Abstract
The stromal vascular fraction of adipose tissue has gained popularity as a source of autologous progenitor cells for tissue engineering and regenerative medicine applications. The aim of this study was to validate a newly developed, automated procedure to isolate adipose-derived mesenchymal stem/stromal cells (ASCs) from adult human lipoaspirates in a closed and clinical-grade device, based on the Sepax® technology. Using a total of 11 donors, this procedure was compared with the standard operator-based manual separation in terms of isolation yield, clonogenic fraction, phenotype, and differentiation potential of ASCs. As compared with the manual process, automation resulted in a 62% higher isolation yield, with 2.6±1.2×105 nucleated cells per mL of liposuction, and a 24% higher frequency of clonogenic progenitors. The variability in the isolation yield and clonogenicity across different preparations was reduced by 18% and 50%, respectively. The cytofluorimetric profile and in vitro differentiation capacity into mesenchymal lineages were comparable in the cells isolated using the two procedures. The new Sepax-based process thus allows an efficient isolation of ASCs with higher and more reproducible yields than the standard manual procedure, along with minimal operator intervention. These results are expected to facilitate the use of ASCs for clinical purposes, either within an intraoperative setting or in combination with further in vitro cell expansion/cultivation.
Introduction
Mesenchymal stem/stromal cells (MSCs), a rare population of nonhematopoietic stromal cells, were initially defined within the rodent bone marrow,1 as the adherent population on tissue culture plastic and by their expression of various molecules, including CD90, CD105, and CD73, and the absence of markers like CD34, CD45, and CD14.2,3 Upon adherence, these MSCs have the capacity to form clones, defined as colony-forming unit–fibroblasts (CFU-fs), and to extensively proliferate. MSCs are able to differentiate into mesenchymal lineages and thus generate bone, cartilage, adipose, and muscle tissues. Such properties have made them a promising tool for cell-based tissue repair and tissue engineering approaches.4
Cells with properties similar to bone-marrow-derived MSCs were later derived from other tissues and organs, including brain and muscle,5 skin,6 or adipose.7 Indeed, adipose tissue, when digested with collagenase and centrifuged to remove differentiated adipocytes floating in the aqueous phase, forms a cellular pellet made of a highly heterogeneous population of cells, typically referred to as the stromal vascular fraction (SVF) and includes fibroblastic colony-forming cells, vascular/endothelial cells, erythrocytes, and other hematopoietic cells. These SVF cells are either freshly used for therapeutic applications8,9 or seeded onto tissue culture plastic in order to select the adherent population and then expanded to generate what is generally referred to as adipose derived mesenchymal stem/stromal cells (ASCs). ASCs share several characteristics of bone marrow MSCs and recently became, due to their ease of harvest and availability, a cell source raising great scientific and clinical interest. Numerous preclinical studies, defining various potential applications for ASCs in human therapy and clinical applications, have indeed documented the ability of ASCs to repair not only mesodermal tissues, but also ectodermal and endodermal tissues or organs, in the field of gastroenterology, neurology, orthopedics, reconstructive surgery, and related clinical disciplines (reviewed in Refs.10,11). The first clinical trials with SVF cells and ASCs are ongoing, in the form of phase I (e.g., myocardial infarction, skin ulcer, or graft versus host disease), phase II (e.g., in rectovaginal fistula), phase III (e.g., enterocutaneous fistula), and phase IV (e.g., breast reconstruction) studies.10,11 Such clinical trials in humans require the supply of clinical grade, generally autologous, SVF cells. The preferable solution to provide such cells is to process adipose tissue in a Good Manufacturing Practice (GMP) facility. For that reason, clinical centers aiming to apply adipose-cell-based therapies require access to such a GMP facility, supported by a highly specialized staff of technicians and qualified persons. This greatly limits the potential applications of adipose-cell-based therapies to larger clinical centers capable of housing such facilities and thus results in a cost-ineffective therapeutic approach. The development of closed, aseptic, and automated devices would allow for the isolation of SVF cells outside of a GMP facility, for instance, directly inside an operating theater, thus reducing such current limitations as cost effectiveness and operator intervention and error.
Automated cell isolation systems are currently being developed by several groups to facilitate clinical implementation of cell-based therapies. Among these, the CE-marked device Sepax® (Biosafe SA) has been previously developed to isolate and to concentrate nucleated cells from umbilical cord, peripheral, or bone marrow blood.12,13 In this study, we aimed to validate a newly developed automated procedure based on the Sepax technology to isolate SVF cells from human adipose tissue in a closed, clinical-grade setting. To validate this new procedure, we compared it with the standard operator-based manual separation of the SVF cells in terms of isolation yield, cytofluorimetric profile, and differentiation capacity into mesenchymal lineages. This study was performed independently in two research centers to confirm the reproducibility of the process.
Materials and Methods
Tissue source
Adipose tissue, in the form of tumescent liposuction samples from subcutaneous abdominal fat, was obtained from 11 healthy female donors (age 20–65) following informed consent and according to a protocol approved by the local ethical committee (EKBB, Ref. 78/07 and 2006-192N-MA [extended in 2009]). Liposuction samples were centrifuged and concentrated to remove tumescent solution immediately in the operating room. The same liposuction samples were used as starting material in parallel for the two isolation procedures as described below.
