Abstract
Background
We have explored the role that inhibitory heterotrimeric G-proteins have in ventricular arrhythmia.
Methods and Results
We studied mice with global genetic deletion of Gαi2 (Gαi2 (−/−)) and find that they have a prolonged QT interval on the surface ECG when awake and studied with telemetry. We used in-vivo electrophysiology studies and found that the Gαi2 (−/−) mice have a reduced ventricular effective refractory period and a predisposition to ventricular tachycardia when challenged with programmed electrical stimulation. Neither control nor mice with combined global deletion of Gαi1 and Gαi3 showed these abnormalities. There was no evidence for structural heart disease at this time point in the Gαi2 (−/−) mice as assessed by cardiac histology and echocardiography. The absence of Gαi2 thus leads to a primary electrical abnormality and we explored the basis for this. Single isolated ventricular cells studied using patch clamping showed that Gαi2 (−/−) mice had a prolonged ventricular action potential duration but steeper action potential shortening as the diastolic interval was reduced in restitution studies. Gene expression studies showed increased expression of L-type Ca2+ channel subunits and patch clamping revealed an increase in these currents in Gαi2 (−/−) mice. There were no changes in K+ currents.
Conclusion
Thus the absence of inhibitory G-protein signaling in particular that mediated via Gαi2 is a substrate for ventricular arrhythmias.
Keywords: Inhibitory G-proteins, long QT syndrome, ventricular tachycardia, calcium channel
Introduction
Sudden cardiac death resulting from ventricular tachyarrhythmia represents a major healthcare issue. An estimated 300,000 cases per year are recorded in the United States stressing the importance of accurate prediction and treatment of those at particular risk 1. Implantable cardiac defibrillators for the management of ventricular tachycardia have proven benefits, however precise calculation of those at greatest risk remains crude 2. Pharmacological suppression of ventricular tachycardia has additionally been largely ineffective, and in some cases pro-arrhythmic 3. Thus there is a pressing need to better understand the molecular pathways that determine the induction and persistence of ventricular tachycardia in an effort to develop treatment strategies.
The action of the sympathetic and parasympathetic systems on the heart are opposing in nature. The sympathetic system acting via β-adrenoreceptors and the stimulatory G-protein Gs is positively inotropic, chronotropic and lusitropic. In contrast, acetylcholine released from the vagus nerve acting via muscarinic receptors (largely M2) and inhibitory G-proteins is negatively chronotropic and inotropic. The ventricle expresses M2 muscarinic receptors at ~300 fmol\mg of protein 4. The density of innervation from the parasympathetic nervous system is less in the ventricle than in supraventricular structures but is nevertheless present. At the cellular level Gi\o-proteins are involved in inhibition of adenylyl cyclase, activation of PI-3 kinase and modulation of K+ and Ca2+ channels 5. The heterotrimer consists of a Gα subunit that defines the particular family and a Gβγ subunit. There are multiple isoforms of inhibitory G-proteins (Gαi1, Gαi2, Gαi3, Gαo with splice variants Gαo1 and Gαo2) and more than one member is often expressed in a single tissue 5. In general acetylcholine has no effect on basal L-type Ca2+ currents in the ventricle but it strongly antagonizes the increase on the application of a catecholamine or forskolin 6. In contrast the α subunit of Gs activates adenylyl cyclase 7 and may also positively regulate the voltage-gated calcium channel by direct action on the α1 subunit independent of protein kinase A 8.
There is also evidence for pathological changes in the cardiovascular system occurring with an imbalance of signalling through stimulatory and inhibitory heterotrimeric G-proteins. For example, in the failing heart β1 -adrenergic receptors are down regulated and Gi\o α subunit expression, in particular Gαi2, is significantly increased 9. Activation of the sympathetic nervous system whilst acutely beneficial is maladaptive in chronic heart failure. Chronic adrenergic receptor activation promotes cardiac myocyte apoptosis, ion channel remodelling and is pro-arrhythmic, partly through cAMP triggered activity 10. This pathological process may be counteracted by signalling via cardiac Gαi-coupled GPCR’s within the heart 11. Interestingly whilst β1 adrenoreceptor receptor overexpression results in a dilated cardiomyopathy, β2 overexpression (which couples via both Gαs and Gαi/o mechanisms) has a much more modest phenotype suggesting that Gαi/o coupled signaling may be cardioprotective 10;12. Gαi2 has also been directly implicated in ventricular tachycardia induction from within pathological foci in the setting of right ventricular outflow tract tachycardia. A somatic cell mutation (F200L) from within the arrhythmia focus results in loss of function of Gαi2 resulting in failed suppression of forskolin stimulated cAMP production 13. It is known that increases in sympathetic drive can initiate ventricular tachyarrhythmias and that vagal nerve activation in an opposing fashion can terminate ventricular tachycardia and increase the ventricular fibrillation threshold 14–16. Furthermore, lethal arrhythmias can often be precipitated by exercise and stress because of the activation of β-adrenergic pathways in defined arrhythmic syndromes and this is particularly the case in some variants of the long QT syndrome (LQT1 and LQT2)17. In this study we address the question of whether signalling via inhibitory heterotrimeric G-proteins is pro- or antiarrhythmic. We show that the genetic deletion of Gαi2 in mice leads to a proarrhythmic substrate in part accounted for by increased L-type Ca2+ channel activity.
Materials and Methods
Experimental Animals
Mice with global deletion of Gαi2 (Gαi2 (−/−)) and the combined global deletion of Gαi1 and Gαi3 were generated by homologous recombination on a 129SvEv background. The gene targetting strategy and confirmation of selective Gαi2 deletion have previously been published 18;19. Gαi1/Gαi3 double (−/−) were obtained from Gαi1/Gαi3 double (−/−) intercrosses. Gαι2 (−/−) mice were obtained from Gαi2 (+/−) intercrosses. Age-matched controls (Gαi2 (+/+) genotype) were generated as littermates in Gαi2 (+/−) intercrosses. The genotyping details are given in Supplementary Methods. Mice were housed up to 4 per cage with free access to standard rodent chow/water with 12hr day/night cycles and in accordance with British Home Office animal welfare guidelines (PPL 70/6732). At the time of study mice (equal sex distribution) were 12–14 weeks of age, weighing 20–25g.
Gene Expression
The methods for the gene expression arrays and quantitative real time PCR are given in Supplementary Methods.
