Abstract
Background
Erythropoietin (EPO) was shown to reduce tumor survival in recent trials, however, its mechanisms of action are unclear. Efforts to measure tumor EPO receptor (EPOR) are limited by the promiscuity of EPOR antibodies, and concerns as to whether EPOR mRNA measurements are confounded by heterogeneity of tumor vasculature, a known EPOR source.
Materials and Methods
This study compared mRNA levels of EPOR and JAK2 in 11 breast tumor epithelial versus endothelial dissections.
Results
In nine tumors EPOR mRNA was 2.6 (1.2–5.7)-fold lower in the epithelial fraction, however, this reduction was less than the reduction of endothelial markers. In two tumors, EPOR mRNA was 2.9 (1.7–4.0)-fold higher in the epithelial fraction. The inter-tumor variation in EPOR levels exceeded the intra-tumor variation between fractions. Similar results were obtained for JAK2.
Conclusion
Tumor vasculature is not the sole source of EPOR and JAK2, and tumors can be segregated by EPOR and JAK2 levels for correlative analysis with clinical outcomes.
Keywords: Erythropoietin, breast cancer, erythropoietin receptor, erythropoiesis stimulating agents
Erythropoietin (EPO), secreted primarily by the kidneys, is required for erythropoiesis. EPO receptor (EPOR) levels on erythroid progenitors in the marrow peak at approximately 1,100 homodimers per cell (1). EPOR is expressed at markedly lower levels in non-erythroid tissues, nonetheless, EPO has been linked to angiogenesis, and to cytoprotection in non-erythroid tissues including the brain, heart, and kidney (for review see reference 2). Adverse effects of EPO on patient survival in recent phase III trials led to major revisions in guidelines for EPO use in chemotherapy-induced anemia (3). However, mechanisms for the adverse effects of EPO remain unclear and preclinical studies have produced disparate results (for review see reference 4). At issue is whether expression of EPOR on tumor cells or tumor blood vessels is sufficient to impart biologic effects in response to EPO. Previous studies in diverse carcinomas indicate that EPOR and EPO expression are not elevated in tumor compared to healthy tissue, suggesting that EPOR and EPO are not oncogenes (5). However, this does not exclude the possibility that low levels of EPOR in tumors might allow them to respond to EPO. Measuring EPOR levels in the archival tumors of participants in completed and ongoing clinical trials of EPO provides an opportunity to determine whether worse outcomes in patients randomized to EPO occur disproportionately among patients whose tumors express higher EPOR levels.
Efforts to assess EPOR protein in tumors have been confounded by the absence of specific antibodies that can detect the low levels of EPOR present in non-erythroid cells (6, 7). Strikingly, elegant 125I-EPO internalization studies in neuroblastoma cells showed that fewer than 50 EPOR homodimers on the cell surface can inhibit apoptosis in response to EPO (8). Thus, although low levels of EPOR can be functional, there are no reagents to assess EPOR protein in primary tumors by immunohistochemistry. Even a sensitive antibody, recently developed by Amgen, exhibited non-specific staining in negative control cells in this application (9).
In contrast, EPOR mRNA can be measured specifically by quantitative RT-PCR. However, three issues confront the use of EPOR mRNA: (i) degradation that characterizes RNA extracted from formalin-fixed, paraffin-embedded (FFPE) tumors, (ii) concerns as to whether EPOR mRNA correlates with surface protein levels, and (iii) uncertainty as to whether EPOR expression in endothelial cells, a known source of EPOR, confounds the characterization of tumors for their level of EPOR, since vasculature is known to be heterogeneous between different regions of the same tumor.
