Abstract
Previously, we have shown that IL-21R-/- splenocytes ameliorate graft-versus-host disease (GVHD) as compared to wild type splenocytes. Here, we investigated whether or not IL-21R-/- splenocytes diminish the graft-versus-leukemia (GVL) effect. Surprisingly, IL-21R-/- splenocytes efficiently eliminate leukemic cells as well as wild type splenocytes, suggesting the retention of GVL effects in the absence of IL-21 signaling. To compare the GVL effect between IL-21R-/- and wild type cells, we titrated the number of splenocytes required for the elimination of leukemic cells and found that the threshold of GVL effect was obtained between 5 × 105 and 5 × 106 with both types of splenocytes. Co-transplantation with CD8-depleted splenocytes but not with purified CD8 T-cells resulted in a significant reduction in anti-leukemic effect of IL-21R-/- cells compared to wild type cells, suggesting that the lack of IL-21 signaling primarily impairs CD4 T-cell rather than CD8 T-cell function and the comparable GVL effect with IL-21R-/- bulk splenocytes results from cooperative compensation by CD8 T-cells.
Keywords: Cytokine, GVL, Interleukin-21, IL-21
Introduction
IL-21 is a pleiotropic cytokine belonging to the common cytokine receptor γ chain family of cytokines1-3. IL-21 is mainly produced by activated CD4+ T cells4 and acts on T-, B-, NK-cells, and other lineages3-5. IL-21 has a critical role in immunoglobulin production6 and the lack of both IL-21 and IL-4 signals impairs normal germinal center formation6. Anti-tumor effects of IL-21 have been reported 7-9, with ongoing clinical trials10-13. Moreover, IL-21 can drive Th17 differentiation14-16. IL-21 also contributes to the development of autoimmune disease has been reported in mouse models17-20, possibly related to Th17 differentiation.
Graft-versus-host disease (GVHD) is one of major complications after hematopoietic stem cell transplantation. and the same graft simultaneously induces the graft-versus-leukemia (GVL) effect, which reduces the relapse rate. In clinical practice, there is no GVHD-specific treatment, and all currently available immunosuppressive reagents attenuate GVL effect as well as GVHD. In mouse models, possible strategies were reported to specifically suppress GVHD21-28, but nothing is available in clinical settings at this point.
Previously, we have shown that IL-21R-/- (KO) splenocytes ameliorate GVHD as compared to wild type splenocytes29, indicating a critical role of IL-21 in GVHD. Bucher et al. reported that IL-21 neutralization resulted in the same results as ours and they found that it did not attenuate GVL effect30. However, they did not titrate the required T-cells for GVL effect, making it impossible to determine if the GVL strength was similar in the normal and the IL-21-neutralized conditions. Here, we sought to quantify and compare the strength of GVL effect between WT and KO splenocytes, and moreover, to analyze the contributions of CD4 and CD8 cells to GVL effect.
Materials and methods
Mice
IL-21R-/- (KO) mice were generated previously6 and mice were C57BL/6 background29. IL-21R+/+ (WT) mice were interbred progeny from the same littermate. C57BL/6-DBA2-F1 mice were purchased from Clea Japan (Tokyo, Japan). All mice were used between 6-12 weeks old and housed in a small animal facility in Jichi Medical University, which is regulated by an intramural small animal committee, and were treated in accordance with the Jichi Medical University guidelines.
GVL models
We have used two different models of leukemic cells.
GVL group 1 compared transplantation with the P815 leukemic cell line, T-cell depleted wild type bone marrow cells (TCD-BM), and either KO-SP vs. WT-SP. C57BL/6-DBA F1 strain mice were used as recipients. The number of splenocytes was 5 × 107, and P815 leukemic cells were co-infused (2 × 104). T cell depletion from BM was performed using Thy1.2 microbeads and an autoMACS (Myltenyi Biotech, Auburn, CA). P815 cells (mast cell leukemia line, H-2d) were used as a model of residual leukemic cells, and C57BL/6 mice were used as donors (H-2b). P815 was originally derived from DBA2 mice (H-2d); therefore, P815 can cause lethal leukemic conditions in C57BL/6-DBA2 F1 recipients 21.
GVL group 2 compared transplantation with either an IL-21 decoy receptor or empty vector transduced BM in the presence of WT-SP. C57BL/6-DBA F1 strain mice were used as recipients. The number of wild type splenocytes was 2 × 107, and P815 leukemic cells were co-infused (2 × 104). Either decoy receptor or empty vector transduced BM cells (starting dose of BM was 5 × 105) were transplanted instead of T cell depleted BM cells.
GVL group 3 compared transplantation with p185 bcr-abl transduced recipient-BM, TCD-BM, and either KO-SP or WT-SP. Transplantation was performed as in the GVL group 1 except for the co-infusion of p185 bcr-abl transduced recipient-BM cells (starting dose of BM was 5 × 105) instead of P815 cells, and the number of splenocytes (2 × 107)31.
CD8+ T cell depletion from splenocytes was performed using CD8 magnetic-microbeads and an autoMACS (Myltenyi Biotech, Auburn, CA). The average proportion of CD8+ T cells in spleen was 12-13 % and after purification it declined to 3.4-3.6 % in average. CD8+ T cell purification from splenocytes was performed as above not with negative selection but with positive selection. The purity was ~85%.