Telemetry implantation
Details of telemetry system (TEA-F20, DSI, St Pauls) implantation have previously been described 20. Recording leads were tunnelled subcutaneously in a conventional “Lead II” ECG configuration to continuously record surface ECG after a 2 week period of surgical recovery. Single lead telemetry data was recorded continuously over 48hrs to look for evidence of spontaneous arrhythmia. In order to record standard surface ECG parameters, consecutive individual ECG complexes recorded over 2 minutes during sinus rhythm at high sampling frequency (2000Hz) were analysed (>1000 ECG complexes/mouse) using an ECG analysis extension module of CHART 4.0 software (ADIntruments, Oxford).
Histology and Isolation of cardiomyocytes
The protocols for the histological study of the heart and the isolation of single cardiomyocytes are given in the Supplementary Methods.
Single-cell electrophysiology
Patch-clamp current recordings were performed with an Axopatch 200B amplifier (Axon Instruments) using fire-polished pipettes with a resistance of 3–4 MΩ, pulled from filamented borosilicated glass capillaries (Harvard Apparatus, 1.5 mm OD × 1.17 mm ID). Data were acquired and analysed by using a Digidata 1322A interface (Axon Instruments) and pCLAMP software (version 10, Axon Instruments). All experiments were done at room temperature. Drugs were applied by a gravity driven system. Further details are given in the Supplementary Methods.
In vivo electrophysiological and programmed ventricular stimulation protocols
Mice were anaesthetised with continuous 2.0% isoflurane gas. After needle electrodes were inserted to record surface ECG, an octapolar 1.1F ultraminature cardiac electrophysiology (EPR-800, Millar Instruments) was inserted into the right ventricular apex through the right internal jugular vein to pace the heart. Cardiac pacing using extrastimulation protocols was performed using a S88-Grass stimulator (Grass technologies, USA) to record in vivo cardiac electrophysiological parameters. These included: sinus node recovery time (SNRT600), AV nodal effective refractory period (AVNERP600), 2:1 AV block coupling interval from atrial pacing and ventricular effective refractory period (VERP600). Finally a ventricular stimulation protocol was performed whereby successive extrastimuli (up to 4 (S2-S3-S4-S5)) were added to the basic drive train (15 paced beats at 100ms coupling interval) to try and provoke ventricular arrhythmia. If unsuccessful 0.1mg/kg intraperitoneal isoprotenerol was administered and the procedure repeated. Ventricular tachycardia (VT) was defined as at least 4 beats of pacing induced broad complex tachycardia that was qualitatively different from sinus rhythm (atrial electrograms confirmed AV dissociation during tachcayrdia. VT > 10 cycle lengths was defined as sustained ventricular tachycardia (i.e. a positive study). These definitions of VT are identical to those recently published 21.
Echocardiography
Mice were placed in the supine position and transthoracic echocardiography performed under 1.0% isoflurane anaesthesia using a commercial echocardiography machine (Vivid 7 Dimension™, GE Healthcare, Bedford, UK) with a 14 MHz probe recording at a depth of 0–1 cm. End diastolic and systolic dimensions were determined from a parasternal short-axis view by M-mode at the papillary muscle level. Measurements were taken of the internal dimension of the cavity using the leading-edge-to-leading-edge convention. Aortic blood flow velocities were determined by pulsed-wave Doppler in aortic arch before the bifurcation of the right carotid artery. The direction of blood flow was confirmed by colour Doppler. Stroke volume was determined as the product of the velocity time integral and the vessel cross-sectional area (π × [0.5 × diameter]2). Prior studies in mice of this age showed the aortic diameter to be 1.34 mm, thus a cross sectional area of (0.67)2 × π was assumed for all animals studied. The peak aortic blood flow velocity was measured as the average maximum velocity from 6 velocity-time traces. Heart rate was determined by measuring the time between 6 consecutive cycles from the start of each Doppler trace. Cardiac output was calculated as the product of heart rate and stroke volume.
Statistical analysis
Continuous data are presented as mean ± SEM and categorical data as percentages. The following statistical tests were used: Student’s t-test to compare two groups, one way ANOVA with a Bonferroni post-hoc test to compare three groups or two way ANOVA to compare the data in Figures 4B, 4C and 5D (GraphPad Prism v4.0). Fischer’s Exact test of proportions was used to compare the induction of VT between Gαi2 (−/−) and control. Standard linear regression was used (GraphPad Prism v4.0) and a correlation coefficient and slope are shown for the data in Figure 4. The slopes are compared using a method equivalent to the analysis of covariance (GraphPad Prism v4.0). A p-value of <0.05 was taken to be statistically significant.
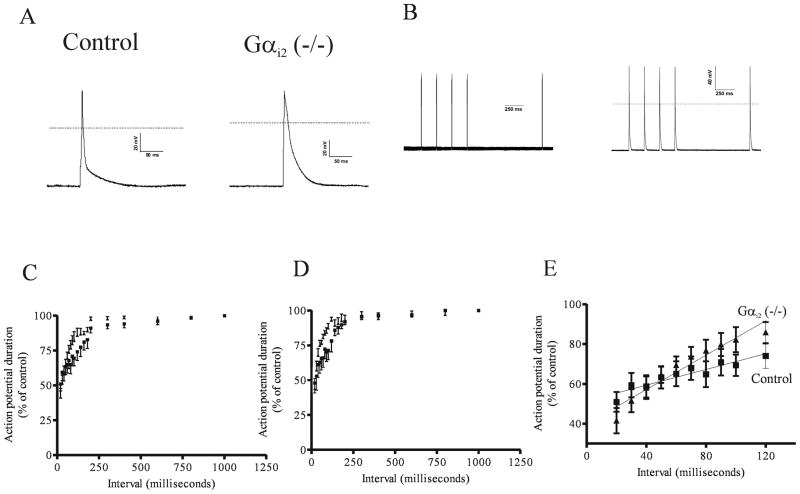
Figure 4. The action potential duration and its’ rate dependence in control and Gαi2 (−/−) mice.
A. Ventricular action potentials in control and Gαi2 (−/−) mice. Representative traces of an action potential measured after stimulation of the cells by a 5 ms pulse after pacing at 1 Hz for 60 seconds. B. An illustration of the protocol for restitution with a 1000 millisecond test interval. C. Restitution curve under basal conditions (■ = wild type mice ▲ = Gαi2 (−/−) mice). D. Restitution curve in the presence of 10 μmol\L isoprenaline (■ = wild type mice ▲ = Gαi2 (−/−) mice). E. Regression analysis of the linear portion of the restitution curve under basal conditions (■ = wild type mice ▲ = Gαi2 (−/−) mice).
