Abstract
Purpose
Several options exist for the surgical correction of male stress urinary incontinence including periurethral bulking agents, artificial urinary sphincters and the recently introduced male urethral slings. We investigated contemporary trends in the use of these treatments.
Materials and Methods
Annualized case log data for incontinence surgeries from certifying and re-certifying urologists were obtained from the American Board of Urology, ranging from 2004 to 2010. Chi-squared tests and logistic regression models were used to evaluate the association between surgeon characteristics (type of certification, annual volume, practice type, and practice location) and the use of incontinence procedures.
Results
Among 2,036 non-pediatric case logs examined, the number of incontinence treatments reported for certification has steadily increased over time (p = 0.008) from 1,936 to 3,366 treatments per year from 2004 to 2010. Nearly one-fifth of urologists reported placing at least one sling. The proportion of endoscopic procedures decreased from 80% of all incontinence procedures in 2004 to 60% in 2010, but they remained the exclusive incontinence procedure performed by 49% of urologists. An urologist’s increased usage of endoscopic treatments was associated with a decreased likelihood of performing a sling procedure (OR=0.5, p<0.0005). Artificial urinary sphincter usage remained stable accounting for 12% of procedures.
Conclusions
Incontinence procedures are on the rise. Urethral slings have been widely adopted and account for the largest increase among treatment modalities. Endoscopic treatments continue to be commonly performed and may represent over usage in the face of improved techniques. Further research is required to validate these trends.
Keywords: Urinary Sphincter, Artificial, Suburethral sling, Urinary Incontinence, Practice Patterns
Introduction
Men who suffer from stress urinary incontinence (SUI) after radical prostatectomy (RP) experience a significant decrease in quality of life.1, 2 The prevalence of post-RP SUI has been reported to be anywhere from 2.5% to 87% but varies greatly according to the definition and time period studied.3 Urinary continence typically improves within the first 1 to 2 years after RP,4 and conservative therapy consisting of pelvic floor muscle training may be helpful to men with mild SUI.5 However, it is estimated that 8% to 20% of men who have undergone RP will ultimately require surgical intervention for incontinence.3, 4, 6 Fortunately, a number of advances in the surgical management of incontinence have led to several treatment options.
Endoscopic injection of periurethral bulking agents is commonly used as a first-line treatment because of urologists’ familiarity with this technique and its relative ease of use. The type of injection material has evolved from polytetrofluorane to more biocompatible materials such as collagen.7 However, success rates have been reported to be as low as 20% in patients with mild incontinence.8 This technique may have limited utility in comparison to other available options.
The artificial urinary sphincter (AUS) is the most established procedure and has long been considered the gold standard for post-RP incontinence since its introduction in the 1970s by American Medical Systems, Inc. (AMS, Minetonka, MN).9, 10 The AUS is effective in treating moderate to severe SUI.10, 11 However, the AMS 800 is a complex, multi-component prosthetic that can be challenging for surgeons to place and for patients to use.12 Revision and reoperations may be required if urethral atrophy or mechanical failure occur. Optimal results require patient education and manual dexterity to operate the sphincter.11
As a minimally-invasive alternative, male slings have recently been popularized.11 In 1998, Schaeffer et al reported a 75% success rate for post-RP SUI using bulbourethral slings made of synthetic bolsters, a procedure extrapolated from the one used for female intrinsic sphincter deficiency.13 In the past decade, male slings have become simplified with a number of commercially available kits that either cause compression of the anterior urethra or suspend the posterior urethra.14 Early short-term and mid-term data show promising results.11
The increased recognition of post-RP SUI and the advent of novel techniques to treat this condition has led us to investigate contemporary trends in the treatment of SUI. Specifically, we characterized changes in the use of endoscopic injections, AUS, and slings by focusing on individual surgeon factors including annual volume, surgeon age, practice type, and practice area size. We examined how widely the recently introduced male slings were used and how this impacted the use of other procedures.
