Abstract
Several nonthyroidal illnesses in euthyroid dogs can affect the results of thyroid function testing, making interpretation of the results more difficult with an increased risk of overdiagnosing hypothyroidism. The purpose of this study was to evaluate the effect of chronic, moderate to severe, osteoarthritis on canine thyroid function. Ninety-six, healthy, client-owned dogs, 65 of which were suffering from moderate to severe osteoarthritis and 31 euthyroid dogs without any physical evidence of osteoarthritis, were used in this study. Blood samples were collected to evaluate serum basal total thyroxine (TT4), free thyroxine (FT4), and thyrotropin (TSHc) concentrations. Basal serum TT4 concentration was not affected by osteoarthritis in dogs. Mild, but statistically significant, differences were noticed in FT4 and TSHc concentrations among the 2 groups. However, this had limited clinical relevance, since virtually all values were within their reference range, and no dogs would have been misdiagnosed as hypothyroid. Therefore, based on the results of our study, osteoarthritis does not need to be considered a factor influencing thyroid function evaluation in dogs.
Introduction
Hypothyroidism is the most frequently diagnosed endocrinopathy in dogs and also frequently overdiagnosed. The clinical signs are numerous, variable, often nonspecific, and rarely pathognomonic (1,2,3,4,5). Therefore, thyroid function is routinely evaluated in this species. Several diagnostic tests are available for assessing canine thyroid function. No single test that is currently available is singly definitive for the disease (1,2,3,4,5,6,7,8,9,10,11,12,13). Moreover, several non-thyroidal illnesses (euthyroid sick syndrome) and various drugs can affect thyroid function testing, making interpretation difficult (14,15,16,17,18,19,20,21,22,23,24,25,26,27,28). Stress induced by nonthyroidal illnesses may increase endogenous glucocorticoid production and alter thyroid function (20,24,25,26,27,28,29).
Osteoarthritis is a common progressive degenerative disease of synovial joints affecting approximately 20% of the canine population over 1 y of age (29,30,31,32,33). This condition is characterized by the destruction of articular cartilage and bony remodeling, resulting in pain, disability, and lameness. Although entirely hypothetical, it is plausible that chronic pain due to moderate to severe chronic osteoarthritis could induce sufficient stress to alter thyroid function.
Canine hypothyroidism typically affects medium to large breeds from 2 to 6 y of age (1,2,3,4,5). This population of dogs is also more at risk for osteoarthritis. In the eventuality that osteoarthritic dogs are presented with clinical signs compatible with hypothyroidism, it is essential, in order to obtain an accurate interpretation, to know if osteoarthritis affects the assessment of thyroid function tests. To the best of the author's knowledge, the effect of osteoarthritis on thyroid function has not previously been evaluated in dogs. The objective of this study was to evaluate the effects of moderate to severe osteoarthritis on canine thyroid function.
Materials and methods
Dogs
Ninety-six, client-owned dogs were used in this study, which consisted of 2 groups; group I: healthy but osteoarthritic dogs (n = 65), and group II: healthy dogs without clinical evidence of osteoarthritis (n = 31). Dogs entered in the study had to weigh more than 20 kg body weight (BW) and be older than 1.5 y. They had to be healthy (except for osteoarthritis in group I), based on the anamnesis, a physical examination, a complete blood cell (CBC), count and a biochemical profile in order to rule out systemic illnesses. In addition, to be accepted in the study, dogs must not have received any medication other than that for flea and heartworm prophylaxis and routine vaccination. Pregnant bitches were excluded.
A complete physical and an orthopedic examination were performed on each dog at the Centre Hospitalier Universitaire Vétérinaire (CHUV) of the Faculté de médecine vétérinaire of the Université de Montréal by at least one of the authors.
Group I: osteoarthritic dogs
The dogs included in this group were part of another clinical study evaluating a nutraceutical, carprofen, and meloxicam for the treatment of dogs afflicted with osteoarthritis (34). To be included in the study, dogs had to show clinical and radiographic evidence of moderate to severe osteoarthritis in 1 or 2 elbow, 1 or 2 stifle, or 1 or 2 hip joints. The pathologic condition had to be the cause of lameness, as determined by a complete orthopedic examination. The algetic gait reported by the owner had to be chronic (> 3 mo) and stable. Surgery on the affected joint, if performed, had to have occurred more than 1 y prior to the presentation.
Dogs presenting with lameness caused by a neurological or a muscular disease, a bleeding disorder, a fracture, a tumor, an infectious or immune-mediated process were rejected. The osteoarthritic dogs were included, only if they had not received injectable glucocorticoids within the previous 3 mo; oral glucocorticoids within the last 3 wk; injection of slow-acting disease modifying osteoarthritis agents within the last 6 mo; oral slow-acting disease modifying osteoarthritis agents (oral nutraceutical such as chondroitin sulfate and glucosamine sulfate) within the last months or oral nonsteroidal anti-inflammatory drugs (NSAID) within the last 2 wk.
