Abstract
We previously found that selective restriction of amino acids inhibits invasion of two androgen-independent human prostate cancer cell lines, DU145 and PC3. Here we show that the restriction of tyrosine (Tyr) and phenylalanine (Phe), methionine (Met) or glutamine (Gln) modulates the activity of G proteins and affects the balance between two actin-binding proteins, cofilin and profilin, in these two cell lines. Selective amino acid restriction differentially reduces G protein binding to GTP in DU145 cells. Tyr/Phe deprivation reduces the amount of Rho-GTP and Rac1-GTP. Met deprivation reduces the amount of Ras-GTP and Rho-GTP, and Gln deprivation decreases Ras-GTP, Rac-GTP, and Cdc42-GTP. Restriction of these amino acids increases the amount of profilin, cofilin and phosphorylation of cofilin-Ser3. Increased PAK1 expression and phosphorylation of PAK1-Thr423, and Ser199/204 are consistent with the increased phosphorylation of LIMK1-Thr508. In PC3 cells, Tyr/Phe or Gln deprivation reduces the amount of Ras-GTP, and all of the examined amino acid restrictions reduce the amount of profilin. PAK1, LIMK1 and cofilin are not significantly altered. These data reveal that specific amino acid deprivation differentially affects actin dynamics in DU145 and PC3. Modulation on Rho, Rac, PAK1, and LIMK1 likely alter the balance between cofilin and profilin in DU145 cells. In contrast, profilin is inhibited in PC3 cells. These effects modulate directionality and motility to inhibit invasion.
The relative specific amino acid dependency is one of the metabolic abnormalities of malignant cells including prostate cancer cells (Fu et al., 1999; Scott et al., 2000; Dillon et al., 2004). We previously found that selective restriction of amino acids inhibits invasion of two human prostate cancer cell lines, DU145 and PC3. However, the mechanisms by which specific amino acid restriction affects invasion of prostate cancer cells are poorly understood.
Tumor cell invasion is a complex process including repeated adhesion to and detachment from the extracellular matrix (ECM), release or activation of proteases that degrade ECM, and direct migration through ECM (Slack et al., 2001). Specific amino acid restriction does not inhibit release or activation of proteases (unpublished results). Therefore, the present study focuses on how specific amino acid restriction affects cell attachment, directionality and motility.
Prostate cancer cells are adhesion-dependent and attach to ECM by cell surface integrins that bind to ECM proteins like fibronectin and laminin. Integrins also interact via their cytoplasmic domains to components of the actin cytoskeleton and signaling molecules within the cell (Aplin et al., 1998; Giancotti and Ruoslahti, 1999). Focal adhesion kinase (FAK) is a major mediator of integrin signaling and a key regulator of focal adhesion dynamics and cell movement (Lipfert et al., 1992; Schaller et al., 1992; Juliano and Haskill, 1993; Parsons et al., 2000; Hsia et al., 2003). FAK and its interacting partners have a major impact on migration of prostate cancer cells (Sumitomo et al., 2000; Slack et al., 2001). We showed previously that specific amino acid restriction modulates the integrin/FAK pathway and actin cytoskeleton remodeling of melanoma and inhibits FAK in prostate cancer cells (Fu et al., 2003, 2004). We are extending those studies to examine the effects of amino acid restriction on cell surface integrins and their intracellular binding partners, paxillin and talin.
The integrin/FAK pathway activates small GTPases (G proteins) including Ras, Rho, Rac and Cdc42 (Sahai and Marshall, 2002), which direct cell movement and regulate actin cytoskeleton arrangement (Hall, 1998; Kraynov et al., 2000; Kulkarni et al., 2000; Katoh et al., 2001; Meili and Firtel, 2003). Additionally, Ras and Rho signaling influence the binding of integrins to laminin and fibronectin (Bar-Sagi and Hall, 2000; Parise et al., 2000), and this controls the activation of integrins (Hynes, 2003). Recent studies reveal the connection between the activities of G protein signaling and invasion, migration and progression of prostate cancer (Hodge et al., 2003; Weber and Gioeli, 2004; Chen et al., 2005; Yao et al., 2006; Zheng et al., 2006; Zhou et al., 2006). The present study elucidates the activity of Ras, Rho, Rac and Cdc42 G proteins in DU145 and PC3 cells during specific amino acid restriction.
The motility of prostate cancer cells is dependent on intracellular actin dynamics. Two actin-binding proteins, cofilin and profilin, are major mediators that regulate this process. Cofilin induces F-actin depolymerization, and this function is inhibited by phosphorylation on the Ser3 residue by LIM kinase 1 (LIMK1) (Schmidt and Hall, 1998; Niwa et al., 2002). The activity of LIMK1 is regulated by distinct members of the Rho family of G proteins (Rho, Rac and Cdc42), and LIMK1 is essential for the invasion of prostate cancer cells (Davila et al., 2003). Moreover, activation of LIMK1 is mediated by PAK1, one of the 21 kDa activated kinases that phosphorylates LIMK1 at the Thr508 residue (Davila et al., 2003; Misra et al., 2005). Earlier we showed that specific amino acid restriction inhibits invasion of solid tumor cells including prostate cancer cells (Pelayo et al., 2001; Fu et al., 2003, 2004), and the present study examines kinetic changes in cofilin and profilin in DU145 and PC3 cells during specific amino acid restriction. The alterations of LIMK1 and PAK1, two upstream signaling molecules that regulate activity of cofilin, are also determined.
We found in DU145 and PC3 prostate cancer cells that specific amino acid restriction affects attachment to and spreading on ECM. Selective amino acid deprivation differentially modulates the activity of G proteins and affects the balance between cofilin and profilin in these two cell lines. These effects modulate the directionality and motility of prostate cancer cells to inhibit invasion.
Materials and Methods
Cell culture conditions
Two human prostate cancer cell lines, DU145 and PC3 (American Type Tissue Collection, Rockville, MD), were maintained in suitable media (DMEM for DU145 and RPMI 1640 for PC3) containing 10% fetal bovine serum (Equitech-Bio, Inc., Kerrville, TX). Amino acid-deprived media were prepared from Selectamine kits or by custom manufacture (Life Technologies, Grand Island, NY) as described previously (Fu et al., 1999, 2003). In experiments, an individual amino acid or two combined amino acids (Tyr/Phe) were deleted completely. Cells were initially cultured in complete medium until they became 30–40% confluent. Then, the media were replaced with amino acid-deficient media. All cells used for experiments were harvested when they reached about 70% confluency. Before harvesting, cells were washed with phosphate buffered saline twice to remove dead cells. For the cell attachment assay and cell surface integrin staining, the cells were harvested with 2 mM ethyleneglycotetraacetic acid (EGTA) in phosphate buffered saline to avoid the membrane-altering effect of trypsin. Unless otherwise stated, the results in the figures are representative of findings from three separate experiments.
Cell attachment assays
The cell attachment assays followed published methods (Frisch et al., 1996; Fu et al., 2004). Briefly, cells cultured in complete or in amino acid-deprived medium were washed with phosphate buffered saline twice to remove dead cells. Then, 1 × 105 viable cells suspended in DMEM or specific amino acid-free medium containing 1% bovine serum albumin were plated onto six-well plastic dishes that were pre-coated with either 50 μg/ml fibronectin, 50 μg/ml laminin, or 50 μg/ml Growth Factor Reduced Matrigel (Collaborative Biomedical Products/Becton Dickinson, Bedford, MA). The incubation time was the minimum during which a maximal number of prostate cancer cells attached to the substrates. Thus, DU145 and PC3 cells were allowed to attach for 4–8 h in a CO2 incubator at 37°C. Cells then were fixed and stained with Diff-Quik (Baxter Healthcare Corporation, McGaw Park, IL). The attached cells were quantified by counting six randomly selected fields in each well under a light microscope at 200×. The data from duplicate wells were averaged, and the results presented as the percentage of cells grown in complete medium. Each experiment was repeated three times.
