Abstract
Damage to normal, nontumor bone tissue following therapeutic irradiation increases the risk of fracture among cancer patients. For example, women treated for various pelvic tumors have been shown to have a greater than 65% increased incidence of hip fracture by 5 years postradiotherapy. Another practical situation in which exposure to ionizing radiation may negatively impact skeletal integrity is during extended spaceflight missions. There is a limited understanding of how spaceflight-relevant doses and types of radiation can influence astronaut bone health, particularly when combined with the significant effects of mechanical unloading experienced in microgravity. Historically, negative effects on osteoblasts have been studied. Radiation exposure has been shown to damage osteoblast precursors. Damage to local vasculature has been observed, ranging from decreased lumen diameter to complete ablation within the irradiated volume, causing a state of hypoxia. These effects result in suppression of bone formation and a general state of low bone turnover. More recently, however, we have demonstrated in pre-clinical mouse models, a very rapid but transient increase in osteoclast activity after exposure to spaceflight and clinically relevant radiation doses. Combined with long-term suppression of bone formation, this skeletal damage may cause long-term deficits. This review will present a broad set of literature outlining our current set knowledge of both clinical therapy and space exploration exposure to ionizing radiation. Additionally, we will discuss prevention of the initial osteoclast-mediated bone loss, the need to promote normal bone turnover and long-term quality of bone tissue, and our hypothesized molecular mechanisms.
Keywords: Osteoporosis, Fracture, Ionizing radiation, Radiation therapy, Spaceflight, Microgravity, Space radiation, Osteoclasts, Bone, Inflammation
Introduction
The spaceflight environment presents numerous challenges to astronauts’ bone health, the most significant of which are the effects of microgravity and space radiation. Decreases in bone strength that result from a loss of bone mass or architectural stability could impact mission success by increasing the risk of a serious fracture. A robust system of countermeasures that can limit bone loss, and return bone strength to baseline levels postflight, is required to ensure astronauts’ long-term skeletal health and the productivity of extended missions.
Bone damage that occurs after absorbing therapeutic radiation has been documented for decades. Recent animal models are now beginning to demonstrate that spaceflight-relevant doses and qualities of radiation represent a risk to skeletal health due to an acute increase in bone resorption followed by the suppression of bone formation [1, 2]. Skeletal unloading, due to extended periods of bed rest [3] or the reduced gravity environment of space [4], is a well-known cause of bone loss in both humans and rodent models [5]. Unloading-induced bone loss is characterized by both increased bone resorption and decreased bone formation. This stands in contrast to traditional postmenopausal osteoporosis, which sees bone resorption and formation moving in parallel, albeit with the former increasing to a relatively greater degree. The combination of microgravity unloading and spaceflight radiation may interact to enhance bone loss [6–8]. As the mechanisms leading to radiation-induced bone loss are less well established than unloading-induced bone loss, this review will focus on the current state of knowledge regarding the influence of clinical and spaceflight radiation on the development of osteoporosis and bone health in the adult skeleton.
Radiation and Biological Damage
Radiation is generated by radioactive atoms or a radiation generating source, like a clinical linear accelerator, and can be either nonionizing or ionizing. Nonionizing radiation is not energetic enough to eject electrons from an atom, while ionizing radiation can remove electrons from atoms, thus potentially breaking molecular bonds that can lead to biological damage (e.g., DNA, RNA, and cell organelles). Bonds can be broken directly by radiation or indirectly through radiation-generated reactive oxygen species that result from the ionization of water molecules. Cells have a remarkable ability to repair radiation damage, but some cells inevitably die or propagate damage to progeny. In everyday life, we are exposed to natural, background sources of radiation (e.g., environment, space, medical imaging, and internal radioisotopes) at doses that do not affect our long-term health at a measurable level. However, as exposures increase, damage can lead to greater levels of death or radiation-induced phenotypic changes that may be heritable. For our research in ionizing radiation-induced osteoporosis, we are primarily interested in the death of bone marrow cells and its effect on the activation of osteoclasts.
Absorbed dose of radiation (D) is measured in energy per unit mass with the SI unit being Gray (Gy = Joule/kg). It is also common to hear dose expressed in the unit of rad, where 100 rad = 1 Gy (1 rad = 1 cGy). The degree of biological damage can vary depending on the type (quality) of radiation: alpha particles, beta particles, gamma rays (X-rays), neutrons, or heavy ions. For example, exposure to 10 cGy of neutrons will be more damaging than exposure to 10 cGy of X-rays. To account for these varying degrees of damage to humans, Sievert (Sv) is the SI unit for absorbed dose equivalent (H) and is the quality factor (Q) (relative biological effectiveness: RBE) times the dose (H = D * Q). Generally, 1 Gy of X-rays is equal to 1 Sv (100 rad = 100 rem).
Clinical Therapy and Spaceflight Radiation Environments
Normal tissues (noncancerous) will absorb ionizing radiation during both radiation therapy (RT) as a part of cancer therapy and during long-duration space missions. The dose of radiation absorbed by normal tissues surrounding or near tumors during RT can be substantial. For example, treatment regimens for gynecological tumors commonly prescribe the administration of thirty 1.8 Gy fractions over 6 weeks, for a total dose of approximately 54 Gy to the tumor. During the irradiation procedure, normal tissues in each hip can receive as much as half of each fraction (0.9 Gy), totaling 27 Gy. While the paramount concern in radiotherapy has always been curative treatment of the primary tumor, the incidental irradiation of normal tissues is not insignificant. Primary consideration has always been given to radiation effects on normal nervous tissue, reproductive structures, and hollow organs (i.e., esophagus, bowel), while concern for irradiation of bone was not a priority. However, advances in surgical excision, concurrent chemotherapy, and modern RT modalities have afforded greater freedom to focus on minimizing the risk to bone and the potential for increased fracture risk.
