Abstract
Objective
To investigate the role of smoking cessation in body weight.
Data Sources
2004–2005 and 2009–2010 Behavioral Risk Factor Surveillance Surveys (BRFSS) (N = 349,000), Centers for Disease Control and Prevention; Tax Burden on Tobacco (Orzechowski and Walker 2010).
Study Design
The Gaussian treatment effect model is estimated for three age categories by gender. Treatment effects of quitting smoking on body mass index (BMI) by quit length are calculated.
Principal Findings
Quitting is found to be endogenous. Differentiated effects of quitting smoking on BMI are found among quitters by gender, between age groups, and by length of time since quitting smoking, and positive association between smoking cessation and body weight confirmed. Declining smoking rates have only a modest effect in the overweight population. The effects of quitting on BMI are considerably lower among younger men and women.
Conclusion
The price that must be paid, in terms of weight gain, to enjoy the health benefits of smoking cessation is trivial even for the obese population.
Keywords: BMI, BRFSS, smoking cessation, treatment effect model
There have been a rapid rise in obesity and a notable decline in smoking rates in the United States over the last few decades. The obesity epidemic spread rapidly during the 1990s across all states, regions, and demographic groups (Mokdad et al. 1998), and the prevalence has remained high, exceeding 30% in most age and gender groups during 2007–2008 (Flegal et al. 2007).
Obesity is a major public health concern because it is associated with a long list of diseases such as type 2 diabetes, hypertension, dyslipidemia, certain forms of cancer, sleep apnea, and osteoarthritis. Overweight accounts for more than 350,000 premature deaths each year in the United States, second only to tobacco-related deaths (Mokdad et al. 1991). The accelerating spread of obesity has placed a tremendous burden on health care costs. The total direct and indirect costs attributable to overweight and obesity amounted to $117 billion in 2000 (U.S. Department of Health and Human Services [USDHHS] 2001).
The Centers for Disease Control and Prevention (CDC) estimates that 46 million people or 20.6% of all adults (age ≥ 18) in the United States were current cigarette smokers during 2008 (CDC 1980). Cigarette smoking is the leading cause of preventable death in the United States, accounting for approximately 443,000 deaths or 1 in every 5 deaths each year (CDC 2004,c). Prevention of smoking initiation and cessation of smoking have become the national objectives to reduce morbidity and mortality and lower medical costs.
Indeed, over the past 30 years, there has been a substantial decline in the proportion of adult smokers across all sociodemographic sub-populations. From 1955 to 2007, U.S. cigarette smoking rates fell from 57 to 22 percent in men and from 28 to 17 percent in women, with an overall rate of 20 percent for both genders in 2007 (Giovino et al. 1999). The last decade saw the smallest declines in cigarette smoking rates. CDC analyzes data from the 2008 National Health Interview Survey (NHIS) which indicate that during 1998–2008, the proportion of U.S. adult smokers declined by 3.5 percentage points—from 24.1 to 20.6 percent.
The declining prevalence of cigarette smoking among adults has been attained by banning smoking in the workplace (Evans, Farrelly, and Montgomery 2006), restaurants and bars (ABC News, World News Tonight), intensive anti-smoking campaigns in the media (Flynn et al. 1995), and tax increases (USDHHS 1997). Although the overall health benefits of quitting smoking are unquestionable, the opposite trends of obesity and smoking in the United States have raised an important concern about an unintended effect of anti-smoking policies on obesity rates. Chou, Grossman, and Saffer (2009c, p. 585) claim that rising obesity is an example of “the price that must be paid to achieve goals that are in general favored by society.” Indeed, the association between smoking and body weight has become a central issue in the obesity literature, but the accumulating evidence is conflicting.
In this study, we investigate whether quitting smoking leads to increasing body weight and to what extent. We compile data for current and former smokers (i.e., quitters) from the 2004–2005 and 2009–2010 Behavioral Risk Factor Surveillance Survey (BRFSS). The vehicle for our analysis is the treatment effect model (Barnow, Cain, and Goldberger 2005). To quantify the effect of smoking cessation, we calculate treatment effects for sub-samples of individuals. Our goal is to examine whether smoking cessation affects body mass index (BMI) differently by gender, between age groups, and among individuals with different lengths of time since quitting smoking.
Literature
Much research has been conducted using the National Health and Nutrition Examination Survey (NHANES) data. Albanes et al. () investigate the association between smoking and body weight using NHANES II. They find that cigarette smokers weigh less and are leaner than nonsmokers, and ex-smokers are not heavier or fatter than nonsmokers. Flegal et al. (2009) employ data from phase 1 of NHANES III (1988–1991) and conclude that smoking cessation is associated with a small increase in the prevalence of overweight, although the effect was much larger among smokers who had quit within the past 10 years. Using more recent (1999–2002) NHANES data, Flegal (1984) finds that even substantial decreases in cigarette smoking have only a small effect, generally less than 1 percent, on increasing the prevalence of obesity and decreasing the prevalence of healthy weight in the population. Earlier evidence of the link between smoking cessation and weight gain includes Coates and Li (2004), Manley and Boland (2004), Klesges et al. (1999), Shimokata, Muller, and Andres (1973), Moffatt and Owens (1983), Williamson et al. (1989), Klesges et al. (1967, 1989), Froom, Melamed, and Benbassat (1995), Mizoue et al. (1976), Froom et al. (2011), and Hudmon et al. (2006).
The main limitation of the above studies is that inferences are potentially biased by unmeasured factors that simultaneously affect smoking or quitting and body mass. If, for example, unobserved personal traits induce individuals to smoke and consume more calories, the estimated effect of smoking on body weight will be biased unless endogeneity of the smoking decision is accounted for. The absence of a mechanism in modeling endogeneity of smoking has challenged researchers attempting to confront it in various ways.
