs in the absence of CD8 costimulation (as a consequence of stage specific CD8 down-regulation) promoting CD8 lineage commitment (20). We have shown that MHC Class II restricted thymocytes are redirected to the CD8 lineage in Themis1−/− mice consistent with an impairment of signaling at the immature SP stage (1). We also observed that upregulation of CD5, CD69 and Gata-3 are not affected in positively selected DP thymocytes but are reduced in transitional CD4+CD8lo thymocytes in Themis1−/− mice (1). Our current results are therefore consistent with a mechanism whereby Themis1 functions primarily to sustain signaling initiated by the TCR in transitional CD4+CD8lo thymocytes, either directly or by affecting the expression or activity of other factors involved in the TCR signaling response.
Themis1 and Themis2 bind constitutively to Grb2 and to the GTP/GDP exchange factor Vav1. The phosphorylation of Vav1 and its activity (data not shown) are down regulated in Themis1−/− CD4 and CD8 SP thymocytes and are restored by re-expression of Themis1 or by expression of Themis2. Interestingly, mice conditionally deficient for Grb2 in thymocytes and “knock-in” mice expressing catalytically inactive Vav1 display a phenotype strikingly similar to that of Themis1−/− mice (i.e., decreased numbers of CD4 or CD8 SP thymocytes and T cells) (21, 22). This suggests that a signaling complex formed by Themis1-Grb2-Vav1 may play a critical role during positive selection of thymocytes. Vav1 catalytic activity is required for the production of Rac1-GTP in thymocytes. Rac1-GTP in turn is important for the activation of the MAPKs JNK and P38 which have been implicated in negative selection (23–25). P38 phosphorylation was decreased in Themis1−/− CD4 SP and CD8SP thymocytes suggesting that reduced Vav1-mediated activation of P38 may explain the defect in negative selection in Themis1−/− mice (1, 3). We also found that ERK phosphorylation was reduced in Themis1−/− CD4 SP thymocytes and sustained activation of ERK has been shown to be important for positive selection in thymocytes (26). The catalytic domain of Vav1 is not required for ERK activity but, interestingly, ERK activation is dramatically impaired in Vav1-deficient thymocytes (11). This raises the possibility that Themis1 could regulate Vav1 adaptor functions, for instance by controlling the recruitment of Vav1 into TCR-dependent signaling complexes and thereby affect ERK activation. Although our data indicate that Themis1 and Themis2 may regulate Vav1 activity in sub-membranous signaling complexes, the possibility remains that they also regulate Vav1 in the nuclear compartment. Methylated forms of Vav1 have been shown to be selectively translocated to the nucleus after TCR+CD28 engagement and to participate in IL-2 synthesis in T cells (27). Although, the bi-partite NLS sequence of Themis proteins is not completely conserved in Themis1 and Themis2, we found that both proteins are partly localized in the nucleus in resting cells. It is therefore possible that Themis proteins play a dual role affecting both sub-membranous and intranuclear signaling events in lymphocytes, although this remains to be tested experimentally.
Finally, our current data provide potential insight into the role played by Themis2 in B cells. Conditional knock-out mice deficient for Grb2 in B cells and Vav1−/−Vav2−/− mice show a dramatic reduction of recirculating mature B cells in the bone marrow and a strong reduction of mature B cells in the spleen and the blood (28, 29). It is possible that mice deficient for Themis2 will have a similar phenotype. However, a potential caveat to this reasoning is that analogous T and B cell signaling proteins have often been shown to have a dissymmetrical impact on T cell and B cell development, respectively. Like Themis1 and Themis2, the related adaptor proteins LAT and LAB/NTAL, or SLP76 and BLNK/SLP65, are selectively expressed in T cells (8, 30) and B cells (31, 32), respectively. Although these molecules share conserved motifs/domains and are localized in similar signaling complexes, they exhibit functional/physiological differences. LAT deficient mice display a profound block in T cell development (33) whereas B cell development appears normal in LAB/NTAL−/− mice (34, 35) even though the transgenic expression of LAB/NTAL in T cells completely restores T cell development in LAT−/− mice (36). Similarly, T cell development is completely blocked in SLP76 deficient mice (37, 38) whereas B cell development is only partially impaired in the absence of SLP65 (the homolog of SLP76 in B cells). In the latter case, it has been shown that the combined deletion of LAT and SLP65 genes severely impairs B cell development. The same study showed that LAT and SLP76 are expressed at early stages of B cell development and act cooperatively with SLP65 to promote B cell maturation (39). Themis1 expression has not been extensively examined B cells. If expressed in immature B cell progenitors it is possible that Themis1 could function redundantly with Themis2 to regulate early stages of B cell development.
Supplementary Material
Acknowledgments
The authors thank B.J. Fowlkes (NIAID, NIH) and R. Bosselut (NCI, NIH) for helpful discussions and advice. This research was supported by the Intramural Research Program of the NIH: Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Cancer Institute.
