Abstract
Background
Asthma prevalence is increasing worldwide in most populations, likely due to a combination of heritable factors and environmental changes. Curiously, however, some European farming populations are protected from asthma, which has been attributed to their traditional lifestyles and farming practices.
Objective
We conducted population-based studies of asthma and atopy in the Hutterites of South Dakota, a communal farming population, to assess temporal trends in asthma and atopy prevalence and describe risk factors for asthma.
Methods
We studied 1325 Hutterites (ages 6–91 years) at two time points from 1996 to 1997 and from 2006 to 2009 using asthma questionnaires, pulmonary function and methacholine bronchoprovocation tests, and measures of atopy.
Results
The overall prevalence of asthma increased over the 10 to 13 year study period (7.5% to 11.1%, P = 0.049), whereas the overall prevalence of atopy did not change (45.0% to 44.8%, P = 0.95). Surprisingly, the rise in asthma was only among females (5.8% to 11.2%, P = 0.02); the prevalence among males remained largely unchanged (9.4% to 10.9%, P = 0.57). Atopy, which was not associated with asthma risk in 1996 to 1997, was the strongest risk factor for asthma among Hutterites studied in 2006 to 2009 (P = 0.003).
Conclusions
Asthma has increased over a 10 to 13 year period among Hutterite females and atopy has become a significant risk factor for asthma, suggesting a change in environmental exposures that are either sex-limited or that elicit a sex-specific response.
Keywords: asthma, atopy, farming exposures, prevalence
INTRODUCTION
The prevalence of asthma has increased in most developed countries over the past 30 years.1, 2 In the United States, the prevalence of asthma rose from 3.1% to 7.4% between 1980 and 2007, with increases in both children (4.3% to 9.1%) and adults (2.8% to 7.3%).3–7 This rise has been variously attributed to concurrent increases in immunization and antibiotic use,8–12 obesity and sedentary lifestyles,13, 14 pro-inflammatory diets, 15–17 and air pollution,18, 19 and to decreases in exposure to microbes in infancy, 20, 21 duration of breastfeeding,22–24 and family sizes, 25–27 among other factors.
In contrast, children raised on traditional European farms are protected from asthma,28–32 suggesting that farming exposure in early life offers protection from asthma and atopy.33 This farm-specific protection has been attributed to endotoxin exposure in utero and in early childhood,29, 34, 35 and possibly to exposure to cattle barns and raw (unpasteurized) milk in particular.29, 31, 36, 37 Curiously, this protective effect has not been consistently observed in children raised on U.S. farms,38, 39 possibly due to differences in farming practices in the U.S. and Europe that include type of livestock, residential proximity to farm animals, farm size, use of antibiotics in livestock feed, and other farming practices that may be relevant to asthma susceptibility.33, 39
In this paper we report the results of a cross-sectional study of asthma and allergic sensitization at two time-points spanning 10 to 13 years in a U.S. farming community, the Hutterites of South Dakota. The Hutterites are a religious isolate that originated in the South Tyrol in the 1500’s and, after migrations throughout Europe over the next 300 years, settled in the United States in the 1870’s. This population offers unique advantages for genetic studies because they live on large communal farms (called colonies), which ensures that environmental exposures are relatively uniform among individuals. Relevant to the study of pulmonary disease, smoking is prohibited (and rare) in this population. Although their farming practices are automated and state-of-the-art, the Hutterites retain a traditional lifestyle that has changed little over many decades. For example, TVs, radios, and internet access are forbidden, nearly all food is raised or grown on the colony, family sizes are large, and children are educated through the 8th grade in 2-classroom schoolhouses on the colony.
We have been conducting genetic studies of asthma in the Hutterites since 1993 and have reported significant sex-specific genetic architecture for asthma-associated quantitative traits in this population.40–44 Here we describe sex- and age-specific changes in asthma prevalences between 1996 and 2009 and describe the changing role of atopy in determining asthma risk.
METHODS
Study Population
Approximately 900 Hutterites migrated from Europe in the 1870’s to what is now South Dakota, and settled on three communal farms, called colonies.45 The Hutterites have remained reproductively isolated since arriving in the United States; however, due to a high natural fertility rate and desire for large families, they subsequently spread across the upper midwestern United States and western Canada.45–48 Today there are more than 35,000 Hutterites living on approximately 350 colonies, each of which is comprised of 20 to 30 families. The more than 1200 subjects in our studies live in South Dakota and are related to each other in a 13-generation, 3,671-person pedigree with 64 founders. The mean kinship coefficient among these individuals is 0.035, slightly greater than that of first cousin once removed.
The Hutterites of South Dakota are highly productive poultry, pork, beef and dairy farmers, though colonies vary with respect to the number and variety of livestock raised and farm acreage. The average colony encompasses 4000 square acres of land, and in addition to the farm facilities, also includes the Hutterite homes, communal kitchen and dining room, laundry, mechanical shops, schoolhouse and church. Unlike European farmers, Hutterite women, infants, and young children rarely visit the animal barns, which are located at a distance from their homes, school, church, and dining room.
Although early life exposures, particularly in infancy, are very similar among boys and girls, Hutterite children begin the transition to sex-specific roles on the colony during their school years, with women eventually assuming responsibility for childcare, cooking, cleaning, sewing, and gardening and men taking on different jobs that include working with livestock (hogs, beef and dairy cows) or poultry (turkeys and chickens), raising crops (soybean, wheat and corn), or performing technical or administrative jobs.