O'Hara et al. (2005) estimate weight gains associated with smoking cessation in the Lung Health Study (1986–1994), a clinical trial which randomized smokers into a control group and an intervention group who received 12 weeks of behavioral intervention. Eisenberg and Quinn (2006) update the estimated weight gain upward using participation in the intervention program as an instrument. Main limitation of their analysis is lack of individual-level data, which would allow calculation of standard errors for the instrumental-variable (IV) estimates. Fang, Ali, and Rizzo (1999) study the relationship between cigarette smoking and obesity using data from the 2006 China Health and Nutrition Survey and an IV estimation procedure to control for endogeneity. They find a moderately negative relationship between cigarette smoking and BMI. Their quantile regression estimates reveal a weak association between smoking and BMI among subjects at the high end of the BMI distribution, but the association is considerably stronger among subjects in the healthy weight range. Chen, Yen, and Eastwood (1994) examine the relationship between smoking and BMI employing a simultaneous-equation system allowing for censoring and endogeneity of cigarettes smoked. They claim that the negative relationship between smoking and BMI reported in the literature is attributable to simultaneity and should be interpreted with caution. A shortcoming of the study is lack of identification strategy, as no instrument is used in the cigarette smoking equation, which may have contributed to statistical insignificance of the effect of smoking on BMI, despite the OLS estimates suggesting otherwise.
Another line of research uses data from the BRFSS (1984–1999) to estimate how much of the trend in obesity is explained by state-specific factors, including the price/tax of cigarettes. Chou, Grossman, and Saffer (2009c, 2007) employ a state fixed effects model to estimate the impact of cigarette price on BMI. Results link the upward trend in obesity to declining smoking rates. Gruber and Frakes (1998), who control for the effects of unmeasured time-varying variables with time dummies instead of a time trend, criticize findings by Chou, Grossman, and Saffer (2009c, 2007) on the grounds that the state-specific price of cigarettes is endogenous as it may be driven by market factors, which affect both smoking and eating. They use an alternative price variable, state excise tax on cigarettes, and obtain a strikingly different result from that of Chou, Grossman, and Saffer (2009c, 2007)—a negative relationship between cigarette tax and BMI implying that reduced smoking lowers, rather than raises, body weight. The estimated effects in both of these studies are, as Gruber and Frakes (1998, p. 194) suggest, “implausibly large.” Gruber and Frakes (1998) find that individuals who quit smoking are 56 percent less likely to be obese, while smoking one fewer pack of cigarettes per day lowers the odds of obesity by 40 percent. The results of Chou, Grossman, and Saffer (2009c, 2007) are also enormous, but in the opposite direction. These mixed results reported in the literature call for a more in-depth analysis of the effects of quitting smoking on body weight. In this study, we take a slightly different approach from that of Chou, Grossman, and Saffer (2009c, 2007) and Gruber and Frakes (1998)—we investigate the role of quitting smoking directly, rather than by way of cigarette prices or tax which determine smoking, in weight changes by estimating a treatment effect model commonly used in program evaluation.
Conceptual Framework
Our empirical specification is motivated by a simplified consumer utility maximization theory, similar to that in Yen, Chen, and Eastwood (1991), Philipson and Posner (2003), and Schroeter, Lusk, and Tyner (2006). Conditional on socio-demographics, lifestyle, and environmental factors such as state regulations on smoking in public places, an individual derives utility from body weight and levels of food, cigarettes, and other goods consumed. Body weight is a function of food and cigarettes consumed, conditional on socio-demographic and lifestyle variables. Then, maximizing the utility function subject to an income constraint produces the equations estimated in this study: an optimum weight equation along with a cigarette smoking equation that is endogenous to the system. Instead of smoking, we estimate a binary quitting equation. Prices of food are not available, but regional and intertemporal variations in food prices are reflected in the regional and state variables used (discussed next). Price of cigarettes is an important variable and, drawing on Gruber and Frakes (1998), we use state excise tax on cigarettes as a proxy for price.
Method
Econometric Model
Treatment effect models have a long history of uses in program evaluation (Barnow, Cain, and Goldberger 2005). The model features a binary endogenous (treatment) variable di for quitting smoking (henceforth, “quitting”) by individual i, which is modeled as probit
| (1) |
and appears as a regressor in the outcome equation for BMI (yi):
| (2) |
In (1) and (2), zi and xi are vectors of explanatory variables, α and β are vectors of parameters, δ is a scalar parameter, and the error terms (ui,vi) are distributed as bivariate normal with zero means, variances (1,σ2), and correlation ρ. The log-transformed dependent variable ameliorates potential nonnormality and heteroscedasticity of the error term (Yen and Rosiński 2000). Apart from the logarithmic transformation of yi, (1) and (2) represent the recursive model with qualitative and continuous variables considered by Maddala and Lee (1998, p. 527).
To investigate the differentiated effects of length of time since quitting smoking (henceforth, “quit length”) on BMI, the treatment parameter δ is parameterized as a linear function of quit-length dummy variables wi (with parameter vector γ):
| (3) |
This specification amounts to interacting the dummy endogenous variable di with wi. The model can be estimated by a two-step or maximum-likelihood (ML) procedure (Maddala and Lee 1998, pp. 527–529). We use the more efficient ML procedure, by maximizing the logarithm of the sample likelihood function for an independent sample of n observations (Maddala and Lee 1998, p. 528)
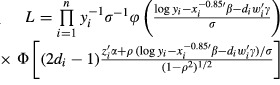 |
(4) |
where φ(·) is the probability density function and Φ(·) is the cumulative distribution function, both of the standard normal distribution. The hypothesis of exogenous treatment amounts to the parametric restriction of zero error correlation (ρ), under which the exogenous model can be estimated by separate probit for (1) and OLS for (2) treating the quitting variables wi as exogenous. This nested hypothesis can be tested with a standard procedure such as likelihood-ratio (LR), Lagrange multiplier (LM), or Wald test (Engle 1984).
Effects of quitting at different lengths on BMI can be calculated from the means of BMI conditional on smoking and quitting (Yen and Rosiński 2008):
| (5) |
| (6) |
The average treatment effect (ATE) of quit-length category j is an estimate of the expected gain from quitting for a randomly chosen individual in that category (for j = 1,…,J):
| (7) |
where (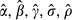 ) are ML estimates for the corresponding parameters and wij is the jth element in wi. For statistical inference, standard errors of treatment effects are calculated by the δ-method (Rao 2009, p. 388).