Manual isolation of the cells
Adipose samples were digested for 60 min at 37°C in 0.15% (W/V) collagenase NB 6 GMP Grade from Clostridium histolyticum (0.12 U/mg collagenase; SERVA Electrophoresis GmbH) diluted in phosphate-buffered saline (PBS; Gibco). After centrifugation at 190 g for 10 min, the lipid-rich layer was discarded and the cellular pellet was washed once with PBS. For analysis, red blood cells were lysed by incubation for 2 min in a solution of 0.15 M ammonium chloride, 1 mM potassium hydrogen carbonate (both from Merck; www.merck-chemicals.com), and 0.1 mM EDTA (Fluka Analytical, Sigma-Aldrich Chemie GmbH). The resulting SVF cells were then resuspended in complete medium (CM), consisting of alpha-minimum essential medium (α-MEM) supplemented with 10% of fetal bovine serum (FBS), 1% HEPES, 1% sodium pyruvate, and 1% of penicillin-streptomycin-glutamin (100×) solution (all from Gibco). For the experiments testing proliferation rate, cells were expanded in Dulbecco's minimal essential medium (DMEM) containing 10% FBS or 10% human AB serum (HS).14
Sepax-based automated isolation of the cells
Liposuction samples were introduced in 400 mL transfer bags (Terumo) and NB 6 GMP Grade collagenase diluted in PBS was added at a final concentration of 0.15% (W/V). The bag was placed into an incubator at 37°C for 60 min. After digestion (Fig. 1), the bag was connected to a CS-490.1 kit (Biosafe SA) specifically modified for this purpose and the kit was installed on a Sepax device (Biosafe SA; www.biosafe.ch) for automated digestion. After priming and straining of the digested adipose tissue into the separation chamber, a centrifugation step was performed to remove the supernatant. It was followed by a washing procedure of the pellets with saline solution. When more than 200 mL of adipose was processed, a two-phase digestion by the machine was necessary, which slightly increased the time for procedure. For cell collection, the system diluted the residual pellets (≈10 mL) and extracted the content into the final bag, and then automatically rinsed the chamber twice for an optimal cell recovery. The minimum final volume used was 40 mL. The re-suspension medium was as described for the manual isolation process.
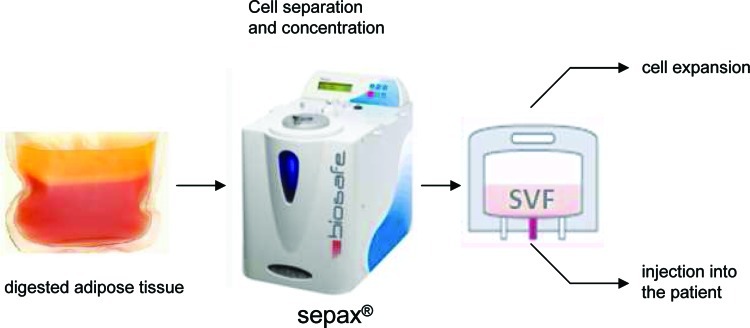
FIG. 1.
Schematic description of the isolation process. The adipose tissue sample is digested with collagenase in a transfer bag. The bag is connected to the Sepax kit and the kit is installed on the device. The output bag contains the stromal vascular fraction (SVF) that can be used for in vitro expansion or infusion into a patient. Color images available online at www.liebertonline.com/tec
Cell count and characterization by flow cytometry
Nucleated cells were counted using a Neubauer chamber after staining with Crystal Violet (Sigma), whereas cell viability was determined by counting the blue (dead) and transparent (alive) cells after trypan blue (Sigma) staining. The phenotype of SVF cells was determined by cytofluorimetric analysis with fluorochrome-conjugated antibodies to human CD105 (from AbD Serotec), CD90, CD31, CD34, CD45, or CD73. Isotype IgGs were used as control (all from BD Biosciences). About 105 cells in suspension per condition were incubated for 30 min with the different antibodies at 4°C in CM, washed with PBS, resuspended in FACS buffer (PBS, 0.5% human serum albumin, and 0.5 mM EDTA), and analyzed with an FACSCalibur flow cytometer (BD Biosciences). 7-Amino-actinomycin D (7-AAD) was used to determine the number of dead cells inside the isolated SVF cell population. Expanded ASCs were analyzed after the first passage, investigating 7-AAD-negative cells for expression of CD105 (eBioscience), CD144 (Beckman Coulter), CD44, CD29 (Biolegend), CD90, and CD73 (BD Biosciences).