51Cr release assays (cytotoxic T cell lysis assays)
As previously described21, T cells were purified with CD90 microbeads (Miltenyi) from splenocytes of C57BL/6-DBA-F1 recipient mice 14 days after bone marrow transplantation, and T cells from three mice were combined in each group. The percentage of CD8+ cells was determined by flow cytometric analysis, and counts were normalized for CD8+ T cell numbers. Cell lines, P815 (H-2d) and EL4 (H-2b), were used as allogeneic and syngeneic targets, respectively. Target cells (2 × 106) were labeled with 100 μCi of 51Cr for 1.5 hours. After washing twice, target cells were plated at 1 × 104 per well in U-bottom 96-well plates. T cells were added to each well at different target/effector ratios, as indicated (Fig. 7A) in quadruplicates and incubated 4-5 hours. 51Cr in the supernatants after incubation was determined using LumaPlate™-96 and TopCount NXT (PerkinElmer, Massachusetts, USA).
Figure 7.
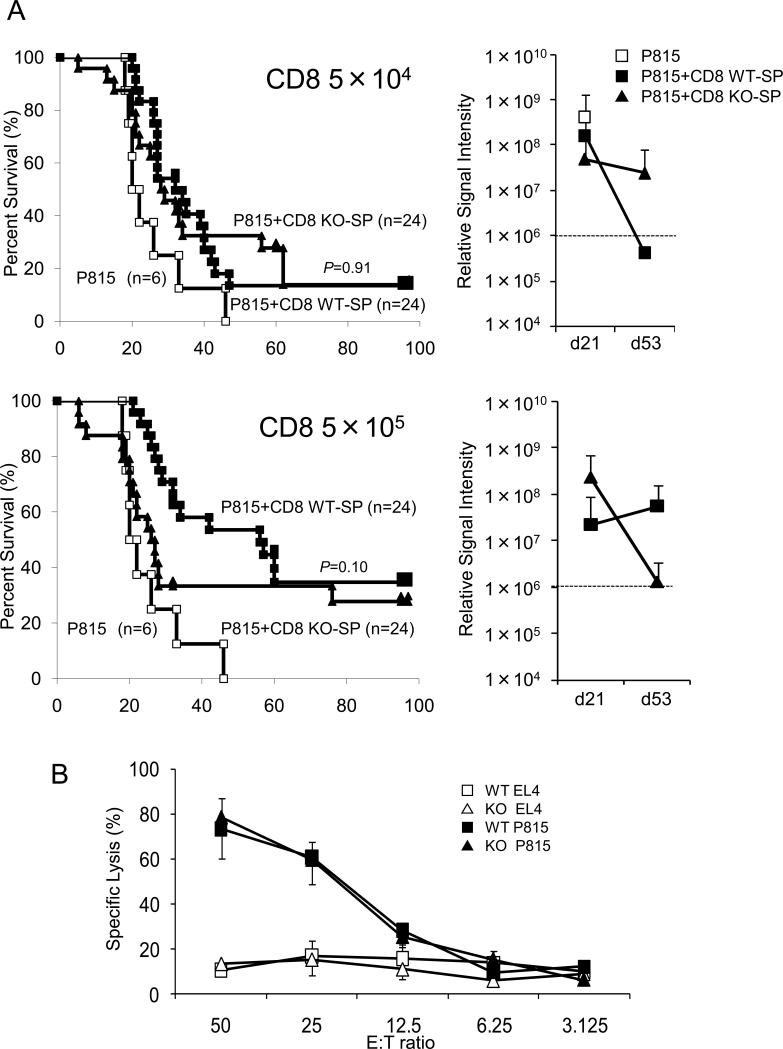
Survival curve in dose-reduction experiments with CD8-purified splenocytes and cytotoxic T cell assay. (A) Experiments were the same as in Fig. 5, but the donor cells were CD8-purified splenocytes. The doses of purified CD8 T cells were 5 × 104 and 5 × 105. Open squares, filled squares, and filled triangles indicate transplantations without splenocytes, with CD8 purified WT-SP, and with CD8 purified KO-SP, respectively. Right panels indicate the average of signal intensity of luminescence in survivors at indicated days after transplantation. (B) 51Cr release assay. Two weeks after co-transplantation with splenocytes from either KO or WT mice, splenocytes from recipient C57BL/6-DBA-2 F1 mice were incubated with 51Cr-preloaded allogeneic (P815, H-2d) and syngeneic (EL4, H-2b) target cells. Specific lysis was calculated as follows: (sample count – background) / (maximum count – background) (%). Error bars are ± S.E.M.
Mixed lymphocyte reaction and ELISA
T cells and CD8+ T cells were purified using CD90 and CD8 microbeads (Miltenyi), and 1 × 105 cells were co-cultured with 30 Gy irradiated splenocytes (1 × 105) from either C57BL/6-DBA2-F1 or C57BL/6 mice, as allogeneic or syngeneic stimulator in U-bottom 96-well plates. Culture medium was the same as described previously32. After 6 days of culture, concentrations of IFN-γ and TNF-α in the supernatants were determined by ELISA (BD Phamingen) per the manufacturer's instructions.
Flow cytometric analysis
Fc-block® (BD Biosciences-Pharmingen, San Diego, CA) was used to prevent non-specific antibody binding to Fc receptors. Anti-CD4, CD8, H-2b, and H-2d antibodies were purchased from BD Biosciences-Pharmingen. An LSR flow cytometer (BD Biosciences-Immunocytometry Systems, San Jose, CA) was used for data collection, and the data were analyzed using CellQuest software (BD Biosciences-Immunocytometry Systems).