Patients and Methods
Study Cohort and Data Source
Since 1985, all eligible urologists who seek initial certification by the American Board of Urology (ABU) must submit case logs containing current procedural terminology (CPT) codes for each procedure done within the prior consecutive 6-month period.15 This process is then repeated every ten years for maintenance of certification.16 Thus, each year the ABU receives case log data representing the surgical volume of roughly 10% of the estimated 6,000 urologists who have certified since 1985.17 The number of urologists certifying per year has remained stable. For our study, de-identified electronic case log data between 2004 and 2010 were obtained from the ABU. Certifying urologists’ annualized case numbers for each CPT code for the treatment of male urinary incontinence were analyzed (table 1). Revision codes were queried, and procedures with gender non-specific CPT codes were included only if patients were male. The etiology of urinary incontinence was unknown, but the majority of procedures were assumed to be for treatment of post-RP SUI. Urologists self-identified their practice type and were designated as hospital-based if designated as salaried by a hospital. Lastly, urologists who identified themselves as pediatric urologists were excluded from our study as they often address SUI stemming from congenital defects.
Table 1.
Summary of physician characteristics
| Exclusive Use |
Combination | |||
|---|---|---|---|---|
| Endoscopic Injection |
Slings | Sphincters | ||
| No. Urologists | 1,000 | 177 | 255 | 604 |
| Median age (IQR) | 43 (40,51) | 44 (36,51) | 44 (36,52) | 43 (38,51) |
| Median annual incontinence procedure vol (IQR) | 4 (2,10) | 2 (2,4) | 2 (2,4) | 8 (4,16) |
| No. male gender (%) | 885 (89) | 169 (95) | 243 (95) | 558 (92) |
| No. yr of (re)certification (%): | ||||
| 2004 | 127 (13) | 11 (6) | 22 (9) | 37 (6) |
| 2005 | 146 (15) | 17 (10) | 45 (18) | 81 (13) |
| 2006 | 142 (14) | 26 (15) | 48 (19) | 97 (16) |
| 2007 | 137 (14) | 25 (14) | 38 (15) | 79 (13) |
| 2008 | 149 (15) | 25 (14) | 32 (13) | 93 (15) |
| 2009 | 142 (14) | 32 (18) | 39 (15) | 95 (16) |
| 2010 | 157 (16) | 41 (23) | 31 (12) | 122 (20) |
| No. specialty (%): | ||||
| Andrology | 3 (less than 1) | 1 (1) | 9 (4) | 8 (1) |
| Endourology | 29 (3) | 6 (3) | 3 (1) | 20 (3) |
| Female | 48 (5) | 2 (1) | 8 (3) | 68 (11) |
| General | 875 (88) | 160 (90) | 218 (85) | 481 (80) |
| Infertility | 0 (0) | 0 (0) | 1 (less than 1) | 0 (0) |
| Oncology | 26 (3) | 5 (3) | 13 (5) | 20 (3) |
| Urolithiasis | 19 (2) | 3 (2) | 3 (1) | 7 (1) |
| No. practice type (%): | ||||
| Private | 716 (72) | 129 (73) | 156 (61) | 372 (62) |
| Hospital | 46 (5) | 4 (2) | 5 (2) | 14 (2) |
| Academic | 58 (6) | 12 (7) | 26 (10) | 89 (15) |
| Multiple sites/unknown | 180 (18) | 32 (18) | 68 (27) | 129 (21) |
| No. certification type (%): | ||||
| Initial | 259 (26) | 57 (32) | 94 (37) | 186 (31) |
| Second | 441 (44) | 67 (38) | 71 (28) | 242 (40) |
| Third | 300 (30) | 53 (30) | 90 (35) | 176 (29) |
| No. practice area size (%): | ||||
| Less than 100,000 | 136 (14) | 15 (8) | 25 (10) | 49 (8) |
| 100,000–250,000 | 149 (15) | 25 (14) | 26 (10) | 81 (13) |
| 250,001–500,000 | 117 (12) | 22 (12) | 31 (12) | 66 (11) |
| 500,001–1,000,000 | 108 (11) | 28 (16) | 30 (12) | 81 (13) |
| Greater than 1,000,000 | 195 (20) | 53 (30) | 67 (26) | 192 (32) |
| Unknown | 295 (30) | 34 (19) | 76 (30) | 135 (22) |
Statistical Methods
Our aim was to describe the trends in treatment for incontinence among urologists submitting case logs for board certification by the ABU. We hypothesized that there would be an increased incidence of male incontinence over time, which would manifest in an increase of incontinence treatments. We further hypothesized that older urologists would be less likely to use surgical interventions for incontinence (sling and AUS) and that AUS would be placed by higher-volume surgeons.