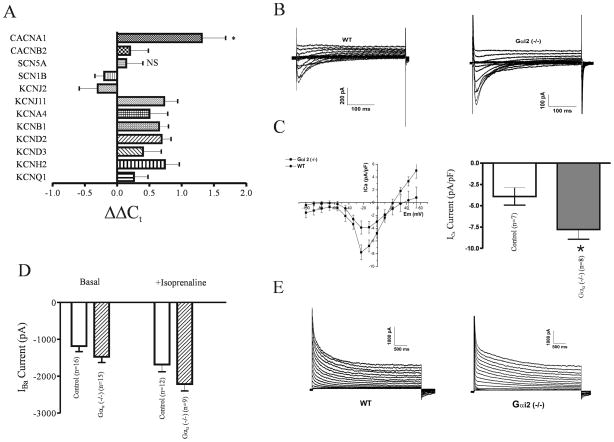
Figure 5. Electrophysiological properties in control and Gαi2 (−/−) mice.
A. Quantitative real-time PCR data comparing transcript expression between control and Gαi2 (−/−) mice (four mice in each group with each assay performed in triplicate). The data are expressed as ΔΔCt i.e. −(ΔCt(Gαi2 (−/−) − ΔCt(control)). ΔCt is measured relative to the house keeping gene GAPDH. Data are shown as mean±SEM. *=p<0.05 and NS=not significant. ΔΔCt of 1 is equivalent to two fold increase and 2 a four fold increase etc. B. Representative traces of calcium current in calcium solutions (see methods) C. Mean current-voltage relationships and a bar graph of current density. The Gαi2 (−/−) mice show asignificant increase in basal current density (Table 3). D. In Ba2+ containing solutions a similar increase was observed in basal and isoprenaline induced currents. E. Representative recordings of outward K+ currents in control and Gαi2 (−/−) mice. The mean current parameters are summarised in Table 3 but there were no significant differences between the two groups.
Results
Gαi2 (−/−) mice have spontaneous ventricular ectopy and QT interval prolongation
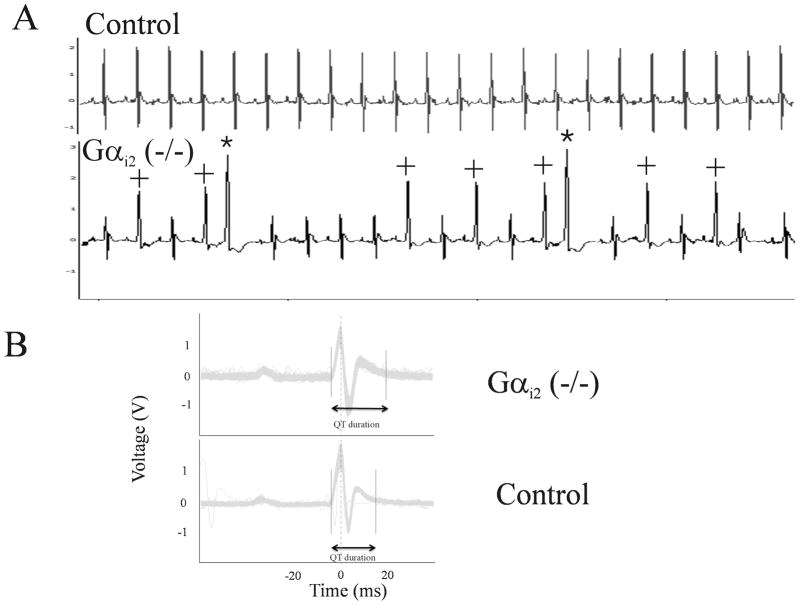
We have recently reported that Gαi2 (−/−) mice have impaired heart rate variability and lose the negative chronotropic response to carbachol 20 probably because Gαi2 acts as the key molecular link between the M2 muscarinic receptor and the G-protein gated K+ channel in sinoatrial nodal cells. In the course of these telemetry studies in conscious and ambulatory Gαi2 (−/−) mice, we noted that two of the six mice studied, under basal conditions, exhibited significant spontaneous ventricular ectopic activity and aberrantly conducted beats – an example is shown in Figure 1A. This prompted us to closely examine the surface ECG parameters in all of these mice. We noted that the Gαi2 (−/−) mice unexpectedly had QT interval prolongation despite relative tachycardia (Figure 1B) and this was reflected in a prolonged QTc 20 (Table 1). These abnormalities (and spontaneous ectopy) were not seen in control or a separate group of mice with the combined global genetic deletion of Gαi1 and Gαi3 (Table 1).
Figure 1. Single lead II surface ECG characteristics recorded from conscious freely moving mice under basal conditions using implantable telemetry.
A. Evidence of unprovoked spontaneous ventricular ectopic activity was observed in 2/6 Gαi2 (−/−) mice. No ventricular ectopics were observed in any littermate controls or in mice with the combined knockout of Gαi1 and Gαi3. The morphology of ectopic beats (highlighted by an asterix) suggests a ventricular origin. The complexes (highlighted by a +) are either ventricular in origin or are aberrantly conducted sinus beats. B. Representative signal averaged ECG recording from a Gαi2 (−/−) (TOP) mouse showing evidence of QT prolongation compared with control (BOTTOM). Signal recorded and averaged from 900 successive sinus beats per mouse.
TABLE 1.
Mean ECG parameters
| Control (n=6) | Gαi2 (−/−)(n=6) | Gαi1\3 (−/−, −/−) (n=6) | P value | |
|---|---|---|---|---|
| R-R interval | 109±4 ms | 92.4±4.1 ms (*) | 121±4 ms | 0.0006 |
| PR interval | 36.9±1.4 ms | 33.9±0.6 ms | 35.6±1.1 ms | 0.18 |
| QRS duration | 10±0.7 ms | 9.8±0.4 ms | 10.7±0.7 ms | 0.57 |
| QTc | 63.4±2.3 | 81.1±3.7 (*) † | 50.1±5.3 | 0.0004 |
All observations are mean ± S.E.M. One way ANOVA with a Bonferroni post hoc test. Significant in post-hoc test = *. The post-hoc test compares control with Gαi2 (−/−) and control with Gαi1\3 (−/−, −/−). QTc =QT/((R-R/100)^0.5).
- n=5 as the program was unable to measure the QT interval in one mouse.