Phenotyping Studies
Phenotyping studies were conducted in 1996 to 1997 and in 2006 to 2009 during visits to South Dakota colonies in the winter months (end of October through early March), the least busy season for farmers. Nine colonies were visited during each phase, of which eight were visited during both phases (a total of 10 colonies). All Hutterites age 6 years and older who were present on the days of our visits were invited to participate in these studies. Participation in each colony was greater than 95%, providing an unbiased cross-section of the Hutterite population (age 6 years and older). In addition, Hutterites from other colonies in South Dakota, Minnesota, and North Dakota who were visiting one of the 10 colonies on the days of our visits were invited to participate in our studies. In 1996 to 1997, 597 individuals completed asthma studies and 687 individuals completed skin prick testing; in 2006 to 2009, 841 individuals completed asthma studies and 937 individuals completed skin prick testing. Of these individuals, 309 were studied for asthma and 361 were studied for atopy at both time points. Methacholine bronchial challenges were not performed on pregnant or lactating women or if there were other medical contra-indications (e.g., prescribed beta blockers), and, therefore, asthma status could not be determined in these individuals. The combined sample size is 1187 Hutterites from 10 communal farms (colonies) in South Dakota studied between 1996 and 2009 and 138 visitors to those colonies (total number with asthma or atopy diagnosis N = 1325).
Subjects were evaluated using a modified protocol from the Collaborative Study on the Genetics of Asthma (CSGA).40, 49 All Hutterites age 15 years and older and mothers of children under the age of 15 years were interviewed by a pulmonologist or asthma nurse using a standardized symptom and clinical history questionnaire for asthma and atopy. A diagnosis of asthma required the following three elements: i) the presence of at least two symptoms (wheezing, cough, or shortness of breath), ii) a positive methacholine bronchial challenge test (PC20 less than or equal to 10 mg/mL) or 15% improvement in baseline FEV1 following inhalation of albuterol, and iii) a doctor’s diagnosis either prior to or at the time of our studies. Of the 45 diagnosed asthmatics in 1996–7, 25 (56%) had a prior physician’s diagnosis of asthma and of the 59 individuals diagnosed in 2006–2009, 17 (29%) had a prior physician’s diagnosis of asthma.
The presence of atopy was determined by a positive skin test reactivity to one or more of the tested allergens (Greer; Lenoir, North Carolina).40 The allergen panel included dust mites (Dermatophagoides pteronyssinus, Dermatophagoides farinae), pollen/grasses (Ambrosia artemisiifolia, Artemisia vulgaris, Lolium perenne, Quercus alba, Betula verrucosa [1996 to 1997 only] or Poa pratensis, Betula nigra [2006 to 2009 only]), pet danders (Felis domesticus, Canis familiaris), cockroaches (Blattella germanica, Periplaneta americana), and molds (Aspergillus fumigatus, Cladosporium herbarum, Alternaria tenuis); as well as negative (saline) and positive (histamine) controls. The area from skin prick wheal reactions (15 minutes after puncturing of the skin) was estimated by calculating the elliptical area using the widest diameter measured and the diameter of the perpendicular axis. Significant skin reactivity was defined as a wheal area greater than 9mm2 greater than that observed for the negative control in the presence of a visible response to histamine.
Prior to initiating our studies, consent was obtained from all adults and from mothers of children ages 18 years or younger and assent was obtained from Hutterites 18 years and younger. These studies were approved by the University of Chicago Institutional Review Board.
Statistical Analysis
The overall, sex-, and age-specific (by decade) prevalences of asthma and atopy were compared using Fisher’s exact tests. P- values (threshold <0.05) are considered significant. P-values from analyses comparing prevalences between the two time points are not corrected for the relatedness between individuals in the sample and are, therefore, slightly anti-conservative. To ensure that the two samples were not interdependent, participants who were studied at both time points were included in the 1996 to 1997 sample but not in the 2006 to 2009 sample in all analyses. This also resulted in samples of similar sizes, making comparisons more readily interpretable.
To gauge the effects of risk factors on asthma, we analyzed both data sets (1996 to 1997 and 2006 to 2009) separately using a binary variance component method. This method was developed in the context of general linear mixed models using a logit link function and is specifically designed to estimate the effects of relevant factors on the risk of a binary trait using data from large pedigrees with a complex lineage.50 We modeled the effects of age, sex, atopy, number of older siblings, and genetics on the development of asthma using a backwards, stepwise selection method (threshold P-value <0.05) to identify the model that best fit each data set. In addition, we examined interaction effects between the significant risk factors. For each time period in our study, the estimates of the parameters from the best fitting model were then used to estimate the risk of asthma.