Urologists could have either used endoscopic, sling, or AUS treatments exclusively, or a combination of these procedures. The data were analyzed using 2 separate outcomes for each procedure: 1) any use and 2) exclusive use (ie, physician only reported using this type of incontinence procedure).
We used chi-squared tests and logistic regression models to evaluate the association between several physician and practice factors (physician age, type of recertification, practice type, and practice area size) and procedure type (both as any use and exclusive use). All statistical analyses were conducted using STATA 11.0 (StataCorp, College Station, TX).
Results
In total, 2,036 non-pediatric urologists submitted case logs for initial certification or recertification that included at least one procedure for incontinence between 2004 and 2010. Approximately 60% of urologists did not report any use of incontinence procedures. This proportion of nonusers remained stable during the study period. Table 2 shows the characteristics of physicians performing endoscopic treatments, slings, or sphincters exclusively, or a mixture of techniques. Median age of surgeons applying for the initial, second, and third certifications were 35, 43, and 52 years old, respectively.
Table 2.
Characteristics of physicians providing any endoscopic, sling or sphincter operations
| Overall | Any Endoscopic Treatments | Any Sling | Any Sphincters | |
|---|---|---|---|---|
| No. urologists | 2,036 | 1,389 | 419 | 592 |
| Median annual vol (IQR) | 4 (2,10) | 6 (4,12) | 6 (4,14) | 6 (4,14) |
| No. practice type (%): | ||||
| Private | 1,373 (67) | 947 (68) | 279 (67) | 351 (59) |
| Hospital | 69 (3) | 55 (4) | 16 (4) | 13 (2) |
| Academic | 185 (9) | 122 (9) | 49 (12) | 88 (15) |
| Multiple sites/unknown | 409 (20) | 265 (19) | 75 (18) | 140 (24) |
| No. certification type (%): | ||||
| Initial | 596 (29) | 366 (26) | 130 (31) | 190 (32) |
| Second | 821 (40) | 610 (44) | 176 (42) | 206 (35) |
| Third | 619 (30) | 413 (30) | 113 (27) | 196 (33) |
| No. practice area size (%): | ||||
| Less than 100,000 | 225 (11) | 163 (12) | 31 (7) | 46 (8) |