Decoy receptor of IL-21
We used the retrovirus-vector previously described29. Briefly, the primers, 5’-TTCTAGCTACCAGCTGCAGGT-3’ and 5’-TCCTGAAGTTCCTCATATTCA-3’, were used to produce a truncated IL-21R lacking the region from box 1 to the C-terminus5,29. Cell surface expression of this truncated receptor was confirmed by flow cytometric analysis using anti-IL-21-receptor polyclonal antibody (R&D Systems, Minneapolis, MN) and a secondary antibody conjugated with PE (R&D Systems).
Retrovirus mediated transduction into BM
A retrovirus construct containing p185 bcr-abl or the decoy IL-21R, was transfected into the packaging cell line, PLAT-E, and the produced viruses were transduced into pre-stimulated BM using Retronectin® according to the manufacturer's instructions (Takara, Osaka, Japan).
In vivo imaging
Luciferase gene was transduced into the P815 cell line using a lentiviral vector, which also includes modified GFP gene. GFP positive cells were purified using FACS sorter, FACSAria Cell-Sorting System, (BD Biosciences, San Jose, CA). At indicated time points, after transplantation, 3 mg of luciferin, a substrate of luciferase, was injected into mice intravenously. Detection was performed with IVIS® imaging system (Xenogen, Alameda, CA) and the strength of light was measured by analyzing software “Living ImageR ver2.5”.
Statistical analysis
Kaplan-Meier plots were used to compare survival rates. The Logrank test was used to evaluate p values. Statistical analyses were performed using “Stat Mate ver. 6” (ATMS, Tokyo, Japan). All error bars in this study are S.D.
Results
IL-21R-/- splenocyte (KO-SP) retained GVL effect against P815 leukemic cell line
To evaluate the potential clinical utility of IL-21, we wished to determine whether the loss of an IL-21 signal abolished the GVL effect. To this end, we transplanted T-cell depleted wild type bone marrow cells (TCD-BM) together with P815 leukemic cells (H-2d, DBA2 mouse derived) into recipient C57BL/6-DBA2 F1 mice. Only mice transplanted with splenocytes survived beyond 4 weeks, suggesting that the leukemic cells were eliminated by either wild type splenocytes (WT-SP) or IL-21R-/- splenocytes (KO-SP) (Fig. 1). KO-SP transplanted mice survived longer than WT-SP transplanted mice (Fig. 1). The transplanted mice without splenocytes died within 4 weeks, all with paralyzed hind legs, and almost half with leukemic nodules on the liver. In contrast, the mice with probable graft-versus-host disease (GVHD) did not show such phenotypes and suffered from diarrhea, ruffled hair, a hunched posture, and weight loss. GVHD appeared to be the cause of death for these mice. Our previous results29 suggest that the worse survival of recipients of WT-SP is due to GVHD.
Figure 1.
Survival curve with the leukemic cell line, P815. C57BL/6-DBA2 F1 recipients (H-2d/H-2b) were transplanted with 2 × 104 P815 leukemic cells (H-2d), 5 × 106 TCD-BM (H-2b), and either 5 × 107 WT-SP or KO-SP (H-2b). Open squares, filled squares, and filled triangles indicate transplantations without splenocytes (n=15), with WT-SP (n=15), and with KO-SP (n=15), respectively. The combined results of two independent experiments are shown. P value was calculated by the Logrank method.
IL-21 decoy receptor retained GVL effect against P815 cell line
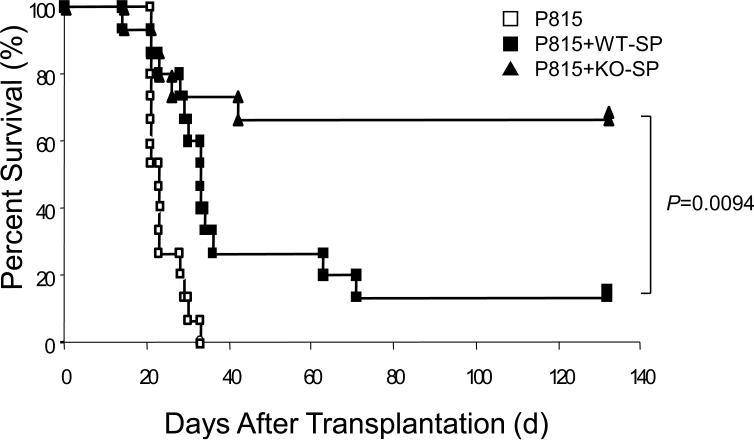
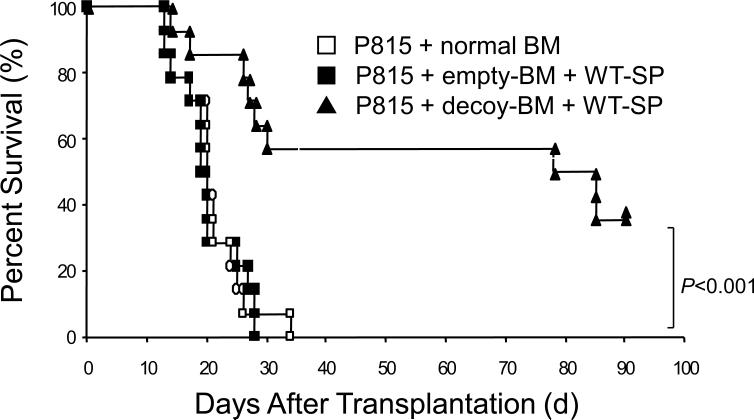
To confirm these results, we used a previously constructed decoy receptor for IL-2129, in which the major cytoplasmic domain encompassing from box 1 through C-terminus was deleted. Theoretically, it can bind to IL-21 ligand but cannot evoke signals from the receptor, and in fact, the decoy receptor was shown to attenuate GVHD in the previous study29. To confirm that the decoy receptor does not abrogate the GVL effect analogous to KO-SP, we performed GVL experiments with P815 leukemic cells. As shown in Fig. 2, P815 efficiently killed recipient mice without splenocytes as in Fig. 1, and the decoy receptor transduced BM prolongs survival, suggesting that the blockade of IL-21 signal retains GVL effect.
