Over the last two decades silk produced by the silkworm Bombyx mori has found new utility as a sustainable material platform for high-technology applications encompassing photonics, electronics and optoelectronics [1-4]. Silk fibers have been used as an FDA approved medical suture material for decades [5] due to their biocompatibility and mechanical properties [6]. These properties, along with the inherent biodegradability of silk, has driven the use of this protein for biological studies [6]. Native silk fibers can be solubilized and reprocessed into an aqueous silk fibroin protein solution [7], which can then be used to generate a multitude of new material formats [5] such as hydrogels [8], foams [9], electrospun mats [10] and sponges [11]. These new forms of silk are finding utility in drug delivery, cell culture and tissue engineering applications. Silk films with excellent optical properties (> 90% transmission in the visible spectrum) [12] are currently being explored for applications in optics and biophotonics [3, 4]. Additionally, the environmentally benign, all-aqueous processing conditions and the chemistry of silk allow bioactive components, such as enzymes to be stabilized in the protein matrix [13].
Due to the excellent mechanical properties [14], micro- and nano patterning of silk films can be achieved across a wide range of feature sizes. Nanoimprinting and nano-casting [4, 15] techniques have been used to pattern silk films for photonic applications [16].
These films possess useful properties that allow interfacing with metals and thin-film semiconductor devices, and the opportunity to develop biocompatible hybrid silk devices. This was recently demonstrated by using silk-based electrodes to measure neural activity in-vivo [17]. Additionally, microfabricated metamaterial (MM) silk composites were demonstrated, with electromagnetic resonance responses in the THz frequency regime, with potential applications for in vivo bio sensors [18].
Device manufacturing with this protein would benefit from fabrication methods that simplify patterning techniques by avoiding prolonged times of sample preparation, elevated temperature or high vacuum [18], which, aside from providing more complexity, would also limit the use of biologically active species [13].
In this communication, we report a simple fabrication technique, which in a single step transfers metal micro patterns to free standing silk films under ambient processing conditions. We refer to this process as “Silk Transfer Applied Micro Pattering” or STAMP for short. Additionally, this method adds versatility and utility to silk protein device fabrication by allowing the use of the patterned films as hard masks for oxygen based reactive ion etching (RIE). RIE is a widely used tool for versatile and high throughput micro- and nano patterning. However, its utility for biopolymers is limited [19] due in part to the lack of convenient methods to apply etching masks to biopolymer films.
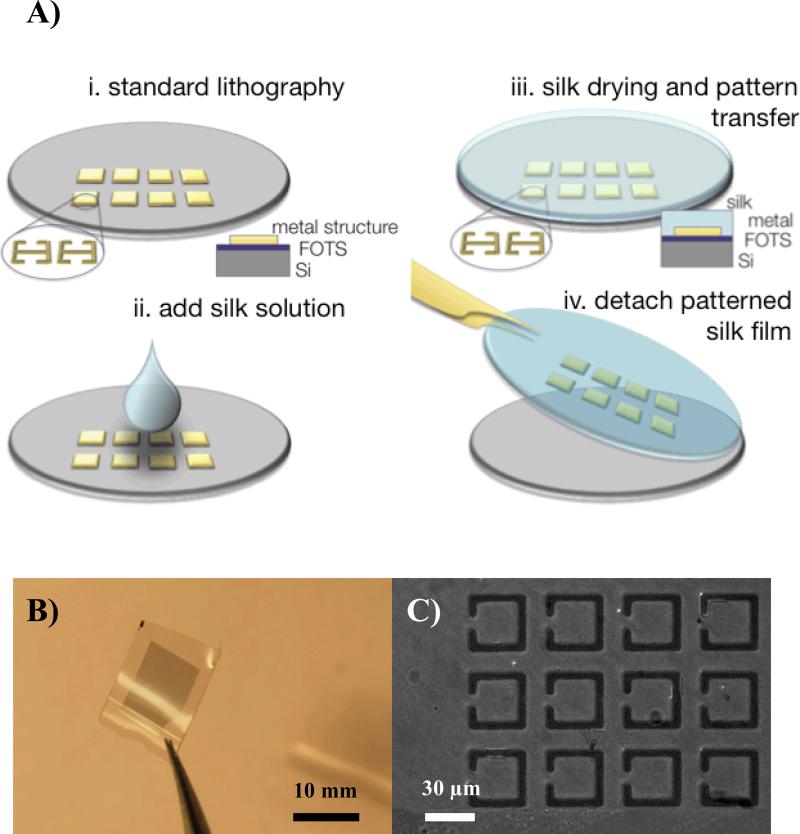
Fig. 1 illustrates the fabrication process that allows the direct transfer of microscale patterns onto the surface of the silk film. The effectiveness of the technique is demonstrated by manufacturing large-area silk film-based THz metamaterial (MM) structures composed of split-ring resonators (SRR) made with aluminum (Al) or gold (Au) at ambient pressure and temperature. The THz MM SRR structures employed have been described previously [18, 20]. Silicon (Si) wafers were first treated with a silanizing agent in order to reduce the adhesion of the metal to the Si surface and allow for easy pattern transfer to the silk film. Microscale patterns were deposited on the Si wafer either by using standard photolithography techniques or, alternatively, with a shadow masking approach [18].