Gαi2 (−/−) mice have a proarrhythmic substrate
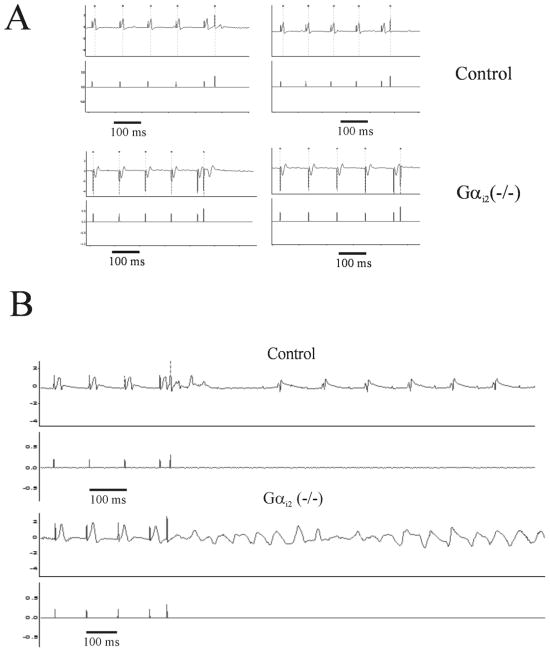
We observed occasional and unexpected death in several Gαi2 (−/−) mice although we were not able to record terminal arrhythmia and this has also been previously observed 22. To investigate further this potential substrate we used in-vivo electrophysiological studies in anaesthetised mice. The results of these studies are shown in Table 2. In particular we noted that the minimum ventricular S1–S2 coupling interval capable of ventricular capture (VERP) was significantly shorter in Gαi2 (−/−) mice compared with control mice (Table 2 and Figure 2A). A reduction in VERP is pro-arrhythmic in situations of both re-entry and triggered activity. These abnormalities were not seen in control or mice with the combined global genetic deletion of Gαi1 and Gαi3 (Table II). Gαi2 (−/−) mice had an increased incidence and duration of induced ventricular tachycardia (Figure 2B). We were able to induce sustained VT in 7 out of 11 Gαi2 (−/−) mice (63.6%) compared with 2 out of 12 (16.7%) littermate controls (Figure 2C, p-value=0.023, Fisher’s exact test). Additionally a significant increase in mean VT duration was seen in Gαi2 (−/−) mice compared with control mice (Figure 2D).
TABLE 2.
Mean Electrophysiology Parameters
| Control (n=12) | Gαi2 (−/−)(n=11) | Gαi1\3 (−/−, −/−) (n=4) | P value | |
|---|---|---|---|---|
| SNRT600 | 149±9 ms | 160±3 | 164±21 ms | 0.50 |
| AVNERP600 | 51.6±3 ms | 52.1±3.2 ms (n=10†) | 59.7±6.1 ms (n=3†) | 0.78 |
| 2:1 block | 55.1±2.5 ms | 48.9±1.3 ms | 56.7±0.7 ms | 0.049 |
| VERP600 | 38.2±2.4 ms | 29.8±2.2 ms (*) | 41±4.5 ms | 0.023 |
| VT stim | 2/12 | 7/11 (p=0.03) | 1/4 (p=0.61) | * |
| VT duration | 205±141 ms | 963±308 ms (*) | 90±90 ms | 0.04 |
All observations are mean ± S.E.M. One way ANOVA with a Bonferroni post hoc test. Significant in post-hoc test = *. The post-hoc test compares control with Gαi2 (−/−) and control with Gαi1\3 (−/−, −/−).
we were not able to determine this parameter in one mouse.
Calculated using Fishers Exact Test not one way ANOVA and p-value given in relevant column.
Figure 2. In vivo murine cardiac EP data and VT induced by programmed electrical stimulation.
A. The measurement of VERP and this was significantly reduced in Gαi2 (−/−) mice. B. Example of programmed electrical stimulation with one extrastimulus (S2) demonstrating VT induction. Control (TOP) shows non sustained VT induction at very short S1–S2 coupling. Gαi2 (−/−) (Bottom) mice develop sustained VT using a similar induction protocol.
There is a primary electrical abnormality in Gαi2 (−/−) mice
One possibility is that mice studied at this age (12–14 weeks) have developed pathological heart disease and the arrhythmic phenotype is secondary to this. However, there was no evidence of functional impairment in contractile function or other abnormalities as assessed using echocardiography in Gαi2 (−/−) mice compared to control wildtype mice (Figure 3 and Supplementary Data Table S1). Furthermore, when we harvested the organs there was no evidence of gross hypertrophy when comparing the ratio of heart weight to body weight or tibial length. In control mice the ratio of heart weight (mg) to body weight (g) was 5.02±0.20 (n=4) and in Gαi2 (−/−) it was 5.34±0.07 (n=4, P=0.18). In control mice the ratio of heart weight (mg) tibial length (mm) was 5.37±0.34 (n=4) and in Gαi2 (−/−) it was 5.20±0.23 (n=4, P= 0.69). Finally at cardiac histological examination ventricular dimensions were normal (Supplementary data, Figure S1A), myocytes were not hypertrophied and there was no evidence of fibrosis in Gαi2 (−/−) mice (Supplementary Data, Figure S1B).
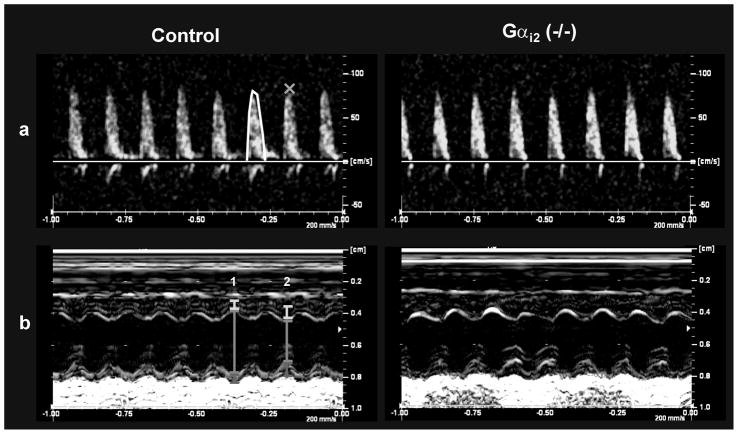
Figure 3. Echocardiography in wild type and Gαi2 (−/−) mice.
A. Pulsed-wave Doppler in the aortic arch. Velocity-time integral represents the area under each envelope (used to calculate stroke volume). Peak blood flow velocity is denoted by the orange cross (x). B. Parasternal short axis view of the left ventricle during M-mode. Dimensions of the anterior wall (yellow bars), left ventricle (green bars) and posterior wall (red bars) during diastole (1) and systole (2).