Figure 2.
Survival curve with decoy receptor and P815 leukemic cells. C57BL/6-DBA2 F1 recipients were transplanted with WT-SP (2 × 107), 2 × 104 P815 leukemic cells, and either empty-vector or decoy-receptor transduced BM (starting dose: 5 × 105). Filled squares, filled triangles, and open squares indicate transplantions with empty-vector transduced BM (n=14), decoy-receptor transduced BM (n=14), and no splenocytes (n=14), respectively. The combined results of two independent experiments are shown. P value was calculated by the Logrank method.
IL-21R-/- splenocyte retained GVL effect against bcr/abl transformed recipient-BM cells
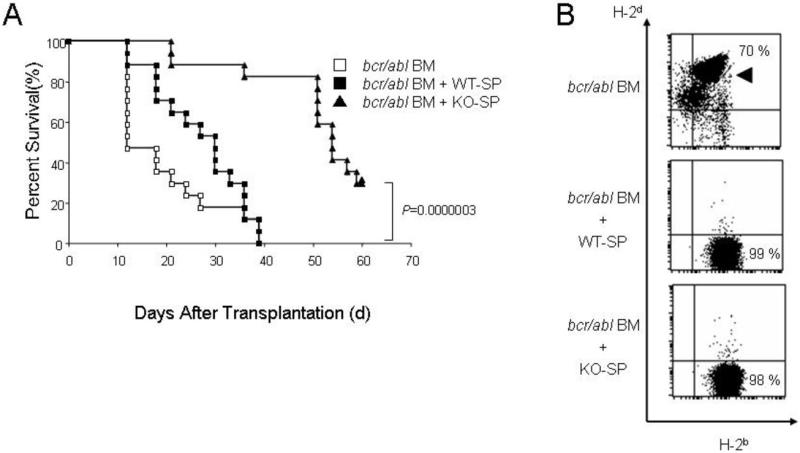
To further confirm the above results, we used another leukemic model, bcr-abl transduced recipient-BM, instead of the established P815 leukemic cell line. Consistent with the results above, KO-SP transplanted mice survived longer than WT-SP transplanted mice (Fig. 3A). In this model, two weeks after transplantation, peripheral blood cells showed the H-2b (C57BL/6) complete donor-phenotype (Fig. 3B, middle and lower panels), suggesting an eradication of leukemic cells by both types of splenocytes. Whereas peripheral blood from the recipients without splenocytes showed the H-2b/H-2d (C57BL/6-DBA2 F1) recipient-phenotype (Fig. 3B, upper panel, arrowhead), suggesting the proliferation of bcr-abl transduced recipient BM-derived leukemic cells. All mice we analyzed demonstrated the same pattern in peripheral blood. These results together with transplantation with P815 leukemic cells suggest that the lack of IL-21 signaling does not abolish the GVL effect. In this model, we found late relapse, suggesting that the GVL effect might not be sustained (Fig. 3A, KO-SP).
Figure 3.
Survival curve with bcr-abl transduced BM as leukemic cells. C57BL/6-DBA2 F1 recipients were transplanted with bcr-abl transduced recipient BM (H-2d/H-2b) (starting dose: 5 × 105), 5 × 106 TCD-BM (H-2b), and 2 × 107 WT-SP or KO-SP (H-2b). Open squares, filled squares, and filled triangles indicate transplantation without splenocytes (n=17), with WT-SP (n=17), and with KO-SP (n=17), respectively. The combined results of three independent experiments are shown. P value was calculated by the Logrank method. (B) Peripheral blood cell phenotypes two weeks after transplantation. H-2b single positive phenotype depicts donor-derived cells. Double positive population indicates recipient-derived bcr-abl transduced leukemic cells (arrowhead).