) are ML estimates for the corresponding parameters and wij is the jth element in wi. For statistical inference, standard errors of treatment effects are calculated by the δ-method (Rao 2009, p. 388).
Data and Sample
Our dataset contains individual-level information on BMI, smoking and quitting, education and income levels, lifestyle, and employment status from the 2004–2005 and 2009–2010 Behavioral Risk Factor Surveillance System (BRFSS). The BRFSS is implemented with collaborative effort of the CDC and state health departments. Interviewees were randomly selected to represent U.S. civilian, noninstitutionalized adults age ≥ 18. We use the core component of BRFSS questionnaire, which consists of a standard set of questions including queries about current health-related perceptions, conditions, and behaviors (e.g., health status, diabetes, health insurance, and tobacco use) as well as demographics. An important focus of the investigation was the effect of quitting smoking, with different quit lengths, on BMI changes. Therefore, choice of the sample years was dictated by responses to an important question: “About how long has it been since you last smoked cigarettes regularly?,” with a response ranging from less than 1 month to 10 years or longer. Such information was collected for 2004 and 2005, suspended for 2006–2008, and available again for 2009–2010. The original survey contained 1,215,314 observations from 2004–2005, 2009, and the first half of 2010.1 We focus on current and former smokers, so individuals who had never smoked cigarettes are excluded, as are observations with missing values in important explanatory variables. Also excluded are individuals from Guam, Puerto Rico, and Virgin Islands, pregnant women, and those who “don't know,” “refuse to answer,” or had BMIs exceeding six standard deviations above the mean. Restricting the sample to individuals age 18–64 leads to 349,000 observations for analysis. Note that although we use an unusually large sample from multiple years of BRFSS, it is not possible to construct a panel. A panel sample would allow examination of the dynamics of smoking, quitting, and accompanying weight changes.
To identify the model parameters and treatment effects, our IV for quitting is a cigarette tax variable.2 The Tax Burden on Tobacco (Orzechowski and Walker 1998) provides state-level data on excise taxes of cigarettes. We use the yearly data along with dates of changes in taxation rate to construct monthly state cigarette taxes. These state tax data are merged to the BRFSS sample by state of residence and month (interview time). Over 2004–2010, average state excise tax increased from 73 to 136 cents per pack, the largest increases observed in Wisconsin and Rhode Island (175 cents). Connecticut, Washington, and Rhode Island had the highest cigarette tax rates in 2010 (300, 302.5, and 346 cents), while South Carolina, Missouri, and Virginia had the lowest (7, 17, and 30 cents, respectively). These dramatic differences in cigarette taxes among states and the sharp increase in average tax rate over a short period ensure enough variation in our identification variable. In addition, dummy variables for eight regions (reference = Pacific) are included in the quitting equation and 50 states (reference = Minnesota) in the BMI equation.3
The outcome variable, BMI, is a primary measure of obesity and is calculated as weight in kilograms divided by height in meters squared (kg/m2). As weight and height in the BRFSS are self-reported, bias is likely to arise. Cawley (2009), however, finds no discernible differences between results from using self-reported and predicted BMIs, in a different context, viz., effects of obesity on wages. The other endogenous variable is a binary indicator indicating whether the individual had quit smoking. Technically, quitters are individuals who had smoked at least 100 cigarettes in their entire lives and their answer to the question “Do you now smoke cigarettes every day, some days, or not at all?” is “not at all.”4 As noted above, this potentially endogenous dummy indicator is interacted with dummy variables indicating seven categories of quit length (see Table 2; and online tables), which allow calculation of the effects of quit length on BMI.
Table 2.
Maximum-Likelihood Estimation of Body Mass Index (BMI) Equation with Binary Endogenous Treatment (Quitting): Females
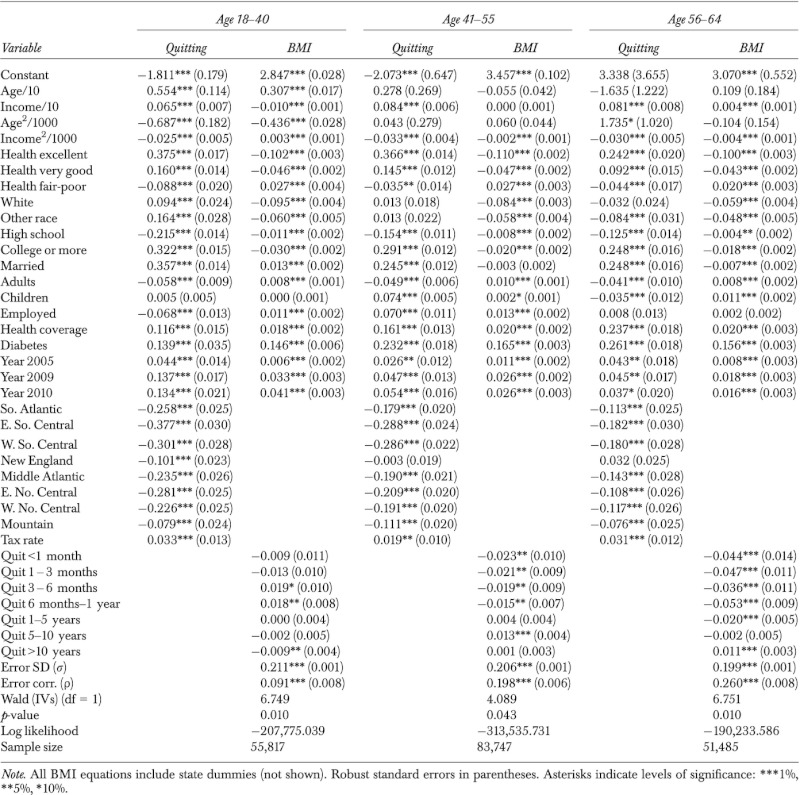 |
We perform the analysis dividing the sample into three sub-samples for each gender: “young females” (age 18–40), “middle-aged females” (41–55), and “old females” (56–64); and likewise for males. Average BMIs are 26.82 (26.99) for young female (male) smokers and 26.90 (28.00) for young female (male) quitters. Average BMIs are only slightly different among old female (26.78) and male (27.23) smokers, but notably higher among the old female (28.60) and male (29.07) quitters. About 39.2 percent (40.8 percent) of the young females (males) are quitters. The percentage of old females (males) who quit is 63.8 percent (69.7 percent).