| 100,000–250,000 | 281 (14) | 203 (15) | 59 (14) | 73 (12) |
| 250,001–500,000 | 236 (12) | 164 (12) | 51 (12) | 66 (11) |
| 500,001–1,000,000 | 247 (12) | 163 (12) | 60 (14) | 79 (13) |
| Greater than 1,000,000 | 507 (25) | 309 (22) | 147 (35) | 173 (29) |
| Unknown | 540 (27) | 387 (28) | 71 (17) | 155 (26) |
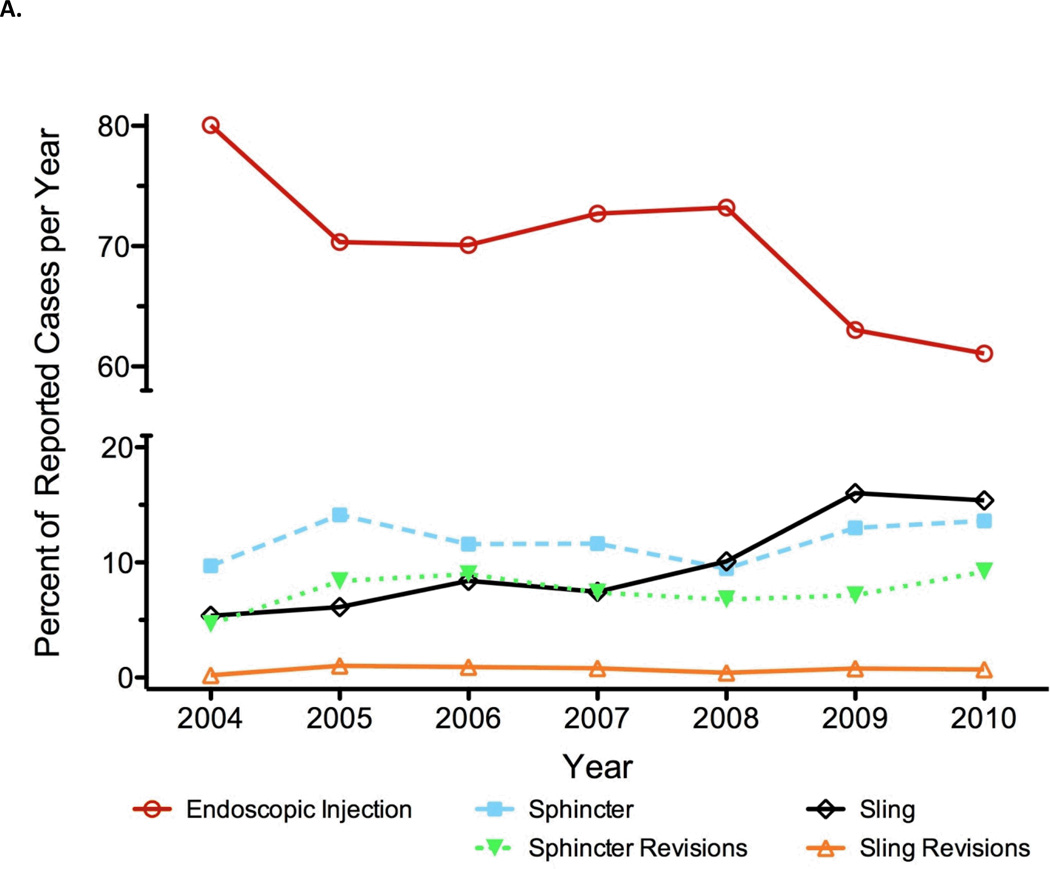
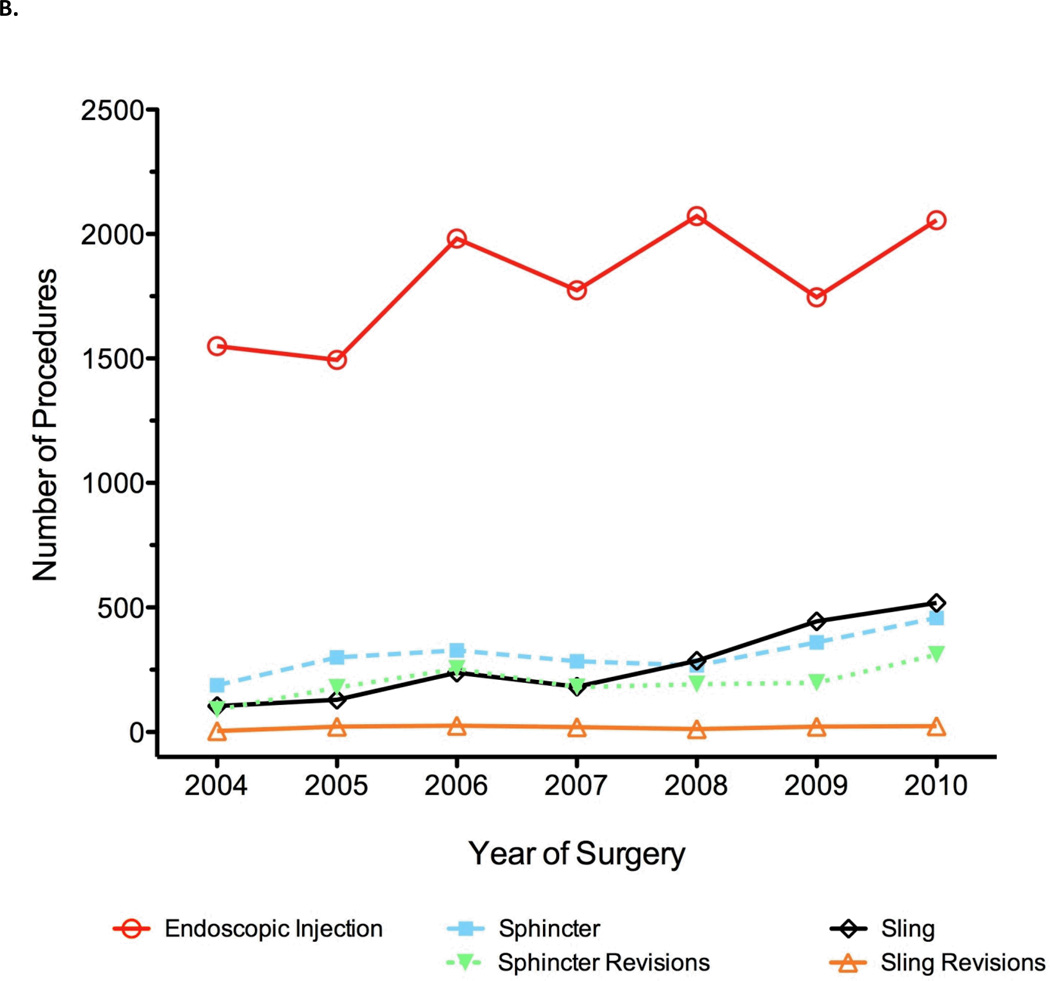
Our first question was whether the use of incontinence procedures has changed over time. Overall, the number incontinence treatments being reported for certification has increased over time (p = 0.008), from 1,936 to 3,366 treatments per year from 2004 to 2010 despite a stable number of certifying urologists. We evaluated whether there were differences in the use of endoscopic treatments, slings, and sphincters over time. Figure 1 shows the number of each procedure over time. The rate of endoscopic treatments declined from being used for 80% of all incontinence procedures in 2004 (1,550 out of 1,936) to 61% in 2010 (2,056 out of 3,366). In contrast, the use of slings increased from 5% to 15% over the same time period (fig. 1a). In 2010, 518 slings were placed as compared to 104 placed in 2004 (fig. 1b). AUS usage was largely stable with a mean volume of 12% of cases per year. Sphincter revisions accounted for a mean of 8% of cases, and sling revisions made up less than 1% of cases per year. We did not observe a corresponding increase in the rate of sling or sphincter revision procedures, although these numbers were low overall.
Figure 1.
Incontinence procedures reported by certifying urologists as percentage of total number per year (A) and absolute number logged per year (B).
Our second question was whether physician or practice characteristics were associated with the patterns of incontinence procedures performed. The majority of urologists performing incontinence procedures reported performing at least one endoscopic procedure (68%; n=1,389), and nearly half (49%; n=1,000) reported performing endoscopic injections exclusively (table 2). Physicians working in hospital settings were more likely to report exclusive use of endoscopic treatments than those in private