The space radiation environment consists of a complex mix of ions from solar particle events (SPEs), which are large mass ejections from the sun commonly known as “solar flares”, and galactic cosmic radiation (GCR), a type of background radiation that originates from outside the solar system. The majority of GCR flux is from protons. While only 1% of GCR is composed of ions heavier than helium, due to the high LET (linear energy transfer) of these high charge (Z) and energy (E) particles (HZE), approximately 41% of the dose equivalent is predicted to be from HZE particles with approximately 13% being from iron alone [9]. LET is the rate of energy lost per length of a particle track and varies as the charge squared divided by the velocity squared. During extended missions in space, estimated tissue dose rates from GCR would be about 0.4–0.8 mGy/day and 1–2.5 mSv/day, respectively [10]. SPE dose rates may reach as high as 50 mGy/h inside a shielded vehicle and between 250 mGy/h for an astronaut exposed during extra-vehicular activity in deep space. The cumulative GCR doses on a deep space mission of 400 days to a near-Earth asteroid would be 0.16–0.32 Gy or 0.4–1.0 Sv. For a large SPE lasting 8–24 h, the whole body cumulative doses could reach the 1- to 2-Gy level for protons (1.0 to 2.0 Sv) depending upon the tissue site. For comparison, cancer patients will receive 1.8–2.0 Gy targeted locally to the tumor each day, delivered over a period of a few minutes.
Fractures of Irradiated Bones After Clinical Exposure
Ionizing radiation is an important and effective modality for the treatment of malignancies and has been a critical factor in the reduction in cancer mortality rates; however, more effective treatment has meant that survivors increasingly experience the long-term side effects caused by radiation damage to normal tissues near the tumor. Bones within the irradiated volume can absorb dose and have an increased fracture risk [11–15]. Rib fractures have been documented in patients receiving treatment for breast cancer, with fracture rates ranging from 1.8% [16] to as high as 19% [17]. Similarly, patients receiving radiotherapy for pelvic tumors are at increased risk for hip fracture, which include fractures of the femoral neck, sacral ala, pubis, and acetabulum [11, 18, 19]. A retrospective analysis of more than 6,400 postmenopausal women receiving RT for cervical, rectal, and anal cancers revealed increased relative risks of hip (primarily femoral neck) fracture of 65, 66, and 214%, respectively, compared with women receiving non-RT cancer treatment such as surgery or chemotherapy [11]. It is important to note that these fractures were localized to the area of the irradiated hip and did not occur at distant sites, such as the wrist. Baxter et al. showed that the effects of radiation on bone are limited to the radiation field, with no increase in fracture risk to nonirradiated bone.
Deterioration of Bone Quantity and Quality After Irradiation
Bone deterioration after exposure to radiation is thought to be associated with both traumatic and spontaneous fractures of bone [11, 20, 21]. Reduction in bone mass and overall bone quality is dependent on several factors, including the dose absorbed, the energy of the radiation beam, the fraction size of the radiation dose, and the age and developmental stage of the patient [17–19]. Cancer patients receiving doses considerably higher than space-flight exposures, osteopenia is often reported in patients 1-year posttherapy, though the observed timing and degree of reduced bone mass is variable [18, 22]. Despite this, demineralization of bone, thinning of bones, sclerosis, and loss of trabecular connections has been described following radiotherapy [18, 20–22]. Thickening of trabeculae within the irradiated volume can be observed within the irradiated volume [19]. This coarsening of trabeculae is also observed from the marrow cavity of various animal models within weeks of exposure [23, 24]. Quantitatively, a significant (~30%) reduction in bone mineral density (BMC) was observed in patients with uterine cervix carcinoma using QCT from the third lumbar vertebrae within 5 weeks following pelvic irradiation with either 45 Gy or 22.5 Gy total dose of high-energy photons [25]. There was no recovery of BMC 12 months after RT.
Recent studies have identified a rapid loss of bone that occurs in animal models with subclinical doses after exposure to either gamma or proton ionizing radiation (Fig. 1). This bone loss can occur after exposure to doses and types of radiation relevant for long-duration missions [6, 26], and a long-term suppression of bone formation after exposure [26]. A long-term reduction in trabecular bone quantity and quality occurs following a whole body 2 Gy dose γ-rays, protons, carbon, or iron ions [6]. Bone loss persists at 4 months after irradiation with doses of protons as low as 1 Gy (modeling a solar flare) and at 9 weeks after modeled galactic radiation of heavy ions <0.5 Gy [9, 26]. Functional bone loss has been identified as early as 3 days after a 2-Gy dose of gamma rays [1]. In addition, there has been observed an increase in early osteoclast activity when mice are irradiated with a 0.5-Gy dose of iron ions during limb disuse, modeling the reduced loading of the spaceflight environment [27]. Six weeks after exposure to high doses modeling clinical therapy in a rat (16 Gy delivered in four doses of 4 Gy each to a single limb), loss of trabecular bone is visibly apparent compared with the nonirradiated contralateral limb [28] (Fig. 2).
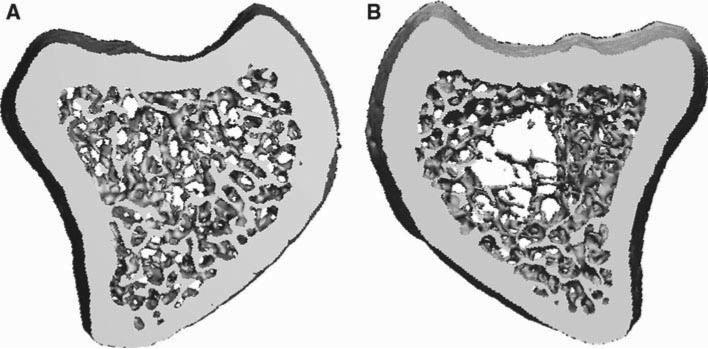
Fig. 1.
MicroCT images of trabecular bone from the proximal tibia of female C57BL/6 mice a not irradiated, b exposed to a 2 Gy (2 Sv) whole body dose of gamma, or c a 2 Gy (2 Sv) dose of proton radiation. Mice were irradiated at 9 weeks of age and tissue collected 4 months later
Fig. 2.