Regarding the first issue, using intact RNA from snap-frozen breast tumors versus degraded RNA from FFPE pieces of the same tumor and an optimized quantitative RT-PCR assay, a high concordance was found in EPOR mRNA determinations, despite the degradation of FFPE-derived RNA (10). Regarding the second issue, using a semi-quantitative Western blot and a panel of 66 cancer cell lines, Swift et al. recently reported a correlation between EPOR mRNA and protein in some, but not all, cancer cell lines (11). The eight cell lines containing the highest levels of EPOR protein were among the eleven lines with the highest levels of EPOR mRNA. The highest EPOR mRNA levels were observed in NCIH661 lung cancer cells, which also contained cell surface EPOR as determined by 125I-EPO binding. A correlation has also been reported between EPOR mRNA and surface protein (using a specific antibody) in some, but not all, cancer cell lines (10). The apparent lack of correlation among many cancer lines may arise from differences in post-transcriptional regulation but may also reflect inaccuracies in measuring the low levels of EPOR protein that characterize most non-erythroid cell types. In the present study, to address the third aforementioned issue, laser capture microdissection (LCM) was used to quantitatively assess EPOR, EPO, and JAK2, an EPOR-associated kinase (for review see reference 12) in epithelial versus endothelial fractions of primary breast tumors.
Materials and Methods
Laser capture microdissection
The Institutional Review Board granted permission to access tissue donated by women undergoing breast cancer surgery (Department of Defense grant DAMD 17-02-1-0691). Written informed consent was obtained. Samples were frozen within 20–60 minutes of devascularization. Depending on the frozen tumor size, up to 24×9 µm sections were placed on RNase-free polyethylene naphthalate membranes (Leica Microsystems, Wetzlar, Germany). Slides were stained and dehydrated using HistoGene (Molecular Devices, Sunnyvale, CA, USA). An LMD6000 system (Leica) was used to harvest epithelial versus stromal/endothelial fractions. The number of captures per slide varied depending on the size of the tumor sample and the extent to which epithelial cells could be clearly separated from stroma.
Quantitative RT-PCR