RESULTS
The demographic and clinical characteristics of our study population are shown in Table I; the sex- and age-specific prevalences of asthma and atopy in 1996 to 1997 and 2006 to 2009 are shown in Tables II and III, respectively. The overall prevalence of asthma increased over this short time period from 7.5% in 1996 to 1997 to 11.1% in 2006 to 2009 (P = 0.049). However, the increase in the prevalence of asthma was present only in females, in whom the prevalence nearly doubled, rising from 5.8% to 11.2% (P = 0.02). In contrast, the overall prevalence of asthma in males remained largely unchanged (9.4% in 1996 to 1997 and 10.9% in 2006 to 2009, P = 0.57). Although the overall prevalence of asthma was highest among children under age 11 years at both time points (23.7% and 21.3%, respectively, P = 0.81), the largest increases in asthma were observed in the 11 to 30 year old cohorts (increase from 6.2% to 11.6% among 11 to 20 year olds, P = 0.08, and 4.0% to 12.7% among 21 to 30 year olds, P = 0.047). The cohorts age 30 years and older had overall low prevalences of asthma that were similar between the two time points. Thus, the increase rise in asthma prevalence was confined to females and to individuals who were between ages 11 and 30 years old during 2006 to 2009.
Table I.
Demographic and Clinical Characteristics of the Study Samples in 1996 – 1997 and 2006 – 2009. For mean values, the standard deviation is presented in parentheses; for median values, the first and third inter-quartile values are presented in parentheses.
| Unaffected | Symptoms Only |
BHR Only |
Asthma | All | |
|---|---|---|---|---|---|
| A. 1996 – 1997 | N=395 | N=62 | N=73 | N=45 | N=597 |
| % Female | 54 | 48 | 53 | 40 | 52 |
| Age, yrs | 27 (17, 41) | 33 (20, 42) | 17 (12, 33) | 22 (10, 37) | 26 (16, 41) |
| BMI, kg/m2 | 24 (20, 28) | 26 (23, 31) | 23 (17, 27) | 24 (17, 27) | 24 (20, 28) |
| % Predicted FEV1 | 103 (15) | 100 (12) | 96 (18) | 94 (10) | 101 (15) |
| % Predicted FVC | 107 (15) | 104 (11) | 104 (17) | 105 (11) | 106 (14) |
| Mean FEV1/FVC | 82 (8) | 82 (8) | 80 (11) | 78 (9) | 81 (9) |
| Total IgE, kU/L | 20 (9, 50) | 31 (8, 93) | 14 (6, 36) | 96 (24, 350) | 23 (10, 77) |
| N (%) ≥1 +SPT | 161 (41) | 32 (52) | 39 (53) | 20 (45) | 252 (44) |
| N (%) ≥3 +SPT | 76 (19) | 16 (26) | 20 (27) | 12 (27) | 124 (22) |
| N (%) Asthmatic Mother | 20 (8) | 2 (5) | 6 (12) | 7 (22) | 35 (8) |
| N (%) Asthmatic Father | 30 (12) | 8 (18) | 9 (20) | 9 (27) | 58 (15) |
| # Older siblings | 3 (1, 6) | 3 (1, 7) | 3 (2, 6) | 2 (1, 4) | 3 (1, 5) |
| B. 2006 –2009 | N=243 | N=78 | N=119 | N=59 | N=532 |
| % Female | 50 | 64 | 51 | 54 | 54 |
| Age, yrs | 19 (14, 38) | 49 (17, 32) | 11 (9, 16) | 20 (10, 14) | 17 (12, 35) |
| BMI, kg/m2 | 23 (20, 28) | 26 (20, 30) | 19 (17, 23) | 22 (17, 24) | 22 (19, 27) |
| % Predicted FEV1 | 101 (13) | 97 (13) | 95 (12) | 94 (12) | 98 (13) |
| % Predicted FVC | 104 (14) | 98 (14) | 96 (12) | 103 (13) | 102 (14) |
| Mean FEV1/FVC | 85 (7) | 85 (7) | 87 (8) | 83 (8) | 85 (8) |
| Total IgE, kU/L | 8 (18, 44) | 9 (18, 43) | 26 (8, 66) | 62 (23, 298) | 23 (9, 61) |
| N (%) ≥1 +SPT | 105 (43) | 35 (45) | 47 (39) | 36 (61) | 223 (45) |
| N (%) ≥3 +SPT | 71 (29) | 25 (32) | 33 (28) | 35 (59) | 164 (33) |
| N (%) Asthmatic Mother | 9 (6) | 2 (5) | 10 (10) | 13 (32) | 37 (10) |
| N (%) Asthmatic Father | 9 (5) | 3 (7) | 17 (17) | 11 (27) | 42 (11) |
| # Older siblings | 3 (1, 5) | 2 (1, 5) | 2 (1, 4) | 2 (1, 4) | 3 (1, 5) |
BHR = bronchial hyperresponsiveness; BMI = body mass index; FEV1 = forced expiratory volume the first second; FVC = forced vital capacity; SPT = skin prick test.
Table II.
Prevalence of asthma in 1996–1997 and 2006–2009. Individuals studied for asthma at both time points are included only in the 1996–1997 groups (N=309). P-values are calculated using a Fisher’s exact test but are not corrected for relatedness of individuals in the sample. P-values <0.05 are in bold font.
| 1996 – 1997 (# Asthma/ Total) |
Prevalence | 2006 – 2009 (# Asthma/ Total) |
Prevalence | P-val | |
|---|---|---|---|---|---|
| Overall | 45/597 | 7.5% | 59/532 | 11.1% | 0.049 |
| Males | 27/286 | 9.4% | 27/247 | 10.9% | 0.57 |
| Females | 18/311 | 5.8% | 32/285 | 11.2% | 0.02 |
| Age, Yrs | |||||
| ≤10 | 9/38 | 23.7% | 17/80 | 21.3% | 0.81 |
| 11 to 20 | 11/177 | 6.2% | 27/232 | 11.6% | 0.08 |
| 21 to 30 | 5/126 | 4.0% | 7/55 | 12.7% | 0.047 |
| 31 to 40 | 10/97 | 10.3% | 2/65 | 3.1% | 0.126 |
| 41 to 50 | 5/83 | 6.0% | 2/52 | 3.8% | 0.70 |
| >50 | 5/76 | 6.6% | 4/48 | 8.3% | 0.73 |
Table III.