Figure 1.
A) Fabrication scheme for Silk Transfer Applied Micro Patterning (STAMP). i) Standard photolithography and metal lift off with the desired patterns is performed on a FOTS coated Si wafer. ii) wafer is coated with aqueous silk solution. iii) silk solution is dried and metal micro patterns transferred from the Si wafer to the silk film. iv) patterned silk film removed from Si wafer. B) Macroscopic image of aluminum metamaterial micro patterned silk film. C) Light microscopy micrograph of aluminum metamaterial patterns on silk.
Once the patterning step is complete, aqueous silk solution can be applied onto the patterned Si wafer. The silk solution was allowed to dry overnight to self-assemble and form a free-standing silk film, as previously described [21]. In addition, by adjusting the processing conditions during and after drying, the silk films can be rendered water insoluble [21]. During the drying process silk binds to the metallic pattern on the surface causing the metal patterns to be transferred from the wafer onto the silk film. The adhesion between the metal patterns and the silk films is the subject of future investigation. Among possible explanations is the presence of Cysteine (Cys)- and Tyrosine (Tyr) residues (a total of 8 Cys and 55 Tyr amino acids are present in the silk macromolecule [22]), which would affect the surface energy properties of the films, thereby favoring either thiol bonds of the Cys with Au [23] or interactions of Al- oxides with the hydroxyl groups on the Tyr residues [24, 25].
The silanization treatment prevents the silk film from adhering to the Si wafer surface and allows for its manual detachment from the substrate. Once this process is completed, the structures were examined by optical- and scanning electron microscopy (SEM) to verify successful transfer with high-fidelity on the microscale. The MM structures obtained in the STAMP process are shown in Figure 1B which illustrates a free-standing micro patterned silk film. The transparent film was fabricated under ambient conditions and has a consistent smooth surface and thickness across the approximately 8 mm × 8 mm patterned area. Figure 1C shows an optical transmission micrograph of an Al MM patterned silk film. The critical dimensions in this SRR design are approximately 3μm. The features of the SRR structures are found to be consistently maintained during the detachment process. In addition to the facile processing of a large-area free-standing silk based MM, it is worth pointing out that all of the processes described above are carried out under ambient conditions. This is particularly important since the activity of biological dopants, such as enzymes or antibodies, is preserved when they are incorporated in the silk matrix [13]. In order to manufacture hybrid systems with biochemical functionality in the silk films (e.g., pharmaceuticals [26]), the use of ambient temperature and ambient -pressure fabrication processes is important in order to maintain the bioactivity of these compounds. With this method, desired patterns of arbitrary complexity can be fabricated on a separate substrate and subsequently transferred onto the biologically doped silk film, under processing conditions that can preserve biological function.