RNA extraction and genomic DNA digestion were performed using the Absolutely RNA Nanoprep kit (Stratagene, La Jolla, CA, USA). cDNA synthesis, pre-amplification of cDNA using the Taqman cDNA pre-amplification kit (Applied Biosystems, Foster City, CA, USA), quantitative real time PCR, and relative quantification were as described previously (10). All Taqman probes were designed to detect exon junctions. Error bars in relative quantification values represent the standard error of the mean obtained upon normalization to HMBS, IPO8, and B2M, which were used based on their stability among 16 candidates previously evaluated in breast cancer using the Genorm algorithm (10, 13).
Results
Epithelial versus stromal/endothelial lineage fractionation
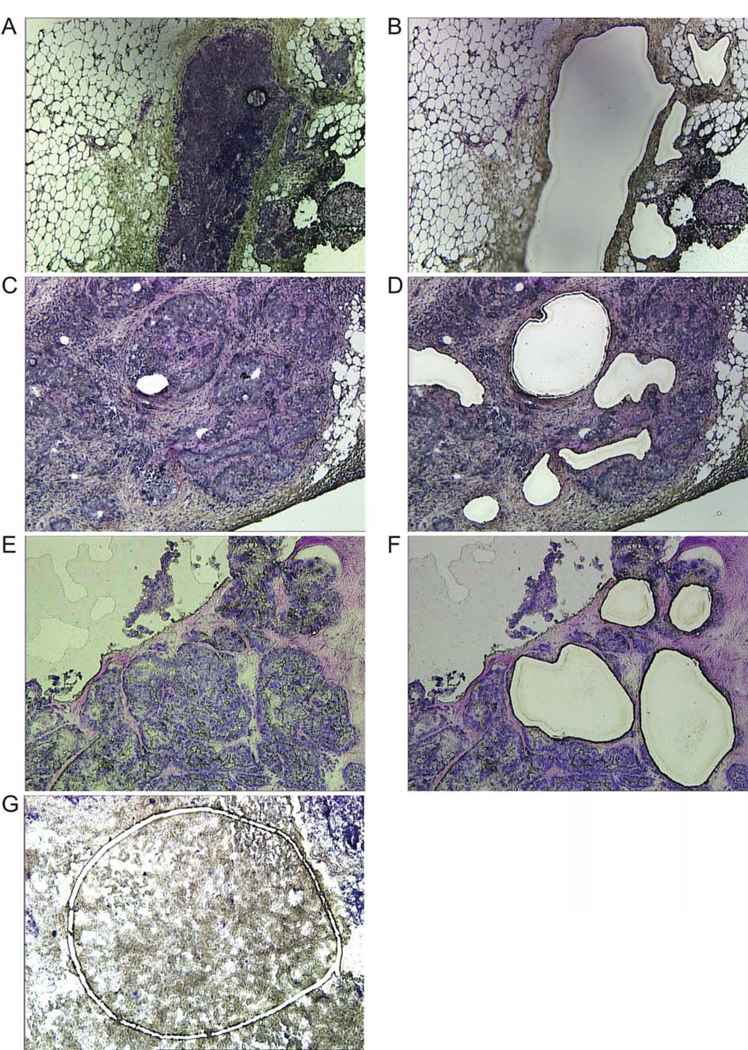
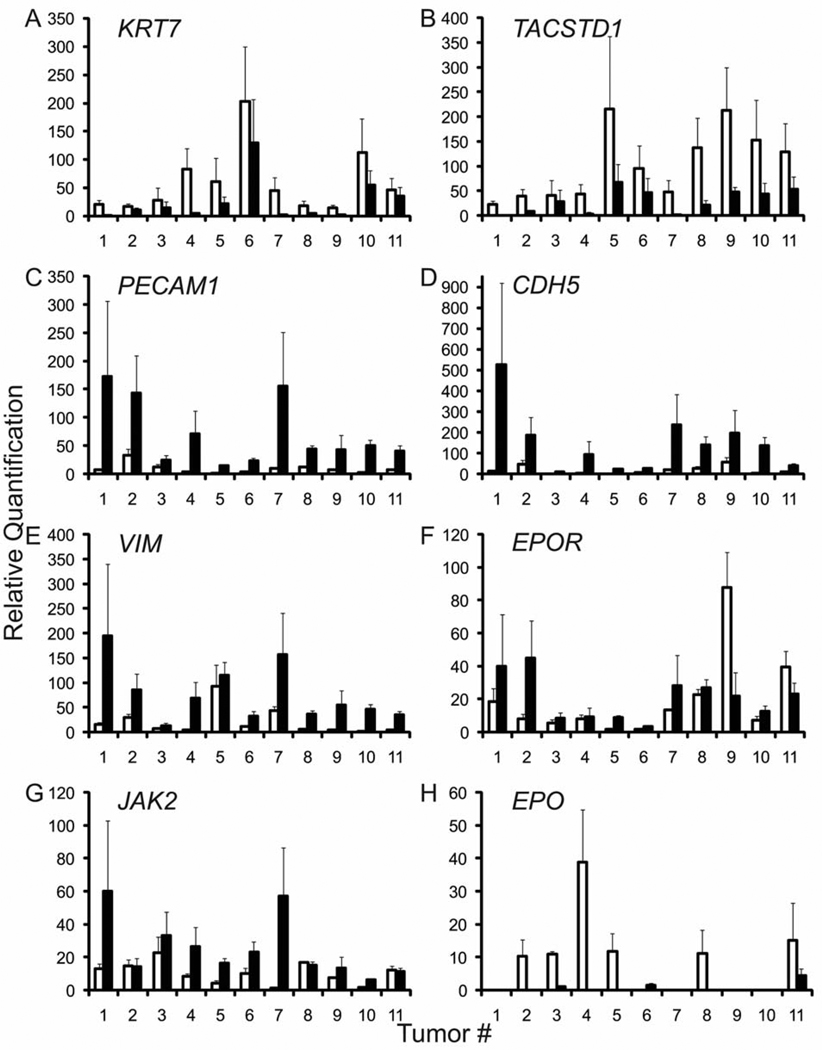
A total of 58 hematoxylin-eosin stained frozen sections were screened to identify tumors with clear separation of epithelial and stromal compartments and 17 of them were selected as being suitable for LCM. Figure 1 delineates representative morphologies of tumor epithelial cell enriched fractions (panels A–F) versus depleted fractions (panel G). Eleven tumor samples yielded sufficient RNA and exhibited successful fractionation of all lineage markers. The stage and histopathological features of these tumors are summarized in Table I. Fractionation was monitored by the epithelial markers KRT7 and TACSTD1, the endothelial markers CDH5 and PECAM1, and the stromal marker VIM. The epithelial markers KRT7 and TACSTD1 were mean (range) 6.8 (1.3–19.2) fold and 7.0 (1.4 –21.9) fold higher in the epithelial fraction compared to the stromal/endothelial fraction (Figure 2A, B). The endothelial markers CDH5 and PECAM1 were reduced by 12.1 (3.4–35.8)-fold and 10.7 (2.0–24.5)-fold in the epithelial fraction compared to the stromal/endothelial fraction (Figure 2C, D), and the stromal marker VIM was reduced by 9.5 (1.2–34.2)-fold (Figure 2E). This successful fractionation provided the opportunity to determine the extent to which EPOR, JAK2, and EPO mRNA segregates as an epithelial versus stromal/endothelial marker in each tumor.
Figure 1.
Morphology of epithelial versus stromal/endothelial fractions used for LCM. Frozen sections (9 µm) were mounted on polyethylene naphthalate membrane slides and stained with HistoGene. Original magnification, ×10. (A–F) Examples of pockets of breast tumor epithelial cells that were harvested from the slides. Sections before and after capture are shown in the left and right panels, respectively. (G) Example of a stromal/endothelial fraction during laser capture.