The action potential duration and its’ rate dependence
The correlation of ventricular action potential depolarisation and repolarisation with the QRS-T complex on the ECG is not as well established in mice as in larger mammals 23. Thus we isolated single cardiac ventricular myocytes and studied the action potential characteristics using patch clamping. We found that the later phases of repolarisation were significantly prolonged in Gαi2 (−/−) mice compared to control as reflected in a significantly prolonged APD50 and APD90 (Figure 4A and Table 3). There was no difference in magnitude of the initial depolarisation or the resting membrane potential between the two groups (Table 3).
TABLE 3.
Mean Single Cell Electrophysiology Parameters
| WT | Gαi2 (−/−) | P Value | |
|---|---|---|---|
| Cell capacitance | 104 ± 4.4 pF, n=37 | 99 ± 4.3 pF, n=35 | 0.42 |
| Em | −64.8 ±2.3 mV, n=16 | −64.4±2.5 mV, n=11 | 0.91 |
| Δ | 100.6 ± 2.6 mV, n=16 | 101.4 ±5.5 mV, n=11 | 0.89 |
| APD50 | 9.9 ±1 ms, n=16 | 16.9 ± 2.2 ms, n=11 | 0.004 |
| ADP90 | 41.2 ± 5.6 ms, n=16 | 72 ±7.1 ms, n=11 | 0.002 |
| Ica | −3.9 ± 1 pA/pF, n=7 | −7.8 ± 1.1 pA/pF, n=8 | 0.022 |
| τ Ica | 38.2 ± 5.9 ms, n=7 | 16.3 ± 2.3 ms, n=8 | 0.003 |
| IK peak | 52.5 ± 5.2 pA/pF, n=8 | 38.2 ± 6.6 pA/pF, n=5 | 0.12 |
| IK plateau | 13.9 ± 2.3 pA/pF, n=8 | 11.7 ± 2.2 pA/pF, n=5 | 0.53 |
| IK τ1 | 142 ± 15.1 ms, n=8 | 166.6 ± 32.3 ms, n=5 | 0.45 |
| IK τ2 | 1122 ± 91.5 ms, n=8 | 1150 ± 126 ms, n=5 | 0.86 |
Electrophysiological parameters recorded in single cell studies from ventricular myocytes of control and Gαi2 (−/−) mice. The following parameters are presented: cell capacitance, resting membrane potential (Em), depolarisation during AP (Δ), 50 % and 90% repolarisation time (APD50, APD90), calcium current with calcium as a carrier (Ica), tau of deactivation of Ica (τ Ica), potassium current measured with 4.5 s pulse at the peak and plateau phases (IK peak, IK plateau), fast and slow inactivation constants of the K+ currents (IK τ1, IK τ2).
We next examined the rate dependence by constructing single cell restitution curves (see Methods and Figure 4B). There were significant differences between the Gαi2 (−/−) mice and control mice under basal conditions and in the presence of isoprenaline (both P<0.01, Two way ANOVA) (Figure 4B and 4C). Specifically the slope of the restitution curve in its linear phase was steeper in the Gαi2 (−/−) mice compared to the control mice (Figure 4E). Under basal conditions: control mice (20 to 120 ms, r2=0.88) slope=0.20±0.03, Gαi2 (−/−) mice (20 to 120 ms, r2=0.93) slope=0.43±0.04, P=0.0002. In the presence of isoprenaline: control mice (20 to 120 ms, r2=0.90) slope= 0.27±0.03, Gαi2 (−/−) mice (20 to 100 ms, r2=0.87) slope= 0.49± 0.07, P=0.01.
Increased L-type Ca2+ currents contribute to the abnormality
The observed abnormalities in repolarisation could be accounted for by increases in inward current in particular via L-type Ca2+ channels or a decrease in outward K+ currents. These could arise through changes in gene expression and concomitantly protein levels or alternatively changes in the surface expression or activity of these channels. Thus we initially ran gene expression arrays and compared Gαi2 (−/−) mice with controls. There is evidence for remodelling of the electrophysiological properties of cardiac myocytes in a number of pathological states. In particular, there have been reports of a decreased expression of K+ channels resulting in a prolonged action potential duration 24. However at the mRNA level, gene array studies comparing control and Gαi2 (−/−) mice showed little change in the relevant K+ channel genes and in some cases their expression was even increased (Table 4). In contrast we did find a significant increase in expression (~2 fold) in Gαi2 (−/−) mice of the calcium channel alpha subunit, CACNA1C (Cav1.2), which underlies the L-type calcium current and SCN5A which underlies the rapidly inactivating sodium current in ventricular myocytes (Table 4). We sought to confirm the changes in gene expression using quantitative real time PCR (Figure 5A). The only ion channel gene with significant increased expression (ΔΔCt>1 corresponding to a two-fold change) was CACNA1C (Cav1.2).
Table 4.
Differential gene expression in the major ion channels involved in the cardiac ventricular action potential in Gαi2 (−/−) and littermate controls.
| Gene | Gαi2 (−\−) (n=3) | Control (n=4) | Adjusted P value |
|---|---|---|---|
| Voltage gated K+ channel | |||
| KCNA5 | 6.0±0.2 | 5.2±0.5 | 0.20 |
| KCNB1 | 3.3±0.2 | 3.6±0.1 | 0.21 |
| KCND2 | 7.4±0.7 | 7.2±0.1 | 0.72 |
| KCND3 | 5.5±0.2 | 5.2±0.1 | 0.23 |
| KCNH2* | 7.7±0.2 | 6.7±0.2 | 0.01 |
| KCNQ1* | 7.5±0.3 | 6.7±0.1 | 0.03 |
| KCNIP2 | 8.5±0.5 | 7.9±0.2 | 0.16 |
| Inward Rectifier K+ channel | |||
| KCNAB2 | 4.4±0.01 | 4.4±0.05 | 0.85 |
| KCNJ2 | 6.9±0.5 | 6.8±0.1 | 0.80 |
| KCNJ3 | 6.9±0.3 | 6.8±0.1 | 0.82 |
| KCNJ11* | 7.1±0.2 | 6.3±0.3 | 0.03 |
| ABCC9 | 10.0±0.3 | 9.3±0.2 | 0.07 |
| Twin Pore K+ channel | |||
| KCNK1 | 3.7±0.05 | 3.9±0.4 | 0.80 |
| KCNK3* | 8.2±0.1 | 7.8±0.1 | 0.03 |
| KCNK6 | 5.8±0.1 | 5.7±0.2 | 0.52 |
| Na+ channel | |||
| SCN5A* | 8.5±0.3 | 7.5±0.1 | 0.02 |
| SCN1B | 5.9±0.1 | 5.8±0.2 | 0.89 |
| Ca2+ channel | |||
| CACNA1C* | 9.1±0.3 | 8.0±0.2 | 0.02 |
| CACNB2 | 7.9±0.2 | 7.5±0.2 | 0.13 |
| Misc | |||
| SLC8A1\NCX1 | 9.1±0.2 | 8.7±0.3 | 0.34 |
Expression data is presented as Mean±S.D. on a logarithmic (base 2) scale. The method for the calculation of the adjusted p value is given in the Supplementary Methods. Therefore an expression differential of 1.0 represents a twofold change in relative expression between groups. Significant differential expression is highlighted in grey.