Cell culture and colony-forming assays
For monolayer expansion, SVF cells were seeded at a density of 2×103 cells/cm2 onto tissue culture plates, cultured in CM supplemented with 5 ng/mL fibroblast growth factor-2 (FGF-2; R&D Systems), and serially replated in new dishes at a density of 3×103 cells/cm2 upon subconfluence. To compare the effects of FBS and HS supplementation, cells were serially replated at a density of 200 cells/cm2. Cumulative population doublings (PD) were calculated using the formula: PD=[log10(NH)−log10(N1)]/log10(2), where N1 is the number of seeded cells and NH is the cell number counted after expansion.15 The number of CFU-fs was determined by plating 5×102 SVF cells per 78 cm2 Petri dishes. Cells were cultured for 10–14 days in CM containing 5 ng/mL FGF-2. After fixation with 4% formalin for 10 min and staining with crystal violet, the colonies consisting of at least 40 cells were counted and the number of colonies was normalized to the number of plated cells.
Osteoblastic differentiation assays
The osteoblastic differentiation capacity of SVF cells was tested after 3 weeks of culture in α-MEM supplemented with 10 mM β-glycerophosphate, 10 nM dexamethasone, and 0.05 M ascorbic acid. Cells cultured with CM containing FGF-2 were used as a control. Deposition of mineralized matrix was detected by a 2% alizarin red S staining, as previously described,16 or by quantification of hydroxyapatite deposition using the Osteoimage Mineralization Assay (Lonza), following manufacturer's instructions.
Chondrocytic differentiation assay
The chondrocytic differentiation capacity of SVF cells was investigated in pellet culture by using a chemically defined, serum-free medium, consisting of DMEM containing 4.5 mg/mL D-glucose, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 10 mM HEPES buffer, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.29 mg/mL L-glutamine (all from Gibco) further supplemented with ITS+1 (10 μg/mL insulin, 5.5 mg/mL transferrin, 5 ng/mL selenium, 0.5 mg/mL bovine serum albumin, and 4.7 mg/mL linoleic acid), 1.25 mg/mL human serum albumin, 0.1 mM ascorbic acid 2-phosphate, and 10−7 M dexamethasone (all from Sigma), and 10 ng/mL TGF-β1 (R&D).17 Aliquots of 5×105 SVF cells were centrifuged at 250 g for 5 min in 1.5 mL polypropylene conical tubes (Saarstedt) to form spherical pellets, which were placed onto a 3D orbital shaker (Bioblock Scientific) at 30 rpm. Pellets were cultured for 2 weeks at 37°C/5% CO2. Some pellets were fixed and paraffin embedded, and then 5 μm sections were serially stained with hematoxylin, Fast Green, and safranin-O as previously described.17 To quantify the sulfated glycosaminoglycan (GAG) content, other pellets were digested with a proteinase K solution, incubated with a dimethylmethylene blue dye, and read spectrophotometrically, with chondroitin sulfate as a standard, as previously described.18 The GAG content was normalized to the deoxyribonucleic acid (DNA) amount that was measured using a CyQUANT cell proliferation assay kit (Molecular Probes), with calf thymus DNA as a standard.
Adipocytic differentiation assay
The adipocytic differentiation was induced by culture of SVF cells on tissue culture plastic, which upon confluence were treated with 10 μg/mL insulin, 1 μM dexamethasone, 100 μM indomethacin, and 0.5 mM 3-isobutyl-1-methylxanthine for 72 h and with 10 μg/mL insulin for 24 h as previously described.17 The 96-h treatment cycle was repeated three times. These adipo-inductive supplements were added to α-MEM containing 10% FBS. At the end of induction, cells were fixed with 4% formalin, stained with oil red O dye (Sigma), and observed with a phase-contrast microscope.
Results
Liposuction samples, in volumes ranging from 40 to 400 mL, were processed in parallel by following either the standard manual procedure described previously16 or the newly developed automated procedure based on the Sepax technology (Fig. 1). Both processes were typically completed within 1.5–2 h, depending on the initial volume of input and including 1 h for the digestion of the sample by collagenase.