Table I.
Summary of clinical characteristics, lineage fractionation, and EPOR/JAK2/EPO mRNA expression*.
| # | Age (years) |
ER | PR | Her2 | Stage | Histology | KRT7 | TACSTD1 | CDH5 | PECAM1 | VIM | EPOR | JAK2 | EPO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | + | + | NA | I | Colloid/Mucinous | Epi 18.5-fold | Epi 21.9-fold | S/VE 35.8-fold | S/VE 24.5-fold | S/VE 12.6-fold | S/VE 2.1-fold | S/VE 4.7-fold | UD |
| 2 | 56 | + | + | NA | IIIC | Lobular | Epi 1.5-fold | Epi 4.2-fold | S/VE 3.8-fold | S/VE 4.4-fold | S/VE 3.0-fold | S/VE 5.7-fold | Epi 1.0-fold | UD |
| 3 | 46 | + | + | + | IIIA | Ductal | Epi 2.0-fold | Epi 1.4-fold | S/VE 3.9-fold | S/VE 2.0-fold | S/VE 1.7-fold | S/VE 1.6-fold | S/VE 1.5-fold | Epi 9.8-fold |
| 4 | 46 | − | − | + | IIA | Ductal | Epi 19.2-fold | Epi 10.2-fold | S/VE 17.5-fold | S/VE 17.3-fold | S/VE 17.1-fold | S/VE 1.2-fold | S/VE 3.1-fold | UD |
| 5 | 56 | − | − | NA | IIB | Ductal | Epi 2.8-fold | Epi 3.2-fold | S/VE 19.7-fold | S/VE 11.6-fold | S/VE 1.2-fold | S/VE 5.5-fold | S/VE 3.9-fold | UD |
| 6 | 64 | − | − | + | IIB | Ductal | Epi 1.6-fold | Epi 2.0-fold | S/VE 3.9-fold | S/VE 6.0-fold | S/VE 3.0-fold | S/VE 2.2-fold | S/VE 2.2-fold | UD |
| 7 | 42 | + | + | NA | IIIA | Ductal | Epi 16.4-fold | Epi 17.8-fold | S/VE 10.6-fold | S/VE 15.4-fold | S/VE 3.7-fold | S/VE 2.1-fold | S/VE 44.6-fold | UD |
| 8 | 48 | + | + | NA | I | Ductal | Epi 4.4-fold | Epi 6.4-fold | S/VE 5.2-fold | S/VE 3.5-fold | S/VE 6.8-fold | S/VE 1.2-fold | Epi 1.1-fold | UD |
| 9 | 62 | + | + | NA | IIB | Ductal | Epi 5.2-fold | Epi 4.4-fold | S/VE 3.4-fold | S/VE 6.2-fold | S/VE 13.9-fold | Epi 4.0-fold | S/VE 1.8-fold | UD |
| 10 | 82 | − | − | NA | IIB | Ductal | Epi 2.0-fold | Epi 3.4-fold | S/VE 24.9-fold | S/VE 21.1-fold | S/VE 34.2-fold | S/VE 1.8-fold | S/VE 4.2-fold | UD |
| 11 | 70 | + | + | NA | IIIC | Lobular | Epi 1.3-fold | Epi 2.4-fold | S/VE 4.0-fold | S/VE 5.9-fold | S/VE 7.7-fold | Epi 1.7-fold | Epi 1.1-fold | Epi 3.4-fold |
ER, Estrogen receptor; PR, progesterone receptor; Her2, human epidermal growth factor receptor 2; NA, not available; Epi, epithelial fraction; S /VE, stromal/vascular endothelial fraction; UD, undetermined.
For each transcript, the fraction with higher expression (Epi versus S/VE) is indicated, followed by the fold enrichment in that fraction compared to the other fraction.