MicroCT images of trabecular bone from the proximal tibiae of a female Sprague–Dawley rat where the a left limb was not irradiated and the b right limb was irradiated with 16 Gy (16 Sv) total dose (four fractions of 4 Gy each) of X-rays. Rats were irradiated at 20 weeks of age with bones collected 6-weeks later
Loss of Bone Strength Animal Models After Irradiation
Changes in bone strength following exposure to radiation would account for the increased fracture risk among those treated for cancer. As a result, animal models have been used to identify any spatial or temporal reduction in bone strength after exposure, generally using higher clinical, rather than spaceflight, dose exposures. Early studies using rabbits and rodents have utilized mechanical testing to identify significant losses in strength after administration of very high radiation doses [29, 30], although no difference in fracture strength after high-dose radiation has been reported in animals [31]. A 2-Gy dose of iron ions causes a loss of vertebral stiffness as tested by compression loading and calculated by finite element analysis (FEA) [7]. Compressive testing of mouse distal femora indicates loss of strength 12 weeks after 5 and 12-Gy acute doses of X-rays [28]. Surprisingly, compressive strength at 2 weeks was increased, which corresponded with an early increase in cortical bone volume and bone mineral content, despite near ablation of trabecular bone parameters [28]. The subsequent loss of strength as determined by compressive testing and estimated by FEA occurred despite a persistent increase in cortical bone mineral content: the bone appeared to be more brittle in nature. Strength changes in bone may thus be influenced by both architectural and material properties after exposure. As mentioned previously, a thickening of trabeculae has been noted early after exposure in certain locations [17], with osteophyte forming where osteoblasts remain after high-dose exposure [32]. An early and transient increase in volumetric bone mineral density [33] and mineral apposition rate [2] has been shown within the first week after exposure in rodent models. These observations suggest an early and dispersed enhancement of bone formation after exposure, although underlying mechanisms are completely unknown. Despite any increase in bone formation, an increased risk of fracture is present at directly irradiated sites [11]. These findings highlight the complicated nature of the skeletal response to ionizing radiation and identify the need for characterizing changes in material properties of bone tissue.
Radiation and Bone Cells
Vasculature
Historically, bone loss following radiotherapy was thought to be a result of physiological changes within the vasculature and bone cells [18, 21, 22, 32, 34–36]. The first report of radiation-induced bone damage (termed “osteitis”) described a reduction in bone vasculature following obliterative endartitis and periartitis [37]. Early loss of vascularization occurs as a result of swelling and vacuolization of endothelial cells within the vascular channels of the osteons [21, 22, 32]. Ultimately, this results in the formation of sclerotic connective tissue within the marrow cavity. Fibrosis occurring in the subintima and replacement of vascular smooth muscle cells with hyaline-like material within the tunica media occur as late injuries, constricting the vessel lumen. Bony elements such as the skull and jaw are considered especially at risk for vascular injury due in part to the paucity of vasculature and their superficial location [19]. Ablation of vasculature has been identified within the bone, including marrow cavity and Haversian systems, in a variety of animal models after exposure [23, 32, 38].
Osteoblasts and Osteocytes
Damage to osteoblasts and osteocytes within the bone microenvironment is thought to be a primary contributor to reduced bone mineral density following irradiation [18, 21, 22, 39]. A reduction in the overall number of osteoblasts occurs following irradiation, with reduced matrix formation [2, 38]. Both in vitro and in vivo data suggest that radiation can impair bone formation by decreasing osteoblast proliferation and differentiation, inducing cell cycle arrest, reducing collagen production, and increasing sensitivity to apoptotic agents [36, 40–42]. Radiation causes a decline in RUNX2 levels, indicative of impaired osteoblast differentiation, in osteoblast cultures stimulated with bone morphogenic protein-2 (BMP-2) [42]. RANKL mRNA levels tended to increase in osteoblasts following exposure to gamma rays, but not to carbon ions [43]. Osteoblast precursors are likely damaged by radiation [1]. Mesenchymal stem cell (MSC) numbers and colony forming ability under osteogenic stimulation are also reduced at the directly irradiated bone after exposure, which would likely delay recovery of osteoblast damage [38]. Oxidative stress appears to contribute to this early damage to osteoprogenitors [1, 38]. However, others have indicated no loss of MSC viability within irradiated bone, but rather terminal differentiation into osteoblasts [44].
The effect of radiation on osteocyte numbers and health are unclear. Some studies have identified loss of osteocytes within irradiated bone following exposure to high doses [18, 21, 32]. Osteocytes were shown to be killed within the irradiated field inside cortical lamellar and Haversian bone of 45 Gy irradiated monkey mandibles, although they were not affected within trabecular bone. Overall, however, osteocytes appear to be relatively radioresistant in several animal models, remaining viable for several months after a single high dose of radiation in mice and rabbits [29, 39, 45, 46].
