Abstract
This case illustrates the ability of electromagnetic tracking navigation to localize difficult targets in real time during biopsy or ablation of lesions that are only transiently apparent on arterial phase computed tomography and may be unapparent on sonography. Readily available technology enabling multimodality registration to sonography allows for the use of positron emission tomographic, magnetic resonance imaging, and computed tomographic information during sonographically guided procedures and examinations.
Keywords: electromagnetic tracking, fusion imaging, multimodality intervention
Biopsy of lesions only transiently apparent on arterial phase computed tomography (CT) can be technically challenging or even impossible, particularly if these lesions are also unapparent on sonography. Under these circumstances, physicians are often faced with a high suspicion for hepatic malignancy but lack the tools to adequately sample the lesion in question. This case illustrates the ability of electromagnetic tracking navigation to localize these difficult targets in real-time during biopsy or ablation. Readily available technology enabling multimodality registration to sonography allows for the use of positron emission tomographic, magnetic resonance imaging, and CT information during sonographically guided procedures and examinations.
Technical Procedure
This procedure was a part of a clinical trial that was approved by the Institutional Investigational Review Board, and this patient gave written informed consent.
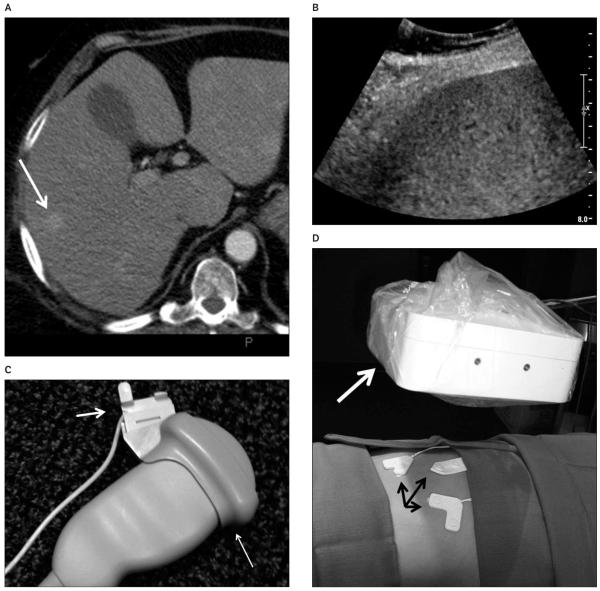
A 61-year-old woman presented with presumed ectopic corticotropin secretion producing Cushing syndrome of an unknown source. Contrast-enhanced CT (256 row; Philips Healthcare, Cleveland, OH) of the abdomen revealed 3 subcentimeter enhancing liver lesions that were apparent only on arterial phase CT. These lesions were not detected on unenhanced or equilibrium (portal venous) phase CT (Figure 1A). Sonography (iU22; Philips Healthcare, Bothell, WA) using a 5-MHz convex transducer showed a possible subtle heterogeneous area in the region of concern but no definite identifiable mass (Figure 1B). Because of the limited and transient conspicuity of these lesions, the patient was scheduled for a biopsy using electromagnetic tracking navigation.
Figure 1.