Prevalence of atopy in 1996 – 1997 and 2006 – 2009. Individuals studied for atoyp at both time points are included only in the 1996–1997 groups (N=361). P-values are calculated using a Fisher’s exact test but are not corrected for relatedness of individuals in the sample. P-values <0.05 are in bold font.
| 1996 – 1997 (# Atopic/ Total) |
Prevalence | 2006 – 2009 (# Atopic/ Total) |
Prevalence | P- val | |
|---|---|---|---|---|---|
| Overall | 309/687 | 45.0% | 258/576 | 44.8% | 0.95 |
| Males | 156/316 | 49.4% | 118/268 | 44.0% | 0.21 |
| Females | 153/371 | 41.2% | 140/308 | 45.5% | 0.28 |
| Age, Yrs | |||||
| ≤10 | 40/70 | 57.1% | 44/132 | 33.3% | 0.001 |
| 11 to 20 | 106/199 | 53.3% | 105/220 | 47.7% | 0.28 |
| 21 to 30 | 69/138 | 50.0% | 26/51 | 50.9% | 1.00 |
| 31 to 40 | 38/119 | 31.9% | 36/72 | 50.0% | 0.02 |
| 41 to 50 | 27/82 | 32.9% | 23/47 | 48.9% | 0.09 |
| >50 | 29/79 | 36.7% | 24/54 | 44.4% | 0.47 |
The observed rise in asthma overall was not coupled with a change in the overall prevalence of atopy in the Hutterites (Table III), which was 45.0% in 1996 to 1997 and 44.8% in 2006 to 2009 (P = 0.95), both lower than the 54.3% prevalence of atopy reported in the U.S. population.51 The prevalence of atopy also did not change significantly in males or females between 1996 and 2009. Surprisingly, however, we saw a dramatic age-specific decrease in atopy prevalence in children ages 10 and younger, with skin prick reactivity dropping from 57.1% in 1996–1997 to 33.3% in 2006–2009 (P = 0.001). In contrast and unlike the trends for asthma, the prevalence of atopy remained relatively unchanged (~50%) among Hutterites ages 11 to 30 but increased in individuals older than 30 years from 32–37% in 1996–1997 to 44–50% in 2006–2009 (Table III).
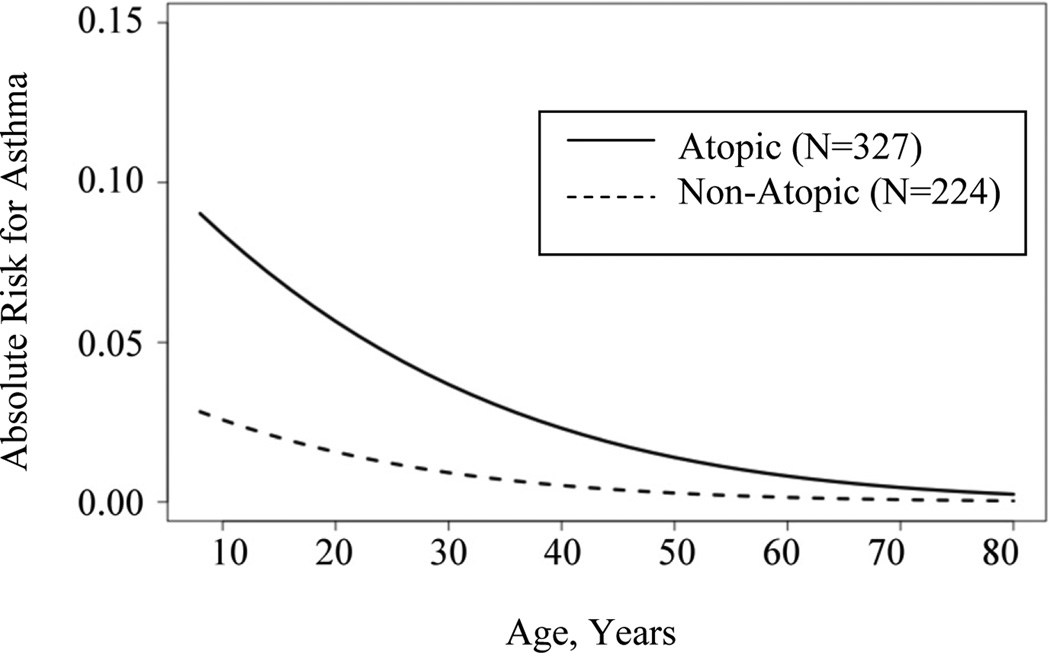
To evaluate whether the effects of risk factors for asthma changed between the two time points, we assessed the effects of age, sex, atopy, and number of older siblings in each sample using a multivariate model that also included a component that models the contribution of genetic factors to the risk of asthma. All analyses at each time point revealed a strong genetic component to the development of asthma (P<0.001). However, other risk factors for asthma differed in the 1996 to 1997 and 2006 to 2009 samples (Table IV). In the earlier study period, being male (P=0.056) and/or having fewer older siblings (P=0.033) contributed to asthma risk; age and atopy were not significant during this time period. However, in the more recent study period, the effect of sex and number of older siblings were no longer significant predictors of asthma, whereas atopy (P=0.003) and younger age (P=0.004) were significant. That sex is not a significant predictor of asthma in 2006–2009 is attributed to the fact that the prevalences of asthma between males and females are similar in the more recent sample (i.e., the females ‘caught up’ to the males during the later time period); the association with ‘younger age’ in the later period reflects the increased prevalence in the 11 to 30 year old cohorts. The lack of association with ‘number of older siblings’ in the later cohort was surprising, but likely reflects the large effects of atopy and age that may have reduced our power to detect the older sibling effect. None of the interaction terms between the significant covariates at either time point were significant. The age-specific risks for Hutterites with and without atopy are shown in Figure I for the 2006–2009 sample.