Figure 2.
mRNA levels in epithelial versus stromal/endothelial fractions. Relative quantification values (y-axis) were normalized to an average of three endogenous control genes (B2M, HMBS, and IPO8). For each tumor (x-axis), the expression level of each marker is shown in the epithelial fraction (open bars) and the stromal/endothelial fraction (closed bars). For each gene, the lowest expressing fraction was assigned a value of 1. Error bars represent the standard error of the mean values obtained upon normalization to three control genes.
EPOR, JAK2, and EPO expression in epithelial versus stromal/endothelial fractions
In 9 out of 11 tumor samples, EPOR mRNA was 2.6 (1.2–5.7)-fold lower in the epithelial fraction compared to the stromal/endothelial fraction (Figure 2F). However, depletion of EPOR mRNA from epithelial fractions was less than the aforementioned extent of depletion of the endothelial markers CDH5 and PECAM1. EPOR depletion from epithelial fractions achieved the same extent as both CDH5 and PECAM1 in only one of these nine tumors (tumor 2, compare Figure 2C, D and F). Moreover, in two tumors, EPOR was higher in the epithelial fraction by 2.9 (1.7–4.0)-fold (tumors 9 and 11, Figure 2F).
Since the tyrosine kinase JAK2 is required for EPOR signaling in erythroid cells and has been implicated in EPOR signaling in non-erythroid cells (14, 15) co-expression of JAK2 is likely a prerequisite for EPOR signaling in tumor cells. Therefore the types of cells within tumors that express JAK2 mRNA were surveyed. In 8 out of 11 tumor samples, JAK2 was 8.3 (1.5–44.6) fold lower in the epithelial fraction compared to the stromal/endothelial fraction (Figure 2G). Excluding tumor 7 (44.6-fold) as an outlier, JAK2 expression in the epithelial fractions of the remaining seven tumors was only 3.1 (1.5–4.7)-fold lower compared to the stromal/ endothelial fraction. Thus, similar to EPOR, the extent of depletion of JAK2 from epithelial fractions was to a lesser extent than the aforementioned extent of depletion of the endothelial markers CDH5 and PECAM1. JAK2 depletion from epithelial fractions was to the same extent as both CDH5 and PECAM1 for only one of these eight tumors (tumor 7, compare Figure 2C, D and G). In three tumors, JAK2 expression was 1.1 (1.0–1.1)-fold higher in the epithelial fraction compared to the stromal/endothelial fraction (tumors 2, 8, and 11, Figure 2G).
