Abstract
Infrared thermography is an imaging modality gaining popularity as a diagnostic aid in the evaluation of equine lameness. Anecdotal reports of skin hyperthermia induced by local anesthesia, detected by thermography, have been made; however, no controlled studies have been reported. The purpose of this study was to examine the effects of perineural anesthesia on infrared thermographic images of the forelimb digits in normal horses. After environmental acclimation, infrared thermographs were made at intervals of 0, 5, 10, 15, 30, and 45 min from administration of mepivacaine hydrochloride or phosphate buffered saline in 6 adult horses with no clinical evidence of abnormality of the forelimb digits. The mean limb surface temperatures were compared by 2-factor ANOVA. Results indicated no significant difference between treatments, time after injection, or an interaction of time and treatment. Infrared thermographic imaging apparently can be performed within 45 min of perineural mepivacaine hydrochloride anesthesia without risk of artifactual changes in limb surface temperature.
Introduction
Infrared thermographic imaging of horses has been used increasingly in equine practice and has been considered a good tool for detection of lesions with the potential to cause lameness. Recent studies have reported the relationship between the detection of early thermographic changes and the onset of clinical lameness (1,2,3). While these studies are very encouraging, most reports review the clinical utilization of infrared imaging and there is very little documentation describing controlled evaluations of thermography as a diagnostic tool.
Infrared thermography and potential veterinary applications for this imaging technique have been described (2,3,4,5,6,7,8,9,10,11). These reports mostly describe thermographic imaging of spontaneous disease and attempts to correlate images to disease or injury diagnosed by other means. Normal thermographic patterns in the horse have been described (4,12). A study in cattle has revealed the successful utilization of thermography in the detection of localized sepsis in the pinna after contaminated growth stimulant pellets had been administered (13). One report describes the potential for thermography to detect early tissue changes in flexor tendons that may precede the frank tendon fiber tearing that is characteristic of a “bowed” tendon (3). This work may lead to improved diagnostic interpretation of infrared images for tendon evaluations and at other sites. In a study in which the correlation between Thoroughbred racehorse trainers' perceptions of potential problems and veterinary diagnoses supported by thermographic assessment was evaluated, it was determined that the correlation was excellent and that, in most cases, there were increases in heat 2 wk before clinical problems were noted (1). While this work is encouraging, most veterinary descriptions of infrared thermography are based on anecdotal impressions rather than on controlled evaluations of the potential for this modality to be an effective diagnostic tool in equine practice.
Current infrared thermographic instrumentation can detect skin temperature differentials of 0.1°C (12). Thermographic information can be detected and mapped with graphic and numerical data to allow the determination of focal temperature changes between 2 regions within an image (10,14). Inflammation in subcutaneous and deeper tissues can be reflected by local tissue temperature changes of ≥ 1°C (12,14). Human hands and fingers can detect temperature differentials on patient skin of ≥ 2°C (14). It has been claimed that modern infrared cameras are at least 10 times more sensitive than human hands in detecting temperature changes in a patient's skin (12). Instrumentation that can reveal tissue inflammatory change associated with injury or disease would be very beneficial in patient evaluation.
Thermography likely has great potential to assist diagnosis in equine lameness. The assessment of subtle temperature changes associated with inflammation could be very important in the detection of early and clinically relevant inflammation associated with pain and lameness. The ability to describe and relate thermographic data to specific injured or inflamed sites in the equine limb has seldom been documented in the veterinary literature (11,15,16). There is a common agreement among practitioners that this is possible, but complete descriptions are lacking. Other considerations are necessary to completely understand the appropriate use of infrared thermography in examinations for equine lameness. The timing of thermography in relation to the other standard components of a lameness examination needs to be thoroughly delineated. Specifically, the creation of artifacts that may confuse interpretation of infrared images should be understood and avoided if possible. Preparation for and utilization of local anesthetic nerve blocks has been speculated to create thermographic artifacts. It has been suggested that regional nerve anesthesia adversely affects infrared thermography by causing a thermal blush, secondary to vasodilation from sympathetic blockade (14,17). Conversely, another author believes nerve injuries with disturbed sympathetic tone may result in an image that reveals thermal cooling (2).