To further demonstrate the convenience of the approach and the suitability of RIE as a manufacturing tool for silk micro- and nano patterning, we used the previously manufactured MM SRR structures as a mask for silk-RIE (Fig. 2). The previously transferred metal patterns provided a hard mask for the subsequent RIE step (Fig. 2A). For this approach we used our smallest size SRR arrays which had feature dimensions of approximately 1.5 μm at the gap, and a line width of the metal forming the resonator equal to 4 μm. The structures obtained from the etching process closely represent the SRR pattern in silk with the metal patterns still present on the etched film surface. In addition, the electromagnetic response of the etched structures was measured to demonstrate functionality of the MM silk composite structures and to illustrate fabrication consistency over the sampled region. Fig. 2B shows a SEM image of the Al MM mask and RIE etched structures. Fig. 2C shows the SEM image of an array of resonators from the RIE-silk film, the structure featured in Fig. 2B. As can be seen in Fig. 2C, the silk features produced with the STAMP method followed by RIE processing were consistent over the 100 × 100 SRR array. Fig. 2D shows an SEM image of Au patterned SRR and RIE etched silk structures. These patterns were fabricated on the Si substrate with a previously described shadow masking technique [18]. The critical feature size in this SRR design was approximately 6 μm at the gap of the resonator. The features fabricated with the Au shadow masking technique were of comparable quality to traditional lithography fabricated features. Considerable difference was observed between RIE processed silk surfaces depending on the metal used. The Au coated specimens showed rough “grassy’ surface structures (insert in Fig. 2D) in contrast to the smoother surfaces observed with the Al masked samples. We attribute the “grassy” surface structure to secondary Au micro masking, which can occur during RIE processing when using masking materials with a low sputtering threshold or a high sputtering yield, such as Au [27]. We chose Al as a mask material for the RIE processing step because of the Al-oxide formation caused when the sample is exposed to oxygen plasma. Such oxides have excellent resistance to sputtering and are less apt to induce micro masking effects and are therefore an excellent masking material for polymers. Fig. 2E shows an SEM micrograph of the etched wall profile of an Al masked sample. The etch depth was approximately 10 μm with vertical sidewalls, indicating the anisotropic nature of the silk dry etching process. As expected, the unmasked surfaces were found to be smooth and showing little residue. We hypothesize that the few features which can be seen on the etch floor in Fig. 2D could be attributed to dissimilar etching rates within the protein itself where the backbone would etch at slower rates than the bulk of the silk material because of its more stable structure [22].
Figure 2.
A) Scheme illustrating the RIE step performed using the previously transferred metal patterns as a hard mask for etching the silk films. B) Enlarged SEM image of aluminum metamaterial masked RIE patterned silk feature. C) SEM image of a RIE fabricated aluminum metamaterial array on silk film. D) SEM image of RIE processed gold metamaterial masked silk film with enlarged view of “grassy” residual etch surface in bottom right corner. E) Detailed SEM image of an aluminum masked and RIE processed silk film etch profile on a separate sample.
Further demonstration of the quality of the etching process and the associated critical dimensions of the resulting structure was provided by measuring the electromagnetic response of the etched Al-MM structures (Fig. 3). The electromagnetic transmission spectrum corresponds to the Al MM structures shown in Fig. 2B and Fig. 2C. The samples were analyzed by terahertz time-domain spectroscopy (THz-TDS) as previously described [28]. A strong resonance response was detected right below 1 THz, indicating the functionality and integrity of the structures after the RIE processing. The THz beam was directed at the center of an 8 mm × 8 mm RIE-etched MM patterned area, probing the resonance response and verify the consistency for two orthogonal polarizations. The MM resonance response is especially sensitive to changes in the SRRs structural dimensions, indicating even slight variation in manufacturing tolerances. We found from statistical analysis (Pearson correlation test) that the effect of the electromagnetic frequency on the transmission did not vary for the two polarizations (correlation coefficient r > 0.99, p < 0.001), indicating excellent symmetry of the structures and corroborating fabrication consistency. We also expect that RIE processed silk-MM sensors could potentially improve sensitivity in comparison to previously fabricated two dimensional structures, because of increased surface area and increased dielectric contrast of the etched three dimensional relief silk structures [18].
Figure 3.
Resonance response of a STAMP fabricated aluminum on silk and RIE processed metamaterial array. Measurements were performed with terahertz time-domain spectroscopy (THz-TDS) for two polarizations. The effect of the electromagnetic frequency on the transmission did not vary for the two polarizations (r > 0.99 and p < 0.001), indicating excellent symmetry of the MM structures and consistency in the fabrication process.