Isolation yields and phenotype
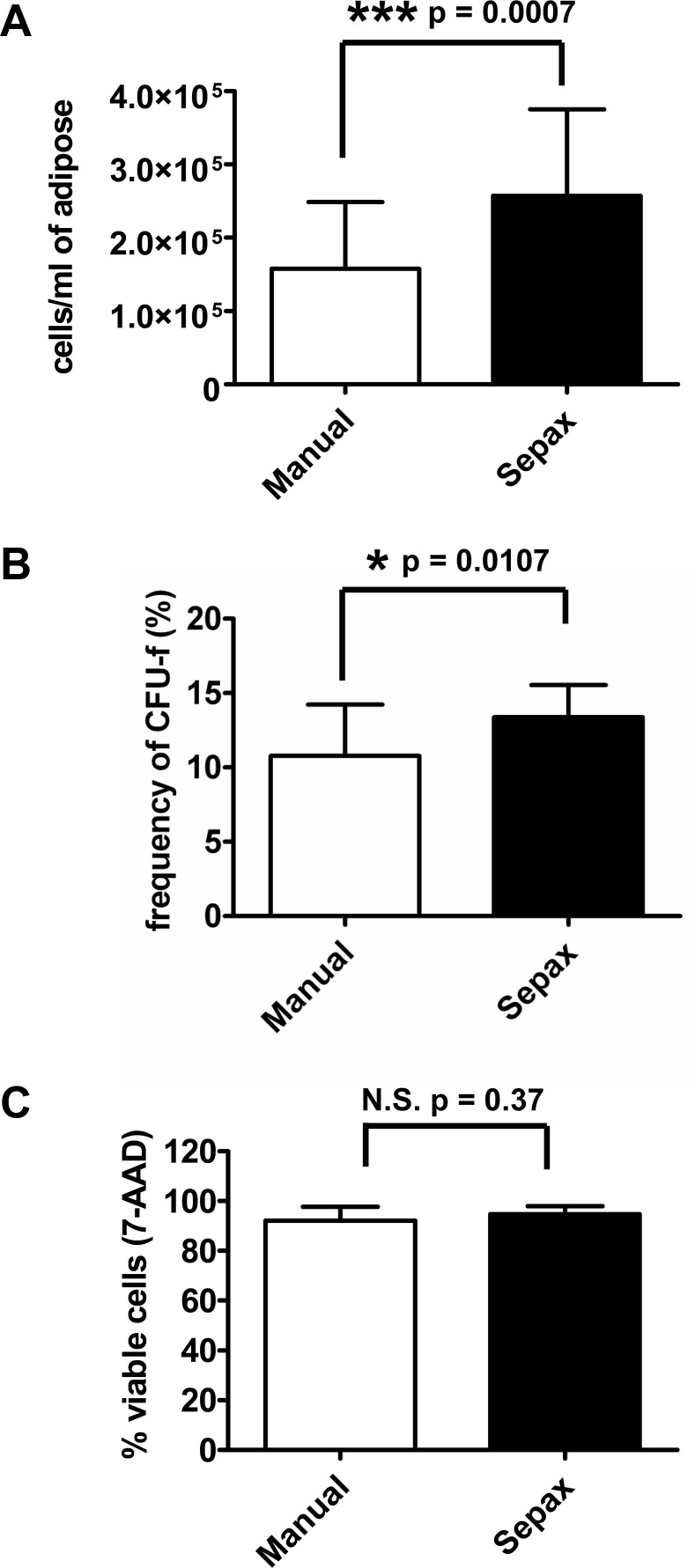
The number of nucleated cells from SVF in the final suspension was evaluated after both procedure types and normalized to the volume of liposuction input processed. The manual procedure yielded 1.6±0.9×105 nucleated cells/mL of liposuction, whereas the Sepax-based procedure isolated 2.6±1.2×105 cells/mL of liposuction (n=6; Fig. 2A). This represents a 62% increase in isolated nucleated cells, and the difference was highly significant as tested by t-test. The yield in terms of isolation of CFU-fs was 24% higher with Sepax (Fig. 2B), which represented a limited but significant difference (n=18, triplicate determinations performed for six donors). The variability in the isolation yield and clonogenicity, that is, the standard deviation as a percentage of the mean, was always lower with Sepax, with a reduction of 18% and 50%, respectively, as compared with the manual procedure. The viability of isolated cells was more than 90% in both conditions as tested by trypan blue and confirmed by 7-AAD staining performed on five donors (Fig. 2C).
FIG. 2.
Isolation yield, clonogenicity, and viability of SVF cells from liposuction samples processed by manual or Sepax procedure. (A) The total number of nucleated cells isolated was counted by using a Neubauer chamber after crystal violet staining and normalized to the volume of the liposuction sample processed. Results are expressed as mean±SD of cell number per mL of processed adipose from six independent donors. (B) The SVF cells obtained from six different donors were plated in triplicate at clonal density (10 cells/cm2) and the cells were allowed to grow colonies, which were counted after staining by crystal violet and normalized to the number of seeded cells. Results are expressed as mean±SD of the colony-forming unit–fibroblast (CFU-f) frequency in the SVF cell. (C) A sample of the SVF derived from five donors was analyzed by cytofluorimetry for 7-amino-actinomycin D (7-AAD). Results are presented as the mean±SD of the percentage of the viable, 7-AAD-negative, cells. Differences were statistically assessed by paired t-tests. N.S., no statistically significant difference.