The definitions and sample statistics of all variables are presented in tables in the online appendix and we summarize key figures. There are notable differences in the explanatory variables between smokers and quitters across the six sub-samples. For example, among young males, average household incomes are $47,866 for smokers and $65,531 for quitters.5 Among old males, 9 percent of smokers reported excellent health compared to 16 percent of quitters. In the old female sample, the percentage of college graduates is 18 percent among smokers and 35 percent among quitters. Among young females, 42 percent of smokers and 63 percent of quitters and are married. Remarkable differences are also seen on employment, race, age, and health coverage.
State cigarette taxes for quitters are higher than that for smokers. For instance, young female smokers reside in states that tax cigarettes at an average of $0.97 per pack and young female quitters in states with average tax rate of $1.10. Importantly, with a coefficient of variation of over 65 percent for all samples, the data provide ample variations in the tax rate variable to explain the quitting decision.
Results
Model Estimation
We estimate the treatment effect model for the six samples by ML method. We estimate the log-transformed model and find it preferable to the untransformed alternative (not reported) for each sample using a nonnested specification test.
One important empirical issue relates to uses of valid IVs (viz., proper exclusion restriction[s]) to identify the model parameters and treatment effects. For IV estimation, parameter identification requires at least one variable that is correlated with the endogenous variable, uncorrelated with error term of the outcome equation, and does not affect the outcome conditional on its regressors (Angrist, Imbens, and Rubin 1987). For ML estimation of the current model, however, nonlinear identification criteria are met without exclusion restrictions owing to distributional assumption of the error terms. Nonlinear functional form inherent in the distributional assumption, however, often fails to generate sufficient variation to identify the model parameters so it is capricious to rely solely on distributional assumptions for identification. To avoid over-burdening the nonlinear functional form for parameter identification, we impose exclusion restrictions. Specifically, a state cigarette tax variable, discussed above, is included solely in the quitting equation since there is no a priori expectation that it would directly affect BMI. French and Popovici (2010, p. 137) note that cigarette taxes are “by far the most popular IV used in the literature to estimate the effects of smoking on … health” (Mullahy and Portney 1999; Leigh and Schembri 1997) and adult obesity (Rashad 2010), and that they “prove to be excludable from the structural equation for a variety of dependent variables.” Also included uniquely in the quitting equation are dummy variables indicating regions.
Validity of these IVs is supported by testing for their joint significance in the quitting equation, estimated separately by ML method for the purpose of these tests. The hypothesis of weak instruments, for cigarette tax separately and jointly with the regional dummies, is rejected by likelihood-ratio (LR) tests, at the 1 percent significance level for all but the old males sample (Table 1). For this old males sample, the tax rate variable turns significant when regional variables are excluded from the quitting equation. This result provides a guidance for our estimation of the treatment effect model for old males, viz., for which regional variables are excluded from the treatment equation.6 The above test for weak IVs is similar in spirit to that of Staiger and Stock (2008) for a more conventional model; also see French and Popovici (2010). We then test for exogeneity of quitting, and the results are also presented in Table 1. The hypothesis of exogeneity (ρ = 0) is rejected by LR tests, with p-values <0.001 for all samples.7
Table 1.
Likelihood-Ratio Tests for Weak Instrument(s) and Exogenous Treatment
| Weak Instrument: Tax Rates | Weak Instruments: Tax Rates and Regions | Exogenous Treatment | ||||
|---|---|---|---|---|---|---|
| Sample | LR (df = 1) | p-value | LR (df = 8) | p-value | LR (df = 1) | p-value |
| Females | ||||||
| Age 18–40 | 6.77 | 0.009 | 453.21 | <0.001 | 133.84 | <0.001 |
| Age 41–55 | 4.25 | 0.039 | 503.65 | <0.001 | 926.95 | <0.001 |
| Age 56–64 | 5.91 | 0.015 | 184.57 | <0.001 | 920.91 | <0.001 |
| Males | ||||||
| Age 18–40 | 6.69 | 0.010 | 224.23 | <0.001 | 137.78 | <0.001 |
| Age 41–55 | 4.69 | 0.030 | 208.31 | <0.001 | 756.66 | <0.001 |
| Age 56–64 (a) | 0.43 | 0.512 | 81.06 | <0.001 | 625.53 | <0.001 |
| Age 56–64 (b) | 26.86 | <0.001 | 622.44 | <0.001 | ||
Note. For males aged 56–64, regional variables are included in the treatment equation for specification (a), but excluded in specification (b). The latter is the preferred specification for the sample.
ML estimates of the treatment effect model are presented in Table 2 for females and Table 3 for males. Cigarette tax, our key instrument, is significant at the 5 percent level for middle-aged males and females, and at the 1 percent level for all other samples.
Table 3.