Osteoclasts
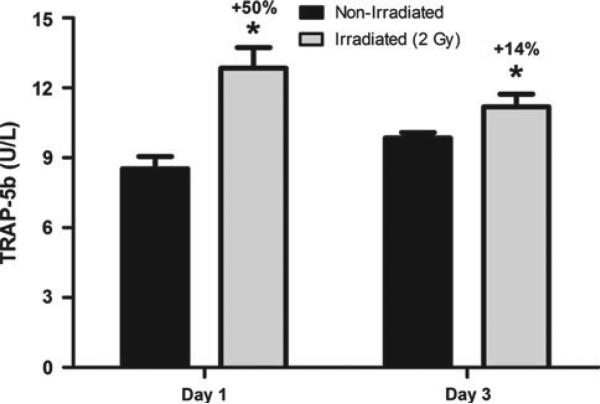
Studies in the last few years show that irradiation results in increased osteoclast number and activity as an early response, which likely contributes to radiation-induced osteoporosis [2]. Serum markers for tartrate-resistant acid phosphatase (TRAP5b), a marker for osteoclast activity, are elevated as early as 24 h after exposure to a whole body dose of X-rays (Fig. 3). An increase in osteoclast number, +213% osteoclast surface normalized to bone surface (Oc.S/BS), and activity has been identified to occur within rodent bones 3 days after exposure [47], with subsequent loss of bone within a week of treatment [1, 2]. Serum chemistry and histological analyses show significant increases in TRAP5b, a marker of bone resorption, as well as osteoblast number and surface, during the first week after treatment. However, in these rodent models, the majority of the bone loss occurs during a time when osteoblast numbers and bone formation are unchanged relative to control [2]. Suppressing osteoclast activity with the bisphosphonate antiresorptive risedronate has been shown to completely block radiation-induced increases in osteoclast activity and subsequent deterioration of bone at multiple skeletal locations [2]. Decreased osteoclast activity has been noted at time points greater than 1 week postexposure, with conflicting reports of recovery [33, 48]. This subsequent decline in both osteoclast and osteoblast activity, if persistent, could sufficiently suppress bone remodeling to impair material properties of bone tissue [49], as described in rodents [28]. Taken together, it can be seen that a combination of an early and acute loss of bone via osteoclast activity, as well as a persistent reduction in bone formation, contribute to osteopenia and bone deterioration after exposure.
Fig. 3.
Serum levels of TRAP5b, a marker for osteoclast activity, are significantly elevated compared with nonirradiated control mice both 1 and 3 days after exposure. Female C57BL/6 mice were exposed whole body to a 2-Gy (2 Sv) dose of X-rays at 13 weeks of age and humanely euthanized to collect blood
Osteoclast Activity and Radiation-Induced Inflammation
The mechanism leading to an acute activation of osteoclasts following exposure to ionizing radiation remains unknown, though radiation-initiated inflammation is suspected. Inflammatory cytokines and reactive oxygen species (ROS) generation within the marrow after exposure to radiation could trigger osteoclast activation. Cell death in the marrow microenvironment results in an increased phagocyte presence at 24 h after exposure to a 4-Gy dose of gamma radiation in mice [50]. The elevated neutrophil and macrophage activity occurs with a coincident, large reduction in the number of hematopoietic cells [50]. Apoptosis could not account for the prolonged presence of activated phagocytes within the irradiated volume; their numbers remained elevated after clearance of apoptotic cells, peaking 24 h after exposure [50]. This suggests an early, exaggerated inflammatory response within irradiated bone marrow.
Inflammation is a common response to exposure to ionizing radiation. For example, inflammatory cytokine concentrations increase rapidly [51–54]. This effect is characterized by the early activation of stress-sensitive kinases, proinflammatory transcription factors, and the up-regulation and production of proinflammatory cytokines, including tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β) [55–58]. Immunohistochemistry of decalcified histological sections for TNFα and IL-1β in female B6 mice 24 h after X-ray exposure of 2 Gy indicates increased concentration of both of these proteins in the marrow (Fig. 5). Gene expression of TNFα and IL-1β in bone marrow is also changed early after exposure: TNFα levels were significantly greater at 4 and 24 h, while IL-1β expression was greater at 24 and 48 h, and IL-6 expression was elevated 24 h after exposure (Fig. 4). Proinflammatory cytokines, such as TNFα, IL-1β, and IL-6, are active stimulators of osteoclastogenesis and are characteristic of many inflammatory diseases that lead to bone loss [59–62]. Collectively, these cytokines induce osteoblast production of receptor activator of nuclear factor kappa-B ligand (RANKL) and stromal-cell expression of macrophage colony stimulating factor (M-CSF) [63] in addition to directly activating osteoclasts. We hypothesize that it is this radiation-initiated inflammatory cascade that is responsible for the early activation of osteoclasts (Fig. 6).
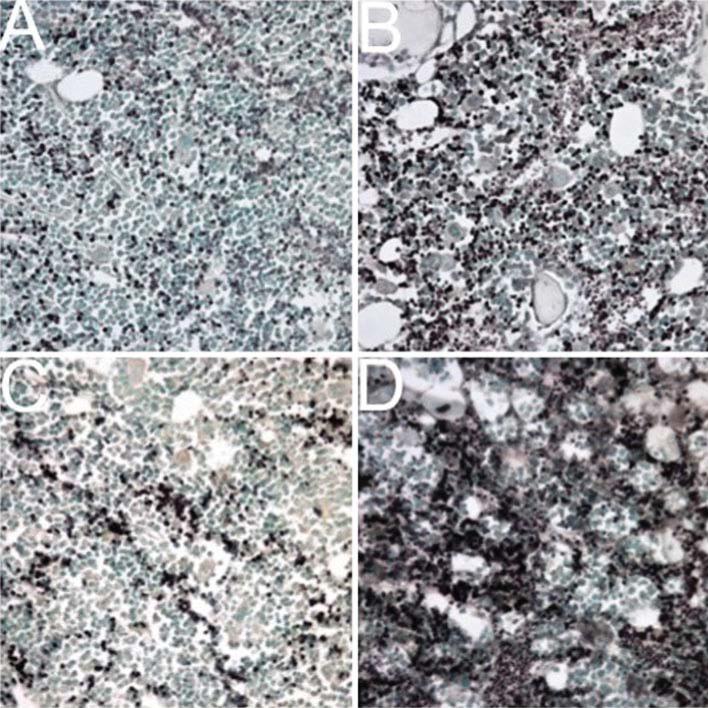
Fig. 5.