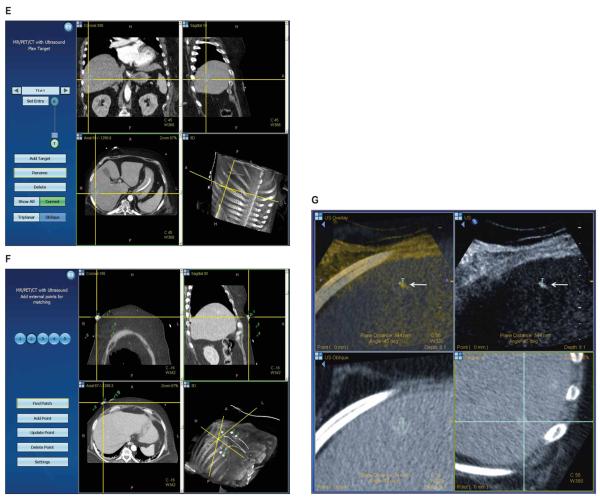
Corticotropin-producing neuroendocrine tumor in a 61-year-old woman. A, Arterial phase computed tomography shows an enhancing liver lesion (arrow). B, Sonography of the liver shows no discernable mass. C, Ultrasound transducer with a disposable electromagnetic tracking sensor (short arrow) attached to a sterilizable needle guide (long arrow). The sterile cover can go above or below the sensor. D, The electromagnetic field generator (white arrow) is mounted on an articulated arm and covered with a sterile drape. Fiducial patches or markers (black arrows) on the skin provide references for registration. E, The graphical user interface shows the lesion in 3 planes, which may be helpful for confirming selection of the center of the target in all planes. F, Skin fiducials (markers) are identified and confirmed to ensure accurate registration, ie, matching of anatomic points on the skin coordinates within the imaging data. G, The tracking screen during needle placement displays the relationship between the targeted lesion (T) and the needle tip (arrow).
The procedure was performed on the CT table, with the sonographic and tracking units positioned next to the table. A procedural setup for using electromagnetic tracking and navigation was followed and has been reported previously.1 First, the abdomen was sterilized and draped, and 3 sterile registration patches (containing 6 fiducials [markers]; Traxtal/Philips Healthcare, Toronto, Ontario, Canada) were placed on the skin. A standard needle guide was attached to a 5-MHz convex transducer, and the ultrasound sensor coil tracker (Traxtal/Philips Healthcare) was attached to the guide in a sterile fashion (Figure 1C). The electromagnetic field generator was covered with a sterile drape and positioned on a portable articulated arm near the patient and registration patches (Figure 1D). A preprocedural dual phase CT scan (3-mm-thick sections with 1.5-mm overlap) was obtained over the liver section of interest as well as the skin fiducial patches. The arterial phase images were uploaded onto the tracking unit workstation via the hospital network. The lesions were identified on the arterial phase CT, and the target was selected for biopsy and entered into the workstation (Figure 1E). The registration patches were semiautomatically identified and verified by the sonographer (Figure 1F), and autoregistration was used to rigidly fuse the sonographic images with the CT images. Uploading the CT images to the Traxtal unit and performing registration took approximately 5 to 10 minutes. Once the images were fused, the interventional radiologist was able to scan semitransparently overlaid sonographic and CT images in real time, allowing identification of the lesion. The two modalities have different color maps, allowing them to be easily distinguished and blended.
Using the guidance system, a tracked introducer with an internal sensor coil embedded in its tip (Traxtal/Philips) was then inserted into the lesion with the help of real-time virtual needle position feedback registered with the arterial phase CT images (Figure 1G). Fine-needle aspirations and core biopsies were obtained from the lesion and evaluated by a cytopathology technologist at the bedside. Anatomic and cytopathologic analysis, including immunohistochemical staining, was diagnostic for a corticotropin-producing neuroendocrine tumor. The total procedure time from preprocedural CT to final biopsy was approximately 60 minutes.
Discussion
Image guidance is essential for procedures such as biopsy and radio frequency ablation, and outcomes may be predicated on the adequacy of target identification and definition. Although standard techniques are adequate most of the time, image guidance may be limited when lesions are difficult to visualize on conventional imaging. This can often lead to increased CT or fluoroscopy exposure in addition to an increased number of needle insertions or repositioning during intervention. It is possible, although speculative, that electromagnetic tracking can enable the physician to perform these procedures without the use of additional ionizing radiation by allowing use of prior CT images instead of requiring new CT images.1–5 The planning phase of the tracking software also allows the radiologist to select the appropriate needle insertion site before beginning the biopsy, which could eliminate additional needle insertions or repeated repositioning.
Electromagnetic tracking and image fusion allow the physician to navigate within a 3-dimensional space precisely, rather than relying primarily on a mental reconstruction of complex anatomic spatial relationships during an intervention. This can be useful when a lesion is only seen for a transient period (during the arterial phase). Localization would normally require the physician to imagine where to best place the needle by mental estimation of the target location based on recognizable nearby anatomy that can be mentally matched. With this technology, real-time referencing of a preprocedural image can be done automatically and with the reproducibility and dependability of a computer.