In conclusion, we have successfully transferred microfabricated patterns to silk biopolymer films under ambient processing conditions by employing a rapid transfer-based micro patterning technique. This method allows parallel fabrication of microstructures on large area, free standing and flexible silk films with high precision and eliminating the need for alignment. We have also demonstrated the use of this technique with various materials as masks for silk biopolymer RIE processing and a variety of SRR MM designs. This approach allows large area fabrication and is amenable to the transfer of different materials beyond metals. Additionally, by leveraging the demonstrated stability of silk based materials in aqueous environments [290, such MM structures can potentially be implemented in fluidic-based detection schemes. Lastly, individual feature sizes can be scaled to larger sizes to manufacture, for instance, metallic electrodes on silk films, or scaled down to the nanoscale for applications in photonic and plasmonic sensor systems [15].
Experimental
Silk extraction and purification
The process to obtain aqueous silk fibroin solution from B. mori cocoons was previously described [7]. Briefly, sericin was removed by boiling the cocoons in an aqueous sodium carbonate solution for 30 minutes. After drying, the fibroin fibers were dissolved in a lithium bromide solution and subsequently the salt was removed by dialysis against deionized water (DI) until the solution reached a concentration of about 8-10 % wt/v. To enhance the purity of the silk, we centrifuged a second time and filtered the solution through a 5 μm syringe filter (5 μm pore size, Millipore Inc, Bedford, MA) [4].
STAMP process
A conventional 4” Si wafer (Nova wafers) was treated with the silanizing agent tridecafluoro-1,1,2,2-tetrahydrooctyl trichlorosilane (FOTS) to reduce sticking of the microfabricated features to the wafer during the transfer process. The Si wafer was placed in a vacuum chamber under house vacuum with a few drops of the FOTS solution to evaporate for approximately 24 hours. Subsequently, a thin layer (between 100 nm and 300 nm) of Al or Au was sputtered onto the FOTS treated Si wafer and standard photolithography was performed with S1813 photoresist (Rohm & Haas). Residual metal was wet etched in an appropriate etching solution and the remaining photoresist was removed, revealing the desired metal patterns. The MM design and mask was used from a previous study [20]. Approximately, 2 ml of 8 % wt/v silk solution was evenly distributed over the whole wafer surface and allowed to dry at room temperature over night. The silk film with the transferred patterns was removed from the wafer surface with the help of a razor blade and tweezers.
RIE
The oxygen RIE process was performed in a custom made research RIE tool [30]. The patterned silk samples were mounted with double sided adhesive cupper tape (Ted Pella) to the cooled chuck to ensure proper thermal conductivity during the subsequent RIE step. The RIE processing conditions were 20 W plate power, < 6 μTorr base pressure and 20 minutes processing time.
Acknowledgements
This material is based upon work supported in part by the U.S. Army Research Laboratory and the U.S. Army Research Office under contract number W911 NF-07-1-0618 and by the DARPA-DSO. Fabrication was partially performed at the Boston University Photonics Center.