Integrin expression
The expression of integrins was measured by flow cytometry. Briefly, cells grown in complete or in amino acid-deprived medium were suspended in phosphate buffered saline and stained with fluorescein isothiocyanate or phycoerythrin-labeled monoclonal antibodies against human integrin α1, α2, α3, α4, α5, α6, αv, β1, β2 and β3 (BD PharMingen, San Diego, CA). Then, 2 × 104 events were analyzed by flow cytometry for each sample (Tsuji et al., 2002; Fu et al., 2004). Appropriately labeled IgG was used to identify non-specific background staining.
Immunoblotting analysis
Total cell lysates were resolved under SDS–PAGE (4–20%) electrophoresis and subjected to immunoblotting as previously described (Fu et al., 2003, 2004). The antibodies against cofilin, PAK1, LIMK1 and anti-phosphorylation residue-specific cofilin, PAK1, and LIMK1 were obtained from Cell Signaling Technology (Beverly, MA). The antibody against profilin was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Monoclonal antibody to FAK was obtained from BD Transduction Laboratories (Lexington, KY). Phosphorylation residue-specific antibodies for FAK were obtained from BioSource International, Camarillo, CA (Slack et al., 2001). Anti-actin antibody (Santa Cruz Biotechnology Inc.) was simultaneously used as an internal control to assess relative protein loading (Fu et al., 2003, 2004).
Rho or Ras activation assay
The GTP binding activity of G proteins including those of the Rho family (Rho, Rac 1, Cdc42) and of Ras was analyzed using activation assay kits from Cytoskeleton (Denver, CO). Briefly, the GTP-bound G proteins were precipitated by specific binding proteins. For example, the Rho binding domain (RBD) of Rho effector protein, Rhotekin, binds specifically to the GTP-bound form of pan-Rho proteins including RhoA, B and C. The glutathione S-transferase-tagged Rhotekin RBD precipitated the GTP-bound Rho protein from the cell lysate, and the amount of activated Rho was determined by an immunoblot with an anti-Rho antibody. An equal amount of protein from cells grown in normal or amino acid restricted media was used in each assay to identify the relative GTP binding activity of each G protein. The Ras activation assay is similar to the Rho activation assay.
Confocal microscopy
Immunofluorescence confocal microscopy was conducted, as previously described (Fu et al., 2003, 2004). Briefly, prostate cancer cells attached to chamber slides (Nalge Nunc International, Napeville, IL) that were pre-coated with fibronectin or laminin were fixed in 4% paraformaldehyde for 5 min at 4°C and then permeabilized with 0.1% Tween 20 for 10 min. Then, the cells were incubated with an anti-paxillin monoclonal antibody (BD Transduction Laboratories, Lexington, KT). Texas-Red-conjugated anti-mouse IgG antibody was used as a secondary antibody (Vector Laboratories, Burlingame, CA). The slides then were mounted with an anti-fade mounting medium with 4′,6′-diamidino-2-phenylindole (DAPI; Vector Laboratories). Fluorescent images were captured in a Leica TCS-4D DMIRBE laser scanning confocal microscope.
Statistical analysis
Differences between group means were analyzed by one-way analysis of variance (ANOVA). The differences in kinetics of the various proteins following amino acid restriction were analyzed by ANOVA with the general linear models computer program. Significant differences were defined as P <0.05.
Results
In this study we show that specific amino acid restriction affects DU145 and PC3 prostate cancer cell attachment to and spreading on ECM components. Selective amino acid deprivation differentially modulates the GTP binding activity of the Rho family and Ras proteins. Moreover, two major mediators of actin dynamics, cofilin and profilin, and two upstream molecules of cofilin, LIMK1 and PAK1, that regulate activity of cofilin are also differentially modulated in these two cell lines by the specific amino acid restriction.
Amino acid restriction differentially inhibits cell attachment of DU145 and PC3
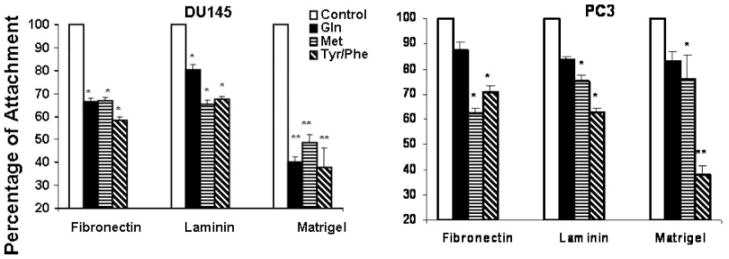
All of the examined amino acid restrictions inhibited cell attachment of DU145 cells to laminin, fibronectin and Matrigel (Fig. 1). In PC3 cells, Tyr/Phe and Met restriction inhibited cell attachment to ECM; however, Gln restriction did not inhibit cell attachment. These results are consistent with the inhibitory effect of these amino acid restrictions on the invasive ability of these two cell lines (Fu et al., 2003).
Fig. 1.
Deprivation of specific amino acids inhibits attachment of DU145 and PC3 cells. Cells were cultured in complete medium or amino acid-deprived medium for 3 days. Then 1 × 105 cells were suspended in 0.1% bovine serum albumin and plated into six-well culture dishes precoated with fibronectin, laminin or Matrigel as described in Materials and Methods Section. Each dish was incubated in a tissue culture incubator for 4 h. Then the attached cells were counted microscopically(Materials and Methods Section). Values represent the mean ± SE of three experiments and are presented as a percentage of the attached cells cultured in complete medium. *P < 0.01, **P < 0.001 compared to cells cultured in normal medium (Control).
Amino acid restriction modulates integrin expression of PC3
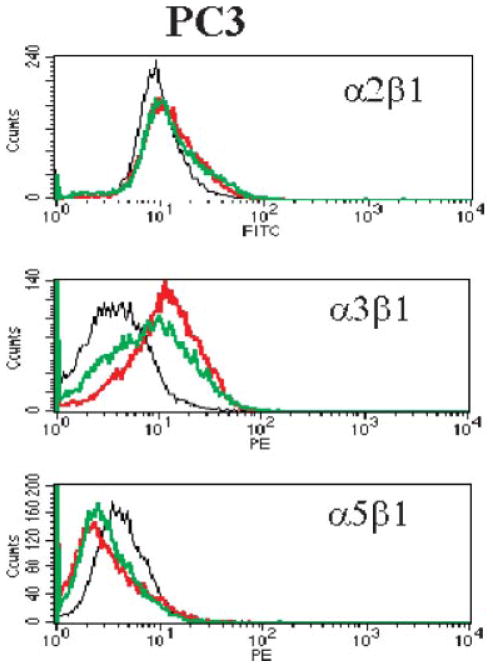
Among the ten integrins examined, Tyr/Phe and Met restriction increased the cell surface density of α3β1 integrin in PC3 cells, and reduced that of α5β1 integrin (Fig. 2). However, neither Tyr/Phe, Met nor Gln restriction significantly altered surface expression of the ten examined integrins in DU145 cells (data not shown).