Dose-reduction experiment with luciferase expressing P815 leukemic cell line
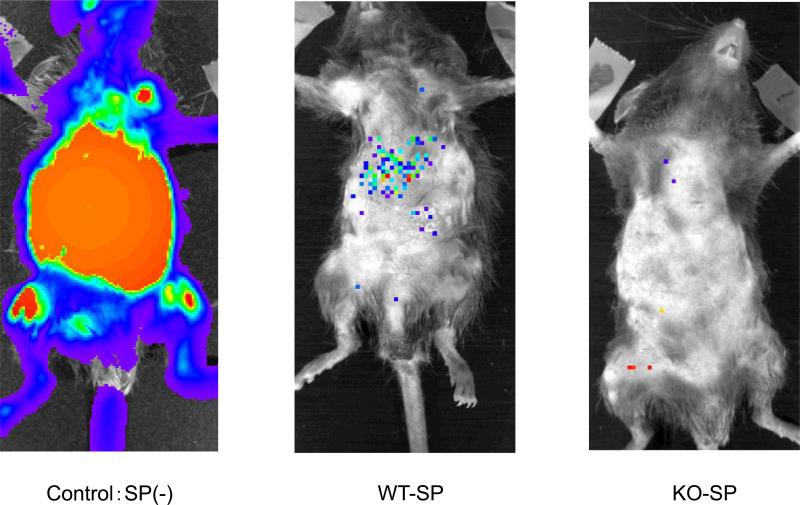
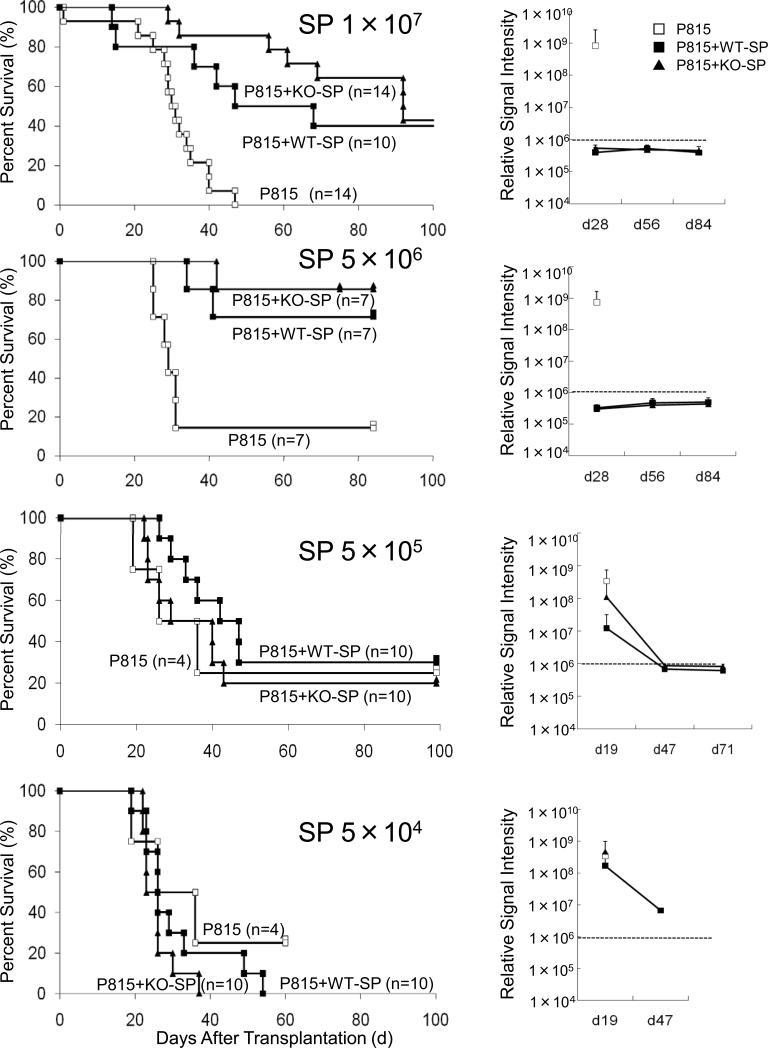
We decided to perform dose-response experiments to determine the number of splenocytes required to eliminate leukemic cells after transplantation, as otherwise it is impossible to compare the strength of GVL effect between the WT and KO cells. To make the leukemia visible in alive mice, we used luciferase transduced P815 cell line and IVIS® imaging system (Xenogen, Alameda, CA). With this system, we could see GVL effect in color (Fig 4). We started with 1 × 107 splenocytes because a dose of 5 × 107 splenocyte was used in Fig. 1. Without co-infusion of splenocytes, control mice died around 30-40 days after transplantation (Fig 5, top left panel) with a marked increase in luciferase activity (Fig 5, top right panel), whereas recipients of both WT-SP and KO-SP demonstrated an eradication of P815 leukemic cells (Fig 5, top right panel). Secondly, we used 5 × 106 splenocytes; with this number, almost no mice died due to GVHD, but the graft still eradicated leukemic cells completely (Fig. 5, second panels from top). Thirdly, we used 5 × 105 and 5 × 104; with these numbers, graft could not eliminate leukemic cells anymore and mice died due to leukemia (Fig. 5, third and bottom panels). The reduction in the relative signal intensity at later two time points resulted from early deaths of highly positive mice until the indicated days (Fig 5. third and bottom right panels). Taken together, the threshold for P815 cells in our experimental conditions was between 5 × 105 and 5 × 106, regardless of genotype of IL-21R.
Figure 4.
GVL effect in vivo. A difference in GVL effect is evident between transplanted mice without splenocytes (control) versus those receiving either wild type or IL-21R-/- splenocytes (WT-SP or KO-SP) at day 28 after transplantation. C57BL/6-DBA2 F1 recipients (H-2d/H-2b) were transplanted with 2 × 104 P815 leukemic cells (H-2d), 5 × 106 TCD-BM (H-2b), and 5 × 106 WT-SP or KO-SP (H-2b).
Figure 5.