Thus, we measured calcium currents in single ventricular myocytes using patch clamping. Representative recordings and current-voltage relationships are shown in Figure 5B. There was a significant increase in L-type calcium currents measured under basal conditions when using Ca2+ as the charge carrier (Figure 5C and Table 3). A similar increase was present using Ba2+ as the charge carrier under basal conditions and with application of isoprenaline, in myocytes isolated from the Gαi2 (−/−) mice compared to control (Figure 5D). In a two way ANOVA if the rows are with and without isoprenaline and the columns are the genotype then the row factor has a P= 0.0009 and the column factor P=0.02. The interaction factor is not significant (P=0.49). Nifedipine blocked the current in cells where it was tested. We also measured outward K+ currents (see methods) and these were not significantly changed in the Gαi2 (−/−) mice compared to control (Figure 5E and Table 3). There were no differences in cell capacitance between the two groups of mice (Table 3).
Discussion
Our findings show that the absence of the inhibitory G-protein, Gαi2 (but not Gαi1/Gαi3) leads to a proarrhythmic substrate in the ventricle resulting in a long QTc interval and a predisposition to ventricular tachycardia. This is reflected at the cellular level with a prolonged ventricular action potential, most likely resulting from changes in expression and\or regulation of L-type Ca2+ currents. Additionally, we and others have observed unexpected sudden death in Gαi2 (−/−) mice but we did not study this prospectively 22. Furthermore, during short-term telemetry we did not observe a lethal ventricular arrythmia though we did observe qualitatively an increased ventricular ectopic burden that we never saw in controls. However during minimally invasive EP testing Gαi2 (−/−) mice had a shortened VERP, a known proarrhythmic substrate. Additionally we were able to induce VT significantly more easily in Gαi2 (−/−) mice than controls. However not every Gαi2 (−/−) mouse developed VT and it is worth noting that the 129\Sv strain is relatively resistant to ventricular arrhythmia 25. Furthermore, in a proportion of the Gαi2 (−/−) mice cAMP responses are relatively well preserved 26;27.
We performed studies to investigate potential mechanisms for the long QT interval on the ECG and the prolonged action potential duration in single cell patch clamping studies. The latter is an important observation as these measurements were made in the absence of extracellular signalling and agonists for these pathways. This contrasts with the in-vivo situation where tonic regulation of ion channel currents via signalling pathways will also make an important net contribution to cardiac cell excitability. It is worth emphasising that the in-vivo phenotype of the Gαi2 (−/−) mice will also be significantly influenced by changes in L-type calcium channel regulation. The absence of muscarinic receptor regulation of this current in Gαi2 (−/−) mice has already been established and potentially in-vivo this will considerably exacerbate the increase in the calcium current as the heart is continuously exposed to varying vagal and sympathetic drive 28. The loss of the muscarinic regulation of Cav1.2 will ensure that Ca2+ currents are larger for any given level of sympathetic-to-vagal balance. However our data suggest that the change in action potential duration is due to a long-term change in channel density at the membrane due to changes in expression or trafficking. Our studies revealed an increase in calcium channel expression and currents in the Gαi2 knockout mice. Previous studies have seen no significant change though there was a trend towards this in the data and the experimental conditions were different 28. That defects in L-type calcium channels might be associated with a long QTc is very much an emerging theme. Timothy syndrome is characterised by a mutations in CACNA1C and prolonged QTc, predisposition to sudden death, autism and a variety of defects in other tissues 29. RAD, a ras related GTPase, is important in governing Cav1.2 channel trafficking. Overexpression of a dominant negative construct in mice and guinea pigs led to a doubling of L-type calcium current (a magnitude of change comparable to ours) and severe prolongation of the QTc together with an arrhythmogenic phenotype 30. Finally, recent large scale human genetic studies have shown linkage of variations in the population QTc interval to variants within the NOS1AP gene that encodes an adaptor protein for nitric oxide synthase 1 31. Subsequent electrophysiological studies have revealed that the protein can regulate the expression of calcium currents in cardiac myocytes 32. The exact link between inhibitory G-protein signalling and ion channel gene expression is unclear and we are actively investigating this. For example, it is known that the promoter for the α1C calcium channel contains a cAMP response element 33 and this pathway involving activation of CRE binding protein is an obvious candidate.
We also examined the rate dependence of action potential duration and found that as the test interval decreased the action potential shortened more steeply in the Gαi2 (−/−) mice than in controls. This is inherently proarrhythmic as it may lead to repolarisation alternans a known marker for ventricular arrhythmia 34. It also may help to rationalise how a shortened VERP and prolonged QT interval can both be present. Thus at normal heart rates the action potential duration (and QTc interval) are relatively prolonged in the Gαi2 (−/−) mice. However at higher rates the slope of the restitution curve in the linear phase is steeper in these mice and thus action potential duration shortens more resulting ultimately in a reduced VERP. The decrease in VERP would support re-entrant circuits by shortening the potential path length. We have not seen evidence for early or delayed afterdepolarisations in these experiments and thus think there is not a prominent substrate for triggered activity. Both of these can occur because of abnormalities in Ca2+ handling 35. For example administration of an L-type Ca2+ channel agonist can lead to early afterdepolarisations 36. However, myocardial contractility as measured using echocardiography in our study was not different. Furthermore other investigators have examined Ca2+ transients and contractility in Gαi2 (−/−) mice and found no differences under control conditions or after the addition of isoprenaline 37. We saw no evidence for structural heart disease in these mice but we only examined this at a relatively young age and it is not clear what happens as the mice age.