Several studies have reported that EPO secreted by tumor cells supports cell growth (16, 17). Therefore, the types of cells within tumors that express EPO mRNA were surveyed. Owing to low expression levels of this gene together with the limiting amounts of RNA, EPO was detectable in only 6 out of 11 epithelial fractions and 3 out of 11 stromal/endothelial fractions. In two tumors for which EPO levels were determined in both fractions, EPO was 9.8- and 3.4-fold higher in the epithelial fraction compared to the stromal/endothelial fraction (tumors 3 and 11, Figure 2H).
Inter-tumoral versus intra-tumoral variation in EPOR and JAK2 levels
Among all eleven tumors, the intra-tumor variation in EPOR mRNA levels between epithelial versus stromal/ endothelial compartments was maximally 5.7-fold-with a mean of 2.6-fold (Figure 2F), whereas the inter-tumor range of overall EPOR expression among all 11 unfractionated tumors was as much as 12.7-fold, with a mean of 6.3-fold (not shown). For JAK2, the intra-tumor variation was maximally 44.6-fold, but all of the remaining tumors exhibited intra-tumor variation of less than 4.7-fold, with a mean of 2.5-fold (Figure 2G). The inter-tumor variation in JAK2 levels was 15.6-fold, with a mean of 8.6 fold (data not shown). Thus, the inter-tumor variation in EPOR and JAK2 levels between different patient tumor samples exceeded the intra-tumor variation between epithelial versus stromal/endothelial fractions.
Discussion
While the current study was limited to 11 tumor samples due to the difficulties in obtaining sufficient RNA and the unsuitable morphology of most tumor specimens for lineage fractionation, this was sufficient to reveal that in 10 out of the 11 tumors the endothelial cells were not able to account for all of the EPOR and JAK2 expression. The basis for elevated EPOR and JAK2 expression in epithelial fractions compared to stromal/endothelial fractions in certain tumors, and the basis for the inter-tumor variation in EPOR and JAK2 levels across patients remain unknown. While tissue processing can affect mRNA levels (18) this study minimized differences in tissue processing, and our results likely reflect biological variation between different patient tumor specimens.
Recently, Liang and others demonstrated that EPO stimulated SRC activation and PTEN inactivation in human breast cancer cell lines, and markedly inhibited the response of HER2+ breast cancer cells to trastuzamab in a murine tumor xenograft model (19). Furthermore, in trastuzamab-treated patients with HER2+ metastatic breast cancer, EPO use correlated with reduced progression-free and overall survival. These results suggest that EPO can act directly on breast tumor epithelial cells. This is consistent with the present demonstration that EPOR and JAK2 are not limited to the endothelial fraction, but are also co-expressed in the epithelial fraction of primary breast tumors.
The observed expression of EPOR and JAK2 in the stromal/endothelial cell fraction is consistent with reports documenting the expression of EPOR mRNA and protein in endothelial cells and with reports of the effects of EPO on tumor angiogenesis (20, 21). In this study it was not possible to determine whether stromal cells express EPOR and JAK2 mRNA, or whether the source of EPOR and JAK2 mRNA in the stromal/endothelial fraction was solely due to endothelial cell expression. Future studies aimed at fractionating endothelial versus stromal cells using fluorescent-labeled antibodies directed against endothelial and stromal markers will help resolve this issue.