Images of immunohistochemistry (IHC) stained bone marrow from the proximal tibia of either nonirradiated or 2 Gy (2 Sv) X-ray whole body irradiated mice for the proinflammatory cytokines TNFα or IL-1. a IHC staining (dark brown) for TNFα in nonirradiated mice, compared with (b) much greater TNFα levels in bone marrow from irradiated mice. c IHC staining for IL-1 in nonirradiated mice, compared with (d) increased levels of IL-1 in X-ray exposed mice. Bones were collected from 13-week-old female C57BL/6 mice 24 h after radiation exposure
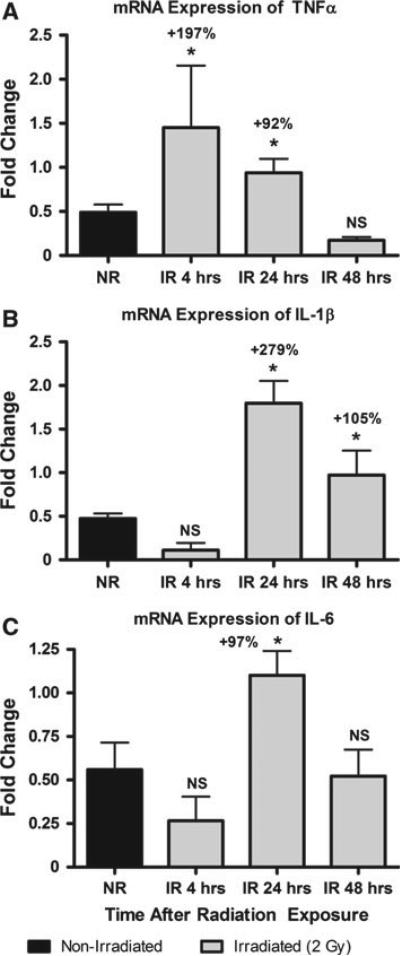
Fig. 4.
In bone marrow from mice exposed to a 2 Gy (2 Sv) whole body dose of X-rays (IR), a mRNA expression of TNFα is significantly increased 4 and 24 h after exposure compared with nonirradiated (NR) mice. Similarly, b with the same exposure profile IL-1 expression in bone marrow is significantly greater 24 and 48 h after exposure, and c IL-6 expression is significantly increased at only the 24 h postirradiation time point
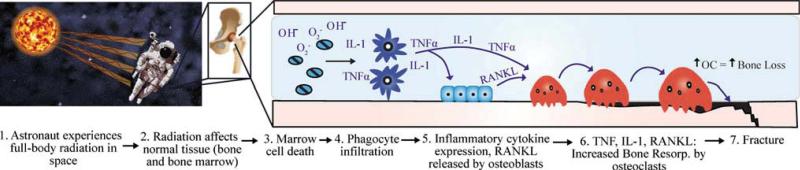
Fig. 6.
Hypothesized mechanisms for the rapid activation of osteoclastic bone resorption leading to increased fracture risk in astronauts exposed to space radiation. The cascade initiates with: 1 acute and chronic exposure of an astronaut to space radiation from solar particle events and galactic cosmic sources, respectively; 2 normal, healthy bone and bone marrow is exposed; 3 immature and rapidly dividing cells within the bone marrow are damaged and die from the ionization of atoms or the creation of reactive oxygen species; 4 macrophages and neutrophils are recruited to remove the dead marrow cells; 5 proinflammatory cytokine levels increase due to macrophage activation and infiltration. These cytokines stimulate osteoclasts or stromal-derived cells (preosteoblasts, fibroblasts, and MSCs). TNFα and IL-1 activate osteoclasts directly and induce the expression of RANKL in stromal cells; 7 osteoclastic bone resorption is initiated, causing a rapid loss of bone that leads to an increased lifetime risk of fracture
Conclusion
Exposure to radiation represents a significant concern for the skeletal health of astronauts in the spaceflight environment and for patients receiving radiotherapy as part of their cancer treatment. Astronauts on long-duration missions could be exposed to low-dose radiation from galactic cosmic radiation and solar flares. These exposures could compromise bone strength and lead to mission-critical fractures, especially in conjunction with the similar responses due to gravity unloading. From the clinical perspective, advances in the treatment of cancer, with radiation, chemotherapy, and surgical modalities, have made concern for incidental radiation damage to nontumor tissues, including bone, a growing concern and clinicians are now considering normal tissue complication probabilities as part of the treatment planning process. It should be noted that radiation exposures outside the treatment volume may substantially exceed those predicted for astronauts and potentially at risk volumes of bone need to be considered as critical structures rather than as a neutral structures. Limited research has been conducted to define the magnitude and mechanisms behind bone loss associated with radiation exposure. It is clear that an inflammatory response does occur in response to radiation and may be the molecular mechanisms that cause early activation of osteoclast-mediated bone resorption. Ultimately, bone formation is also suppressed. Bone strength is thus compromised, which could dispose individuals to significant fracture risks with accompanying mortality as a late sequela, especially in geriatric patients. There is a clear need for effective countermeasures to radiation-induced bone loss, both for the astronaut population and the ever growing population of individuals taking advantage of highly effective cancer radiotherapy. More work is required to define the precise molecular and cellular mediators of this response and to define targets for novel therapeutics. Once in place, these countermeasures will be able to augment the impressive advances in cancer treatment and reduce the long-term morbidity and mortality associated with cancer treatment.
Acknowledgments
This research is supported by the National Space Biomedical Research Institute through NASA NCC 9-58 (BL01302 TAB; PF01403 JSW), NASA Cooperative Agreements NCC9-79 and NCC9-149 (GAN), and the National Institutes of Health (NIAMS R21AR054889 TAB). Support was also provided by the National Institutes of Health (T32 CA113267 JSW). We want to thank Procter and Gamble Pharmaceuticals for providing an unrestricted grant (TAB JSW) and to the MD/PhD Program at The Pennsylvania State University College of Medicine for partial funding support (SAJL).