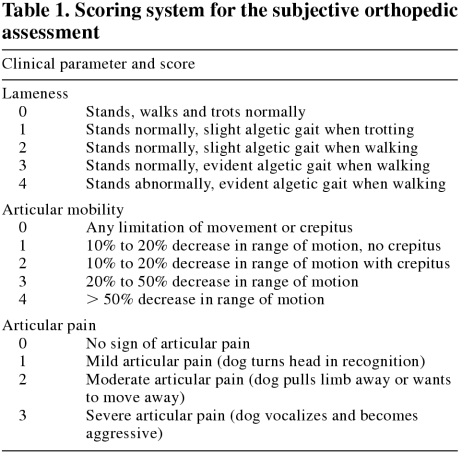
The severity of arthritic clinical signs was assessed and graded by a veterinary orthopedic surgeon using a scoring system (Table 1). This score was attributed to the limb with the most signs. Clinical parameters evaluated were lameness (score of 0 to 4), articular mobility (score of 0 to 4), and articular pain (score of 0 to 3) (34). The score for each of the 3 clinical parameters was added to generate a cumulative score. Dogs were then divided into 2 subgroups. Group Ia consisted of 30 dogs with the lowest cumulative scores (0 to 6) and group Ib consisted of 35 dogs with the highest cumulative scores (7 to 11).
Table 1.
Group II: control dogs
Dogs selected to this group had to demonstrate exemplary health status. The complete physical and orthopedic examinations had to reveal no abnormalities of any joints and absence of lameness.
The thyroid function evaluation had to demonstrate euthyroid status. At least 2 of the 3 following parameters needed to be within the reference values: serum total thyroxine concentration (TT4), free thyroxine concentration (FT4), and canine endogenous thyrotropin (TSHc). In addition, 10 of the 31 healthy dogs had had previous hip (n = 10) and elbow (n = 7) radiographs, with no evidence of osteoarthritis.
Specimen collection and storage
Blood samples for a CBC count, biochemical profile, serum thyroglobulin antibodies, and the measurement of serum TT4, FT4, and TSHc concentrations were taken by jugular venipuncture on each dog. All samples obtained for endocrine assay were centrifuged immediately after clot formation, and the serum was frozen at −20°C until assayed.
Endocrine assays
Total T4 was measured by using a commercially available solid-phase radioimmunoassay kit (Clinical Assays Gammacoat M Total T4 125I RIA Kit; DiaSorin Inc, Stillwater, Minnesota USA). Buffer solutions, T4 radioligand, antibody-coated polypropylene tubes, and standards were supplied in the kit. Specificity data provided by the manufacturer identified 92% cross-reactivity with D-thyroxine, 2.1% cross-reactivity with D- and L-triiodothyronine, and less than 0.1% cross-reactivity with other iodothyronines. Reagents were prepared following the manufacturer's protocol. The following modifications were made to the assay protocol, in part to enhance the sensitivity of the assay. The volume of sample or standard was increased from 10 μL to 25 μL. A “low” standard of 6.5 nmol/L was made by mixing equal volumes of 0- and 13-nmol/L standards. The 257-nmol/L standard provided by the manufacturer was discarded, leaving the 156-nmol/L standard as the highest in the standard curve of the assay. After pipetting sample/standard and 1 mL of radioligand solution into antibody-coated tubes, the assay mixture was incubated for 3 h at room temperature (~22°C). The sensitivity of the assay, defined as the concentration of T4 at 90% specific binding, was 3 nmol/L (data from 10 assays). When l-thyroxine (Sigma Chemical, St. Louis, Missouri USA) was added to a canine serum pool to achieve increases of 26, 52, or 78 nmol/L, 117%, 105%, and 106% of added T4 was measured in the assay. A pool of canine serum having a high concentration of T4, 104 nmol/L, was diluted at rates of 50% and 25% in “0” standard or protein buffer. The respective recovery rates of T4 were 100% and 108% of expected results in “0” standard and 88% and 92% in protein buffer-diluted serum. Intraassay repeatability was assessed in 3 pools of canine serum assembled to have low (10 nmol/L), middle range (32 nmol/L), and high (86 nmol/L) concentrations of T4. The respective intraassay coefficients of variation (CV) for 10 replicates of each pool were 0.093, 0.092, and 0.120. In 10 assays, the interassay CV for canine serum pools having T4 concentrations of 15 and 63 nmol/L were 0.046 and 0.041, respectively. The reference range of TT4 of euthyroid dogs is 15 to 67 nmol/L.