Table IV.
Multivariate analysis of risk factors for asthma in 1996–1997 and 2006–2009. Only covariates close to the significance threshold (P-value <0.05) are listed.
| 1996 – 1997 | ||||
|---|---|---|---|---|
| Covariates | Estimate (SE) | O.R. (95% C.I.) | P-val | |
| Constant | −3.45 (0.83) | <0.001 | ||
| Sex | −0.97 (0.51) | 0.38 (0.14, 1.03) | 0.056 | |
| No. Older Siblings | −0.18 (0.09) | 0.83 (0.71, 0.98) | 0.033 | |
| Genetic Component† | 2.58 (0.58) | <0.001 | ||
| 2006 – 2009 | ||||
| Covariates | Estimate (SE) | O.R. (95% C.I.) | P-val | |
| Constant | −6.82 (1.62) | <0.001 | ||
| Age | −0.08 (0.03) | 0.92 (0.87, 0.98) | 0.004 | |
| Atopy†† | 2.22 (0.73) | 9.22 (2.19, 38.85) | 0.003 | |
| Genetic Component† | 3.79 (0.84) | <0.001 | ||
The genetic component is an estimate of the variability in asthma risk that can be attributed to genetics.
Atopy is defined a positive skin prick test response to any of 13 tested aero-allergens (see Methods).
Figure I.
Absolute age-specific risks for asthma by atopic status in 2006–2009.
DISCUSSION
The prevalence of asthma has risen over the past decade in the Hutterites, similar to other ‘Westernized’ populations.2 In the Hutterites, the rise in asthma was disproportionately in females and in the subset of the population between the ages of 11 and 30 years. The female-specific rise in asthma prevalence mirrors data from the National Health and Nutrition Examination Survey (NHANES) data,5, 6 as well as from other international epidemiological studies.52–55 In NHANES, U.S. asthma prevalences have consistently been higher in females than in males (4.6% vs. 3.3%, respectively, in 1996–1997 based on symptoms, and 8.6% vs. 6.9%, respectively, in 2006 to 2008 based on a physician’s diagnosis).3 However, in contrast to NHANES, there was virtually no change in asthma rates among Hutterite males during this time period.
In the Canadian National Public Health Study, Ghosh et al. investigated asthma prevalence by sex, age, and urban/rural residence from 1996 to 2001. They also reported a trend toward increased asthma in women over time, particularly in the 15 to 34 years age group, whereas boys in the 0 to 14 years age group consistently had the highest overall prevalence of asthma.53 Female sex was also associated with a greater incidence of new asthma cases in the European Community Respiratory Health Survey of young adults who were unaffected at age 20 to 44 years in 1990 to 1995, but developed asthma in the interval between 1998 to 2003. However, atopy was not a significant risk factor for new-onset asthma in that study, in contrast to our findings in the Hutterites.52 In all of the aforementioned studies, both female sex and young to mid-adulthood appear to be important elements allowing for the development of asthma.
A genetic component contributing to asthma risk in the Hutterites was highly significant in the multivariate analyses of both the 1996 to 1997 and 2006 to 2009 samples (P < 0.001). Although the multivariate method used in this study does not allow a direct measure of the changes in genetic risk, the strong genetic predisposition to asthma and the increased prevalence observed in this population over a relatively short time period are similar findings to those of a recent Danish twin cohort study.56 In that study, an increase in the heritability of asthma over a similarly short (decade-long) time period was observed, in particular among females. The investigators proposed a change in the penetrance of asthma risk alleles as a result of changing environmental exposures as a plausible mechanism. We suggest a similar mechanism for the sex- and age-specific changes in asthma prevalence in the Hutterites over a 10 to 12 year interval.
For example, a change in common practices (such as a shared occupational exposure among women) may underlie the increase of asthma and allergic sensitization over the past 10–12 years. Although we do not know the specific exposure, it is notable that during this same time period the Hutterite women stopped using homemade soaps for cleaning their houses and started to purchase commercial cleaners, which have been associated with occupational asthma in other populations.57 For instance, exposure to quaternary ammonium compounds, such as those found in many common cleaners (egs., Pine-Sol® and Lysol®), induced a non-specific IgE response to common aeroallergens and concurrent respiratory symptoms compatible with asthma in a study of pig farmers.58 Cleaning chemicals are thought to act as low molecular weight haptens which complex to other proteins (including allergens) and thus drive a non-specific IgE response.59 This would support an immunologic basis for the rise in asthma prevalence in women and may represent an unmasking of an underlying genetic predisposition to asthma.