or academic settings (67% vs 52% and 31%, respectively; p < 0.001). Physicians in larger practice areas were significantly less likely to report any use of endoscopic treatments (61% of those in areas with populations over 1,000,000 vs 72% of those in areas with populations less than 100,000; p = 0.001) or exclusive use (38% vs 60%, respectively; p < 0.001) (Table 3).
Approximately 20% (n = 419) of physicians reported placing at least one sling and nearly 10% (n = 177) reported placing slings exclusively. For those performing sling procedures, the median number per year was 2 (IQR 2 to 4). Five percent (n = 97) of urologists placed more than 5 slings in one recertification year, and only 1% (n = 24) placed more than 10 slings. There was no evidence of a difference in the proportion of physicians placing slings by practice type (p = 0.14 and p = 0.4 for any use or exclusive use) or recertification type (p = 0.2 and p = 0.6). Physicians in larger practice areas appeared more likely to place slings: 29% of physicians in areas with populations over 1,000,000 compared to 14% of physicians in areas with populations less than 100,000 reported placing at least one sling (p < 0.001; Table 3). Similar trends were seen for placing slings exclusively, although these differences were not statistically significant (p = 0.096).
Overall, 29% (n = 592) of physicians performed at least one sphincter procedure, and 13% (n = 255) performed sphincter procedures exclusively. However, among urologists who placed any sphincters, the median number of such cases was only 2 per year (IQR 2 to 4). Only 4% (24 of 592) of urologists who reported any use of sphincters placed at least 10. Physicians in academic settings were more likely to report use of sphincters than those in private or hospital settings (48% vs 26% and 19% reported any use, and 14% vs 11% and 7% reported exclusive use; p < 0.001 and p = 0.018, respectively). Physicians in larger practice areas were significantly more likely to report some use of sphincter procedures than those in smaller areas (p = 0.005), but they were not significantly more likely to place sphincters exclusively (p = 0.5).