The purpose of this study was to evaluate the effects of regional nerve anesthesia on thermographic imaging of the digit in the forelimbs of normal horses.
Materials and methods
Horses
Six adult horses with no abnormalities on physical examination of the fetlock and pastern regions were used in this study. Horses were housed in 12' 3 12' stalls or paddocks for a minimum of 3 h prior to experimentation in order to eliminate the effects of exercise. Each horse was fed to meet National Research Council requirements and each was routinely vaccinated for protection against eastern equine encephalitis, western equine encephalitis, influenza, and tetanus. Each horse was routinely dewormed. The research protocol performed on these horses was submitted to and accepted by the Kansas State University Institutional Animal Care and Use Committee, prior to the initiation of the study.
Preimaging preparation
Gross debris was removed distal to the proximal aspect of the metacarpus on each forelimb. Each forelimb was cleansed and prepared for routine perineural local anesthetic injection over the palmar digital neurovascular structures at the abaxial sesamoid regions on both medial and lateral aspects of the limb. Chlorhexidine gluconate 4% and isopropyl alcohol 90% were utilized for topical disinfection. A minimum of 60 min was allowed for the skin surface and hair to dry before study injections were performed.
One forelimb was randomly selected on each horse for local anesthetic injection. Mepivacaine hydrochloride (HCl) (3 mL) (Carbocaine; Pharmacia & Upjohn, Kalamazoo, Michigan USA) was injected into the SC tissue plane at the medial and lateral abaxial sesamoid sites over the palmar digital neurovascular tissues, using a 2-cm, 23g needle and a 3-mL syringe. The contralateral limb was injected similarly at the abaxial sesamoid sites, as an injection control, with 3 mL of phosphate buffered saline (PBS). All perineural injections were subsequently tested by pressure application around the coronary band with a blunt instrument to assess that anesthesia was appropriately attained.
Thermal imaging
All thermographic data and images were collected in a closed, covered, indoor environment following a minimum 3 h period of acclimation to this environment for the horses under study. The horses were restrained with a halter and lead rope. Neither sedatives nor tranquilizers were utilized for restraint, due to the vasodilatory properties of these pharmaceuticals. Imaging was performed in all instances at the same time of day under similar ambient temperature conditions, as recorded and measured by the thermographic camera and analytical software. The horses were fasted for 2 h prior to and during the imaging procedure to avoid postprandial thermal variation. Infrared thermograms of the skin temperatures of the lateral and medial surfaces of the forelimbs at the fetlock and pastern regions, centered at the disinfected abaxial sesamoid regions, were obtained. To establish baseline, thermal images were taken on 3 consecutive days before the injections began. Then, thermal images were collected immediately after local anesthetic and saline injections (time 0) and at the following temporal increments: 5, 10, 15, 30, and 45 min or until baseline measurements were resumed. Images were repeated at 24 h in 3 of the horses. The thermal imaging equipment consisted of a high resolution, short wave (3 to 5 mm), radiometric infrared (IR) camera (PM-280 ThermaCam; Inframetrics, North Billerica, Massachusetts USA). The IR camera is equipped with a 16° field of view lens with images displayed in a focal plane array arrangement of 256 3 256 pixels. The camera is calibrated annually by the manufacturer to maintain precision of 0.2°C per pixel, and tested against a known heat source at 1.0 emissivity to assure accuracy. Images were taken at a distance of 2 m, perpendicular to the lateral and medial surfaces of the study sites. This imaging distance results in a single pixel area of 2.2 mm2 (2 m 3 17.5 mrad 3 16° lens/256 pixels = 2.187 mm/pixel; actual measurement using IR camera on a 1'' object was 25 mm/11 pixels = 2.27 mm) on the limb surface.