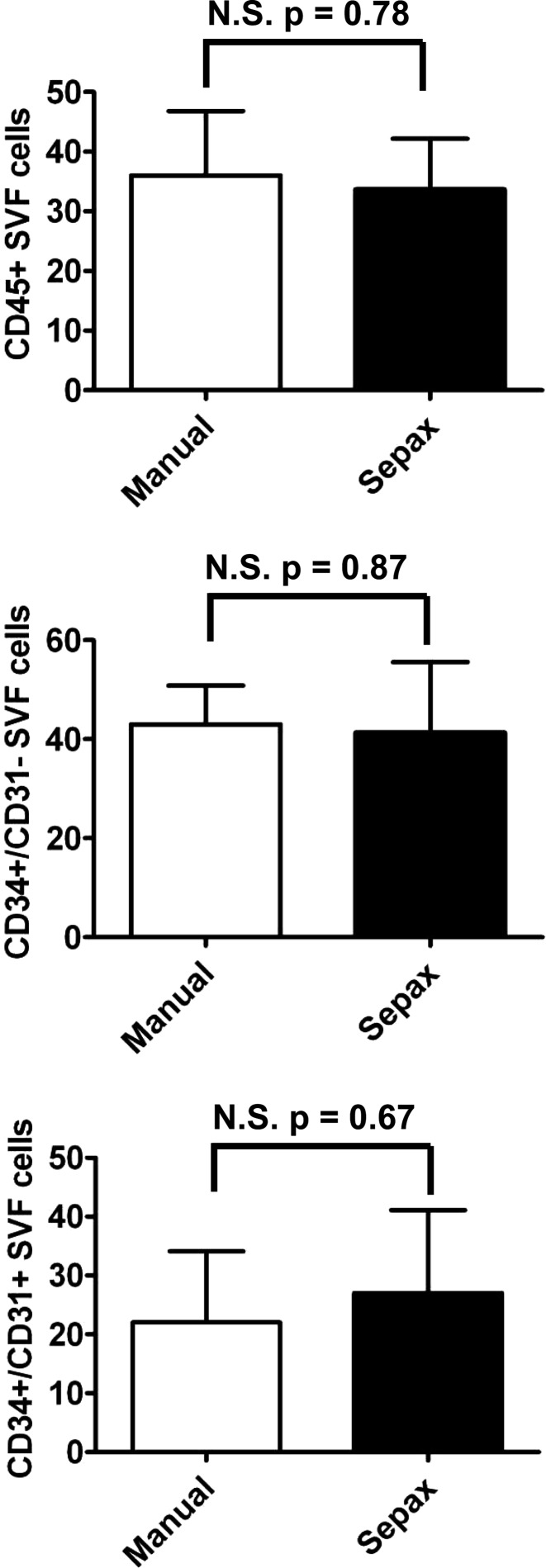
The SVF is composed of a highly heterogeneous population of cells.19 To investigate whether the two procedures led to the isolation of specific subpopulations of cells of interest, the different cell types inside the SVF were analyzed by cytofluorimetry, by using a set of markers that we recently validated.20 Based on this set of markers, we identified the presence of about 35% of hematopoietic cells (CD45-positive cells); 40% of CD34+/CD31− cells, previously shown to contain the CFU-fs and the pericytic populations within SVF cells; and 25% of CD34+/CD31+ cells, previously characterized as endothelial cells (Fig. 3). No significant enrichment in any of the three populations between the two experimental conditions was observed and paired t-tests showed no significant difference of cell types between the two isolation procedures (n=3). To complete this analysis, the percentages of SVF cells expressing CD31, CD34, CD45, CD73, CD90, or CD105 after the manual procedure were normalized by the respective percentages for each marker after Sepax procedure. For every marker, this ratio was not significantly different from 1 (a ratio of 1 is indicative of the same percentage of cells expressing a marker with both isolation techniques), as tested by Wilcoxon signed rank test (n=5 different donors).
FIG. 3.
Cytofluorimetric analysis of SVF cells obtained from liposuction samples processed by manual or Sepax procedure. SVF cells obtained from three different donors were analyzed by cytofluorimetry for the expression of CD45 (top graph) and CD34/CD31 (middle and bottom graphs). Results are expressed as mean±SD of the positivity for the indicated markers. N.S. in percentage of cells was found between the two procedures as tested by paired t-tests.
Expansion and differentiation potential
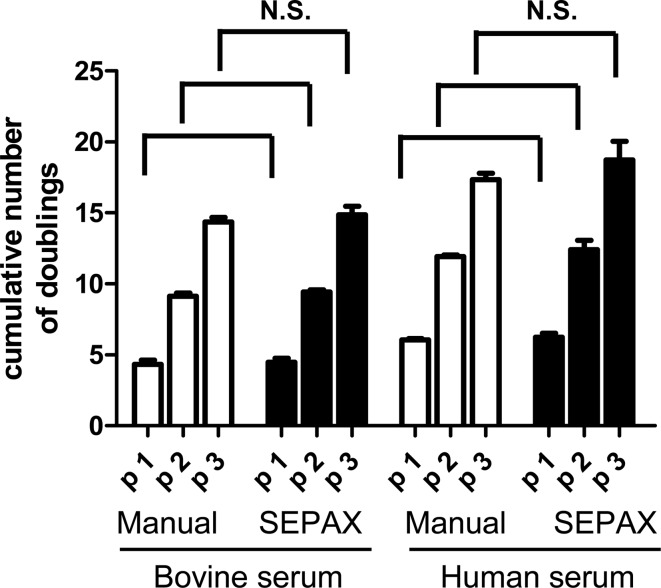
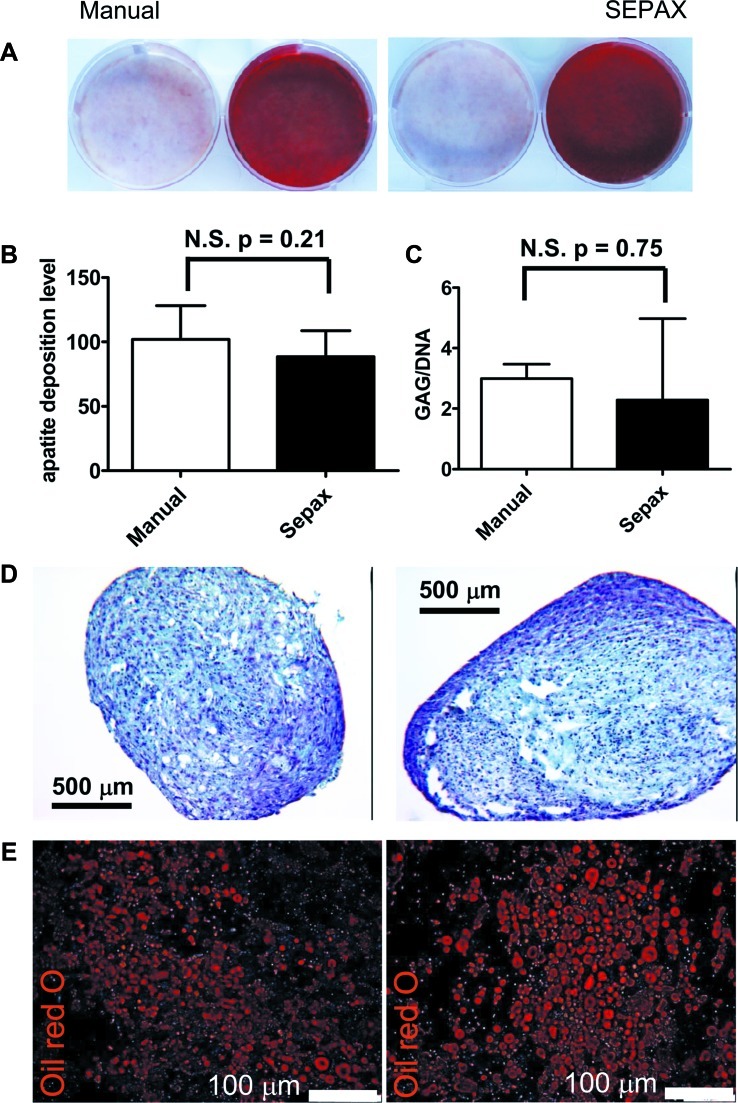