Contributor Information
Jeffrey S. Willey, Section of Molecular Medicine and Department of Radiation Oncology, Comprehensive Cancer Center, Wake Forest School of Medicine, Radiation Biology 405 NRC, Medical Center Blvd., Winston-Salem, NC 27157, USA jwilley@wfubmc.edu
Shane A. J. Lloyd, Department of Orthopaedics and Rehabilitation, Division of Musculoskeletal Sciences, The Pennsylvania State University College of Medicine, 500 University Drive, Hershey, PA 17033, USA slloyd@hmc.psu.edu
Gregory A. Nelson, Department of Radiation Medicine, Loma Linda University, 11175 Campus St., CSP A1010, Loma Linda, CA 92354, USA gnelson@dominion.llumc.edu
Ted A. Bateman, Departments of Biomedical Engineering and Radiation Oncology, University of North Carolina, 152 MacNider Hall, CB 7575, Chapel Hill, NC 27599-7575, USA bateman@unc.edu
References
- 1.Kondo H, Searby ND, Mojarrab R, Phillips J, Alwood J, Yumoto K, Almeida EA, Limoli CL, Globus RK. Total-body irradiation of postpubertal mice with (137)Cs acutely compromises the microarchitecture of cancellous bone and increases osteoclasts. Radiat Res. 2009;171(3):283–9. doi: 10.1667/RR1463.1. [DOI] [PubMed] [Google Scholar]
- 2.Willey JS, Livingston EW, Robbins ME, Bourland JD, Tirado-Lee L, Smith-Sielicki H, Bateman TA. Risedronate prevents early radiation-induced osteoporosis in mice at multiple skeletal locations. Bone. 2010;46(1):101–11. doi: 10.1016/j.bone.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spector ER, Smith SM, Sibonga JD. Skeletal effects of long-duration head-down bed rest. Aviat Space Environ Med. 2009;80(5 Suppl):A23–8. doi: 10.3357/asem.br02.2009. [DOI] [PubMed] [Google Scholar]
- 4.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19(6):1006–12. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 5.Bikle DD, Halloran BP. The response of bone to unloading. J Bone Min Metab. 1999;17(4):233–44. doi: 10.1007/s007740050090. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton SA, Pecaut MJ, Gridley DS, Travis ND, Bandstra ER, Willey JS, Nelson GA, Bateman TA. A murine model for bone loss from therapeutic and space-relevant sources of radiation. J Appl Physiol. 2006;101(3):789–93. doi: 10.1152/japplphysiol.01078.2005. [DOI] [PubMed] [Google Scholar]
- 7.Alwood JS, Yumoto K, Mojarrab R, Limoli CL, Almeida EA, Searby ND, Globus RK. Heavy ion irradiation and unloading effects on mouse lumbar vertebral microarchitecture, mechanical properties and tissue stresses. Bone. 2010;47(2):248–55. doi: 10.1016/j.bone.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Kondo H, Yumoto K, Alwood JS, Mojarrab R, Wang A, Almeida EA, Searby ND, Limoli CL, Globus RK. Oxidative stress and gamma radiation-induced cancellous bone loss with musculo-skeletal disuse. J Appl Physiol. 2010;108(1):152–61. doi: 10.1152/japplphysiol.00294.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandstra ER, Thompson RW, Nelson GA, Willey JS, Judex S, Cairns MA, Benton ER, Vazquez ME, Carson JA, Bateman TA. Musculoskeletal changes in mice from 20–50 cGy of simulated galactic cosmic rays. Radiat Res. 2009;172(1):21–9. doi: 10.1667/RR1509.1. [DOI] [PubMed] [Google Scholar]
- 10.NCRP . Report No. 132—radiation protection guidance for activities in low-earth orbit, in 2000. National Council on Radiation Protection and Measurements; Bethesda, MD: 2000. [Google Scholar]
- 11.Baxter NN, Habermann EB, Tepper JE, Durham SB, Virnig BA. Risk of pelvic fractures in older women following pelvic irradiation. JAMA. 2005;294(20):2587–93. doi: 10.1001/jama.294.20.2587. [DOI] [PubMed] [Google Scholar]
- 12.Brown SA, Guise TA. Cancer treatment-related bone disease. Crit Rev Eukaryot Gene Expr. 2009;19(1):47–60. doi: 10.1615/critreveukargeneexpr.v19.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guise TA. Bone loss and fracture risk associated with cancer therapy. Oncologist. 2006;11(10):1121–31. doi: 10.1634/theoncologist.11-10-1121. [DOI] [PubMed] [Google Scholar]
- 14.Florin TA, Fryer GE, Miyoshi T, Weitzman M, Mertens AC, Hudson MM, Sklar CA, Emmons K, Hinkle A, Whitton J, Stovall M, Robison LL, Oeffinger KC. Physical inactivity in adult survivors of childhood acute lymphoblastic leukemia: a report from the childhood cancer survivor study. Cancer Epidemiol Bio-markers Prev. 2007;16(7):1356–63. doi: 10.1158/1055-9965.EPI-07-0048. [DOI] [PubMed] [Google Scholar]
- 15.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS, Schwartz CL, Leisenring W, Robison LL. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 16.Pierce SM, Recht A, Lingos TI, Abner A, Vicini F, Silver B, Herzog A, Harris JR. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys. 1992;23(5):915–23. doi: 10.1016/0360-3016(92)90895-o. [DOI] [PubMed] [Google Scholar]
- 17.Overgaard M. Spontaneous radiation-induced rib fractures in breast cancer patients treated with postmastectomy irradiation. A clinical radiobiological analysis of the influence of fraction size and dose-response relationships on late bone damage. Acta Oncol. 1988;27(2):117–22. doi: 10.3109/02841868809090331. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell MJ, Logan PM. Radiation-induced changes in bone. Radiographics. 1998;18(5):1125–36. doi: 10.1148/radiographics.18.5.9747611. quiz 1242-3. [DOI] [PubMed] [Google Scholar]