Images were stored on high-resolution SVHS videotape for postimaging processing and evaluation with analytical software (ThermaGram Pro 95; Inframetrics). Effective mean limb surface temperature (MLST) for the targeted site was calculated from an approximately 3000-pixel area selected over the pastern and fetlock region of each forelimb image. The analytical software utilizes the mean of the pixels composing the targeted area within each image to determine the surface temperature of the limb. Effective mean temperature for the areas was calculated to allow comparison of changes in MLST, as affected by the time after the injection protocol. Regional surface temperature changes in each limb in relation to the baseline images were noted and recorded.
A minimum washout period of 72 h was allowed for each horse. Then, the limb that received mepivacaine HCl was injected with PBS and the limb that received PBS was injected with local anesthetic. Infrared thermographic imaging, imaging storage, and analysis were similarly repeated.
Statistical analysis
All results are the mean of 3 replicate thermal images of both medial and lateral views at each time interval after injection. Each forelimb injection was considered a separate treatment for purposes of analysis. Data analysis was completed by using a commercial statistical software package (SAS, version 8; SAS Institute, Cary, North Carolina USA). Residual plots were used to evaluate data for normality and variance before and after logarithmic transformation. Normal distribution and equal variance occurred in the residual plots of logarithmically transformed data. Two-factor ANOVA with repeated measures was used to determine the effects of anesthetic injection, time after injection, and their interactions on MLST. Covariance was evaluated by first-order autoregression. Dunnett's test was applied to compare all groups to the control for separation of time after injection. Significance was established at P , 0.05.
Results
Each horse tolerated the chlorhexidine scrub and alcohol applications, local anesthetic and saline injections, and infrared imaging without complication or resistance. No environmental complications were encountered and ambient temperatures were held steady at 20° to 22°C. Infrared images were obtained successfully for each horse at all time intervals.
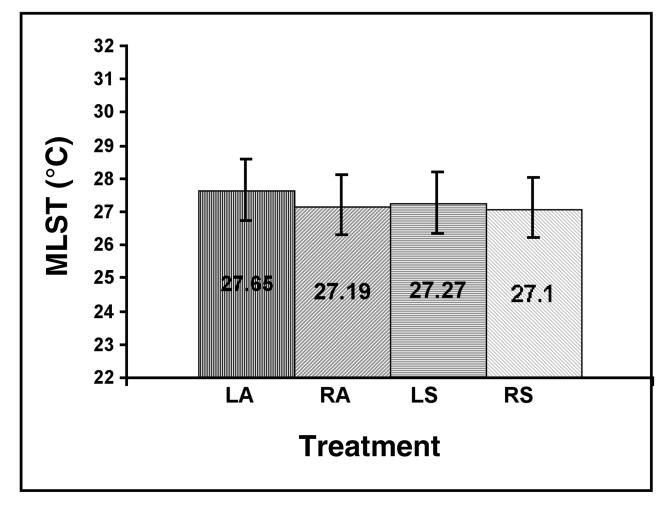