The rate of proliferation of ASCs derived from the two isolation procedures was identical when expanded as monolayer on tissue culture plastic in either FBS or HS, from the first to the third passage (Fig. 4). The previously demonstrated trend showing higher number of ASCs with HS than with FBS at the different passages14 was valid similarly for both the manual and Sepax procedures. For both isolation procedures, until the third passage, the viability of ASCs was higher than 97%±1% (n=3) as indicated by the percentage of 7-AAD-negative cells (data not shown). These cells were characteristic ASCs in every experimental condition tested, as shown by >99% positivity for the mesenchymal markers CD29, CD44, CD73, CD90, and CD105 and absence of hematopoietic markers (data not shown). The capacity of ASCs to differentiate into the three mesenchymal lineages was assessed. Following osteoblastic induction, ASCs derived from both isolation types were able to produce matrix in vitro and to mineralize it, as evidenced by a strong positive staining for alizarin red (Fig. 5A). Apatite production by these cells was also evaluated quantitatively, and demonstrated no significant difference between manual- and Sepax-isolated ASCs (Fig. 5B). ASCs poorly differentiated into the chondrocytic lineage in pellet cultures, as assessed by safranin-O staining (Fig. 5D) for GAG and consistent with previous studies.21,22 Quantification of GAG, normalized to DNA content, confirmed a limited GAG deposition but demonstrated no difference between the two isolation processes (Fig. 5D). After adipocytic induction, both types of ASCs differentiated into adipocytes and exhibited large cytoplasmic lipid droplets, as stained by oil red O (Fig. 5E).
FIG. 4.
Expansion of adipose-derived mesenchymal stem/stromal cells (ASCs) derived from liposuction samples processed by manual or Sepax procedure. ASCs were grown in different media, as indicated, and the cumulative number of cell population doublings was established at every passage. N.S. in growing rates was found between the two procedures using either serum source, as tested by ANOVA with Tukey's post hoc tests.
FIG. 5.