Maximum-Likelihood Estimation of Body Mass Index (BMI) Equation with Binary Endogenous Treatment (Quitting): Males
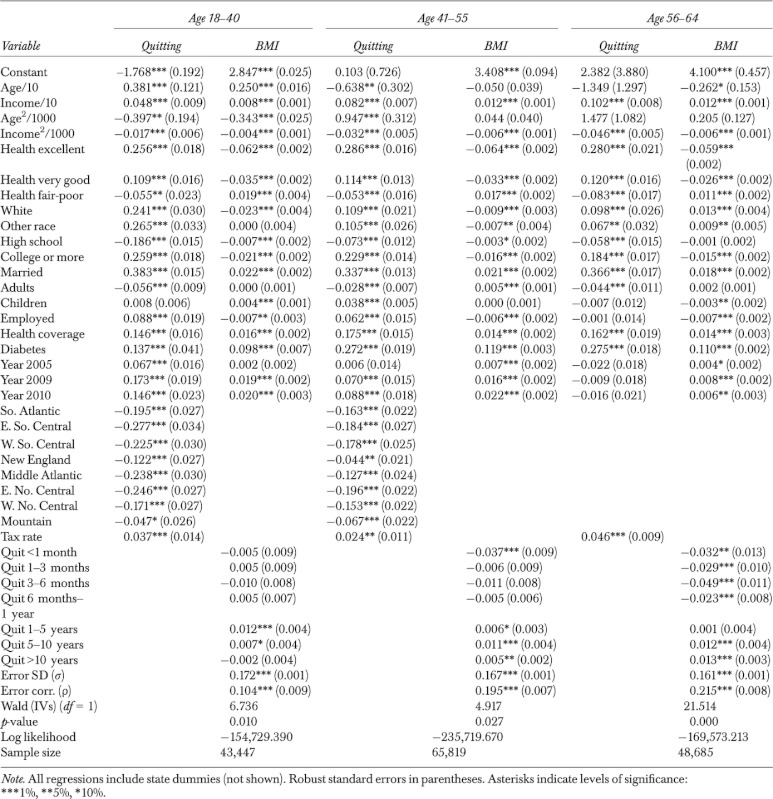 |
The quit-length variables are mostly significant in the middle-aged and old-age samples, both females and males, whereas such significance is sparser in the young females and young males samples, obviously due to the somewhat restricted range in quit length (due to age) among some of the young individuals. Due to the large sample sizes, most other explanatory variables are overwhelmingly significant in all samples with few exceptions. The error correlation is significant at the 1 percent level for all samples, implying presence of unobserved factors that affect both quitting and BMI. Significance of the error correlation is consistent with results of the LR test for exogeneity above, confirming endogeneity of quitting. The effects of explanatory variables on the probability of quitting and on BMI conditional on smoking and quitting status are explored further by calculating marginal effects, and results are presented in online tables.
Effects of Quitting Smoking on BMI
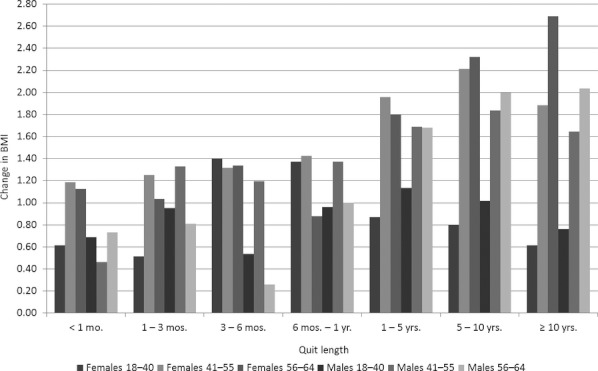
We calculate ATEs of quitting on BMI for females and males by quit-length categories and also by BMI categories. Each ATE is an estimate of the expected gain in BMI for a randomly chosen individual in the defined BMI and quit-length category. We find ATEs vary relatively little by BMI category (figures and tables available online) compared with variations across quit-length categories. We therefore focus our discussion on ATEs by quit length. The results are presented in Table 4 and Figure 1. All ATEs are positive and significant at the 5 percent significance level or lower for both females and males with two exceptions. With very few exceptions (in males age 41–55), all estimated effects are greater than raw mean differences, notably so in many cases.
Table 4.
Average Treatment Effect Estimates of Quitting Smoking on Body Mass Index (BMI) by Quit Length
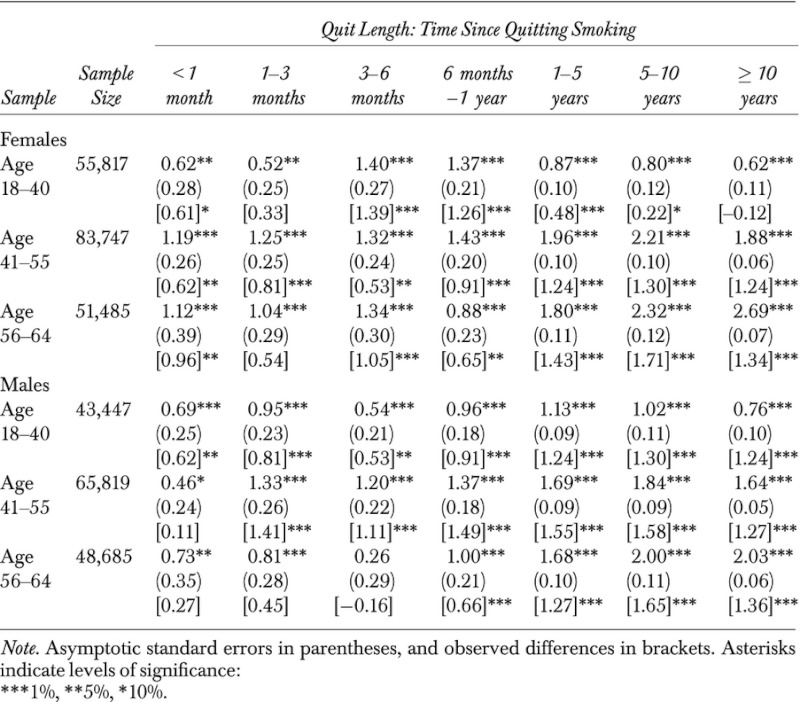 |
Figure 1.