Lastly, we note the unexpected observation of a significant decrease in atopy in children ages ten years and younger (from 57.1% to 33.3%, Table III) between 1996 to 1997 and 2006 to 2009, without a corresponding change in the prevalence of asthma (Table II). This observation suggests that the trigger for asthma in the youngest age group is not directly tied to an allergic response as measured by the skin prick test. A similar discordance was seen in the increase in asthma prevalence among 11 to 30 year olds without a corresponding change in the prevalence of atopic sensitization. These observations underscore that atopic sensitization remains a relatively common condition in the Hutterites. Although sensitization did not correlate with asthma risk in the earlier study, it became an important risk factor for development of asthma in the 2006 to 2009 sample, particularly in the younger age groups (Figure I). These combined observations reflect the fact that the overall decrease in atopy in children in the later time period occurred disproportionally in the non-asthmatic children.
While the causes of the increased prevalence of asthma in this farming community are at present still unknown, our observation of the increased asthma prevalence that is confined solely to females and individuals ages 11 to 30 years of age is intriguing. Whether this represents a response to an exposure that is unique to this community, or an exposure that is common to many populations (such as household cleaners) remains to be determined, but nonetheless extends the spectrum of environmental factors that may be contributing toward the worldwide rise in asthma prevalence developed countries. Furthermore, this epidemiological study underscores the importance of future efforts to untangle important gene by environment interactions that may bear on sex- and development-specific observations of disease prevalence.
KEY MESSAGES.
Asthma has increased significantly in a South Dakota farming population, but only in females.
Hutterite females and males are now similarly affected by a high prevalence of asthma.
Atopy and younger age were independent risk factors for asthma among Hutterites studied in 2006 to 2009 compared to 1996 to 1997.
Acknowledgements
We thank Erika von Mutius, Anne Sperling, and Dan Nicolae for helpful discussions, Gaixin Du and Ying Sun for computational assistance, members of our field trip team (Gorka Alkorta Aranburu, Maitane Arruabarrena Orbegozo, Elizabeth Anderson, Rebecca Anderson, Sally Cain, Minal Çalişkan, Jessica Chong, Kristina Davis, Marcy deTineo, Sandy Koch, Susan Kuldanek, James Klocksieben, Gülüm Kosova, Dagan Loisel, Leslie Martin, Kathleen Murray, Jayant Pinto, Natasha Philips, Robert Stanaker, Emma Thompson, Kathleen Shanovich, Preeti Sharma, Shilpy Sharma, and Melody Young), and the Hutterites for their continued participation in our studies.
Funding: NIH Grants U01 HL49596, P50 HL56399, R01 HL085197 to C.O.; C.A.M. supported by K12 HL090003.
ABBREVIATIONS
- BHR
Bronchial hyperresponsiveness
- CSGA
Collaborative Study on the Genetics of Asthma
- FEV1
Forced exhaled volume in the first second
- FVC
Forced vital capacity
- IgE
Immunoglobulin E
- NHANES
National Health and Nutrition Examination Survey
- PC20
Methacholine concentration producing a 20% decrease in FEV1
- SPT
Skin prick test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 2.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 3.Asthma and chronic obstructive pulmonary disease: U.S., 1999–2007.: Centers for Disease Control.] Available from http://www.cdc.gov/nchs/hdi.htm.
- 4.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. National surveillance for asthma--United States, 1980–2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 5.Summary Health Statistics for U.S. Adults: National Health Interview Survey. Hyattsville (MD): U.S. Department of Health and Human Services. Center for Disease Control and Prevention. National Center for Health Statistics.]; 2008. [Google Scholar]
- 6.Summary Health Statistics for U.S. Children: National Health Interview Survey. Hyattsville (MD): U.S. Department of Health and Human Services. Center for Disease Control and Prevention. National Center for Heatlh Statistics.]; 2008. [Google Scholar]
- 7.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma--United States, 1980–1999. MMWR Surveill Summ. 2002;51:1–13. [PubMed] [Google Scholar]
- 8.Marra F, Lynd L, Coombes M, Richardson K, Legal M, Fitzgerald JM, et al. Does antibiotic exposure during infancy lead to development of asthma?: a systematic review and metaanalysis. Chest. 2006;129:610–618. doi: 10.1378/chest.129.3.610. [DOI] [PubMed] [Google Scholar]
- 9.Wickens K, Ingham T, Epton M, Pattemore P, Town I, Fishwick D, et al. The association of early life exposure to antibiotics and the development of asthma, eczema and atopy in a birth cohort: confounding or causality? Clin Exp Allergy. 2008;38:1318–1324. doi: 10.1111/j.1365-2222.2008.03024.x. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz EL, Morgenstern H. Effects of diphtheria-tetanus-pertussis or tetanus vaccination on allergies and allergy-related respiratory symptoms among children and adolescents in the United States. J Manipulative Physiol Ther. 2000;23:81–90. [PubMed] [Google Scholar]