Differentiation into mesodermal lineages of SVF cells obtained from liposuction samples processed by manual or Sepax procedure. (A) Alizarin red staining after manual (left picture) and Sepax (right picture) procedures; SVF cells were grown to subconfluence and induced with either standard medium (left well) or osteoblastic induction medium (right well) for 3 weeks. (B) Apatite deposition by ASCs obtained either by manual isolation or by Sepax and cultured for 3 weeks with osteoblastic induction medium quantified by using the Osteoimage kit. Results are expressed as mean±SD of 10 fluorescence intensity values obtained from three independent donors. No significant difference in fluorescence intensity was found between the two procedures as tested by unpaired t-test. (C) GAG/DNA quantification and (D) safranin-O staining on sections of samples from ASCs obtained either by manual isolation (left) or by Sepax (right) and cultured for 2 weeks with chondrogenic induction medium. (E) Fluorescence microscopy pictures after oil red O staining of ASCs obtained either by manual isolation (left) or by Sepax (right) and cultured for 2 weeks with adipogenic induction medium. GAG, glycosaminoglycan. Color images available online at www.liebertonline.com/tec
Discussion
In this study, we validated a new automated procedure to isolate SVF cells from human adipose tissue samples based on the Sepax technology and compared it with the current standard, namely, the manual isolation of SVF. The automated procedure resulted in higher and more reproducible isolation yields of SVF cells, with phenotypic and functional characteristics similar to those isolated by the manual procedure.
The isolation yield of SVF cells not only depends on how the tissue is processed, but also on various factors, including interdonor variability, anatomical harvest site, aspiration procedure, storage time, sample processing prior to digestion (e.g., washing or concentration by centrifugation), and concentration/type of enzyme used. Those factors explain the high variability of yields described in the literature, from as low as 4×104 cells/mL23 to around 3×105 cells/mL of adipose tissue.24 Another factor affecting yield variability is whether the adipose tissue is in the form of a solid resection or a liposuction sample, whether or not tumescent solution was used for liposuction, and/or whether or not centrifugation is performed after collection. All of which greatly modify the characteristics of the source material used for isolation. Typically, our standard manual isolation procedure results in average yields of 1.5–2×105 cells/mL of adipose tissue. To avoid these intradonor (liposuction vs. resection) and technical sources of variability, we divided a given tissue sample into two equal fractions that were then processed via the automated or manual procedure. The average isolation yield achieved with Sepax was in the range of 2.6×105 nucleated cells per mL of liposuction sample. This average yield is comparable with the previously described Celution™ system from Cytori Therapeutics,25 for which an average yield of 2.9×105 cells per mL of adipose was reported. The reported frequency of CFU-fs in SVF also varies in the literature, ranging from 0.3% to 15%, likely due to different clonal densities of seeding, different media used, or a discrepancy in the definition of a CFU-f colony.8,24,26 Frequencies between 10% and 15% of CFU-fs are reported here, with only a slight difference between manual and automated processing for a given donor. The variability seen in the manual processing was reduced with the automated procedure, likely due to a better standardized performance of the critical steps (e.g., removal of the supernatant after centrifugation).
The investigation of the phenotypical and functional characteristics of the isolated cells showed no difference between ASCs isolated manually or by Sepax. We recently identified CD34+/CD31− as the population containing CFU-fs and pericytic cells within SVF, with CD34+/CD31+ and CD45+ cells as the endothelial and hematopoietic fractions, respectively.20 No difference between manual and Sepax processing was observed in the percentage of the different cell populations or in the expression of other mesenchymal markers (CD73+ or CD90+, data not shown). From a functional standpoint, ASCs from SVF proliferated and differentiated similarly toward the three mesenchymal lineages (adipo-, osteo-, and chondrocytic), independently of the isolation procedure. The results of the present study therefore validate Sepax as an automated alternative to manual processing of adipose tissue, leading to higher yields of SVF cells of comparable quality.
The potential benefit of the automated device/process is manifold. (1) For research purposes, it could help to standardize and simplify the processing of adipose material. This could allow for a better comparison of the results generated in different experimental runs or research laboratories. (2) For clinical purposes, the presented approach would enable the direct coupling of the technology with another bioreactor system for cell expansion/culture, resulting in a streamlined and perhaps fully automated approach27 to generate adipose-cell-based grafts. (3) Alternatively, the Sepax system may be employed for intraoperative transplantation of SVF cells. This approach is already in clinical use for cell-assisted lipotransfer in breast augmentation and breast reconstruction,9 but so far relies on a manual isolation of the SVF cells.28 In this latter context, our group is currently planning to clinically test the efficacy of osteogenic/vasculogenic grafts8 intraoperatively generated by cells automatedly isolated from autologous adipose tissue for bone defect repair in osteoporotic patients.
Acknowledgments
The authors acknowledge financial support by the Swiss National Science Foundation (Grant 310030-120432) to A.S. and the 7th EU-Framwork programs “CASCADE” (No. 223 236) to K.B. We thank Benjamin Pippenger (Basel, Switzerland) for reviewing the article for English language.
Disclosure Statement
The present study was partially funded and supported by Biosafe SA (grant to I.M. and free supply of a Sepax device and related kits).