Assay of FT4 by equilibrium dialysis was done by using a commercially available kit (Free T4 by equilibrium dialysis; Nichols Institute Diagnostics, San Juan Capistrano, California USA) that was previously validated in this laboratory for canine serum (25). The reference range of FT4 of euthyroid dogs is 6 to 42 pmol/L.
Canine TSH was measured with a commercially available immunoradiometric assay (Coat-A-Count canine TSH IRMA; Diagnostic Products Corporation, Los Angeles, California USA). Initial evaluation of assay performance demonstrated negligible cross-reactivity with other canine pituitary gonadotropins, good repeatability, and dilutional parallelism (11,12). In this laboratory, 2 canine serum pools having concentrations of TSHc of 0.21 and 1.33 ng/mL were established for repeatability studies. For 10 replicates of each pool, the intraassay CVs were 0.106 and 0.052, respectively. Among 10 assay runs, the interassay CV for each pool was 0.139 and 0.071, respectively. A canine serum pool of high TSHc (4.67 ng/mL) was made for dilutional studies. When serum from this pool was diluted at rates of 50%, 25%, and 12.5% in “0” standard, 84%, 89%, and 86% of expected TSHc concentrations were measured. When serum from the high pool was diluted in protein buffer solution at rates of 50%, 25%, and 12.5%, 95%, 99%, and 103% of expected TSHc results were obtained. The reference range of TSHc of euthyroid dogs is 0 to 0.6 ng/mL.
Canine thyroglobulin autoantibody (TgAA) assays were performed with a commercially available ELISA kit (Oxford Biomedical Research, Oxford, Michigan USA) with a previously defined diagnostic utility (13). The reference range of TgAA of euthyroid dogs is < 200%.
Data analysis
Data for each of the parameters (basal serum TT4, FT4, and TSHc) were analyzed by a one-way analysis of variance (ANOVA). A Tukey-Kramer multiple comparison test was used to compare each group (osteoarthritic dogs (group I), control dogs (group II), moderately affected dogs (group Ia), and severely affected dogs (group Ib)) when a significant difference occurred in data. A value of P < 0.05 was considered significant. Data are reported as mean ± standard deviation (s).
Results
Group I: osteoarthritic dogs
This group was constituted of 65 client-owned dogs with moderate to severe osteoarthritis. There were 33 females (22 spayed) and 32 males (21 neutered). Age ranged from 1.5 to 13 y (mean 6.8 y). Different breeds were represented: Labrador retriever (n = 18), mixed breed (n = 14), German shepherd (n = 10), Golden retriever (n = 7), Bernese mountain dog (n = 6), Saint-Bernard (n = 2), and 1 each of briard, standard poodle, collie, boxer, rottweiler, Newfoundland, Akita, and English shepherd. Body weight ranged between 26 and 58 kg BW (mean 39.1 kg).
The progressive degenerative process in these dogs was due to developmental arthropathies such as elbow dysplasia (n = 14) and hip dysplasia (n = 26), and acquired arthropathies caused by cranial cruciate ligament rupture (n = 25). The 30 dogs of group Ia and the 35 dogs of group Ib had a mean cumulative orthopedic assessment score of 4 (range 1 to 6) and 8 (range 7 to 11), respectively.
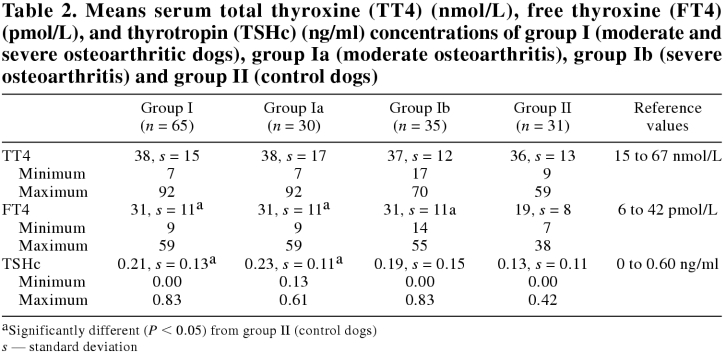
The CBC counts and the biochemistry profiles were all within normal reference ranges. Results of thyroid function evaluation are reported in Table 2.
Table 2.
Group II: control dogs
This group consisted of 31 client owned healthy dogs. There were 17 females (10 spayed) and 14 males (9 neutered). Age ranged from 1.8 to 10.2 y (mean 3.8 y). Different breeds were represented: Golden retriever (n = 7), German shepherd (n = 6), Labrador retriever (n = 5), Australian shepherd (n = 3), mixed breed (n = 3), Bernese mountain dog (n = 2), and 1 each of Airedale, Dalmatian, Doberman pinscher, Great Dane, and griffon. Body weight ranged between 20.4 and 59.0 kg BW (mean 30.6 kg).