With respect to physician age, we found no evidence of an association between age and a tendency to perform one procedure over another (p > 0.3 for slings, sphincters, and endoscopic treatments combined). We did find an association between injection-specific volume and a lower tendency to use slings. After adjusting for the year of certification, we found that the more endoscopic injections a surgeon performed, the less likely he was to perform sling procedures (per 10 additional injections performed OR for placing a sling: 0.73 (95% CI 0.62–0.85; p < 0.001). However, we did not find any significant evidence that a higher caseload of injections was associated with decreased use of sphincters (per 10 additional injections performed OR for placing a sling: 0.95 (95% CI 0.88–1.03; p = 0.2). The probability of placing a sling was 24%, 18%, and 1% among those who performed no injections, those who placed 10 annually, and those who placed 100, respectively. The corresponding predicted probabilities for sphincter procedures were 30%, 29%, and 21%, respectively.
Discussion
During the past decade, the annual number of RPs being performed in the United States has steadily increased, and we hypothesized that there would be a subsequent greater need for corrective procedures for SUI. 10, 18 Through the analysis of case logs submitted by American urologists, we have shown that there has indeed been a steady increase in these surgeries. We found a consistent increase in sling usage (absolute numbers and percentage of total incontinence procedures) during the study period, 2004–2010. Approximately 10% of urologists performing incontinence procedures placed slings exclusively. Male slings appear to have been widely adopted by surgeons of all private, academic, and hospital-based practices, and by surgeons of all ages (ie, whether applying for their initial, second, or third certification). While AUS usage remained stable as a fraction of all procedures during the increasing trend in sling use, endoscopic incontinence procedures decreased during the study period.
The male urethral sling is a welcome addition to the urologists’ armamentarium and has now emerged as the preferred initial treatment for many patients.19 Long-term outcomes are unavailable, but intermediate-term data in several series show a 40%–80% success rate. 20–22 Kumar et al reported that when offered a choice, 92% of patients chose a sling over AUS to avoid a mechanical device.23 However, less is known about the surgeons’ decision process in choosing between these devices. It is unknown if the sling’s increasing popularity is due to the prevalence of patients with the mild-moderate SUI or due to the attractiveness of its minimally invasive nature. The fact that sling procedures appear to be performed at the expense of bulking agents suggests that surgeons equate the minimally invasive nature of bulking agents and slings. The ability to salvage a failed sling with a subsequent AUS may also factor into the urologist’s decision process.24 The simplicity of the sling presents a steep learning curve, but this has yet to be studied. We were unable to study the prevalence of sling revision surgeries as the number of logged cases was too low.
The decreasing trend of endoscopic procedures is reassuring given that many reports have shown poor outcomes for the injection of periurethral bulking materials.11, 25 Early failure rates are approximately 50%, and repeat endoscopic injections are often necessary.26 Outcomes are generally thought to be inferior to slings.27 Thus, we were surprised that up to 50% of certifying urologists reported the exclusive use of these procedures. We found that exclusive use of endoscopic procedures was highest among urologists practicing in hospital settings and urologists in practice areas with populations less than 1,000,000. This may reflect an absence specialized expertise in treating incontinence.
Lastly, our study supports the works of others who have shown that most urologists are inexperienced with AUS placement. Sandhu et al have shown that the majority of sphincters are implanted by urologists who have implanted less than 25 of them.12 This is worrisome because the risk of reoperation for surgeons with 5 prior implants was 24% and decreased to 18.1% for surgeons with 100 prior implants. In the present study, the median number of AUS devices placed per year was two.
The strength of this study is that the data represent the contemporary experience of urologists from all geographic locations and practice types in the United States, and the data allow for the analysis of trends over time. However, several limitations of the data need to be mentioned. About 5,000 urologists who were board certified before 1985 are not required to submit case logs for recertification.17 Thus, the data are skewed towards younger urologists’ practices. Different injectable materials and types of urethral slings (e.g. bone-anchored, bulbourethral, transobturator) are currently available, but we were unable to distinguish them by CPT code. Also, we could not differentiate whether multiple incontinence procedures were performed on individual patients. Lastly, we could not study the impact of fellowship training or geographical trends as this information could potentially lead to identifying specific urologists from the data set and was thus unavailable.