References
- 1.Friedenstein A.J. Petrakova K.V. Kurolesova A.I. Frolova G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230. [PubMed] [Google Scholar]
- 2.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 3.Caplan A.I. Bruder S.P. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 4.Parekkadan B. Milwid J.M. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang Y. Vaessen B. Lenvik T. Blackstad M. Reyes M. Verfaillie C.M. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30:896. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 6.Gorio A. Torrente Y. Madaschi L. Di Stefano A.B. Pisati F. Marchesi C. Belicchi M. Di Giulio A.M. Bresolin N. Fate of autologous dermal stem cells transplanted into the spinal cord after traumatic injury (TSCI) Neuroscience. 2004;125:179. doi: 10.1016/j.neuroscience.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 7.Zuk P.A. Zhu M. Mizuno H. Huang J. Futrell J.W. Katz A.J. Benhaim P. Lorenz H.P. Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 8.Muller A.M. Mehrkens A. Schafer D.J. Jaquiery C. Guven S. Lehmicke M. Martinetti R. Farhadi I. Jakob M. Scherberich A. Martin I. Towards an intraoperative engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. Eur Cell Mater. 2010;19:127. doi: 10.22203/ecm.v019a13. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura K. Sato K. Aoi N. Kurita M. Hirohi T. Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gimble J.M. Guilak F. Bunnell B.A. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010;1:19. doi: 10.1186/scrt19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizuno H. Adipose-derived stem and stromal cells for cell-based therapy: current status of preclinical studies and clinical trials. Curr Opin Mol Ther. 2010;12:442. [PubMed] [Google Scholar]
- 12.Aktas M. Radke T.F. Strauer B.E. Wernet P. Kogler G. Separation of adult bone marrow mononuclear cells using the automated closed separation system Sepax. Cytotherapy. 2008;10:203. doi: 10.1080/14653240701851324. [DOI] [PubMed] [Google Scholar]
- 13.Zinno F. Landi F. Scerpa M.C. Aureli V. Lanti A. Ceccarelli S. Caniglia M. Miele M.J. Daniele N. Landolfo A. Cometa A.M. Locatelli F. Isacchi G. Processing of hematopoietic stem cells from peripheral blood before cryopreservation: use of a closed automated system. Transfusion. 2011;51:2656. doi: 10.1111/j.1537-2995.2011.03180.x. [DOI] [PubMed] [Google Scholar]
- 14.Kocaoemer A. Kern S. Kluter H. Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 2007;25:1270. doi: 10.1634/stemcells.2006-0627. [DOI] [PubMed] [Google Scholar]
- 15.Cristofalo V.J. Allen R.G. Pignolo R.J. Martin B.G. Beck J.C. Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc Natl Acad Sci U S A. 1998;95:10614. doi: 10.1073/pnas.95.18.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherberich A. Galli R. Jaquiery C. Farhadi J. Martin I. Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells. 2007;25:1823. doi: 10.1634/stemcells.2007-0124. [DOI] [PubMed] [Google Scholar]
- 17.Barbero A. Ploegert S. Heberer M. Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- 18.Miot S. Gianni-Barrera R. Pelttari K. Acharya C. Mainil-Varlet P. Juelke H. Jaquiery C. Candrian C. Barbero A. Martin I. In vitro and in vivo validation of human and goat chondrocyte labeling by green fluorescent protein lentivirus transduction. Tissue Eng Part C Methods. 2010;16:11. doi: 10.1089/ten.TEC.2008.0698. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerlin L. Donnenberg V.S. Pfeifer M.E. Meyer E.M. Peault B. Rubin J.P. Donnenberg A.D. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guven S. Mehrkens A. Saxer F. Schaefer D.J. Martinetti R. Martin I. Scherberich A. Engineering of large osteogenic grafts with rapid engraftment capacity using mesenchymal and endothelial progenitors from human adipose tissue. Biomaterials. 2011;32:5801. doi: 10.1016/j.biomaterials.2011.04.064. [DOI] [PubMed] [Google Scholar]
- 21.Hennig T. Lorenz H. Thiel A. Goetzke K. Dickhut A. Geiger F. Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 22.Winter A. Breit S. Parsch D. Benz K. Steck E. Hauner H. Weber R.M. Ewerbeck V. Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 23.Aust L. Devlin B. Foster S.J. Halvorsen Y.D. Hicok K. du Laney T. Sen A. Willingmyre G.D. Gimble J.M. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell J.B. McIntosh K. Zvonic S. Garrett S. Floyd Z.E. Kloster A. Di H.Y. Storms R.W. Goh B. Kilroy G. Wu X. Gimble J.M. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 25.Lin K. Matsubara Y. Masuda Y. Togashi K. Ohno T. Tamura T. Toyoshima Y. Sugimachi K. Toyoda M. Marc H. Douglas A. Characterization of adipose tissue-derived cells isolated with the Celution system. Cytotherapy. 2008;10:417. doi: 10.1080/14653240801982979. [DOI] [PubMed] [Google Scholar]
- 26.Fraser J.K. Wulur I. Alfonso Z. Hedrick M.H. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Martin I. Smith T. Wendt D. Bioreactor-based roadmap for the translation of tissue engineering strategies into clinical products. Trends Biotechnol. 2009;27:495. doi: 10.1016/j.tibtech.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura K. Shigeura T. Matsumoto D. Sato T. Takaki Y. Iba-Kojima E. Sato K. Inoue K. Nagase T. Koshima I. Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]