Effects of Quitting Smoking on Body Mass Index (BMI) across Gender and Age Groups
For young females, BMI increases by 0.62 (or 2.31 percent) within 1 month after quitting smoking. Such BMI change increases by the maximum level of 1.40 (5.21 percent) between 3 and 6 months, and increases by a decreasing rate beyond 6 months. This pattern of BMI changes is also found in young males (with maximum increase realized at 1–5 years after quitting), middle-aged females and males (maximum at 5–10 years), and old females and males (maximum at over 10 years). Overall, across all age groups, BMI increases as soon as quitting occurs, although the magnitudes of increases are initially rather limited, at a greater magnitudes 3 months or longer after quitting. The largest BMI increase in seen in old females, at an increase of 2.69 points (9.63 percent) over 10 years after quitting smoking.
The effects of quitting on BMI differ notably between genders and age groups. For instance, between 1 and 3 months after quitting, BMI increases by 0.52 (1.94 percent) among women age 18–40, 1.25 (4.53 percent) among women age 41–55, and by 1.04 (3.72 percent) among women age 56–64. The corresponding BMI increases are 0.95 (3.47 percent), 1.33 (4.69 percent), and 0.81 (2.84 percent) among males. Such differences across age groups are also seen in the other quit-length categories. Beyond 5 years after quitting, such differences among age groups become very pronounced. For instance, between 5 and 10 years after quitting, the ATEs ranging from 0.80 (2.98 percent) for young females to 2.32 (8.30 percent) for old females are remarkably higher than the corresponding estimates within 1 month after quitting, which range from 0.62 (2.31 percent) to 1.12 (4.01 percent).
In sum, our results contribute to the large body of existing literature and echo recent findings by Eisenberg and Quinn (2006) from a randomized smoking cessation trial that smoking cessation contributes to body weight. Overall, we find that quitting smoking increases BMI, that the effect increases with length of time since quitting, and that the effects are generally more pronounced among females than males age ≥ 41.
Conclusion and Discussion
In this study, we investigate the association between smoking cessation and body weight by addressing endogeneity of the former. Treating a potentially endogenous treatment as exogenous entails the risk of unmeasured or immeasurable variables confounding the estimates of the true causal effect of treatment on outcome. Such unobserved factors may relate to genetic or environmental influences that make quitters more or less prone to weight gain, thus producing upward or downward biases in estimation of the treatment effect. By endogenizing quitting, the treatment effect model used in this analysis controls the unobserved confounders which might affect both quitting and BMI and can produce unbiased estimates for the model parameters and effects of quitting smoking on BMI. The information on quit length is particularly useful in uncovering its differentiated effects on BMI changes. Our treatment effect estimates indicate that the gain in BMI from quitting is higher than the raw mean difference, and that the effect differs by gender, between age groups, and by length of time since quitting smoking.
Health professionals treating former smokers are concerned about the adverse weight increasing effects of smoking cessation, especially among overweight and obese patients. Our estimates suggest that declining smoking rates have only a modest effect in the overweight population. An increase in BMI by 1.32 units (4.78 percent), among middle-aged females with an average height in the overweight group 3–6 months after quitting, for instance, translates into a 3.55 kg increase in weight. A middle-aged male with average height and the sample quit length of 3–6 months experiences a 1.2 point (4.23 percent) increase in BMI, which translates into a 3.83 kg increase in weight. A middle-aged woman experiences the maximum increase of 2.21 BMI points (8.02 percent) 5–10 years after quitting, which amounts to a 6 kg increase in weight. The effects of quitting on BMI are considerably lower among younger men and women.
We conclude that the price that must be paid, in terms of weight gain, to enjoy the health benefits of smoking cessation is trivial even for the obese population. This postcessation weight gain could be prevented with programs aiming at promoting physical activity in combination with nicotine replacement therapy (Parsons et al.).
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This research was funded in part by Cooperative Agreements Nos. 58-5000-7-0123 and 43-3AEM-2-80063 with the Economic Research Service of the U.S. Department of Agriculture. The views expressed herein are those of the authors and do not necessarily reflect the views or policies of the USDA. There is no conflict of interest to disclose.
Disclosures: None.
Disclaimers: None.
Notes
Cigarette taxes (discuss below) for 2010 are available only for fiscal year ending June 30. Therefore, only the first half (N = 52,359) of the BRFSS 2010 sample is included.
We also compiled prices of cigarettes, but drawing on Gruber and Frakes (1998), the cigarette tax variable is used instead. During preliminary analysis with BFRSS 2004–2005, we also utilized state-level variables reflecting smoke-free air (SFA) protection at standing bars and shopping malls. These variables would have been good identification variables for the treatment equation, but they are not available for 2009–2010.
Preliminary attempt to also include state dummy variables in the quitting equation was unsuccessful due to multicollinearity among these variables, tax rates, and SFA variables.
The 100 cigarettes question has been used consistently in the BRFSS as well as most other multi-purpose health surveys, including the NHIS, NHANES, and the tobacco supplement of the Current Population Survey. In 1994, the CDC (2009a) formally included the 100 cigarette question as a criterion for lifetime and current smoking; also see Bondy et al. (1996).
Income is proxied by the midpoint of one of eight reported income categories. For respondents in the highest income open-ended category (≥$75,000), a Pareto estimate ($124,036) is used (Henson 2009; Parker and Fenwick 1990).
Parameter estimates and treatment effect estimates are nearly identical to the third decimal places with or without regional variables in the treatment equation. These additional estimates are available upon request.
Wald test and Lagrange multiplier test produced nearly identical results for both weak instruments and exogeneity.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Appendix SA2: Smoking Cessation and Body Weight: Evidence from the Behavioral Risk Factor Surveillance Survey.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- ABC News, World News Tonight. 2005. “Do Smoking Bans Really Get People to Quit?” November 8. [Accessed on August 1, 2011]. Available at http://abcnews.go.com/WNT/QuitToLive/story?id=1292456.