Fig. 2.
Specific amino acid deprivation modulates the expression of surface integrin molecules in PC3 cells. Specific culture and staining conditions are described in Materials and Methods Section. Cells were suspended and stained with fluorescently labeled anti-integrin antibodies. Twenty thousand events for each sample were collected by and analyzed. The X axis represents the density of fluorescence. The Y axis represents the cell count. Black line: cells cultured in complete medium; red line: cells cultured in Tyr/Phe-deprived medium; green line: cells cultured in Met-deprived medium. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Amino acid restriction inhibits cell spreading of DU145 and PC3
Immunofluorescence confocal microscopy was used to visualize the inhibitory effect of amino acid restriction of prostate cancer cell spreading on fibronectin or laminin. Like the effect on cell attachment, restriction of Tyr/Phe and Met inhibited cell spreading of PC3 (Fig. 3), and all of the restrictions examined inhibited cell spreading of DU145 cells. These results are consistent with the inhibitory effect of the restrictions on invasion (Fu et al., 2003). During cell spreading, F-actin accumulates at the leading edges of the protrusions, and F-actin forms networks for shaping the cell. Thus, the inhibitory effect of amino acid restriction on prostate cancer cell spreading indicates that actin cytoskeleton remodeling is affected by the amino acid restrictions in these prostate cancer lines.
Fig. 3.
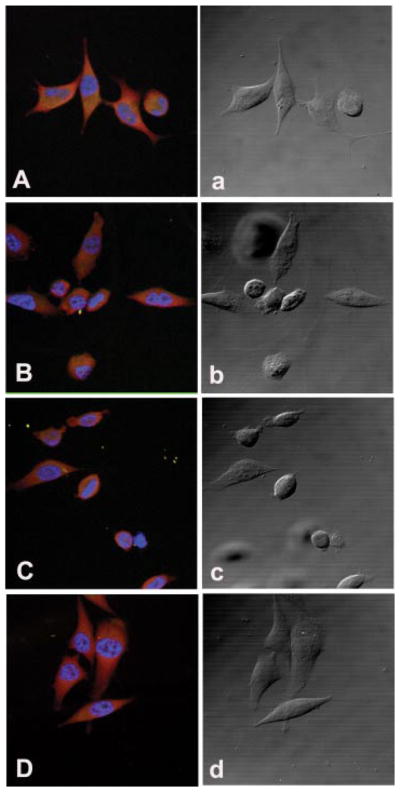
Confocal fluorescence microscopy of paxillin in PC3 cells during cell attachment. Cells were cultured in complete medium, or Tyr/Phe-, Gln-, or Met-restricted medium for 3 days and then allowed to attach to fibronectin coated four-well slides for 4 h. The slides were incubated with an anti-paxillin monoclonal antibody and then stained with a Texas Red-conjugated horse anti-mouse IgG antibody. The slides were then mounted with an anti-fade mounting medium containing DAPI to show nuclear staining. A and a: Confocal fluorescence (A) and phase contrast (a) images in PC3 cells cultured in normal medium. B and b: Cells cultured in Tyr/Phe-free medium for 3 days. C and c: Cells cultured in Met-free medium for 3 days. D and d: Cells cultured in Gln-free medium for 3 days. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Unlike melanoma cell lines (Fu et al., 2004), the inhibitory effect of amino acid restriction on cell attachment and spreading of DU145 is not related to alterations in surface integrins. Since specific amino acid restriction modulates the integrin/FAK pathway in melanoma and inhibits FAK in prostate cancer cells (Fu et al., 2003, 2004), we expected that the activity of integrin intracellular binding partners, such as paxillin and talin, would be inhibited and that the activation of down-stream events in the integrin/FAK pathway, such as Rho family and Ras G proteins, would be modulated by amino acid restriction. We found that Tyr/Phe, Met and Gln restriction did not alter the expression of talin, or the expression and phosphorylation of paxillin in DU145 and PC3 cells.
Amino acid restriction differentially inhibits GTP binding activity of Rho and Ras G proteins in DU145 and PC3
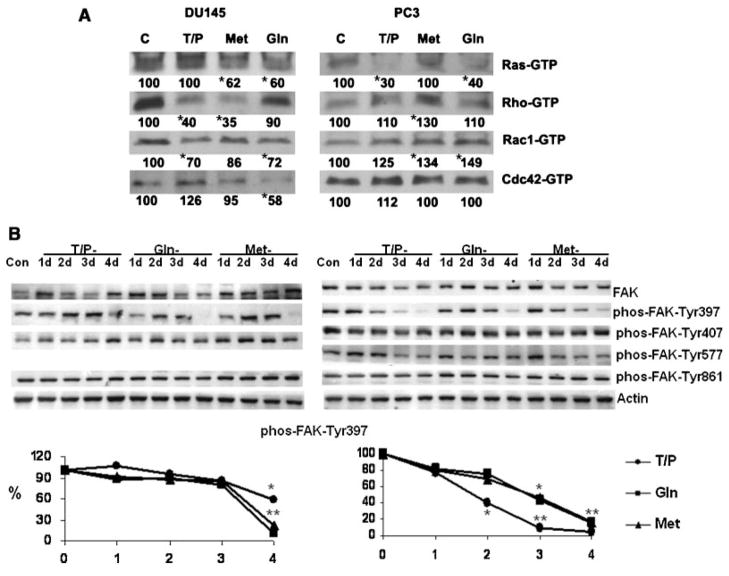
Selective amino acid deprivation differentially modulates the GTP binding activity of the Rho family of proteins (Rho, Rac, Cdc42) and Ras G proteins in DU145 and PC3 cells. The effect of specific amino acid restriction on GTP binding of Rho, Rac, Cdc42 and of Ras proteins is shown in Figure 4A. The total amounts of these proteins were not altered by selective amino acid deprivation. In DU145 cells, Tyr/Phe deprivation reduced the amount of Rho-GTP and Rac1-GTP. Met deprivation reduced the amount of Ras-GTP and Rho-GTP, and Gln deprivation decreased Ras-GTP, Rac1-GTP, and Cdc42-GTP. In PC3 cells, Tyr/Phe and Gln deprivation reduced the amount of Ras-GTP. None of the amino acid restrictions reduced GTP binding of Rho family (Rho, Rac Cdc42) proteins and in some cases binding increased. Moreover, all amino acid restrictions inhibited the adhesion-dependent autophosporylation of FAK-Tyr397 in DU145 and PC3 cells (Fig. 4B).
Fig. 4.