Survival curve and leukemic-tumor-burden in dose-reduction transplantation with bulk splenocytes. C57BL/6-DBA2 F1 recipients were transplanted with 2 × 104 P815 leukemic cells expressing luciferase (H-2d), 5 × 106 TCD-BM (H-2b), and 1 × 107 WT-SP or KO-SP (H-2b). Then, the dose of bulk splenocytes was reduced to 5 × 106, 5 × 105, and 5 × 104. Open squares, filled squares, and filled triangles indicate transplantations without splenocytes, with WT-SP, and with KO-SP, respectively. Right panels indicate the average of signal intensity of luminescence in survivors at indicated days after transplantation, which correlates with leukemic-tumor-burden. Empirically determined positive-negative threshold indicated in broken line was 106 relative signal intensity per mouse.
CD8-depleted grafts showed attenuated GVL effect
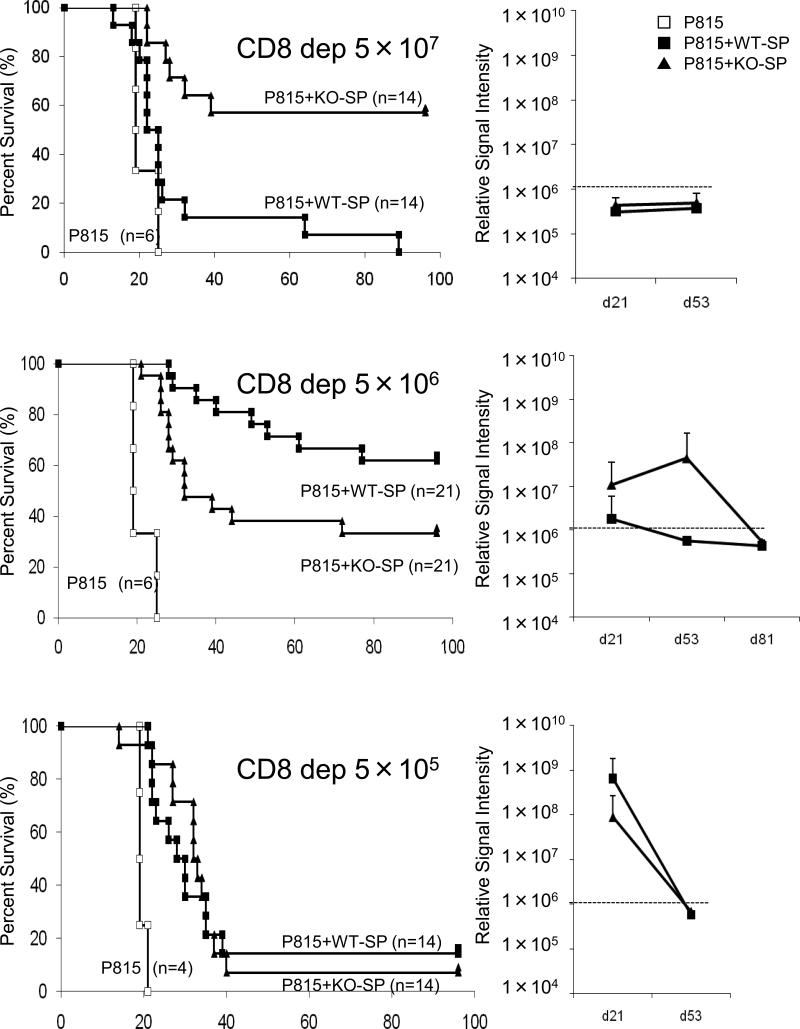
Our previous experiments demonstrated that IL-21R-/- CD4+ T-cells are defective in GVH reaction after transplantation32. It was therefore of great interest to determine whether IL-21R-/- CD4+ T-cells are also defective in GVL effect. To evaluate the contribution of CD4+ T-cells to GVL effect, we performed transplantation with CD8-depleted splenocytes with dose-reduction as above. Because CD8 T-cells compose only ~10% of splenocyte, we chose CD8-depletion rather than CD4 purification, so we could use the same dose of CD8-depleted splenocytes as in the case of bulk splenocytes. As shown in Fig. 6, CD8-depleted KO-SP at the dose of 5 × 107 demonstrated attenuated GVHD and prolonged survival (top left panel), consistent with our previous experiments above29 (Fig. 1). No increase of luciferase activity indicated that all deaths were primarily due to GVHD (top right panel). However, interestingly, CD8-depleted KO-SP at the dose of 5 × 106 demonstrated diminished GVL effect. Higher luciferase activity (middle right panel) and more deaths (middle left panel) in the KO-SP group indicated leukemic deaths. According to luciferase activity, with CD8-depleted KO-SP at the dose of 5 × 106 cells, 10 out of 21 recipients showed leukemic growth at day 21, whereas for CD8-depleted WT-SP, only 3 out of 21 mice showed leukemic growth at day 21 (middle right panel); only 7 out of 21 mice survived in recipients of KO-SP, but 13 out of 21 mice survived in recipients of WT-SP at day 100 after transplantation (middle left panel), indicating that IL-21R-/- CD4 cells have lower GVL effect than wild type CD4 cells.
Figure 6.
Survival curve and leukemic-tumor-burden in dose-reduction experiments with CD8-depleted splenocytes. Experiments were the same as in Fig. 5 but the donor splenocyte was CD8-depleted. Like the bulk splenocyte experiment (Fig. 5), the starting dose was 5 × 107 WT-SP or KO-SP (H-2b). Then, the dose of CD8-depleted splenocytes was reduced to 5 × 106, and 5 × 105, gradually. Open squares, filled squares, and filled triangles indicate transplantations without splenocytes, with WT-SP, and with KO-SP, respectively. Right panels indicate the average of signal intensity of luminescence in survivors at indicated days after transplantation, which correlates with leukemic-tumor-burden. Empirically determined positive-negative threshold indicated in broken line was 106 relative signal intensity per mouse.