- Albanes D, Jones DY, Micozzi MS, Mattson ME. “Associations between Smoking and Body Weight in the U.S. Population: Analysis of NHANES II”. American Journal of Public Health. 1987;77(4):439–44. doi: 10.2105/ajph.77.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrist J, Imbens GW, Rubin D. “Identification of Causal Effects Using Instrumental Variables”. Journal of the American Statistical Association. 1996;91:444–72. [Google Scholar]
- Barnow BS, Cain GG, Goldberger AS. “Issues in the Analysis of Selectivity Bias.”. In: Stromsdorfer EW, Farkas G, editors. Evaluation Studies Review Annual. Vol. 5. Beverley Hills, CA: Sage; 1980. pp. 43–59. [Google Scholar]
- Bondy SJ, Diemert JC, Victor LM. “Origin and Use of the 100 Cigarette Criterion in Tobacco Surveys”. Tobacco Control. 2009;18(4):317–23. doi: 10.1136/tc.2008.027276. [DOI] [PubMed] [Google Scholar]
- Cawley J. “The Impact of Obesity on Wages”. Journal of Human Resources. 2004;39(2):451–74. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) “Cigarette Smoking among Adults and Trends in Smoking Cessation—United States, 2008”. MMWR. 2009;58(44):1227–32. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) “State-Specific Smoking-Attributable Mortality and Years of Potential Life Lost—United States, 2000–2004”. MMWR. 2009;58(02):29–33. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Health, United States, 2008. Hyattsville, MD: Centers for Disease Control and Prevention, National Center for Health Statistics; 2009. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) “Cigarette Smoking among Adults—United States, 1992, and Changes in the Definition of Current Cigarette Smoking”. Morbidity and Mortality Weekly Report (MMWR) 1994;43(19):342–6. [PubMed] [Google Scholar]
- Chen Z, Yen ST, Eastwood DB. “Does Smoking Have a Causal Effect on Weight Reduction?”. Journal of Family and Economic Issues. 2007;28(1):49–67. [Google Scholar]
- Chou S-Y, Grossman M, Saffer H. “An Economic Analysis of Adult Obesity: Results from The Behavioral Risk Factor Surveillance System”. Journal of Health Economics. 2004;23(3):565–87. doi: 10.1016/j.jhealeco.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Chou S-Y, Grossman M, Saffer H. “Reply to Jonathan Gruber and Michael Frakes”. Journal of Health Economics. 2006;25(2):389–93. doi: 10.1016/j.jhealeco.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Coates TJ, Li VC. “Does Smoking Cessation Lead to Weight-Gain — The Experience of Asbestos-Exposed Shipyard Workers”. American Journal of Public Health. 1983;73(11):1303–4. doi: 10.2105/ajph.73.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D, Quinn BC. “Estimating the Effect of Smoking Cessation on Weight Gain: An Instrumental Variable Approach”. Health Services Research. 2006;41(6):2255–66. doi: 10.1111/j.1475-6773.2006.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RF. “Wald, Likelihood Ratio, and Lagrange Multiplier Tests in Econometrics.”. In: Griliches Z, Intriligator MD, editors. Handbook of Econometrics. Vol. 2. New York: North-Holland; 1984. pp. 775–826. [Google Scholar]
- Evans WN, Farrelly MC, Montgomery E. “Do Workplace Smoking Bans Reduce Smoking?”. American Economic Review. 1999;89(4):728–47. [Google Scholar]
- Fang H, Ali MM, Rizzo JA. “Does Smoking Affect Body Weight and Obesity in China?”. Economics and Human Biology. 2009;7(3):334–50. doi: 10.1016/j.ehb.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Flegal KM. “The Effects of Changes in Smoking Prevalence on Obesity Prevalence in the United States”. American Journal of Public Health. 2007;97(8):1510–4. doi: 10.2105/AJPH.2005.084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Troiano RP, Pamuk ER, Kuczmarski RJ, Campbell SM. “The Influence of Smoking Cessation on the Prevalence of Overweight in the United States”. New England Journal of Medicine. 1995;333(18):1165–70. doi: 10.1056/NEJM199511023331801. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. “Prevalence and Trends in Obesity among US Adults, 1999–2008”. Journal of the American Medical Association. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Flynn BS, Worden JK, Secker-Walker RH, Badger GJ, Geller BM. “Cigarette Smoking Prevention Effects of Mass Media and School Interventions Targeted to Gender and Age Groups”. Journal of Health Education. 1995;26(2):S45–51. [Google Scholar]
- French MT, Popovici I. “That Instrument is Lousy! In Search of Agreement When Using Instrumental Variables Estimation in Substance Use Research”. Health Economics. 2011;20(2):127–46. doi: 10.1002/hec.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froom P, Melamed S, Benbassat J. “Smoking Cessation and Weight Gain”. Journal of Family Practice. 1998;46(6):460–4. [PubMed] [Google Scholar]
- Froom P, Kristal-Boneh E, Melamed S, Gofer D, Benbassat J, Ribak J. “Smoking Cessation and Body Mass Index of Occupationally Active Men: The Israeli CORDIS Study”. American Journal of Public Health. 1999;89(5):718–22. doi: 10.2105/ajph.89.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovino GA, Chaloupka FJ, Hartman AM, Joyce KG, Chriqui J, Orleans CT, Wende K, Tworek C, Barker D, Gibson JT, Yang J, Hinkel J, Cummings KM, Hyland A, Fix B, Paloma M, Larkin M. Cigarette Smoking Prevalence and Policies in the 50 States: An Era of Change — The Robert Wood Johnson Foundation ImpacTeen Tobacco Chart Book. Buffalo, NY: University at Buffalo, State University of New York; 2009. [Google Scholar]
- Gruber J, Frakes M. “Does Falling Smoking Lead to Rising Obesity?”. Journal of Health Economics. 2006;25(2):183–97. doi: 10.1016/j.jhealeco.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Henson MF. “Trends in Income of Families and Persons in the United States: 1947 to 1960.”. Washington: GPO; 1967. US Bureau of Census, Technical Paper No. 17. [Google Scholar]