A: Specific amino acid restriction differentially modulates the activity of G proteins. Prostate cancer cells were cultured in complete or amino acid-free medium for 3 days. GTP binding activity of each G protein was analyzed using the activation assay kits from Cytoskeleton (Denver, CO). Briefly, the GTP-bound G proteins were precipitated by specific binding proteins. For example, the Rho binding domain (RBD) of Rho effector protein Rhotekin, binds specifically to the GTP-bound form of Rho. The glutathione S-transferase-tagged Rhotekin RBD precipitated the GTP-bound Rho protein from the cell lysate and the amount of activated Rho was determined by an immunoblot with a Rho specific antibody. An equal amount of protein from cells cultured in normal or amino acid restricted conditions was used in each assay to precipitate each GTP-bound G protein. The blots show the relative activation of each G protein. Since selective amino acid restriction does not alter the total amount of these proteins in DU145 and PC3, the relative activation of each G protein in cells cultured in amino acid-free media is shown as a percentage of control cells cultured in complete medium (Numbers at the bottom of the blot). Lane C: lysates from cells cultured in complete medium; Lane T/P: lysates from cells cultured in Tyr/Phe-free medium; Lane Gln: lysates from cell cultured in Gln-free medium. *P < 0.01 compared to cells cultured in complete medium. Statistical analysis was conducted on the results from three experiments. B. Immunoblots of FAK in DU145 and PC3 during amino acid restriction (center part) and densitometry of phos-FAK-Tyr397 (bottom). Cells were cultured in complete (Con) or in amino acid-free medium. Immunobloting was conducted as described in Materials and Methods Section. Lane C: lysate from cells grown in complete medium. Lanes 1d–4d: cells cultured from 1 to 4 days respectively in Tyr/Phe (T/P), Gln, or Met restricted media. Each blot was also probed with an anti-actin monoclonal antibody that served as a protein loading control. We did not detect phos-FAK-Tyr577 in DU145 cells. The autophosphorylation of FAK at Tyr397 is adhesion-dependent (Slack et al., 2001). The ratio of the integrated absorbance of the phos-FAK-Tyr397band to the FAK band was used as an index of its phosphorylation. The relative phosphorylation of phos-FAK-Tyr397 from cells cultured in amino acid-free media is expressed as the ratio to cells cultured in complete medium and is expressed on the Y-axis as a percentage (%) in the bottom panel. *P < 0.01, **P < 0.001 compared to cells cultured in complete medium. Statistical analysis was conducted on the results of three experiments. (●) Tyr/Phe-, (■) Gln-, and (▲) Met-restriction.
Amino acid restriction differentially modulates proteins regulating actin dynamics in DU145 and PC3
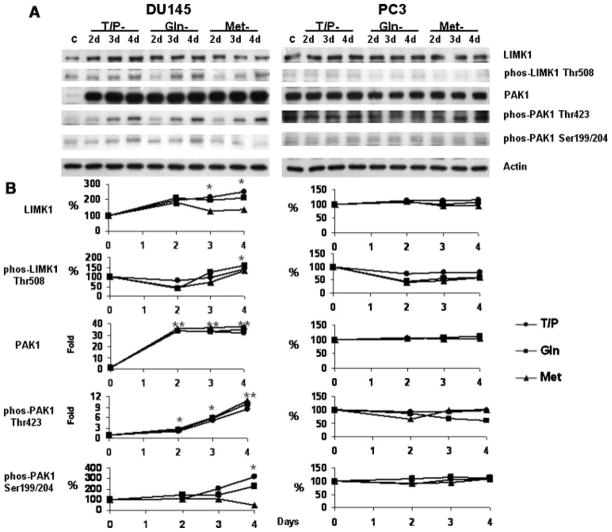
Our earlier studies showed that specific amino acid restriction inhibits motility of melanoma and prostate cancer cell lines (Pelayo et al., 2001; Fu et al., 2003, 2004). Motility of prostate cancer cells is dependent on dynamic changes in actin polymerization/depolymerization that are regulated by different actin binding proteins. For example, the actin polymerization is regulated by cofilin and profilin. Actin nucleation is mediated by several proteins such as talin, vinculin, actinin, paxillin, tensin, and FAK (Schmidt and Hall, 1998). We first examined the kinetic alterations of these proteins in DU145 and PC3 cells under the specific amino acid restrictions and found that among these proteins, only FAK, profilin and cofilin were modulated (Fig. 5 and Fu et al., 2003). The regulatory function of cofilin on actin depolymerization is inhibited by phosphorylation at the Ser3 residue by LIMK1 (Schmidt and Hall, 1998; Niwa et al., 2002). The activity of LIMK1 is mediated by PAK1, which phosphorylates LIMK1 at the Thr508 residue (Davila et al., 2003; Misra et al., 2005). The kinetic changes of LIMK1 and PAK1 in DU145 and PC3 during specific amino acid restriction are shown in Figure 6.
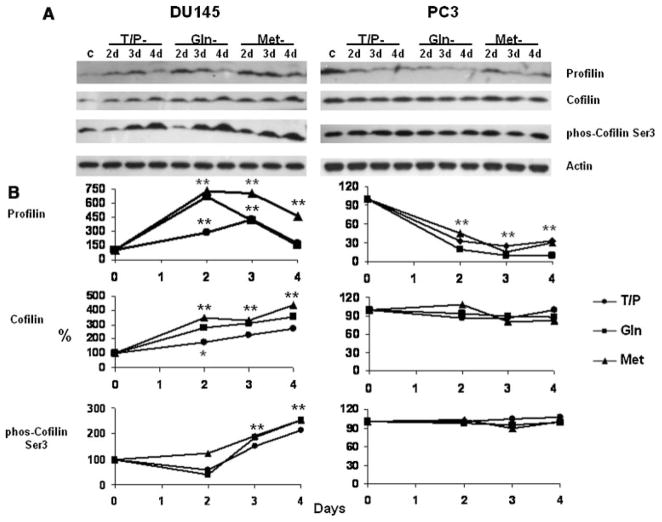
Fig. 5.
A: Immunoblots of profilin and cofilin in DU145 and PC3 during amino acid restriction. Cells were cultured in complete or in amino acid-free medium. The proteins on the immunoblots were analyzed as described in Materials and Methods Section. Lane C: lysate from cells grown in complete medium. Lanes 2d–4d: cells cultured from 2 to 4 days respectively in Tyr/Phe (T/P), Gln, or Met restricted media. Each blot was also probed with an anti-actin monoclonal antibody that served as a protein loading control. B: Densitometry of A. The ratio of the integrated absorbance of the profilin or cofilin band to that of the actin band is used as an index of its expression. The relative amount of each protein in cells cultured in aminoacid-free media is expressed as a percentage of control cells. The ratio of the integrated absorbance of the phosphorylated cofilin band to the cofilin band is used as an index of its phosphorylation. The relative phosphorylation of each protein from cells cultured in aminoacid-free media is expressed as the ratio to cells cultured in complete medium. *P < 0.01, **P < 0.001 compared to cells cultured in complete medium. Statistical analysis was conducted on the results from three experiments. (●) Tyr/Phe-, (■) Gln-, and (▲) Met-restriction.
Fig. 6.
Immunoblots of LIMK1 and PAK1 in DU145 and PC3 under aminoacid restriction. A: Cells were cultured in complete or in aminoacid-free medium. Lane C: lysate from cells grown in complete medium. Lanes 2d–4d: cells cultured from 2 to 4 days respectively in Tyr/Phe (T/P), Gln, or Met restricted media. Each blot was also probed with an anti-actin monoclonal antibody that served as a protein loading control. B: Densitometry of A. The ratio of the integrated absorbance of the LIMK1 and PAK1 band to that of the actin band is used as an index of its expression. The relative amount of each protein in cells cultured in amino acid-free media is expressed as a percentage or fold of control cells. The relative phosphorylation of each protein from cells cultured in amino acid-free media is expressed as a percentage or fold of control cells. *P < 0.01, **P < 0.001 compared to cells cultured in complete medium. Statistical analysis was conducted on the results from three experiments. (●) Tyr/Phe-, (■) Gln-, and (▲) Met-restriction.