Conclusions
The number of incontinence procedures has steadily increased from 2004 to 2010. AUSs are largely placed by a small group of surgeons, and AUS usage has remained relatively stable. Urethral slings have been widely adopted by surgeons of all ages and practice location. The sling’s popularity may be due to its less invasive nature, decreased need for revision, and perceived relative ease of learning. The overall use of endoscopic procedures has decreased, but a significant number of urologists continue to use these procedures exclusively. Further research is required to fully elucidate the urologists’ decision process in the use of incontinence procedures and to clarify a possible overuse of endoscopic treatments.
Acknowledgments
Funding
This work was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers and David H Koch through the Prostate Cancer Foundation. SP and JLS are supported by the NCI T32 CA082088-11 training grant. The study sponsors had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Appendix. CPT Codes for Incontinence Surgery
| Insertion: | |
| 51715 | Endoscopic injection of implant material into the submucosal tissues of the urethra and/or bladder neck |
| 53440 | Male sling placement |
| 53445 | Insertion of inflatable urethral/bladder neck sphincter, including placement of pump, reservoir, and cuff |
| Revision/Removal: | |
| 53442 | Revision/removal male sling |
| 53444 | Insertion of tandem cuff (dual cuff) |
| 53446 | Removal of inflatable urethral/bladder neck sphincter, including pump, reservoir, and cuff |
| 53447 | Removal and replacement of inflatable urethral/bladder neck sphincter, including pump, reservoir, and cuff at same operative session |
| 53448 | Removal and replacement of inflatable urethral/bladder neck sphincter, including pump, reservoir, and cuff through an infected field at the same operative session including irrigation and debridement of infected tissue |
| 53449 | Repair of inflatable urethral/bladder neck sphincter, including pump, reservoir, and cuff |
Footnotes
Data Source/IRB
Data were acquired from the American Board of Urology. All data were de-identified, and therefore, IRB approval was not required. The authors are responsible for the content of this paper, and the presented views do not reflect endorsement by the ABU.
Authors’ contributions
The study was conceived by SP and JLS. All authors were responsible for the overall study design. CS and AM conducted statistical analyses. All authors contributed to writing the manuscript and approved the final version.
Contributor Information
Stephen A. Poon, Department of Surgery, Sidney Kimmel Center for Prostate and Urologic Cancers, Memorial Sloan-Kettering Cancer Center, New York, NY
Jonathan L. Silberstein, Department of Surgery, Sidney Kimmel Center for Prostate and Urologic Cancers, Memorial Sloan-Kettering Cancer Center, New York, NY
Caroline Savage, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY.
Alexandra C. Maschino, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY
William T. Lowrance, Department of Surgery, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT
Jaspreet S. Sandhu, Department of Surgery, Sidney Kimmel Center for Prostate and Urologic Cancers, Memorial Sloan-Kettering Cancer Center, New York, NY.