CD8-purified grafts did not show significant reduction in GVL effect
To further evaluate the contribution of CD8+ T-cells to GVL effect, we performed transplantation with CD8-purified splenocytes with dose-reduction as above. As shown in Fig. 7A, CD8-purified KO-SP at the dose of 5 × 105 did not demonstrate a significant increase in the incidence of leukemic cell death (p=0.1), but if anything, CD8-purified KO-SP might have a slight decrease in GVL effect (Lower left panel). Consistent with the survival, both CD8-purified KO-SP and WT-SP showed increased luciferase activity at day 21 (Lower right panel). To confirm these results, we performed standard cytotoxic T cell assay using 51Cr. Splenocyte from recipients of C57BL/6-DBA-2-F1 mice demonstrated similar and comparable killing activity against allogeneic cells but not syngeneic cells (Fig. 7B). Cytokine production, at least, IFN-γ from T cell and CD8+ T cell in mixed lymphocyte reaction was comparable between KO and WT (Supplemental figure S1).
Discussion
Here we demonstrated that IL-21R-/- splenocytes (KO-SP), which ameliorate graft-versus-host disease (GVHD), do not attenuate graft-versus-leukemia (GVL) effect even in the splenocyte-dose-titration. In the further detailed analysis with CD8-depleted and CD8-purified splenocytes, GVL effect with IL-21R-/- CD8-depleted splenocytes but not CD8-purified splenocytes was significantly diminished compared to that with wild type, suggesting that IL-21R-/- CD4 cells have lower GVL activity than wild type cells but IL-21R-/- CD8 cells have no or only tiny decrease in GVL effect. These results suggested that no reduction in the GVL effect with bulk splenocytea was due to compensatory effect of IL-21R-/- CD8 cells on GVL effect. This conclusion is reasonable in the view of the fact that IL-21R-/- CD4 cells or splenocytes induce the less severe GVHD29, 32. Because no immunosuppressive reagent specific for GVHD but not GVL effect is clinically available, the specific suppression of GVHD in the presence of both CD4 and CD8 cells is of interest and could contribute to the development of GVHD treatment.
During this manuscript preparation, Bucher et al. reported similar results30 with our present and published data29, 32. The authors concluded that the GVL effect was maintained even after IL-21 neutralization, however, we believe that titration experiments are required in order to determine if GVL strength is the same between normal and IL-21 neutralized conditions, because the threshold of the required number of T-cells for GVL is much less than for GVHD as we have shown in this study. One must titrate the dose of splenocytes to compare the GVL strength between the two groups, but this was not done by Bucher et al.30.
We found that there were different thresholds and time frames between GVHD and GVL. Under our experimental conditions, 5 × 106 bulk splenocytes did not induce death from GVHD but the dose can completely eliminate P815 leukemic cells. Thus, the required number of splenocytes was apparently less for the GVL effect than for GVHD. As we have shown, timings of GVHD and GVL effects appeared to be different. Two weeks after transplantation, leukemic cells in peripheral blood had been already eradicated (Fig 3) but symptoms of GVHD only became clinically obvious 3-4 weeks after transplantation. Our previous study demonstrated that CD4 dysfunction occurs only after transplantation32, suggesting the possibility that at the very early phase of GVHD, T cells still have normal GVL capacity to eliminate leukemic cells, but GVL capacity gradually diminished later on. However, our present study revealed that the incidence of leukemia was hgher in the KO group than in the WT group at day 21 at the dose of 5 × 106 CD8-depleted splenocytes. This experiment was performed with the same dose and analyzed at the same time point, suggesting that cause of the diminished GVL effect may be due to CD4 dysfunction itself rather than differences in dose and timing.
In summary, our results revealed the attenuated GVL effect of IL-21R-/- splenocytes in the absence of CD8 T-cells, whereas bulk splenocytes and CD8 purified splenocytes did not demonstrate such a defect, together indicating the importance of CD8 T cells for the effect. Our detailed analysis provides new insights regarding the role for IL-21 in GVL effect.
Supplementary Material
Acknowledgments
We thank Dr. Tanabe (Institute of Medical Science, University of Tokyo, Tokyo) for donating the bcr-abl vector, Dr. Kitamura (Institute of Medical Science, University of Tokyo, Tokyo) for donating PLAT-E, a packaging cell line, and Drs. Miyoshi (Tsukuba Institute, RIKEN, Tsukuba) and Okano (Keio University, Tokyo) for donating the luciferase containing lentivirus vector.
This work was supported in part by grants from the Ministry of Health, Labor and Welfare of Japan, by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by the Intramural Research Program of the National Heart, Lung and Blood Institute, National Institutes of Health (Bethesda, MD), and by a Young Investigator Award from Jichi Medical University, Tochigi, Japan.
Footnotes
The authors have declared there is no conflict of interest to disclose.