The results in Figures 5 and 6 show that the selective restriction of amino acids differentially modulates profilin, cofilin, LIMK1, and PAK1 expression and phosphorylation. In DU145 cells, Tyr/Phe, Gln and Met restriction similarly increased the amount of profilin after 2 days and in some cases expression continued to increase (Tyr/Phe) or remain elevated (Met) at 3 days. By 4 days expression of profilin returned to control levels in Tyr/Phe- and Gln-restricted cells and was decreasing, but still elevated in Met-restricted cells (Fig. 5). The amount of cofilin gradually increased over the 4-day period for all of the amino acid restrictions. After a decrease in phosphorylation of cofilin-Ser3 in Try/Phe- and Gln-restricted cells, phosphorylation increased through day 4 (Fig. 5). Expression of the phosphorylated cofilin was similar. Also in DU145 cells, Tyr/Phe, Gln and Met restriction similarly increased the amount of PAK1 and phosphorylation of PAK1-Thr423 (Fig. 6). Tyr/Phe and Gln restriction increased phosphorylation of PAK1-Ser199/204 by day 4; however, phosphorylation decreased in Met-restricted cells. PAK1 expression similarly increased during Tyr/Phe, Gln, and Met restriction, and the increases are consistent with increased phosphorylation of LIMK1 at Thr508 indicating that LIMK1 is activated during amino acid restriction (Fig. 6). Activated LIMK1 will phosphorylate and inactivate cofilin (Schmidt and Hall, 1998; Niwa et al., 2002). Thus, the increased phosphorylation of LIMK1-Thr508 (Fig. 6) is consistent with the increased phosphorylation of cofilin-Ser3 in DU145 cells (Fig. 5).
Unlike DU145, Tyr/Phe, Gln, and Met restriction reduced the amount of profilin from 2 to 4 days and did not alter the expression of cofilin or phosphorylation of cofilin-Ser3 in PC3 (Fig. 5). Moreover, expression of LIMK1, PAK1 and phosphorylation of these proteins was also not altered in PC3 cells under these amino acid restrictions (Fig. 6). Thus, LIMK1 is not activated by Tyr/Phe, Gln, and Met restriction in PC3 cells.
Discussion
We previously found that selective restriction of amino acids inhibits invasion of two human prostate cancer cell lines, DU145 and PC3 (Fu et al., 2003). Cell invasion is a complex process including repeated adhesion to and detachment from ECM, and directionally mediated migration through ECM. In the present study we found that specific amino acid restriction not only affects cell attachment and spreading of these prostate cancer cells, but also differentially modulates the activity of G proteins and proteins that regulate actin. The results provide important clues into the mechanism(s) by which specific amino acid restriction affects cell attachment, directionality, motility and invasion of prostate cancer cells. The results also indicate that the mechanisms by which restriction of amino acids inhibit invasion are different between DU145 and PC3 cells.
The role of integrins in prostate cancer cell adhesion to and spreading on ECM is well known (Kostenuik et al., 1997; Johnson, 1999; Laidler et al., 2000; Liu et al., 2000; Parise et al., 2000; Hao et al., 2001; Felding-Habermann et al., 2002; Giannelli et al., 2002). Our previous study found that specific amino acid restriction selectively inhibits the expression of cell surface integrins in melanoma cells and indicated a specific integrin-dependent mechanism that is associated with the inhibition of the cell attachment and spreading (Fu et al., 2004). However, the present study shows that Tyr/Phe, Gln and Met restriction does not alter surface expression of integrins in DU145 cells, although the restrictions inhibited cell attachment and spreading. Thus, it appears that the inhibition of cell attachment and spreading are independent of cell surface integrin expression in DU145 cells.
In PC3 cells, however, Tyr/Phe and Met restriction increased the surface density of α3β1 integrin and reduced that of α5β1 integrin (Fig. 2). These integrins are regarded as the receptors for fibronectin and laminin (Aplin et al., 1998; Giancotti and Ruoslahti, 1999). We found that attachment to and spreading on these substrates was inhibited by Tyr/Phe and Met restriction. While information on the role of α3β1 integrin in prostate cancer invasion is rare, one study has shown an inverse correlation between expression of this integrin and invasion in PC3 cells (Dedhar et al., 1993). The role of α5β1 integrin in invasion of PC3 cells is not clear (Romanov and Goligorsky, 1999); however, reduced expression is related to decreased adhesion and invasion in other solid tumor cells (Schmitmeier et al., 2003; Tantivejkul et al., 2003). Thus, the alterations of integrin induced by amino acid restriction could contribute to the inhibitory effects on cell adhesion, spreading, and invasion of PC3 cells.
The inhibition of cell attachment and spreading suggests that specific amino acid restriction interferes with the interactions between integrin–ECM and integrin–FAK since we previously found that specific amino acid restriction inhibits FAK in DU145 and PC3 cells (Fu et al., 2003). In PC3 cells, the inhibition solely of FAK phosphorylation does not affect the formation of integrin–FAK complexes during attachment (Liu et al., 2000). The inhibition of cell attachment in PC3 cells by amino acid restriction could be due to the alteration of integrins and not to FAK phosphorylation. Thus, the inhibition of FAK phosphorylation by specific amino acid restriction most likely inhibits cell spreading and migration since the expression and function of FAK correlates with migratory capacity of PC3 and DU145 cells (Slack et al., 2001). FAK and its interacting partners have a major impact on migration of prostate cancer cells (Sumitomo et al., 2000). During cell attachment, spreading and migration, FAK is involved in the formation of focal adhesion/ complexes. Focal complexes and focal adhesions are actin nucleation sites, which direct membrane-associated actin polymerization (Schmidt and Hall, 1998). On the intracellular side of these complexes, the actin anchoring proteins like paxillin, actinin, talin, vinculin, tensin, and, FAK, are the crucial molecules for actin nucleation. We found that the expression of paxillin, actinin, talin, vinculin and tensin are not altered by specific amino acid restriction in DU145 and PC3 cells. Thus, it is likely that inhibition of FAK by the amino acid restriction impacts actin nucleation, which in turn affects cell attachment (of DU145), spreading and migration (of DU145 and PC3 cells). Additionally, integrins and FAK regulate cell spreading and migration through the Rho-related family of GTPases (Giancotti and Ruoslahti, 1999).
Ras and Rho GTPases (Rho, Rac, CdC42) play important roles in controlling cell directionality, motility and migration (Hall, 1998; Parent and Devreotes, 1999; Bar-Sagi and Hall, 2000; Kraynov et al., 2000; Gu et al., 2003). Activated Cdc42 and Rac facilitate the formation of protrusions at the leading edge of cells to direct migration, and Ras and Rho regulate the disassembly of focal adhesions at the rear of the cell (Bar-Sagi and Hall, 2000; Meili and Firtel, 2003). Activities of these G proteins not only are involved in migration, but also are connected to invasion and progression of prostate cancer (Hodge et al., 2003; Weber and Gioeli, 2004; Chen et al., 2005; Yao et al., 2006; Zheng et al., 2006; Zhou et al., 2006). The present study shows that specific amino acid restriction differentially modulates the activity of Ras, Rho, Rac and Cdc42 in DU145 and PC3 cells (Fig. 4).