- 19.Williams HJ, Davies AM. The effect of X-rays on bone: a pictorial review. Eur Radiol. 2006;16(3):619–33. doi: 10.1007/s00330-005-0010-7. [DOI] [PubMed] [Google Scholar]
- 20.Howland W, Loeffler RK, Starchman DE, et al. Post-irradiation atrophic changes of bone and related complications. Radiology. 1975;117:677–85. doi: 10.1148/117.3.677. [DOI] [PubMed] [Google Scholar]
- 21.Ergun H, Howland WJ. Postradiation atrophy of mature bone. CRC Crit Rev Diagn Imaging. 1980;12(3):225–43. [PubMed] [Google Scholar]
- 22.Hopewell JW. Radiation-therapy effects on bone density. Med Pediatr Oncol. 2003;41(3):208–11. doi: 10.1002/mpo.10338. [DOI] [PubMed] [Google Scholar]
- 23.Furstman LL. Effect of radiation on bone. J Dent Res. 1972;51(2):596–604. doi: 10.1177/00220345720510025901. [DOI] [PubMed] [Google Scholar]
- 24.Sawajiri M, Mizoe J. Changes in bone volume after irradiation with carbon ions. Radiat Environ Biophys. 2003;42(2):101–6. doi: 10.1007/s00411-003-0191-x. [DOI] [PubMed] [Google Scholar]
- 25.Nishiyama K, Inaba F, Higashihara T, Kitatani K, Kozuka T. Radiation osteoporosis—an assessment using single energy quantitative computed tomography. Eur Radiol. 1992;2(4):322–5. [Google Scholar]
- 26.Bandstra ER, Pecaut MJ, Anderson ER, Willey JS, De Carlo F, Stock SR, Gridley DS, Nelson GA, Levine HG, Bateman TA. Long-term dose response of trabecular bone in mice to proton radiation. Radiat Res. 2008;169(6):607–14. doi: 10.1667/RR1310.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yumoto K, Globus RK, Mojarrab R, Arakaki J, Wang A, Searby ND, Almeida EA, Limoli CL. Short-term effects of whole-body exposure to (56)fe ions in combination with musculoskeletal disuse on bone cells. Radiat Res. 2010;173(4):494–504. doi: 10.1667/RR1754.1. [DOI] [PubMed] [Google Scholar]
- 28.Wernle JD, Damron TA, Allen MJ, Mann KA. Local irradiation alters bone morphology and increases bone fragility in a mouse model. J Biomech. 2010;43(14):2738–46. doi: 10.1016/j.jbiomech.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto M, Takahashi S, Toguchida J, Kotoura Y, Shibamoto Y, Yamamuro T. Changes in bone after high-dose irradiation. Biomechanics and histomorphology. J Bone Joint Surg Br. 1991;73(3):492–7. doi: 10.1302/0301-620X.73B3.1670456. [DOI] [PubMed] [Google Scholar]
- 30.Nyaruba MM, Yamamoto I, Kimura H, Morita R. Bone fragility induced by X-ray irradiation in relation to cortical bone-mineral content. Acta Radiol. 1998;39(1):43–6. doi: 10.1080/02841859809172147. [DOI] [PubMed] [Google Scholar]
- 31.Maeda M, Bryant MH, Yamagata M, Li G, Earle JD, Chao EY. Effects of irradiation on cortical bone and their time-related changes. A biomechanical and histomorphological study. J Bone Joint Surg Am. 1988;70(3):392–9. [PubMed] [Google Scholar]
- 32.Rohrer MD, Kim Y, Fayos JV. The effect of cobalt-60 irradiation on monkey mandibles. Oral Surg Oral Med Oral Pathol. 1979;48(5):424–40. doi: 10.1016/0030-4220(79)90074-4. [DOI] [PubMed] [Google Scholar]
- 33.Margulies B, Morgan H, Allen M, Strauss J, Spadaro J, Damron T. Transiently increased bone density after irradiation and the radioprotectant drug amifostine in a rat model. Am J Clin Oncol. 2003;26(4):e106–14. doi: 10.1097/01.COC.0000077934.48841.40. [DOI] [PubMed] [Google Scholar]
- 34.Bliss P, Parsons CA, Blake PR. Incidence and possible aetiological factors in the development of pelvic insufficiency fractures following radical radiotherapy. Br J Radiol. 1996;69(822):548–54. doi: 10.1259/0007-1285-69-822-548. [DOI] [PubMed] [Google Scholar]
- 35.Konski A, Sowers M. Pelvic fractures following irradiation for endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1996;35(2):361–7. doi: 10.1016/0360-3016(95)02139-6. [DOI] [PubMed] [Google Scholar]
- 36.Gal TJ, Munoz-Antonia T, Muro-Cacho CA, Klotch DW. Radiation effects on osteoblasts in vitro: a potential role in osteoradio-necrosis. Arch Otolaryngol Head Neck Surg. 2000;126(9):1124–8. doi: 10.1001/archotol.126.9.1124. [DOI] [PubMed] [Google Scholar]
- 37.Ewing J. Radiation osteitis. Acta Radiol. 1926;6:399–412. [Google Scholar]
- 38.Cao X, Wu X, Frassica D, Yu B, Pang L, Xian L, Wan M, Lei W, Armour M, Tryggestad E, Wong J, Wen CY, Lu WW, Frassica FJ. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc Natl Acad Sci U S A. 2011;108(4):1609–14. doi: 10.1073/pnas.1015350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sams A. The effect of 2000 r of x-rays on the internal structure of the mouse tibia. Int J Radiat Biol Relat Stud Phys Chem Med. 1966;11(1):51–68. doi: 10.1080/09553006614550791. [DOI] [PubMed] [Google Scholar]
- 40.Dudziak ME, Saadeh PB, Mehrara BJ, Steinbrech DS, Greenwald JA, Gittes GK, Longaker MT. The effects of ionizing radiation on osteoblast-like cells in vitro. Plast Reconstr Surg. 2000;106(5):1049–61. doi: 10.1097/00006534-200010000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Szymczyk KH, Shapiro IM, Adams CS. Ionizing radiation sensitizes bone cells to apoptosis. Bone. 2004;34(1):148–56. doi: 10.1016/j.bone.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Sakurai T, Sawada Y, Yoshimoto M, Kawai M, Miyakoshi J. Radiation-induced reduction of osteoblast differentiation in C2C12 cells. J Radiat Res (Tokyo) 2007;48(6):515–21. doi: 10.1269/jrr.07012. [DOI] [PubMed] [Google Scholar]