References
- 1.Herr HW. Quality of life of incontinent men after radical prostatectomy. J Urol. 1994;151:652. doi: 10.1016/s0022-5347(17)35038-3. [DOI] [PubMed] [Google Scholar]
- 2.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 3.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 4.Penson DF, McLerran D, Feng Z, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the Prostate Cancer Outcomes Study. J Urol. 2008;179:S40. doi: 10.1016/j.juro.2008.03.136. [DOI] [PubMed] [Google Scholar]
- 5.Goode PS, Burgio KL, Johnson TM, 2nd, et al. Behavioral therapy with or without biofeedback and pelvic floor electrical stimulation for persistent postprostatectomy incontinence: a randomized controlled trial. JAMA. 2011;305:151. doi: 10.1001/jama.2010.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283:354. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- 7.Comiter CV. Male incontinence surgery in the 21st century: past, present, and future. Curr Opin Urol. 2010;20:302. doi: 10.1097/MOU.0b013e328339b795. [DOI] [PubMed] [Google Scholar]
- 8.Westney OL, Bevan-Thomas R, Palmer JL, et al. Transurethral collagen injections for male intrinsic sphincter deficiency: the University of Texas-Houston experience. J Urol. 2005;174:994. doi: 10.1097/01.ju.0000170237.72750.64. [DOI] [PubMed] [Google Scholar]
- 9.Elliott DS, Barrett DM. Mayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: a review of 323 cases. J Urol. 1998;159:1206. [PubMed] [Google Scholar]
- 10.Lee R, Te AE, Kaplan SA, et al. Temporal trends in adoption of and indications for the artificial urinary sphincter. J Urol. 2009;181:2622. doi: 10.1016/j.juro.2009.01.113. [DOI] [PubMed] [Google Scholar]
- 11.Bauer RM, Gozzi C, Hubner W, et al. Contemporary management of postprostatectomy incontinence. Eur Urol. 2011;59:985. doi: 10.1016/j.eururo.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Sandhu JS, Maschino AC, Vickers AJ. The Surgical Learning Curve for Artificial Urinary Sphincter Procedures Compared to Typical Surgeon Experience. Eur Urol. 2011;60:1285. doi: 10.1016/j.eururo.2011.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaeffer AJ, Clemens JQ, Ferrari M, et al. The male bulbourethral sling procedure for post-radical prostatectomy incontinence. J Urol. 1998;159:1510. doi: 10.1097/00005392-199805000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Madjar S, Jacoby K, Giberti C, et al. Bone anchored sling for the treatment of post-prostatectomy incontinence. J Urol. 2001;165:72. doi: 10.1097/00005392-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Howards SS. Information for applicants and candidates. 58 ed. Charlottesville: American Board of Urology, Inc.; 2011. [Google Scholar]
- 16.Howards SS. Information for applicants for recertification. 20 ed. American Board of Urology, Inc; 2011. p. 36. [Google Scholar]
- 17.Monroe C. Electronic communication. American Board of Urology; 2011. [Google Scholar]
- 18.Stitzenberg KB, Wong YN, Nielsen ME, et al. Trends in radical prostatectomy: centralization, robotics, and access to urologic cancer care. Cancer. 2011 doi: 10.1002/cncr.26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comiter CV. Male incontinence surgery in the 21st century: past, present, and future. Curr Opin Urol. 20:302. doi: 10.1097/MOU.0b013e328339b795. [DOI] [PubMed] [Google Scholar]
- 20.Castle EP, Andrews PE, Itano N, et al. The male sling for post-prostatectomy incontinence: mean followup of 18 months. J Urol. 2005;173:1657. doi: 10.1097/01.ju.0000154782.86431.41. [DOI] [PubMed] [Google Scholar]
- 21.Comiter CV. The male perineal sling: intermediate-term results. Neurourol Urodyn. 2005;24:648. doi: 10.1002/nau.20166. [DOI] [PubMed] [Google Scholar]
- 22.Fischer MC, Huckabay C, Nitti VW. The male perineal sling: assessment and prediction of outcome. J Urol. 2007;177:1414. doi: 10.1016/j.juro.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Litt ER, Ballert KN, et al. Artificial urinary sphincter versus male sling for post-prostatectomy incontinence--what do patients choose? J Urol. 2009;181:1231. doi: 10.1016/j.juro.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Fisher MB, Aggarwal N, Vuruskan H, et al. Efficacy of artificial urinary sphincter implantation after failed bone-anchored male sling for postprostatectomy incontinence. Urology. 2007;70:942. doi: 10.1016/j.urology.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Thuroff JW, Abrams P, Andersson KE, et al. EAU guidelines on urinary incontinence. Eur Urol. 2011;59:387. doi: 10.1016/j.eururo.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Kylmala T, Tainio H, Raitanen M, et al. Treatment of postoperative male urinary incontinence using transurethral macroplastique injections. J Endourol. 2003;17:113. doi: 10.1089/08927790360587450. [DOI] [PubMed] [Google Scholar]
- 27.Onur R, Singla A. Comparison of bone-anchored male sling and collagen implant for the treatment of male incontinence. Int J Urol. 2006;13:1207. doi: 10.1111/j.1442-2042.2006.01531.x. [DOI] [PubMed] [Google Scholar]