It was previously found that EPOR mRNA levels varied 30-fold range across panels of both breast tumors and head and neck tumors (10). In the present study, it was found that the inter-tumor variation in EPOR and JAK2 levels between different patient tumor samples exceeded the intra-tumor variation between epithelial versus stromal/endothelial fractions. Thus, archival tissues from completed and ongoing randomized trials of EPO can be used to determine whether tumors with the highest levels of EPOR and JAK2 mRNA are more susceptible to EPO-induced tumor progression. However, the presence of endothelial cells in tumor sections together with the exquisite sensitivity of quantitative PCR precludes the ability to simply characterize tumors as EPOR or JAK2 positive versus negative (22). Moreover, the heterogeneity of vasculature or other factors such as hypoxia between different tumors, or even between different regions of the same tumor, may influence measurements of EPOR or JAK2 mRNA obtained using single tumor sections. Nonetheless, it was previously shown, using 23 breast tumors, that EPOR and JAK2 measurements in different pieces of the same tumor sample were significantly correlated, while the endothelial markers CDH5 and PECAM1 were not (10). This is consistent with the present demonstration that endothelial cells are not the sole source of EPOR and JAK2 mRNA in tumors. Thus, despite the known heterogeneity in endothelial representation, tumors can be characterized for their overall levels of EPOR and JAK2 mRNA. In addition, tumor epithelial-specific levels of EPOR and JAK2 in single tumor sections may be normalized to the levels of endothelial markers, to adjust for the heterogeneity in tumor vasculature representation.
Acknowledgements
This work was supported by the National Institutes of Health [1R01CA135357 to CAB], Avon [P50 CA083636-S2 to NU] and the American Cancer Society [117682-MRSG-09-268-01-CCE to CPM]. We thank Dr. Keith Loeb, Dr. Kerstin Edlefsen, and Dr. Lynn Goldstein for advice regarding anatomic histopathology and lineage markers, Dr. Marshall Horwitz for access to LCM, Dr. Erica Jonlin for assistance with human subjects issues, Dr. David Beatty for providing tumor samples, Dr. Michel Schummer for assistance with clinical data, Kathy O’Briant and Leah Sabacan for accessing archival tumors, and Marianna Rudakova and Karine Valliant-Saunders for technical assistance.
References
- 1.Broudy VC, Lin N, Brice M, Nakamoto B, Papayannopoulou T. Erythropoietin receptor characteristics on primary human erythroid cells. Blood. 1991;77:2583–2590. [PubMed] [Google Scholar]
- 2.Noguchi CT, Wang L, Rogers HM, Teng R, Jia Y. Survival and proliferative roles of erythropoietin beyond the erythroid lineage. Expert Rev Mol Med. 2008;10:e36. doi: 10.1017/S1462399408000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spivak JL, Bennett CL, Bohlius J, Evanchuk D, Goode MJ, Jakubowski AA, Regan DH, Somerfield MR. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood. 2010;116:4045–4059. doi: 10.1182/blood-2010-08-300541. [DOI] [PubMed] [Google Scholar]
- 4.Arcasoy MO. Erythropoiesis-stimulating agent use in cancer: preclinical and clinical perspectives. Clin Cancer Res. 2008;14:4685–4690. doi: 10.1158/1078-0432.CCR-08-0264. [DOI] [PubMed] [Google Scholar]
- 5.Sinclair AM, Rogers N, Busse L, Archibeque I, Brown W, Kassner PD, Watson JE, Arnold GE, Nguyen KC, Powers S, Elliott S. Erythropoietin receptor transcription is neither elevated nor predictive of surface expression in human tumour cells. Br J Cancer. 2008;98:1059–1067. doi: 10.1038/sj.bjc.6604220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott S, Busse L, Bass MB, Lu H, Sarosi I, Sinclair AM, Spahr C, Um M, Van G, Begley CG. Anti-Epo receptor antibodies do not predict Epo receptor expression. Blood. 2006;107:1892–1895. doi: 10.1182/blood-2005-10-4066. [DOI] [PubMed] [Google Scholar]