The results in DU145 cells indicate that amino acid restriction inhibits invasion through a Rho GTPase-dependent pathway since Tyr/Phe, Gln, and Met deprivation all reduced the GTP binding activity of Rho protein. These findings suggest that the amino acid restrictions slow down the Rho-regulated rear release of cell adhesions. The fact that actin cytoskeleton rearrangement and cell adhesion of DU145 are Rho-dependent (Wells et al., 2005) adds further support for this concept. The inhibition of Rac and Cdc42 by Tyr/Phe and Gln restriction indicates that control of cell directionality is inhibited. Moreover, the inhibitory effect of amino acid restriction on the Rho G protein family in DU145 cells occurs either in conjunction with FAK or is independent from effects on FAK. Tyr/Phe restriction inhibits FAK in these cells (Fu et al., 2003), and this is consistent with inhibition of Rac GTP binding. FAK is not affected by Met or Gln restriction (Fu et al., 2003); however restriction of these amino acids inhibits the Ras and Rho family GTPases. These data help to explain the FAK-independent effect of these amino acid restrictions on inhibition of invasion previously described in DU145 cells (Fu et al., 2003).
Although recent studies suggest a role for the Rho G protein family in migration and invasion in PC3 cells (Hodge et al., 2003; Chen et al., 2005; Zheng et al., 2006; Zhou et al., 2006), we found no decrease in GTP binding of Rho, Rac1, or Cdc42 induced by the amino acid restrictions (Fig. 4). This could suggest that Tyr/Phe restriction and Met restriction inhibit attachment, spreading, invasion and migration in PC3 though a Rho-independent pathway. Additionally, we observed inconsistent effects of the three amino acid restrictions on Ras-GTP in relation to their effects on cell attachment, spreading, migration, and invasion in PC3 cells. For example, Tyr/Phe and Gln deprivation reduced the amount of Ras-GTP (Fig. 4), but Gln restriction did not inhibit the spreading or invasion of these cells (Fig. 3 and Fu et al., 2003). This indicates that specific amino acid restriction differentially modulates the Ras and Rho G protein pathways in DU145 and PC3 cells.
Maintenance of the actin dynamics in an ordered fashion is essential for promotion of cell motility. The regulation of the actin dynamics involves different actin binding and signaling proteins (Hall, 1998; Schmidt and Hall, 1998; Machesky and Insall, 1999; Niwa et al., 2002). Two actin-binding proteins, profilin and cofilin, are major mediators of actin polymerization and depolymerization dynamics. Cofilin induces F-actin depolymerization and this function is inhibited by phosphorylation at the Ser3 residue by LIMK1 (Schmidt and Hall, 1998; Niwa et al., 2002). The activity of LIMK1 is regulated by distinct members of the Rho G protein family (Rho, Rac and Cdc42). For example, Rac activation induces PAK1 to phosphorylate LIMK1 at Thr508 residue (Davila et al., 2003; Misra et al., 2005). LIMK1 is overexpressed in DU145 and PC3 and is essential for the invasion of prostate cancer cells (Davila et al., 2003).
This study shows that the selective restriction of amino acids differentially modulates profilin, cofilin, LIMK1 and PAK1 in DU145 and PC3 (Figs. 5 and 6). In DU145 cells, Tyr/Phe, Gln and Met restriction increased the amount of profilin within 2 days. The amount of cofilin also increased and phosphorylation of cofilin-Ser3 increased at 3 days (Fig. 5). Tyr/Phe, Gln and Met restriction similarly increased the amount of PAK1 and the phosphorylation of PAK1-Thr423. Tyr/Phe, and Gln but not Met restriction increased phosphorylation of PAK1-Ser199/204. Since the alterations in PAK1 are associated with increased phosphorylation of LIMK1-Thr508, this indicates that LIMK1 is activated and that the decreased phosphorylation of PAK1-Ser199/204 by Met restriction does not affect activation of LIMK1. Activated LIMK1 phosphorylates and inactivates cofilin (Schmidt and Hall, 1998; Niwa et al., 2002). The progressive changes of cofilin, phosphorylated-cofilin, and profilin in DU145 cells induced by amino acid restriction alters the balance between cofilin and profilin, and this would affect their regulatory function to maintain actin polymerization and depolymerization dynamics in a proper order leading to reduced cell motility. Interestingly, the enhanced activation of PAK1 and LIMK1 is not consistent with the inhibition of Rac1 GTP by amino acid restrictions in DU145. This suggests that a Rac-independent pathway is involved in activation of PAK1 and LIMK1 by amino acid restriction. Reduced expression of LIMK1 also inhibits invasiveness of prostate cancer cells (Davila et al., 2003). Since the activation of LIMK1 increased during amino acid restriction, this indicates that inhibition of invasion by amino acid restriction in DU145 cells is not due to inhibition of LIMK1. This also suggests that increased LIMK1 inhibits invasion of DU145 by phosphorylating cofilin and by altering the balance between cofilin and profilin to reduce cell motility.
Unlike DU145, Tyr/Phe, Gln, and Met restriction only reduced the amount of profilin in PC3 cells. This suggests that inhibition of profilin alone is sufficient to alter actin dynamics/ cell motility in PC3 cells.
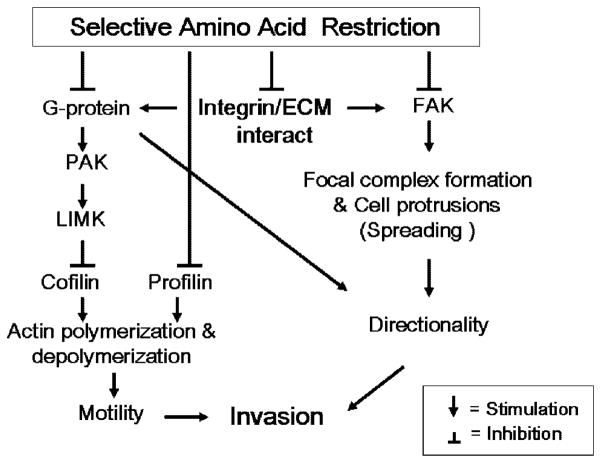
The present study shows that specific amino acid restriction differentially affects multiple steps in cell invasion of DU145 and PC3 such as cell attachment and spreading, cell motility and directionality, and that the mechanisms by which restriction inhibits invasion are different between the two cell lines. Combined with our previous findings on FAK inhibition induced by specific amino acid restriction, the scheme outlined in Figure 7 concisely summarizes these findings and our current understanding of the underlying the effects of specific amino acid restriction on prostate cancer cell invasion.
Fig. 7.
Diagram concisely summarizing the findings and current understanding regarding the effects of specific amino acid restriction on prostate cancer invasion.
In DU145, the three different amino acid restrictions inhibit integrin-mediated cell adhesion/spreading without significantly altering the cell surface integrins. They all inhibit G protein activity to affect cell directionality. They also progressively modulate two actin-binding proteins, profilin and cofilin, the upstream regulators of cofilin, PAK1 and LIMK1, to affect their regulatory function on maintaining actin polymerization and depolymerization dynamics. This reduces cell motility. Moreover, inhibition of FAK by Tyr/Phe restriction contributes to reduced cell adhesion/spreading and directionality. The combined effects on cell motility and directionality inhibit invasion.
In PC3 cells, the inhibition of cell attachment by Tyr/Phe and Met restriction is associated with modulation of surface density of α3β1 and α5β1 integrins. Inhibition of FAK could further contribute to the inhibition of cell spreading and directionality in cells restricted for Met. Cell motility and the subsequent inhibition of invasion are also related to inhibition of profilin. The differences between DU145 and PC3 under amino acid restriction not only suggest different mechanisms by which restriction inhibits invasion of these two cell lines, but also indicate the complexity in specific amino acid dependency of prostate cancer.