- 11.McDonald KL, Huq SI, Lix LM, Becker AB, Kozyrskyj AL. Delay in diphtheria, pertussis, tetanus vaccination is associated with a reduced risk of childhood asthma. J Allergy Clin Immunol. 2008;121:626–631. doi: 10.1016/j.jaci.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Matheson MC, Haydn Walters E, Burgess JA, Jenkins MA, Giles GG, Hopper JL, et al. Childhood immunization and atopic disease into middle-age - a prospective cohort study. Pediatr Allergy Immunol. 2009 doi: 10.1111/j.1399-3038.2009.00950.x. [DOI] [PubMed] [Google Scholar]
- 13.Platts-Mills T. Asthma severity and prevalence: An ongoing interaction between exposure, hygiene and lifestyle. PLoS Medicine. 2005;2:122–126. doi: 10.1371/journal.pmed.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platts-Mills T. Specific and non-specific obstructive lung disease in disease in childhood: Causes of changes in the prevalence of asthma. Environ Health Perspect. 2000;108:725–731. doi: 10.1289/ehp.00108s4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haby MM, Peat JK, Marks GB, Woolcock AJ, Leeder SR. Asthma in preschool children: prevalence and risk factors. Thorax. 2001;56:589–595. doi: 10.1136/thorax.56.8.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyake Y, Sasaki S, Arakawa M, Tanaka K, Murakami K, Ohya Y. Fatty acid intake and asthma symptoms in Japanese children: the Ryukyus Child Health Study. Clin Exp Allergy. 2008;38:1644–1650. doi: 10.1111/j.1365-2222.2008.03074.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Ellwood PE, Asher MI. Diet and asthma: looking back, moving forward. Respir Res. 2009;10:49. doi: 10.1186/1465-9921-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gehring U, Wijga AH, Brauer M, Fischer P, de Jongste JC, Kerkhof M, et al. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med. 2010;181:596–603. doi: 10.1164/rccm.200906-0858OC. [DOI] [PubMed] [Google Scholar]
- 19.McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, et al. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359:386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- 20.von Hertzen L, Haahtela T. Disconnection of man and the soil: reason for the asthma and atopy epidemic? J Allergy Clin Immunol. 2006;117:334–344. doi: 10.1016/j.jaci.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Braun-Fahrlander C. Environmental exposure to endotoxin and other microbial products and the decreased risk of childhood atopy: evaluating developments since April 2002. Curr Opin Allergy Clin Immunol. 2003;3:325–329. doi: 10.1097/00130832-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Sears MR, Greene JM, Willan AR, Taylor DR, Flannery EM, Cowan JO, et al. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet. 2002;360:901–907. doi: 10.1016/S0140-6736(02)11025-7. [DOI] [PubMed] [Google Scholar]
- 23.Oddy WH. The long-term effects of breastfeeding on asthma and atopic disease. Adv Exp Med Biol. 2009;639:237–251. doi: 10.1007/978-1-4020-8749-3_17. [DOI] [PubMed] [Google Scholar]
- 24.Nagel G, Buchele G, Weinmayr G, Bjorksten B, Chen YZ, Wang H, et al. Effect of breastfeeding on asthma, lung function and bronchial hyperreactivity in ISAAC Phase II. Eur Respir J. 2009;33:993–1002. doi: 10.1183/09031936.00075708. [DOI] [PubMed] [Google Scholar]
- 25.Karmaus W, Botezan C. Does a higher number of siblings protect against the development of allergy and asthma? A review. J Epidemiol Community Health. 2002;56:209–217. doi: 10.1136/jech.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strachan DP. Family size, infection and atopy: the first decade of the "hygiene hypothesis". Thorax. 2000;55(Suppl 1):S2–S10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg S, Israeli E, Schwartz S, Shochat T, Izbicki G, Toker-Maimon O, et al. Asthma prevalence, family size, and birth order. Chest. 2007;131:1747–1752. doi: 10.1378/chest.06-2818. [DOI] [PubMed] [Google Scholar]
- 28.Ernst P, Cormier Y. Relative scarcity of asthma and atopy among rural adolescents raised on a farm. Am J Respir Crit Care Med. 2000;161:1563–1566. doi: 10.1164/ajrccm.161.5.9908119. [DOI] [PubMed] [Google Scholar]
- 29.Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 30.Von Ehrenstein OS, Von Mutius E, Illi S, Baumann L, Bohm O, von Kries R. Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy. 2000;30:187–193. doi: 10.1046/j.1365-2222.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 31.Elliott L, Yeatts K, Loomis D. Ecological associations between asthma prevalence and potential exposure to farming. Eur Respir J. 2004;24:938–941. doi: 10.1183/09031936.04.00006404. [DOI] [PubMed] [Google Scholar]
- 32.Klintberg B, Berglund N, Lilja G, Wickman M, van Hage-Hamsten M. Fewer allergic respiratory disorders among farmers' children in a closed birth cohort from Sweden. Eur Respir J. 2001;17:1151–1157. doi: 10.1183/09031936.01.00027301. [DOI] [PubMed] [Google Scholar]
- 33.von Mutius E, Radon K. Living on a farm: impact on asthma induction and clinical course. Immunol Allergy Clin North Am. 2008;28:631–647. ix–x. doi: 10.1016/j.iac.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Douwes J, Cheng S, Travier N, Cohet C, Niesink A, McKenzie J, et al. Farm exposure in utero may protect against asthma, hay fever and eczema. Eur Respir J. 2008;32:603–611. doi: 10.1183/09031936.00033707. [DOI] [PubMed] [Google Scholar]