- 7.Brown WM, Maxwell P, Graham AN, Yakkundi A, Dunlop EA, Shi Z, Johnston PG, Lappin TR. Erythropoietin receptor expression in non-small cell lung carcinoma: a question of antibody specificity. Stem Cells. 2007;25:718–722. doi: 10.1634/stemcells.2006-0687. [DOI] [PubMed] [Google Scholar]
- 8.Um M, Gross AW, Lodish HF. A ‘classical’ homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal. 2007;19:634–645. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Elliott S, Busse L, McCaffery I, Rossi J, Sinclair A, Spahr C, Swift S, Begley CG. Identification of a sensitive anti-erythropoietin receptor monoclonal antibody allows detection of low levels of EpoR in cells. J Immunol Methods. 2010;352:126–139. doi: 10.1016/j.jim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Miller CP, Lowe KA, Valliant-Saunders K, Kaiser JF, Mattern D, Urban N, Henke M, Blau CA. Evaluating erythropoietin-associated tumor progression using archival tissues from a phase III clinical trial. Stem Cells. 2009;27:2353–2361. doi: 10.1002/stem.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swift S, Ellison AR, Kassner P, McCaffery I, Rossi J, Sinclair AM, Begley CG, Elliott S. Absence of functional EpoR expression in human tumor cell lines. Blood. 2010;115:4254–4263. doi: 10.1182/blood-2009-10-248674. [DOI] [PubMed] [Google Scholar]
- 12.Ihle JN, Gilliland DG. JAK2: normal function and role in hematopoietic disorders. Curr Opin Genet Dev. 2007;17:8–14. doi: 10.1016/j.gde.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-research0034. research 0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between JAK2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 15.Breggia AC, Wojchowski DM, Himmelfarb J. JAK2/Y343/STAT5 signaling axis is required for erythropoietin-mediated protection against ischemic injury in primary renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008;295:1689–1695. doi: 10.1152/ajprenal.90333.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar SM, Acs G, Fang D, Herlyn M, Elder DE, Xu X. Functional erythropoietin autocrine loop in melanoma. Am J Pathol. 2005;166:823–830. doi: 10.1016/S0002-9440(10)62303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong JY, Hoxhaj G, Socha AL, Sytkowski AJ, Feldman L. An erythropoietin autocrine/paracrine axis modulates the growth and survival of human prostate cancer cells. Mol Cancer Res. 2009;7:1150–1157. doi: 10.1158/1541-7786.MCR-08-0243. [DOI] [PubMed] [Google Scholar]
- 18.Imbeaud S, Auffray C. ‘The 39 steps’ in gene expression profiling: critical issues and proposed best practices for microarray experiments. Drug Discov Today. 2005;10:1175–1182. doi: 10.1016/S1359-6446(05)03565-8. [DOI] [PubMed] [Google Scholar]
- 19.Liang K, Esteva FJ, Albarracin C, Stemke-Hale K, Lu Y, Bianchini G, Yang CY, Li Y, Li X, Chen CT, Mills GB, Hortobagyi GN, Mendelsohn J, Hung MC, Fan Z. Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via JAK2-mediated SRC activation and PTEN inactivation. Cancer Cell. 2010;18:423–435. doi: 10.1016/j.ccr.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasuda Y, Fujita Y, Matsuo T, Koinuma S, Hara S, Tazaki A, Onozaki M, Hashimoto M, Musha T, Ogawa K, Fujita H, Nakamura Y, Shiozaki H, Utsumi H. Erythropoietin regulates tumour growth of human malignancies. Carcinogenesis. 2003;24:1021–1029. doi: 10.1093/carcin/bgg060. [DOI] [PubMed] [Google Scholar]
- 21.Hardee ME, Cao Y, Fu P, Jiang X, Zhao Y, Rabbani ZN, Vujaskovic Z, Dewhirst MW, Arcasoy MO. Erythropoietin blockade inhibits the induction of tumor angiogenesis and progression. PLoS One. 2007;2:e549. doi: 10.1371/journal.pone.0000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longmore GD. Do cancer cells express functional erythropoietin receptors? N Engl J Med. 2007;356:2447. doi: 10.1056/NEJMp078112. [DOI] [PubMed] [Google Scholar]