Metabolic studies on specific amino acid dependency of prostate cancers are in their infancy. Because DU145 and PC3 cells were originally isolated from metastatic lesions, DU145 from brain and PC3 from bone, it is possible that differences in the microenvironments at the original metastatic sites not only contributed to the ability of the prostate cancer cells to colonize at the metastatic sites, but also contributed to the differences that we observed in amino acid dependency of these cells. However, the current knowledge regarding the genetic and metabolic phenotypes does not suggest any direct connection to specific amino acid dependency. However, the present study indicates that the amino acid dependency and alterations in cellular processes are connected in these cells.
The purpose of this paper was to compare the effects of amino acid restriction on DU145 and PC3, which are invasive prostate cancer cell lines. We also examined the effects of amino acid restriction on LNCaP, a non-invasive prostate cancer cell line. Compared to DU145 and PC3 Tyr/Phe, Gln, and Met restriction had much less of an effect on attachment and spreading on laminin, fibronectin and Matrigel in LNCaP cells (data not shown). Since LNCaP is non-invasive, we were unable to compare the responses of this cell line to amino acid restriction on cell motility and invasion with those of DU145 and PC3 cells. LNCaP is also resistant to apoptosis induced by Tyr/Phe, Gln, and Met restriction; whereas, restriction of these amino acids induces apoptosis in DU145 and PC3 cells (Fu et al., 2003). This further shows the differences in relative amino acid dependency between invasive and non-invasive prostate cancer cells.
It is not known which metabolic alterations connect to the above signaling pathways that control invasion of prostate cancer; however, this study suggests a possible metabolic connection. For example, the lack of cell protrusions during cell spreading by amino acid restriction might indicate either inhibition of F-actin movement controlled by myosins, or inhibition of G-actin transportation to the leading edges of cell protrusions (Zicha et al., 2003). These processes are ATP-dependent, and our previous study showed that specific amino acid restriction reduces the amount of ATP in DU145 and PC3 cells (Fu et al., 2006). It is likely that the metabolic alterations induced by specific amino acid restriction alter actin cytoskeleton remodeling and cell motility. This further indicates that the detailed metabolic alterations induced by the amino acid restriction require elucidation in order to fully understand the mechanisms by which amino acid restriction inhibits invasion of prostate cancer cells. We are actively pursuing this avenue of investigation.
Acknowledgments
Contract grant sponsor: National Cancer Institute; Contract grant number: R01CA101035.
Abbreviations
- ECM
extracellular matrix
- DAPI
4′,6′-diamidino-2-phenylindole
- FAK
focal adhesion kinase
- Gln
glutamine
- LIMK1
LIM kinase 1
- Met
methionine
- Tyr
tyrosine
- Phe
phenylalanine
- RBD
Rho binding domain
Literature Cited
- Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: The role of integrins, cadherins, immunoglobulin–cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50:197–263. [PubMed] [Google Scholar]
- Bar-Sagi D, Hall A. Ras and Rho GTPases: A family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang Y, Yu H, Wang F, Xu W. The cross talk between protein kinase A- and RhoA-mediated signaling in cancer cells. Exp Biol Med. 2005;230:731–741. doi: 10.1177/153537020523001006. [DOI] [PubMed] [Google Scholar]
- Davila M, Frost AR, Grizzle WE, Chakrabarti R. LIM kinase 1 is essential for the invasive growth of prostate epithelial cells: Implications in prostate cancer. J Biol Chem. 2003;278:36868–36875. doi: 10.1074/jbc.M306196200. [DOI] [PubMed] [Google Scholar]
- Dedhar S, Saulnier R, Nagle R, Overall CM. Specific alterations in the expression of alpha 3 beta 1 and alpha 6 beta 4 integrins in highly invasive and metastatic variants of human prostate carcinoma cells selected by in vitro invasion through reconstituted basement membrane. Clin Exp Metastasis. 1993;11:391–400. doi: 10.1007/BF00132982. [DOI] [PubMed] [Google Scholar]
- Dillon BJ, Prieto VG, Curley SA, Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers: A method for identifying cancers sensitive to arginine deprivation. Cancer. 2004;100:826–833. doi: 10.1002/cncr.20057. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B, Fransvea E, O’Toole TE, Manzuk L, Faha B, Hensler M. Involvement of tumor cell integrin alpha v beta 3 in hematogenous metastasis of human melanoma cells. Clin Exp Metastasis. 2002;19:427–436. doi: 10.1023/a:1016377114119. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Ruoslahti E, Chan-Hui P-Y. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y-M, Yu Z-X, Pelayo BA, Ferrans VJ, Meadows GG. Focal adhesion kinase-dependent apoptosis of melanoma induced by tyrosine and phenylalanine deficiency. Cancer Res. 1999;59:758–765. [PubMed] [Google Scholar]
- Fu Y-M, Yu ZX, Li Y-Q, Ge X, Sanchez PJ, Fu X, Meadows GG. Specific amino acid dependency regulates invasiveness and viability of androgen-independent prostate cancer cells. Nutr Cancer. 2003;45:60–73. doi: 10.1207/S15327914NC4501_8. [DOI] [PubMed] [Google Scholar]
- Fu Y-M, Zhang H, Ding M, Li Y-Q, Fu X, Yu Z, Meadows GG. Specific amino acid restriction inhibits attachment and spreading of human melanoma via modulation of the integrin/focal adhesion kinase pathway and actin cytoskeleton remodeling. Clin Exp Metastasis. 2004;21:587–598. doi: 10.1007/s10585-004-5515-y. [DOI] [PubMed] [Google Scholar]
- Fu YM, Zhang H, Ding M, Li YQ, Fu X, Yu ZX, Meadows GG. Selective amino acid restriction targets mitochondria to induce apoptosis of androgen-independent prostate cancer cells. J Cell Physiol. 2006;209:522–534. doi: 10.1002/jcp.20766. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Astigiano S, Antonaci S, Morini M, Barbieri O, Noonan DM, Albini A. Role of the alpha3beta1 and alpha6beta4 integrins in tumor invasion. Clin Exp Metastasis. 2002;19:217–223. doi: 10.1023/a:1015579204607. [DOI] [PubMed] [Google Scholar]
- Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hao J, Jackson L, Calaluce R, McDaniel K, Dalkin BL, Nagle RB. Investigation into the mechanism of the loss of laminin 5 (α3β3γ3) expression in prostate cancer. Am J Path. 2001;158:1129–1135. doi: 10.1016/s0002-9440(10)64060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge JC, Bub J, Kaul S, Kajdacsy-Balla A, Lindholm PF. Requirement of RhoA activity for increased nuclear factor kappaB activity and PC-3 human prostate cancer cell invasion. Cancer Res. 2003;63:1359–1364. [PubMed] [Google Scholar]
- Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, Huang S, Li E, Nemerow GR, Leng J, Spencer KS, Cheresh DA, Schlaepfer DD. Differential regulation of cell motility and invasion by FAK. J Cell Biol. 2003;160:753–767. doi: 10.1083/jcb.200212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Structural biology. Changing partners. Science. 2003;300:755–756. doi: 10.1126/science.1084854. [DOI] [PubMed] [Google Scholar]