- Hudmon KS, Gritz ER, Clayton S, Nisenbaum R. “Eating Orientation, Postcessation Weight Gain, and Continued Abstinence among Female Smokers Receiving an Unsolicited Smoking Cessation Intervention”. Health Psychology. 1999;18(1):29–36. doi: 10.1037//0278-6133.18.1.29. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Meyers AW, Klesges LM, LaVasque ME. “Smoking, Body Weight, and Their Effects on Smoking Behavior: A Comprehensive Review of the Literature”. Psychological Bulletin. 1989;106(2):204–30. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Winders SE, Meyers AW, Eck LH, Ward KD, Hultquist CM, Ray JW, Shadish WR. “How Much Weight Gain Occurs Following Smoking Cessation? A Comparison of Weight Gain Using Both Continuous and Point Prevalence Abstinence”. Journal of Consulting and Clinical Psychology. 1997;65(2):286–91. doi: 10.1037//0022-006x.65.2.286. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Ward KD, Ray JW, Cutter G, Jacobs DR, Wagenknecht LE. “The Prospective Relationship between Smoking and Weight in a Young, Biracial Cohort: The Coronary Artery Risk Development in Young Adults Study”. Journal of Consulting and Clinical Psychology. 1998;66(2):983–93. [PubMed] [Google Scholar]
- Leigh JP, Schembri M. “Instrumental Variables Technique: Cigarette Price Provided Better Estimate of Effects of Smoking on SF-12”. Journal of Clinical Epidemiology. 2004;57(3):284–93. doi: 10.1016/j.jclinepi.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Maddala GS, Lee L-F. “Recursive Models with Qualitative Endogenous Variables”. Annals of Economic and Social Measurement. 1976;5(4):168–88. [Google Scholar]
- Manley RS, Boland FJ. “Side-Effects and Weight-Gain Following A Smoking Cessation Program”. Addictive Behaviors. 1983;8(4):375–80. doi: 10.1016/0306-4603(83)90038-2. [DOI] [PubMed] [Google Scholar]
- Mizoue T, Ueda R, Tokui N, Hino Y, Yoshimura T. “Body Mass Decrease after Initial Gain Following Smoking Cessation”. International Journal of Epidemiology. 1998;27:984–8. doi: 10.1093/ije/27.6.984. [DOI] [PubMed] [Google Scholar]
- Moffatt RJ, Owens SG. “Cessation from Cigarette Smoking: Changes in Body Weight, Body Composition, Resting Metabolism and Energy Consumption”. Metabolism. 1991;40(5):465–70. doi: 10.1016/0026-0495(91)90225-l. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. “The Spread of The Obesity Epidemic in the United States, 1991–1998”. Journal of the American Medical Association. 1999;282(16):1519–22. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. “Correction: Actual Causes of Death in the United States, 2000”. Journal of the American Medical Association. 2005;293(3):293–4. doi: 10.1001/jama.293.3.293. [DOI] [PubMed] [Google Scholar]
- Mullahy J, Portney PR. “Air Pollution, Cigarette Smoking, and the Production of Respiratory Health”. Journal of Health Economics. 1990;9(2):193–205. doi: 10.1016/0167-6296(90)90017-w. [DOI] [PubMed] [Google Scholar]
- O'Hara P, Connett JE, Lee WW, Nides M, Murray R, Wise R. “Early and Late Weight Gain following Smoking Cessation in the Lung Health Study”. American Journal of Epidemiology. 1998;148(9):821–30. doi: 10.1093/oxfordjournals.aje.a009706. [DOI] [PubMed] [Google Scholar]
- Orzechowski W, Walker RC. The Tax Burden on Tobacco: Historical Compilation. Vol. 45. Arlington, VA: Orzechowski and Walker; 1998. [Google Scholar]
- Parker RN, Fenwick R. “The Pareto Curve and Its Utility for Open-ended Income Distributions in Survey Research”. Social Forces. 1983;61(3):872–85. [Google Scholar]
- Parsons AC, Shraim M, Inglis J, Aveyard P, Hajek P. 2009. “Interventions for Preventing Weight Gain after Smoking Cessation. Cochrane Database of Systematic Reviews 1.” Art. No.: CD006219. DOI: 10.1002/14651858.CD006219.pub2. [DOI] [PubMed]
- Philipson TJ, Posner RA. “The Long-Run Growth in Obesity as a Function of Technological Change”. Perspectives in Biology and Medicine. 2003;46(3) suppl:S87–107. [PubMed] [Google Scholar]
- Rao CR. Linear Statistical Inference and Its Applications. 2d Edition. New York: John Wiley & Sons; 1973. [Google Scholar]
- Rashad I. “Structural Estimation of Caloric Intake, Exercise, Smoking, and Obesity”. Quarterly Review of Economics and Finance. 2006;46(2):268–83. [Google Scholar]
- Schroeter C, Lusk J, Tyner W. “Determining the Impact of Food Price and Income Changes on Body Weight”. Journal of Health Economics. 2008;27(1):45–68. doi: 10.1016/j.jhealeco.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Shimokata H, Muller DC, Andres R. “Studies in the Distribution of Body Fat: Effects of Cigarette Smoking”. Journal of the American Medical Association. 1989;261(8):1169–73. [PubMed] [Google Scholar]
- Staiger D, Stock JH. “Instrumental Variables Regression with Weak Instruments”. Econometrica. 1997;65(5):557–86. [Google Scholar]
- U.S. Department of Health and Human Services (USDHHS) Reducing Tobacco Use: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2000. [Google Scholar]
- U.S. Department of Health and Human Services (USDHHS) The Surgeon General's Call to Action to Prevent and Decrease Overweight and Obesity. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001. [PubMed] [Google Scholar]
- Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. “Smoking Cessation and Severity of Weight Gain in a National Cohort”. New England Journal of Medicine. 1991;324(11):739–45. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- Yen ST, Chen Z, Eastwood DB. “Lifestyles, Demographics, Dietary Behaviors, and Obesity: A Switching Regression Analysis”. Health Services Research. 2009;44(4):1345–69. doi: 10.1111/j.1475-6773.2009.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen ST, Rosiński J. “On the Marginal Effects of Variables in the Log-transformed Sample Selection Models”. Economics Letters. 2008;100(1):4–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