- 43.Sawajiri M, Nomura Y, Bhawal UK, Nishikiori R, Okazaki M, Mizoe J, Tanimoto K. Different effects of carbon ion and gamma-irradiation on expression of receptor activator of NF-kB ligand in MC3T3-E1 osteoblast cells. Bull Exp Biol Med. 2006;142(5):618–24. doi: 10.1007/s10517-006-0433-4. [DOI] [PubMed] [Google Scholar]
- 44.Schonmeyr BH, Wong AK, Soares M, Fernandez J, Clavin N, Mehrara BJ. Ionizing radiation of mesenchymal stem cells results in diminution of the precursor pool and limits potential for multilineage differentiation. Plast Reconstr Surg. 2008;122(1):64–76. doi: 10.1097/PRS.0b013e31817743cd. [DOI] [PubMed] [Google Scholar]
- 45.Jacobsson M, Jonsson A, Albrektsson T, Turesson I. Alterations in bone regenerative capacity after low level gamma irradiation. Scand J Plastic Reconstr Surg. 1985;19:231–6. doi: 10.3109/02844318509074508. [DOI] [PubMed] [Google Scholar]
- 46.Rabelo GD, Beletti ME, Dechichi P. Histological analysis of the alterations on cortical bone channels network after radiotherapy: a rabbit study. Microsc Res Tech. 2010;73(11):1015–8. doi: 10.1002/jemt.20826. [DOI] [PubMed] [Google Scholar]
- 47.Willey JS, Lloyd SA, Robbins ME, Bourland JD, Smith-Sielicki H, Bowman LC, Norrdin RW, Bateman TA. Early increase in osteoclast number in mice after whole-body irradiation with 2 Gy X rays. Radiat Res. 2008;170(3):388–92. doi: 10.1667/RR1388.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawajiri M, Mizoe J, Tanimoto K. Changes in osteoclasts after irradiation with carbon ion particles. Radiat Environ Biophys. 2003;42(3):219–23. doi: 10.1007/s00411-003-0204-9. [DOI] [PubMed] [Google Scholar]
- 49.Burr DB, Miller L, Grynpas M, Li J, Boyde A, Mashiba T, Hirano T, Johnston CC. Tissue mineralization is increased following 1-year treatment with high doses of bisphosphonates in dogs. Bone. 2003;33(6):960–9. doi: 10.1016/j.bone.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Lorimore SA, Coates PJ, Scobie GE, Milne G, Wright EG. Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects? Oncogene. 2001;20(48):7085–95. doi: 10.1038/sj.onc.1204903. [DOI] [PubMed] [Google Scholar]
- 51.Robbins ME, Jaenke RS, Bywaters T, Golding SJ, Rezvani M, Whitehouse E, Hopewell JW. Sequential evaluation of radiation-induced glomerular ultrastructural changes in the pig kidney. Radiat Res. 1993;135(3):351–64. [PubMed] [Google Scholar]
- 52.Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33(1):99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 53.Van der Meeren A, Vandamme M, Squiban C, Gaugler MH, Mouthon MA. Inflammatory reaction and changes in expression of coagulation proteins on lung endothelial cells after total-body irradiation in mice. Radiat Res. 2003;160(6):637–46. doi: 10.1667/rr3087. [DOI] [PubMed] [Google Scholar]
- 54.Hosoi Y, Miyachi H, Matsumoto Y, Enomoto A, Nakagawa K, Suzuki N, Ono T. Induction of interleukin-1beta and interleukin-6 mRNA by low doses of ionizing radiation in macrophages. Int J Cancer. 2001;96(5):270–6. doi: 10.1002/ijc.1030. [DOI] [PubMed] [Google Scholar]
- 55.Akmansu M, Unsal D, Bora H, Elbeg S. Influence of locoregional radiation treatment on tumor necrosis factor-alpha and interleukin-6 in the serum of patients with head and neck cancer. Cytokine. 2005;31(1):41–5. doi: 10.1016/j.cyto.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 56.Barcellos-Hoff MH. How do tissues respond to damage at the cellular level? The role of cytokines in irradiated tissues. Radiat Res. 1998;150(5 Suppl):S109–20. [PubMed] [Google Scholar]
- 57.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6(9):702–13. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 58.Vujaskovic Z, Anscher MS, Feng QF, Rabbani ZN, Amin K, Samulski TS, Dewhirst MW, Haroon ZA. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys. 2001;50(4):851–5. doi: 10.1016/s0360-3016(01)01593-0. [DOI] [PubMed] [Google Scholar]
- 59.Walsh MC, Choi Y. Biology of the TRANCE axis. Cytokine Growth Factor Rev. 2003;14(3–4):251–63. doi: 10.1016/s1359-6101(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 60.Walsh NC, Crotti TN, Goldring SR, Gravallese EM. Rheumatic diseases: the effects of inflammation on bone. Immunol Rev. 2005;208:228–51. doi: 10.1111/j.0105-2896.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 61.Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23(31):7897–903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 62.Weitzmann MN, Pacifici R. The role of T lymphocytes in bone metabolism. Immunol Rev. 2005;208:154–68. doi: 10.1111/j.0105-2896.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 63.Kimble RB, Srivastava S, Ross FP, Matayoshi A, Pacifici R. Estrogen deficiency increases the ability of stromal cells to support murine osteoclastogenesis via an interleukin-1 and tumor necrosis factor-mediated stimulation of macrophage colony-stimulating factor production. J Biol Chem. 1996;271(46):28890–7. doi: 10.1074/jbc.271.46.28890. [DOI] [PubMed] [Google Scholar]