There are debates as to what can be learnt from murine models 38 but what are the potential pathophysiological implications of our observations? There has been a substantial focus on the role of aberrant signalling in heart failure via β-adrenergic receptors particularly in light of the beneficial outcomes of β-blockers on survival 11;39. It has been established that there is an enhanced expression of Gαi2 in heart failure 9. Our results would suggest that increased expression may be a potential protective mechanism against malignant ventricular tachyarrhythmias. More broadly, parasympathethic modulation of cardiac rhythm is considered protective against ventricular arrhythmia 40. In contrast parasymapthetic attenuation manifesting as a reduced heart rate variability signal is a powerful predicitor of sudden cardiac death in heart failure 41 and a number of other cardiac pathologies 42. Interestingly pravastatin treatment has been reported to selectively upregulate Gαi2 expression and increase high frequency (HF) power 43. We have previously described increased heart rate and selective loss of HF power in mice deficient for Gαi2 (but not Gαi1, Gαi3 or Gαo) 20. In the setting of hypertrophic cardiomyopathy, an increased of expression of Gαi1 has been proposed to be proarrhythmic 44. Our studies failed to reveal any significant abnormalities in mice with combined global genetic deletion of Gαi1 and Gαi3. Intriguingly, such remodelling may only become important in certain disease settings and different inhibitory G-protein isoforms may exhibit differential effects under these conditions.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by the Wellcome Trust, Medical Research Council and the British Heart Foundation, and by the Intramural Research Program of the NIH (project Z01-ES101643 to LB).
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Noseworthy PA, Newton-Cheh C. Genetic determinants of sudden cardiac death. Circulation. 2008;118:1854–1863. doi: 10.1161/CIRCULATIONAHA.108.783654. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 3.The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N Engl J Med. 1992;327:227–233. doi: 10.1056/NEJM199207233270403. [DOI] [PubMed] [Google Scholar]
- 4.Bohm M, Gierschik P, Jakobs KH, Pieske B, Schnabel P, Ungerer M, Erdmann E. Increase of Gi alpha in human hearts with dilated but not ischemic cardiomyopathy. Circulation. 1990;82:1249–1265. doi: 10.1161/01.cir.82.4.1249. [DOI] [PubMed] [Google Scholar]
- 5.Birnbaumer L. Expansion of signal transduction by G proteins. The second 15 years or so: from 3 to 16 alpha subunits plus betagamma dimers. Biochim Biophys Acta. 2007;1768:772–793. doi: 10.1016/j.bbamem.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischmeister R, Hartzell HC. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilman AG. G proteins: transducers of receptor-generated signals. Annual Review of Biochemistry. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton SL, Codina J, Hawkes MJ, Yatani A, Sawada T, Strickland FM, Froehner SC, Spiegel AM, Toro L, Stefani E, Birnbaumer L, Brown AM. Evidence for direct interaction of Gs alpha with the Ca2+ channel of skeletal muscle. J Biol Chem. 1991;266:19528–19535. [PubMed] [Google Scholar]
- 9.Eschenhagen T, Mende U, Nose M, Schmitz W, Scholz H, Haverich A, Hirt S, Doring V, Kalmar P, Hoppner W. Increased messenger RNA level of the inhibitory G protein alpha subunit Gi alpha-2 in human end-stage heart failure. Circ Res. 1992;70:688–696. doi: 10.1161/01.res.70.4.688. [DOI] [PubMed] [Google Scholar]
- 10.DeGeorge BR, Jr, Gao E, Boucher M, Vinge LE, Martini JS, Raake PW, Chuprun JK, Harris DM, Kim GW, Soltys S, Eckhart AD, Koch WJ. Targeted inhibition of cardiomyocyte Gi signaling enhances susceptibility to apoptotic cell death in response to ischemic stress. Circulation. 2008;117:1378–1387. doi: 10.1161/CIRCULATIONAHA.107.752618. [DOI] [PubMed] [Google Scholar]
- 11.Zheng M, Zhu W, Han Q, Xiao RP. Emerging concepts and therapeutic implications of beta-adrenergic receptor subtype signaling. Pharmacol Ther. 2005;108:257–268. doi: 10.1016/j.pharmthera.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Foerster K, Groner F, Matthes J, Koch WJ, Birnbaumer L, Herzig S. Cardioprotection specific for the G protein Gi2 in chronic adrenergic signaling through beta 2-adrenoceptors. Proc Natl Acad Sci USA. 2003;100:14475–14480. doi: 10.1073/pnas.1936026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lerman BB, Dong B, Stein KM, Markowitz SM, Linden J, Catanzaro DF. Right ventricular outflow tract tachycardia due to a somatic cell mutation in G protein subunitalphai2. J Clin Invest. 1998;101:2862–2868. doi: 10.1172/JCI1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waxman MB, Wald RW. Termination of ventricular tachycardia by an increase in cardiac vagal drive. Circulation. 1977;56:385–391. doi: 10.1161/01.cir.56.3.385. [DOI] [PubMed] [Google Scholar]
- 15.Kolman BS, Verrier RL, Lown B. The effect of vagus nerve stimulation upon vulnerability of the canine ventricle: role of sympathetic-parasympathetic interactions. Circulation. 1975;52:578–585. doi: 10.1161/01.cir.52.4.578. [DOI] [PubMed] [Google Scholar]
- 16.Brack KE, Patel VH, Coote JH, Ng GA. Nitric oxide mediates the vagal protective effect on ventricular fibrillation via effects on action potential duration restitution in the rabbit heart. J Physiol. 2007;583:695–704. doi: 10.1113/jphysiol.2007.138461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moss AJ, Kass RS. Long QT syndrome: from channels to cardiac arrhythmias. J Clin Invest. 2005;115:2018–2024. doi: 10.1172/JCI25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 19.Jiang M, Spicher K, Boulay G, Martin-Requero A, Dye CA, Rudolph U, Birnbaumer L. Mouse gene knockout and knockin strategies in application to alpha subunits of Gi/Go family of G proteins. Methods Enzymol. 2002;344:277–298. doi: 10.1016/s0076-6879(02)44721-0. [DOI] [PubMed] [Google Scholar]