- Johnson JP. Cell adhesion molecules in the development and progression of malignant melanoma. Cancer Met Rev. 1999;18:345–357. doi: 10.1023/a:1006304806799. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Amano M, Onishi H, Kaibuchi K, Fujiwara K. Rho-kinase-mediated contraction of isolated stress fibers. J Cell Biol. 2001;153:569–583. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostenuik PJ, Singh G, Orr FW. Transforming growth factor β upregulates the integrin-mediated adhesion of human prostate carcinoma cells to type I collagen. Clin Exp Metastasis. 1997;15:41–52. doi: 10.1023/a:1018484323210. [DOI] [PubMed] [Google Scholar]
- Kraynov VS, Chamberlain C, Bokoch GM, Schwartz MA, Slabaugh S, Hahn KM. Localized Rac activation dynamics visualized in living cells. Science. 2000;290:333–337. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- Kulkarni SV, Gish G, van der Geer P, Henkemeyer M, Pawson T. The role of p120 Ras-GAP in directed cell movement. J Cell Biol. 2000;149:457–469. doi: 10.1083/jcb.149.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidler P, Gil D, Pituch-Noworolska A, Ciolczyk D, Ksiazek D, Przybylo M, Litynska A. Expression of beta1-integrins and N-cadherin in bladder cancer and melanoma cell lines. Acta Biochim Pol. 2000;47:1159–1170. [PubMed] [Google Scholar]
- Lipfert L, Haimovich B, Schaller MD, Cobb BS, Parsons JT, Brugge JS. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kyle E, Lieberman R, Crowell J, Kelloff G, Bergan RC. Focal adhesion kinase (FAK) phosphorylation is not required for genistein-induced FAK-β–1-integrin complex formation. Clin Exp Metastasis. 2000;18:203–212. doi: 10.1023/a:1006729106034. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Insall RH. Signaling to actin dynamics. J Cell Biol. 1999;146:267–272. doi: 10.1083/jcb.146.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meili R, Firtel RA. Two poles and a compass. Cell. 2003;114:153–156. doi: 10.1016/s0092-8674(03)00553-1. [DOI] [PubMed] [Google Scholar]
- Misra UK, Deedwania R, Pizzo SV. Binding of activated alpha2-macroglobulin to its cell surface receptor GRP78 in 1-LN prostate cancer cells regulates PAK-2-dependent activation of LIMK. J Biol Chem. 2005;280:26278–26286. doi: 10.1074/jbc.M414467200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–246. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- Parent CA, Devreotes PN. A cell’s sense of direction. Science. 1999;284:765–770. doi: 10.1126/science.284.5415.765. [DOI] [PubMed] [Google Scholar]
- Parise LV, Lee JW, Juliano RL. New aspects of integrin signaling in cancer. Semin Cancer Biol. 2000;10:407–414. doi: 10.1006/scbi.2000.0337. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal adhesion kinase: A regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–5613. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- Pelayo BA, Fu Y-M, Meadows GG. Decreased tissue plasminogen activator and increased plasminogen activator inhibitors are associated with inhibition of invasion in human A375 melanoma deprived of tyrosine and phenylalanine. Int J Oncol. 2001;18:877–883. doi: 10.3892/ijo.18.4.877. [DOI] [PubMed] [Google Scholar]
- Romanov VI, Goligorsky MS. RGD-recognizing integrins mediate interactions of human prostate carcinoma cells with endothelial cells in vitro. Prostate. 1999;39:108–118. doi: 10.1002/(sici)1097-0045(19990501)39:2<108::aid-pros5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. Rho-GTPases and cancer. Nature Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK, a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hall MN. Signaling to the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:305–338. doi: 10.1146/annurev.cellbio.14.1.305. [DOI] [PubMed] [Google Scholar]
- Schmitmeier S, Markland FS, Ritter MR, Sawcer DE, Chen TC. Functional effect of contortrostatin, a snake venom disintegrin, on human glioma cell invasion in vitro. Cell Commun Adhes. 2003;10:1–16. doi: 10.1080/15419060302062. [DOI] [PubMed] [Google Scholar]
- Scott L, Lamb J, Smith S, Wheatley DN. Single amino acid (arginine) deprivation: Rapid and selective death of cultured transformed and malingnant cells. Br J Cancer. 2000;83:800–810. doi: 10.1054/bjoc.2000.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JK, Adams RB, Rovin JD, Bissonette EA, Stoker CE, Parsons JT. Alterations in the focal adhesion kinase/Src signal transduction pathway correlate with increased migratory capacity of prostate carcinoma cells. Oncogene. 2001;20:1152–1163. doi: 10.1038/sj.onc.1204208. [DOI] [PubMed] [Google Scholar]
- Sumitomo M, Shen R, Walburg M, Dai J, Geng Y, Navarro D, Boileau G, Papandreou CN, Giancotti FG, Knudsen B, Nanus DM. Neutral endopeptidase inhibits prostate cancer cell migration by blocking focal adhesion kinase signaling. J Clin Invest. 2000;106:1399–1407. doi: 10.1172/JCI10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantivejkul K, Vucenik I, Shamsuddin AM. Inositol hexaphosphate (IP6) inhibits key events of cancer metastasis. II. Effects on integrins and focal adhesions. Anticancer Res. 2003;23:3681–3689. [PubMed] [Google Scholar]
- Tsuji T, Kawada Y, Kai-Murozono M, Komatsu S, Han SA, Takeuchi K, Mizushima H, Miyazaki K, Irimura T. Regulation of melanoma cell migration and invasion by laminin-5 and alpha3beta1 integrin (VLA-3) Clin Exp Metastasis. 2002;19:127–134. doi: 10.1023/a:1014573204062. [DOI] [PubMed] [Google Scholar]
- Weber MJ, Gioeli D. Ras signaling in prostate cancer progression. J Cell Biochem. 2004;91:13–25. doi: 10.1002/jcb.10683. [DOI] [PubMed] [Google Scholar]
- Wells CM, Ahmed T, Masters JR, Jones GE. Rho family GTPases are activated during HGF-stimulated prostate cancer-cell scattering. Cell Motil Cytoskeleton. 2005;62:180–194. doi: 10.1002/cm.20095. [DOI] [PubMed] [Google Scholar]
- Yao H, Dashner EJ, van Golen CM, van Golen KL. RhoC GTPase is required for PC-3 prostate cancer cell invasion but not motility. Oncogene. 2006;25:2285–2296. doi: 10.1038/sj.onc.1209260. [DOI] [PubMed] [Google Scholar]
- Zheng R, Iwase A, Shen R, Goodman OB, Jr, Sugimoto N, Takuwa Y, Lerner DJ, Nanus DM. Neuropeptide-stimulated cell migration in prostate cancer cells is mediated by RhoA kinase signaling and inhibited by neutral endopeptidase. Oncogene. 2006;25:5942–5952. doi: 10.1038/sj.onc.1209586. [DOI] [PubMed] [Google Scholar]
- Zhou C, Ling MT, Kin-Wah Lee T, Man K, Wang X, Wong YC. FTY720, a fungus metabolite, inhibits invasion ability of androgen-independent prostate cancer cells through inactivation of RhoA-GTPase. Cancer Lett. 2006;233:36–47. doi: 10.1016/j.canlet.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Zicha D, Dobbie IM, Holt MR, Monypenny J, Soong DY, Gray C, Dunn GA. Rapid actin transport during cell protrusion. Science. 2003;300:142–145. doi: 10.1126/science.1082026. [DOI] [PubMed] [Google Scholar]