Historically, sonography has been fused to other imaging modalities for surgical procedures using optical, infrared, or electromagnetic tracking.6 Prior reports have shown that this technology can enable biopsy and ablation procedures that otherwise would have been impossible or difficult to perform. Furthermore, needle angles (toward predefined point targets) can be markedly improved with the addition of this multimodality navigation technology. Specifically, the needle angle error was improved by 13.3° ± 6.5°, and the distance between the needle and target was improved from 17.8 ± 17.1 to 3.3 ± 3.1 mm with the use of the technology, compared to standard methods.4
Although sonographically guided biopsy is highly accurate (exceeding 90%) for diagnosis of focal liver disease,7 this does not account for situations in which lesions cannot be seen on sonography. Electromagnetic tracking enables multimodality registration to reference the sonographic image to preprocedural positron emission tomographic, magnetic resonance imaging, and CT images during sonographically guided procedures and examinations. Accurate overlay is required for successful fusion guidance. For small enhancing lesions in the liver that are only apparent during the arterial phase, electromagnetically tracked sonography may enable real-time biopsy or ablation with ultrasound that otherwise would be difficult or impossible with use of a conventional technique alone.
Acknowledgments
We thank Deborah Kim, MRT(N) (Traxtal/Philips Healthcare, Toronto, Ontario, Canada), for technical assistance during the biopsy procedure. This study was supported in part by the Intramural Research Program of the National Institutes of Health (NIH) and was related to a Cooperative Research and Development Agreement between the NIH and Philips Healthcare. The mention of commercial devices or products, their source, or their use in connection with the material reported herein is not to be construed as either an actual or implied endorsement of such products by the NIH, the US Department of Health and Human Services, or the US Public Health Service. Dr Wood and the NIH may have intellectual property in the field.
Abbreviations
- CT
computed tomography
References
- 1.Wood BJ, Kruecker J, Abi-Jaoudeh N, et al. Navigation systems for ablation. J Vasc Interv Radiol. 2010;21(suppl):S257–S263. doi: 10.1016/j.jvir.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crocetti L, Lencioni R, Debeni S, See TC, Pina CD, Bartolozzi C. Targeting liver lesions for radiofrequency ablation: an experimental feasibility study using a CT-US fusion imaging system. Invest Radiol. 2008;43:33–39. doi: 10.1097/RLI.0b013e31815597dc. [DOI] [PubMed] [Google Scholar]
- 3.Kruecker J, Xu S, Glossop N, et al. Electromagnetic tracking for thermal ablation and biopsy guidance: clinical evaluation of spatial accuracy. J Vasc Interv Radiol. 2007;18:1141–1150. doi: 10.1016/j.jvir.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruecker J, Xu S, Venkatesan A, et al. Clinical utility of real-time fusion guidance for biopsy and ablation. J Vasc Interv Radiol. doi: 10.1016/j.jvir.2010.10.033. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos RS, Gupta A, Ebright MI, et al. Electromagnetic navigation to aid radiofrequency ablation and biopsy of lung tumors. Ann Thorac Surg. 2010;89:265–268. doi: 10.1016/j.athoracsur.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Marinho AM, Barua M, Haller J, Ryken TC. Enhanced anatomic visualization with ultrasound-assisted intracranial image-guidance in neurosurgery. Technol Cancer Res Treat. 2002;1:181–186. doi: 10.1177/153303460200100303. [DOI] [PubMed] [Google Scholar]
- 7.Buscarini L, Fornari F, Bolondi L, et al. Ultrasound-guided fine-needle biopsy of focal liver lesions: techniques, diagnostic accuracy and complications—a retrospective study on 2091 biopsies. J Hepatol. 1990;11:344–348. doi: 10.1016/0168-8278(90)90219-h. [DOI] [PubMed] [Google Scholar]