- 20.Zuberi Z, Birnbaumer L, Tinker A. The role of inhibitory heterotrimeric G-proteins in the control of in-vivo heart rate dynamics. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1822–R1830. doi: 10.1152/ajpregu.90625.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gellen B, Fernandez-Velasco M, Briec F, Vinet L, LeQuang K, Rouet-Benzineb P, Benitah JP, Pezet M, Palais G, Pellegrin N, Zhang A, Perrier R, Escoubet B, Marniquet X, Richard S, Jaisser F, Gomez AM, Charpentier F, Mercadier JJ. Conditional FKBP12. 6 overexpression in mouse cardiac myocytes prevents triggered ventricular tachycardia through specific alterations in excitation-contraction coupling. Circulation. 2008;117:1778–1786. doi: 10.1161/CIRCULATIONAHA.107.731893. [DOI] [PubMed] [Google Scholar]
- 22.Gohla A, Klement K, Piekorz RP, Pexa K, vom DS, Spicher K, Dreval V, Haussinger D, Birnbaumer L, Nurnberg B. An obligatory requirement for the heterotrimeric G protein Gi3 in the antiautophagic action of insulin in the liver. Proc Natl Acad Sci USA. 2007;104:3003–3008. doi: 10.1073/pnas.0611434104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danik S, Cabo C, Chiello C, Kang S, Wit AL, Coromilas J. Correlation of repolarization of ventricular monophasic action potential with ECG in the murine heart. Am J Physiol Heart Circ Physiol. 2002;283:H372–H381. doi: 10.1152/ajpheart.01091.2001. [DOI] [PubMed] [Google Scholar]
- 24.Nattel S, Maguy A, Le BS, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 25.Maguire CT, Wakimoto H, Patel VV, Hammer PE, Gauvreau K, Berul CI. Implications of ventricular arrhythmia vulnerability during murine electrophysiology studies. Physiol Genomics. 2003;15:84–91. doi: 10.1152/physiolgenomics.00034.2003. [DOI] [PubMed] [Google Scholar]
- 26.Salama G, London B. Mouse models of long QT syndrome. J Physiol. 2007;578:43–53. doi: 10.1113/jphysiol.2006.118745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudolph U, Spicher K, Birnbaumer L. Adenylyl cyclase inhibition and altered G protein subunit expression and ADP-ribosylation patterns in tissues and cells from Gi2 alpha−/− mice. Proc Natl Acad Sci USA. 1996;93:3209–3214. doi: 10.1073/pnas.93.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen F, Spicher K, Jiang M, Birnbaumer L, Wetzel GT. Lack of muscarinic regulation of Ca(2+) channels in G(i2)alpha gene knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2001;280:H1989–H1995. doi: 10.1152/ajpheart.2001.280.5.H1989. [DOI] [PubMed] [Google Scholar]
- 29.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1. 2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Yada H, Murata M, Shimoda K, Yuasa S, Kawaguchi H, Ieda M, Adachi T, Murata M, Ogawa S, Fukuda K. Dominant negative suppression of Rad leads to QT prolongation and causes ventricular arrhythmias via modulation of L-type Ca2+ channels in the heart. Circ Res. 2007;101:69–77. doi: 10.1161/CIRCRESAHA.106.146399. [DOI] [PubMed] [Google Scholar]
- 31.Arking DE, Pfeufer A, Post W, Kao WH, Newton-Cheh C, Ikeda M, West K, Kashuk C, Akyol M, Perz S, Jalilzadeh S, Illig T, Gieger C, Guo CY, Larson MG, Wichmann HE, Marban E, O’Donnell CJ, Hirschhorn JN, Kaab S, Spooner PM, Meitinger T, Chakravarti A. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38:644–651. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- 32.Chang KC, Barth AS, Sasano T, Kizana E, Kashiwakura Y, Zhang Y, Foster DB, Marban E. CAPON modulates cardiac repolarization via neuronal nitric oxide synthase signaling in the heart. Proc Natl Acad Sci USA. 2008;105:4477–4482. doi: 10.1073/pnas.0709118105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai CT, Wang DL, Chen WP, Hwang JJ, Hsieh CS, Hsu KL, Tseng CD, Lai LP, Tseng YZ, Chiang FT, Lin JL. Angiotensin II increases expression of alpha1C subunit of L-type calcium channel through a reactive oxygen species and cAMP response element-binding protein-dependent pathway in HL-1 myocytes. Circ Res. 2007;100:1476–1485. doi: 10.1161/01.RES.0000268497.93085.e1. [DOI] [PubMed] [Google Scholar]
- 34.Myles RC, Burton FL, Cobbe SM, Smith GL. The link between repolarisation alternans and ventricular arrhythmia: does the cellular phenomenon extend to the clinical problem? J Mol Cell Cardiol. 2008;45:1–10. doi: 10.1016/j.yjmcc.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht: Kluwer Academic Publishers; 2001. Cardiac Inotropy and Ca mismanagement; pp. 273–331. [Google Scholar]
- 36.January CT, Riddle JM, Salata JJ. A model for early afterdepolarizations: induction with the Ca2+ channel agonist Bay K 8644. Circ Res. 1988;62:563–571. doi: 10.1161/01.res.62.3.563. [DOI] [PubMed] [Google Scholar]
- 37.Nagata K, Ye C, Jain M, Milstone DS, Liao R, Mortensen RM. Galpha(i2) but not Galpha(i3) is required for muscarinic inhibition of contractility and calcium currents in adult cardiomyocytes. Circ Res. 2000;87:903–909. doi: 10.1161/01.res.87.10.903. [DOI] [PubMed] [Google Scholar]
- 38.Yutzey KE, Robbins J. Principles of genetic murine models for cardiac disease. Circulation. 2007;115:792–799. doi: 10.1161/CIRCULATIONAHA.106.682534. [DOI] [PubMed] [Google Scholar]
- 39.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 40.Frenneaux MP. Autonomic changes in patients with heart failure and in post-myocardial infarction patients. Heart. 2004;90:1248–1255. doi: 10.1136/hrt.2003.026146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pruvot E, Thonet G, Vesin JM, van-Melle G, Seidl K, Schmidinger H, Brachmann J, Jung W, Hoffmann E, Tavernier R, Block M, Podczeck A, Fromer M. Heart rate dynamics at the onset of ventricular tachyarrhythmias as retrieved from implantable cardioverter-defibrillators in patients with coronary artery disease. Circulation. 2000;101:2398–2404. doi: 10.1161/01.cir.101.20.2398. [DOI] [PubMed] [Google Scholar]
- 42.Pumprla J, Howorka K, Groves D, Chester M, Nolan J. Functional assessment of heart rate variability: physiological basis and practical applications. Int J Cardiol. 2002;84:1–14. doi: 10.1016/s0167-5273(02)00057-8. [DOI] [PubMed] [Google Scholar]
- 43.Welzig CM, Shin DG, Park HJ, Kim YJ, Saul JP, Galper JB. Lipid lowering by pravastatin increases parasympathetic modulation of heart rate: Galpha(i2), a possible molecular marker for parasympathetic responsiveness. Circulation. 2003;108:2743–2746. doi: 10.1161/01.CIR.0000103680.61390.16. [DOI] [PubMed] [Google Scholar]
- 44.Ruan H, Mitchell S, Vainoriene M, Lou Q, Xie LH, Ren S, Goldhaber JI, Wang Y. Gi alpha 1-mediated cardiac electrophysiological remodeling and arrhythmia in hypertrophic cardiomyopathy. Circulation. 2007;116:596–605. doi: 10.1161/CIRCULATIONAHA.106.682773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.