References
- 1.Ozaki K, Leonard WJ. Cytokine and cytokine receptor pleiotropy and redundancy. J Biol Chem. 2002;277:29355–29358. doi: 10.1074/jbc.R200003200. [DOI] [PubMed] [Google Scholar]
- 2.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. Review. [DOI] [PubMed] [Google Scholar]
- 3.Leonard WJ, Zeng R, Spolski R. Interleukin 21: a cytokine/cytokine receptor system that has come of age. J Leukoc Biol. 2008;84:348–56. doi: 10.1189/jlb.0308149. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 5.Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci USA. 2000;97:11439–11444. doi: 10.1073/pnas.200360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 7.Takaki R, Hayakawa Y, Nelson A, Sivakumar PV, Hughes S, Smyth MJ, et al. IL-21 enhances tumor rejection through a NKG2D-dependent mechanism. J Immunol. 2005;175:2167–2173. doi: 10.4049/jimmunol.175.4.2167. [DOI] [PubMed] [Google Scholar]
- 8.Wang G, Tschoi M, Spolski R, Lou Y, Ozaki K, Feng C, et al. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003;63:9016–9022. [PubMed] [Google Scholar]
- 9.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis ID, Skrumsager BK, Cebon J, Nicholaou T, Barlow JW, Moller NP, et al. An open-label, two-arm, phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin Cancer Res. 2007;13:3630–3636. doi: 10.1158/1078-0432.CCR-07-0410. [DOI] [PubMed] [Google Scholar]
- 11.Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS, et al. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. 2008;26:2034–2039. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- 12.Frederiksen KS, Lundsgaard D, Freeman JA, Hughes SD, Holm TL, Skrumsager BK, et al. IL-21 induces in vivo immune activation of NK cells and CD8(+) T cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1439–1449. doi: 10.1007/s00262-008-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis ID, Brady B, Kefford RF, Millward M, Cebon J, Skrumsager BK, et al. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin Cancer Res. 2009;15:2123–2129. doi: 10.1158/1078-0432.CCR-08-2663. [DOI] [PubMed] [Google Scholar]
- 14.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Schluns LMa K, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 15.Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 17.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 18.Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC, 3rd, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB- Yaa mice. Proc Natl Acad Sci USA. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spolski R, Kashyap M, Robinson C, Yu Z, Leonard WJ. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc Natl Acad Sci USA. 2008;105:14028–14033. doi: 10.1073/pnas.0804358105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutherland AP, Van Belle T, Wurster AL, Suto A, Michaud M, Zhang D, et al. IL-21 is required for the development of type 1 diabetes in NOD mice. Diabetes. 2009;58:1144–1155. doi: 10.2337/db08-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teshima T, Hill GR, Pan L, Brinson YS, van den Brink MR, Cooke KR, et al. IL-11 separates graft-versus-leukemia effects from graft-versus-host disease after bone marrow transplantation. J Clin Invest. 1999;104:317–325. doi: 10.1172/JCI7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooke KR, Gerbitz A, Crawford JM, Teshima T, Hill GR, Tesolin A, et al. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest. 2001;107(12):1581–1589. doi: 10.1172/JCI12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy P, Teshima T, Hildebrandt G, Duffner U, Maeda Y, Cooke KR, et al. Interleukin 18 preserves a perforin-dependent graft-versus-leukemia effect after allogeneic bone marrow transplantation. Blood. 2002;100:3429–3431. doi: 10.1182/blood-2002-04-1252. 2002. [DOI] [PubMed] [Google Scholar]
- 24.Yang YG, Qi J, Wang MG, Sykes M. Donor-derived interferon gamma separates graft-versus-leukemia effects and graft-versus-host disease induced by donor CD8 T cells. Blood. 2002;99:4207–4215. doi: 10.1182/blood.v99.11.4207. [DOI] [PubMed] [Google Scholar]
- 25.Clouthier SG, Cooke KR, Teshima T, Lowler KP, Liu C, Connolly K, et al. Repifermin (keratinocyte growth factor-2) reduces the severity of graft-versus-host disease while preserving a graft-versus-leukemia effect. Biol Blood Marrow Transplant. 2003;9:592–603. doi: 10.1016/s1083-8791(03)00230-1. [DOI] [PubMed] [Google Scholar]
- 26.Reddy P, Maeda Y, Hotary K, Liu C, Reznikov LL, Dinarello CA, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci U S A. 2004;101:3921–3926. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Lou J, Li N, Todorov I, Lin CL, Cao YA, et al. Donor CD8+ T cells mediate graft-versus-leukemia activity without clinical signs of graft-versus-host disease in recipients conditioned with anti-CD3 monoclonal antibody. J Immunol. 2007;178:838–850. doi: 10.4049/jimmunol.178.2.838. [DOI] [PubMed] [Google Scholar]
- 28.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 29.Meguro A, Ozaki K, Oh I, Hatanaka K, Matsu H, Tatara R, et al. IL-21 is critical for GVHD in a mouse model. Bone Marrow Transplant. 2010;45:723–729. doi: 10.1038/bmt.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bucher C, Koch L, Vogtenhuber C, Goren E, Munger M, Panoskaltsis-Mortari A, et al. Sivakumar P, Blazar BR. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood. 2009;114:5375–5384. doi: 10.1182/blood-2009-05-221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams RT, Roussel MF, Sherr CJ. Arf gene loss enhances oncogenicity and limits imatinib response in mouse models of Bcr-Abl-induced acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2006;103:6688–6693. doi: 10.1073/pnas.0602030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh I, Ozaki K, Meguro A, Hatanaka K, Kadowaki M, Matsu H, et al. Altered effector CD4+ T cell function in IL-21R-/- CD4+ T cell-mediated graft-versus-host disease. J Immunol. 2010;185:1920–1926. doi: 10.4049/jimmunol.0902217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.