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Lesourne R, et al. Themis, a T cell-specific protein important for late thymocyte development. Nat Immunol. 2009;10:840–847. doi: 10.1038/ni.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson AL, et al. Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nat Immunol. 2009;10:831–839. doi: 10.1038/ni.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu G, et al. Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat Immunol. 2009;10:848–856. doi: 10.1038/ni.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakugawa K, et al. A novel gene essential for the development of single positive thymocytes. Mol Cell Biol. 2009;29:5128–5135. doi: 10.1128/MCB.00793-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrick MS, et al. Gasp, a Grb2-associating protein, is critical for positive selection of thymocytes. Proc Natl Acad Sci U S A. 2009;106:16345–16350. doi: 10.1073/pnas.0908593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nel AE, et al. Ligation of the T-cell antigen receptor (TCR) induces association of hSos1, ZAP-70, phospholipase C-gamma 1, and other phosphoproteins with Grb2 and the zeta-chain of the TCR. J Biol Chem. 1995;270:18428–18436. doi: 10.1074/jbc.270.31.18428. [DOI] [PubMed] [Google Scholar]
- 7.Ye ZS, et al. Binding of Vav to Grb2 through dimerization of Src homology 3 domains. Proc Natl Acad Sci U S A. 1994;91:12629–12633. doi: 10.1073/pnas.91.26.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, et al. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Villar JJ, et al. Phosphorylation of the linker for activation of T-cells by Itk promotes recruitment of Vav. Biochemistry. 2002;41:10732–10740. doi: 10.1021/bi025554o. [DOI] [PubMed] [Google Scholar]
- 10.Crespo P, et al. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds LF, et al. Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos, and RasGRP1. J Biol Chem. 2004;279:18239–18246. doi: 10.1074/jbc.M400257200. [DOI] [PubMed] [Google Scholar]
- 12.Peirce MJ, et al. Themis2/ICB1 is a signaling scaffold that selectively regulates macrophage Toll-like receptor signaling and cytokine production. PLoS One. 2010;5:e11465. doi: 10.1371/journal.pone.0011465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Love PE, et al. Differential effects of zeta and eta transgenes on early alpha/beta T cell development. J Exp Med. 1994;179:1485–1494. doi: 10.1084/jem.179.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greaves DR, et al. Human CD2 3'-flanking sequences confer high-level, T cell-specific, position-independent gene expression in transgenic mice. Cell. 1989;56:979–986. doi: 10.1016/0092-8674(89)90631-4. [DOI] [PubMed] [Google Scholar]
- 15.Bunnell SC, et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barda-Saad M, et al. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat Immunol. 2005;6:80–89. doi: 10.1038/ni1143. [DOI] [PubMed] [Google Scholar]
- 17.Azzam HS, et al. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brockmeyer C, et al. T cell receptor (TCR)-induced tyrosine phosphorylation dynamics identifies THEMIS as a new TCR signalosome component. J Biol Chem. 2011;286:7535–7547. doi: 10.1074/jbc.M110.201236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dower NA, et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 20.Singer A, et al. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang IK, et al. Grb2 functions at the top of the T-cell antigen receptor-induced tyrosine kinase cascade to control thymic selection. Proc Natl Acad Sci U S A. 2010;107:10620–10625. doi: 10.1073/pnas.0905039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saveliev A, et al. Function of the nucleotide exchange activity of vav1 in T cell development and activation. Sci Signal. 2009;2:ra83. doi: 10.1126/scisignal.2000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rincon M, et al. The JNK pathway regulates the In vivo deletion of immature CD4(+)CD8(+) thymocytes. J Exp Med. 1998;188:1817–1830. doi: 10.1084/jem.188.10.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugawara T, et al. Differential roles of ERK and p38 MAP kinase pathways in positive and negative selection of T lymphocytes. Immunity. 1998;9:565–574. doi: 10.1016/s1074-7613(00)80639-1. [DOI] [PubMed] [Google Scholar]
- 25.Salojin KV, et al. ZAP-70 is essential for the T cell antigen receptor-induced plasma membrane targeting of SOS and Vav in T cells. J Biol Chem. 2000;275:5966–5975. doi: 10.1074/jbc.275.8.5966. [DOI] [PubMed] [Google Scholar]
- 26.Mariathasan S, et al. Duration and strength of extracellular signal-regulated kinase signals are altered during positive versus negative thymocyte selection. J Immunol. 2001;167:4966–4973. doi: 10.4049/jimmunol.167.9.4966. [DOI] [PubMed] [Google Scholar]
- 27.Blanchet F, et al. CD28 costimulatory signal induces protein arginine methylation in T cells. J Exp Med. 2005;202:371–377. doi: 10.1084/jem.20050176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ackermann JA, et al. Grb2 regulates B-cell maturation, B-cell memory responses and inhibits B-cell Ca2+ signalling. EMBO J. 2011;30:1621–1633. doi: 10.1038/emboj.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tedford K, et al. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat Immunol. 2001;2:548–555. doi: 10.1038/88756. [DOI] [PubMed] [Google Scholar]
- 30.Clements JL, et al. SLP-76 expression is restricted to hemopoietic cells of monocyte, granulocyte, and T lymphocyte lineage and is regulated during T cell maturation and activation. J Immunol. 1998;161:3880–3889. [PubMed] [Google Scholar]
- 31.Janssen E, et al. LAB: a new membrane-associated adaptor molecule in B cell activation. Nat Immunol. 2003;4:117–123. doi: 10.1038/ni882. [DOI] [PubMed] [Google Scholar]
- 32.Fu C, et al. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, et al. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhu M, et al. Positive and negative regulation of FcepsilonRI-mediated signaling by the adaptor protein LAB/NTAL. J Exp Med. 2004;200:991–1000. doi: 10.1084/jem.20041223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volna P, et al. Negative regulation of mast cell signaling and function by the adaptor LAB/NTAL. J Exp Med. 2004;200:1001–1013. doi: 10.1084/jem.20041213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janssen E, et al. Linker for activation of B cells: a functional equivalent of a mutant linker for activation of T cells deficient in phospholipase C-gamma1 binding. J Immunol. 2004;172:6810–6819. doi: 10.4049/jimmunol.172.11.6810. [DOI] [PubMed] [Google Scholar]
- 37.Clements JL, et al. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 38.Pivniouk V, et al. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- 39.Su YW, et al. LAT links the pre-BCR to calcium signaling. Immunity. 2003;19:295–305. doi: 10.1016/s1074-7613(03)00202-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