- 35.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 36.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 37.Waser M, Michels KB, Bieli C, Floistrup H, Pershagen G, von Mutius E, et al. Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across Europe. Clin Exp Allergy. 2007;37:661–670. doi: 10.1111/j.1365-2222.2006.02640.x. [DOI] [PubMed] [Google Scholar]
- 38.Chrischilles E, Ahrens R, Kuehl A, Kelly K, Thorne P, Burmeister L, et al. Asthma prevalence and morbidity among rural Iowa schoolchildren. J Allergy Clin Immunol. 2004;113:66–71. doi: 10.1016/j.jaci.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 39.Merchant JA, Naleway AL, Svendsen ER, Kelly KM, Burmeister LF, Stromquist AM, et al. Asthma and farm exposures in a cohort of rural Iowa children. Environ Health Perspect. 2005;113:350–356. doi: 10.1289/ehp.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ober C, Tsalenko A, Parry R, Cox NJ. A second-generation genomewide screen for asthma-susceptibility alleles in a founder population. Am J Hum Genet. 2000;67:1154–1162. doi: 10.1016/s0002-9297(07)62946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358:1682–1691. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ober C, Abney M, McPeek MS. The genetic dissection of complex traits in a founder population. Am J Hum Genet. 2001;69:1068–1079. doi: 10.1086/324025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss LA, Pan L, Abney M, Ober C. The sex-specific genetic architecture of quantitative traits in humans. Nat Genet. 2006;38:218–222. doi: 10.1038/ng1726. [DOI] [PubMed] [Google Scholar]
- 44.Ober C, Pan L, Phillips N, Parry R, Kurina LM. Sex-specific genetic architecture of asthma-associated quantitative trait loci in a founder population. Curr Allergy Asthma Rep. 2006;6:241–246. doi: 10.1007/s11882-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 45.Hostetler J. Hutterite Society. Baltimore: John Hopkins University Press; 1974. [Google Scholar]
- 46.Sheps M. An analysis of reproductive patterns in an American isolate. Popul Stud. 1965;19:65–80. [Google Scholar]
- 47.Steinberg A. Genetic studies in an inbred human isolate. In: Crow JaJN., editor. Proc. Third Intl. Congr. of Human Genetics. Baltimore: John Hopkins University Press; 1967. pp. 267–290. [Google Scholar]
- 48.Ober C, Hyslop T, Hauck WW. Inbreeding effects on fertility in humans: evidence for reproductive compensation. Am J Hum Genet. 1999;64:225–231. doi: 10.1086/302198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lester LA, Rich SS, Blumenthal MN, Togias A, Murphy S, Malveaux F, et al. Ethnic differences in asthma and associated phenotypes: collaborative study on the genetics of asthma. J Allergy Clin Immunol. 2001;108:357–362. doi: 10.1067/mai.2001.117796. [DOI] [PubMed] [Google Scholar]
- 50.Papachristou C, Ober C, Abney M. Genetic variance components estimation for binary traits using multiple related individuals. Genet Epidemiol. 2011 doi: 10.1002/gepi.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 52.Anto JM, Sunyer J, Basagana X, Garcia-Esteban R, Cerveri I, de Marco R, et al. Risk factors of new-onset asthma in adults: a population-based international cohort study. Allergy. doi: 10.1111/j.1398-9995.2009.02301.x. [DOI] [PubMed] [Google Scholar]
- 53.Ghosh S, Pahwa P, Rennie D, McDuffie HH. Opposing trends in the prevalence of health professional-diagnosed asthma by sex: a Canadian National Population Health Survey study. Can Respir J. 2008;15:146–152. doi: 10.1155/2008/793913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson DH, Adams RJ, Tucker G, Appleton S, Taylor AW, Ruffin RE. Trends in asthma prevalence and population changes in South Australia, 1990–2003. Med J Aust. 2006;184:226–229. doi: 10.5694/j.1326-5377.2006.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 55.Brogger J, Bakke P, Eide GE, Johansen B, Andersen A, Gulsvik A. Long-term changes in adult asthma prevalence. Eur Respir J. 2003;21:468–472. doi: 10.1183/09031936.03.00056103. [DOI] [PubMed] [Google Scholar]
- 56.Thomsen SF, van der Sluis S, Kyvik KO, Skytthe A, Skadhauge LR, Backer V. Increase in the heritability of asthma from 1994 to 2003 among adolescent twins. Respir Med. 2011 doi: 10.1016/j.rmed.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 57.Kogevinas M, Anto JM, Sunyer J, Tobias A, Kromhout H, Burney P. Occupational asthma in Europe and other industrialised areas: a population-based study. European Community Respiratory Health Survey Study Group. Lancet. 1999;353:1750–1754. doi: 10.1016/s0140-6736(98)07397-8. [DOI] [PubMed] [Google Scholar]
- 58.Preller L, Doekes G, Heederik D, Vermeulen R, Vogelzang PF, Boleij JS. Disinfectant use as a risk factor for atopic sensitization and symptoms consistent with asthma: an epidemiological study. Eur Respir J. 1996;9:1407–1413. doi: 10.1183/09031936.96.09071407. [DOI] [PubMed] [Google Scholar]
- 59.Mapp CE, Boschetto P, Maestrelli P, Fabbri LM. Occupational asthma. Am J Respir Crit Care Med. 2005;172:280–305. doi: 10.1164/rccm.200311-1575SO. [DOI] [PubMed] [Google Scholar]