References
- 1.Li C, Vepari C, Jin H, Kim H, Kaplan D. Biomaterials. 2006;27:3115. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen JS, Lu H, Richmond J, Kaplan DL. Biomaterials. 2003;24:401. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 3.Parker S, Domachuk P, Amsden J, Bressner J, Lewis J, Kaplan D, Omenetto F. Advanced Materials. 2009;21:2411. [Google Scholar]
- 4.Perry H, Gopinath A, Kaplan D, Dal Negro L, Omenetto F. Advanced Materials. 2008;20:3070. [Google Scholar]
- 5.Omenetto F, Kaplan D. Science. 2010;329:528. doi: 10.1126/science.1188936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Biomaterials. 2003;24:401. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 7.Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Journal of Biomedical Materials Research. 2001;54:139. doi: 10.1002/1097-4636(200101)54:1<139::aid-jbm17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Kim U, Park J, Li C, Jin H, Valluzzi R, Kaplan D. Biomacromolecules. 2004;5:786. doi: 10.1021/bm0345460. [DOI] [PubMed] [Google Scholar]
- 9.Kang D, Xu D, Zhang Z, Pal K, Bang D, Kim J. Macromolecular Materials and Engineering. 2009;294:620. [Google Scholar]
- 10.Jin H, Chen J, Karageorgiou V, Altman G, Kaplan D. Biomaterials. 2004;25:1039. doi: 10.1016/s0142-9612(03)00609-4. [DOI] [PubMed] [Google Scholar]
- 11.Chang G, Kim H, Kaplan D, Vunjak-Novakovic G, Kandel R. European Spine Journal. 2007;16:1848. doi: 10.1007/s00586-007-0364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrence B, Cronin-Golomb M, Georgakoudi I, Kaplan D, Omenetto F. Biomacromolecules. 2008;9:1214. doi: 10.1021/bm701235f. [DOI] [PubMed] [Google Scholar]
- 13.Lu S, Wang X, Lu Q, Hu X, Uppal N, Omenetto F, Kaplan D. Biomacromolecules. 2009;10:1032. doi: 10.1021/bm800956n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang C, Wang X, Gunawidjaja R, Lin Y, Gupta M, Kaplan D, Naik R, Tsukruk V. Advanced functional materials. 2007;17:2229. [Google Scholar]
- 15.Amsden J, Perry H, Boriskina S, Gopinath A, Kaplan D, Dal Negro L, Omenetto F. Optics Express. 2009;17:21271. doi: 10.1364/OE.17.021271. [DOI] [PubMed] [Google Scholar]
- 16.Omenetto F, Kaplan D. Nature Photonics. 2008;2:641. [Google Scholar]
- 17.Kim D, Viventi J, Amsden J, Xiao J, Vigeland L, Kim Y, Blanco J, Panilaitis B, Frechette E, Contreras D. Nature Materials. 2010;9:511. doi: 10.1038/nmat2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao H, Amsden J, Strikwerda A, Fan K, Kaplan D, Zhang X, Averitt R, Omenetto F. Advanced Materials. 2010;22:3527. doi: 10.1002/adma.201000412. [DOI] [PubMed] [Google Scholar]
- 19.Lei M, Gu Y, Baldi A, Siegel R, Ziaie B. Langmuir. 2004;20:8947. doi: 10.1021/la048719y. [DOI] [PubMed] [Google Scholar]
- 20.Tao H, Landy N, Bingham C, Zhang X, Averitt R, Padilla W. Opt. Lett. 2007;32:53. [Google Scholar]
- 21.Jin H, Park J, Karageorgiou V, Kim U, Valluzzi R, Cebe P, Kaplan D. Advanced Functional Materials. 2005;15:1241. [Google Scholar]
- 22.Zhou C, Confalonieri F, Jacquet M, Perasso R, Li Z, Janin J. Proteins: Structure, Function, and Bioinformatics. 2001;44:119. doi: 10.1002/prot.1078. [DOI] [PubMed] [Google Scholar]
- 23.Bain C, Troughton E, Tao Y, Evall J, Whitesides G, Nuzzo R. Journal of the American Chemical Society. 1989;111:321. [Google Scholar]
- 24.Murphy A, John P, Kaplan D. Biomaterials. 2008;29:2829. doi: 10.1016/j.biomaterials.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.She H, Malotky D, Chaudhury M. Langmuir. 1998;14:3090. [Google Scholar]
- 26.Pritchard E, Wilz A, Li T, Lan J, Boison D, Kaplan D. Sustained-release silk biomaterials for drug delivery and tissue engineering scaffolds. presented at Bioengineering Conference, 2009 IEEE 35th Annual Northeast; 2009. [Google Scholar]
- 27.Sameoto D, Li Y, Menon C. Advances in Science and Technology. 2009;54:439. [Google Scholar]
- 28.Nishizawa S, Sakai K, Hangyo M, Nagashima T, Takeda M, Tominaga K, Oka A, Tanaka K, Morikawa O. Terahertz Optoelectronics. Springer; Berlin/Heidelberg: 2005. p. 203. [Google Scholar]
- 29.Tsioris K, Tilburey G, Murphy A, Domachuk P, Kaplan D, Omenetto F. Advanced Functional Materials. 2010;20:1083. [Google Scholar]
- 30.Forgotson N, Khemka V, Hopwood J. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures. 2009;14:732. [Google Scholar]