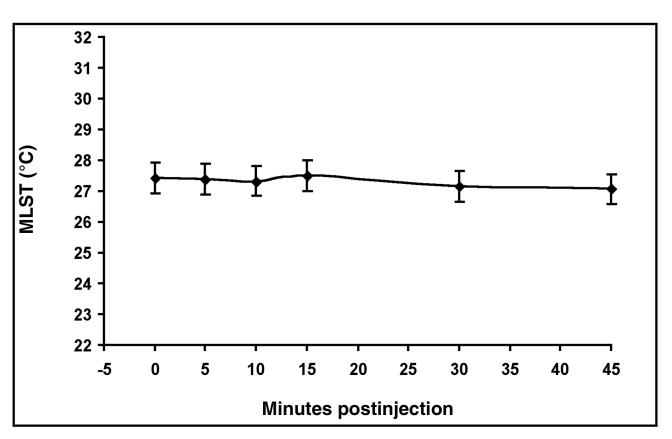
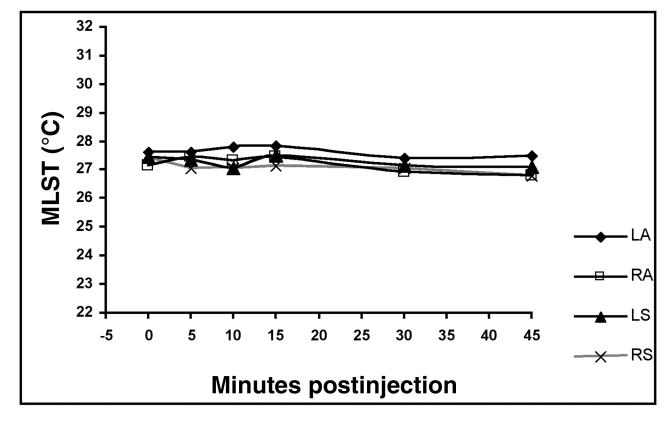
The perineural injection of mepivacaine HCl did not create a significant effect on MLST compared with the perineural injection of PBS (P = 0.8527) (Figure 1). The MLST at each time interval up to 45 min postinjection was not significantly different from the MLST at time 0. The MLST at 30 min (P = 0.2100) and 45 min (P = 0.0666) was less than time 0 and the preceding time intervals (P = 0.9086 to 0.9930) (Figure 2). Images obtained at 24 h on 3 horses indicated that the skin temperature was similar to that at the time of the 45-min images, but they were not included in the statistical analyses. The interaction between mepivacaine HCl or PBS and time after injection displayed no significant effect on MLST (P = 0.8665) (Figure 3 and 4).
Figure 1. Mean limb surface temperature (MLST) (°C) as a result of treatment. No significant (P . 0.05) differences were revealed between perineural anesthetic and perineural phosphate buffered saline (PBS) injections at the abaxial sesamoid sites of the forelimbs in clinically normal horses. LA = left forelimb with anesthetic injection, RA = right forelimb with anesthetic injection, LS = left forelimb with PBS injection, RS = right forelimb with PBS injection. Error bars indicate 95% confidence intervals.
Figure 2. Mean limb surface temperature (MLST) (°C) for all treatments over time. Time after injection had no significant influences on MLST (P . 0.05). Error bars indicate 95% confidence intervals.
Figure 3. Mean limb surface temperature (MLST) (°C) as a result of the interaction between treatment and time. Administration of anesthetic or phosphate buffered saline (PBS) had no significant effect on MLST over time (P . 0.05). LA = left forelimb with anesthetic injection, RA = right forelimb with anesthetic injection, LS = left forelimb with PBS injection, RS = right forelimb with PBS injection.
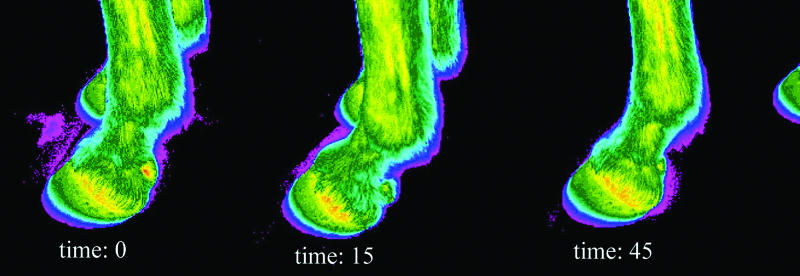
Figure 4. Infrared thermograms of normal forelimb digits at various time intervals in relation to perineural injection of mepivacaine HCl. These images are typical of the results for anesthetized limbs in this study. Time: 0 = image at time of mepivacaine HCl injection, time: 15 = 15 min postinjection of mepivacaine HCl, time: 45 = 45 min postinjection of mepivacaine HCl.
Discussion
The results of this study reveal that local perineural anesthesia with mepivacaine HCl had no effect on mean limb surface temperature, as detected by thermographic imaging. The data indicates that the MLST is not significantly altered within 45 min of performing perineural anesthetic injection. The calculated temperature of the images of the digit at 24 h was quite similar to that of the 45-min images, so further 24-hour images were not made on the remaining 3 horses. The values for 24-hour images were not included in the statistical analyses. Other authors have indicated that abaxial sesamoid nerve blocks can result in elevated limb temperature; however, these observations were made on horses being evaluated for lameness and without having had thermographic images taken prior to local anesthetic injection (2,14). Therefore, an alternative interpretation is that the temperature elevation was due to local inflammation that may have preexisted in the affected forelimbs. In discussing areas of increased temperature on thermographic images, Denoix (2) stated, “Normal thermographic variation also must be considered.”
Denoix (2) observed that local tissue cooling might result from injured nerves with decreased sympathetic tone. Conversely, induced and spontaneous Horner's syndrome in the horse has resulted in thermographic images showing unilateral skin temperature elevation in the neck (18). Murray et al (19) utilized thermography as an aid to diagnose neoplasia at the cervicothoracic ganglion. The presenting signs were similar to those of Horner's syndrome, including unilateral hyperthermia of the neck and forelimb. Elevated skin temperature, unconfirmed by thermography, was also observed in adult horses subjected to cervicothoracic ganglion blockade induced by injection of lidocaine HCl (20). Instillation of local anesthetic agents appears to mimic the signs of naturally occurring sympathetic blockade. However, these reported findings all involved more centrally located neural lesions, where the concentration of sympathetic nerve fibers is known to be much greater.
Myelinated type B fibers originate in the thoracolumbar spinal cord, leave the cord with motor fibers, but soon separate from the spinal nerve to enter the chain ganglia of the sympathetic truck (22). These are the preganglionic fibers of the sympathetic nervous system. Under “fight or flight” stimulation, type B nerve fibers initiate peripheral vasoconstriction, which may allow cooling of the skin temperature. Consequently, sympathetic blockade by local anesthetics could allow increased skin temperature (21). The sympathetic nerve fibers that travel peripherally with the spinal nerves are postganglionic nonmyelinated type C fibers (22). These fibers are the sympathetic supply for blood vessels, erector pili muscles, and sweat glands throughout the dermatome supplied by the spinal nerve. Signs of blockade of type C fibers include “pain relief, loss of temperature, sensation (21).” This might indicate that a decrease in surface temperature of the distal part of the forelimb is more likely after sympathetic blockade.
Local anesthetic agents may have variable influence on local vascular activity (21). Less vasodilator activity is produced when using mepivacaine HCl rather than lidocaine HCl, resulting in a prolonged duration of activity for mepivacaine HCl (21). This could help to explain why no effect was observed in the thermographic images obtained in this study, which evaluated the effects of mepivacaine, a commonly used local anesthetic agent in the diagnosis of equine lameness.
Thermographic imaging has proven to be a valuable diagnostic tool for the evaluation and treatment of lameness in horses. The ability to detect potential underlying injury and associated inflammation from changes in the skin surface temperature changes in a noninvasive manner is very desirable. To combine thermography with other tools of standard lameness evaluation would improve the utilization and understanding of diagnostic capabilities. It appears from this study that infrared thermographic imaging can be performed after administration of perineural anesthesia with mepivacaine HCl without creating artifactual changes of the limb surface temperature. CVJ
Footnotes
Funding provided by the Food Animal Health and Management Center and the Department of Clinical Sciences, College of Veterinary Medicine, Kansas State University, Manhattan, Kansas, USA.
Dr. Holmes' current address is Veterinary Medical Teaching Hospital, College of Veterinary Medicine, Kansas State University, 1800 Denison Avenue, Manhattan, Kansas 66506, USA.
Address all correspondence to Dr. Layne C. Holmes: e-mail: lholmes@vet.ksu.edu
Reprints will not be available from the author.
References
- 1.Turner TA, Pansch J, Wilson JH. Thermographic assessment of racing thoroughbreds. 47th Annu Conv Am Assoc Equine Prac. 2001;47:344–346.
- 2.Denoix JM. Diagnostic techniques for identification and documentation of tendon and ligament injuries. Vet Clin North Am Equine Pract 1994;10:365–407. [DOI] [PubMed]
- 3.Marr CM. Microwave thermography: a non-invasive technique for investigation of injury of the superficial digital flexor tendon in the horse. Equine Vet J 1992;24:269–273. [DOI] [PubMed]
- 4.Purohit RC, McCoy MD. Thermography in the diagnosis of inflammatory processes in the horse. Am J Vet Res 1980;41:1167–1174. [PubMed]
- 5.Vaden MF, Purohit RC, McCoy MD, Vaughan JT. Thermography: a technique for subclinical diagnosis of osteoarthritis. Am J Vet Res 1980;41:1175–1179. [PubMed]
- 6.Turner TA. Thermography as an aid to the clinical lameness evaluation. Vet Clin North Am Equine Pract 1991;7:311–338. [DOI] [PubMed]
- 7.Mogg KC, Pollitt CC. Hoof and distal limb surface temperature in the normal pony under constant and changing ambient temperatures. Equine Vet J 1992;24:134–139. [DOI] [PubMed]
- 8.von Schweinitz DG. Thermographic diagnostics in equine back pain. Vet Clin North Am Equine Pract 1999;15:161–177. [DOI] [PubMed]
- 9.Cockcroft PD, Henson FM, Parker C. Thermography of a septic metatarsophalangeal joint in a heifer. Vet Rec 2000;146:258–260. [DOI] [PubMed]
- 10.van Hoogmoed L, Snyder JR, Allen AK, Waldsmith JD. Use of infrared thermography to detect performance-enhancing techniques in horses. Equine Vet Educ 2000;12:102–107.
- 11.Eddy AL, van Hoogmoed LM, Snyder JR. The role of thermography in the management of equine lameness. Vet J 2001;162:172–181. [DOI] [PubMed]
- 12.Turner TA. Diagnostic thermography. Vet Clin North Am Equine Pract 2001;17:95–113. [DOI] [PubMed]
- 13.Spire MF, Drouillard JS, Galland JC, Sargeant JM. Use of infrared thermography to detect inflammation caused by contaminated growth promotant ear implants in cattle. J Am Vet Med Assoc 1999;215:1320–1324. [PubMed]
- 14.Waldsmith JK. Real-time thermography: a diagnostic tool for the equine practitioner. 38th Annu Conv Am Assoc Equine Pract 1992;38:455–466.
- 15.Turner TA, Fessler JF, Lamp M, Pearce JA, Geddes LA. Thermographic evaluation of horses with podotrochlosis. Am J Vet Res 1983;44:535–539. [PubMed]
- 16.Turner TA. Alternate methods of soft tissue imaging. Dubai Int Equine Symp 1996:165–176.
- 17.Waldsmith JK, Oltmann JI. Thermography: subclinical inflammation, diagnosis, rehabilitation, and athletic evaluation. J Equine Vet Sci 1994;14:8–10.
- 18.Purohit RC, McCoy MD, Bergfeld WA. Thermographic diagnosis of Horner's syndrome in the horse. Am J Vet Res 1980;41:1180–1182. [PubMed]
- 19.Murray MJ, Cavey DM, Feldman BF, Trostle SS, White NA. Signs of sympathetic denervation associated with a thoracic melanoma in a horse. J Vet Intern Med 1997;11:199–203. [DOI] [PubMed]
- 20.Skarda RT, Muir WW, Swanson CR, Hubbell JA. Cervicothoracic (stellate) ganglion block in conscious horses. Am J Vet Res 1986;47:21–26. [PubMed]
- 21.Skarda RT. Local anesthetics and local anesthetic techniques in horses, In: Muir WW, Hubbell JAE, eds. Equine Anesthesia: Monitoring and Emergency Therapy. 1st ed. St. Louis: Mosby — Year Book, 1991:199–246.
- 22.Gilman S, Winans SS, eds. Manter and Gatz's Essentials of Clinical Neuroanatomy and Neurophysiology. 6th ed. Philadelphia; FA Davis, 1982:149–155.