The CBC counts and biochemical profiles were all within normal reference ranges. Results of thyroid function evaluation are reported in Table 2.
Total thyroxine concentrations
There was no statistically significant difference in basal serum TT4 concentrations between the 2 groups (P = 0.73) (Table 2). All serum TT4 concentrations were within the reference range (15 to 67 nmol/L) of euthyroid dog, except for 2 control and 3 osteoarthritic dogs. The 2 control dogs had serum TT4 concentrations of 12 and 9 nmol/L, for which serum FT4 concentrations (10 and 7 pmo/L, respectively) and serum TSHc concentrations (0.19 and 0.13 ng/mL, respectively) were within normal limits.
The 3 osteoarthritic dogs had serum TT4 concentrations of 7, 70, and 92 nmol/L, for which serum FT4 concentrations were 9, 40, and 55 pmol/L, respectively. Serum TSHc concentrations were within normal limits (0.16, 0.14, and 0.30 ng/mL respectively).
Free thyroxine concentrations
There was a significant difference in basal serum FT4 concentrations among the groups (P < 0.0001) (Table 2). Posthoc tests revealed significantly lower FT4 concentrations in group II (19, s = 8 pmol/L) when compared with group I (31, s = 11; P < 0.001), group Ia (31, s = 11; P < 0.001), and group Ib (31, s = 11; P < 0.001). However, all serum FT4 concentrations were within the reference range (6 to 42 pmol/L) of euthyroid dogs, except for 1 control dog and 4 osteoarthritic dogs with mildly elevated results (55, 59, 56, 55, and 52 pmol/L, respectively).
Serum TSHc concentrations
There was a statistically significant difference in serum TSHc concentrations among the groups (P = 0.01) (Table 2). Posthoc tests revealed significantly lower TSHc concentrations in group II (0.13, s = 0.11 nmol/L) compared with group I (0.21, s = 0.13; P < 0.05) and group Ia (0.23, s = 0.11; P < 0.05). However, all serum TSHc concentrations were within the reference range (0 to 0.60 ng/mL) of euthyroid dogs, except for 1 osteoarthritic dog with a serum TSHc concentration of 0.83 ng/mL, which had normal serum TT4 and FT4 concentrations (25 nmol/L and 18 pmol/L, respectively) and no TgAA.
Thyroglobulin antibodies
Serum TgAA were present in 3 out of 65 (4.7%) osteoarthritic dogs (246%, 299%, and 985%, respectively) and 1 out of 31 (3.6%) of the control dogs (768%). However, these 4 dogs had normal serum TT4, FT4, and TSH concentrations. The percentage of dogs in which we detected serum TgAA was in agreement with a previous study in which these TgAA were detected in 3.4% of dogs with nonthyroidal illness (13).
Discussion
Hypothyroidism is one of the most frequently encountered endocrinopathies in the dog. However, due to the diversity of clinical signs and lack of availability of perfect diagnostic tests, hypothyroidism is also commonly misdiagnosed in dogs. The assessment of thyroid function in dogs can be challenging when laboratory findings are equivocal or in the hypothyroid reference range. Indeed, the evaluation of serum TT4 and FT4 concentrations has a high sensitivity but a low specificity (1,2,3,6,7,15). Conversely, the evaluation of serum TSHc and TgAA concentrations has a relatively high specificity, but a low sensitivity (1,2,3,4,6,7,12,13,14,15,16,17,18). In addition, many nonthyroidal factors can affect circulating hormone concentrations, resulting in the misdiagnosis of hypothyroidism in euthyroid dogs. Therefore, the diagnosis of hypothyroidism must rely on a combination of compatible clinical signs, routine laboratory tests (CBC counts, serum biochemical analyses), and specific thyroid test results (TT4, FT4, TSHc, TgAA), coupled with successful long-term response to levothyroxine supplementation (1,2,8,16,18).
Several nonthyroidal illnesses and drugs are known to affect the evaluation of the thyroid function of dogs (1,2,8,16,17). The list of acute and chronic diseases that can alter serum thyroid hormone concentrations in dogs is extensive and includes conditions such as cutaneous and noncutaneous infectious diseases, kidney and liver diseases, cardiac failure, hyperadrenocorticism, diabetes mellitus, and other critical illnesses (1,2). The alteration of the thyroid function can result from phenomena such as a decrease in TSH secretion, synthesis of T4, concentration of serum binding proteins or protein binding affinity for thyroid hormones, inhibition of transformation of T4 to triiodothyronine (T3), etc. (1,2,35,36).
Endogenous or exogenous glucocorticoids can decrease basal thyroid hormone concentrations in dogs. The effects of exogenous glucocorticoid administration have previously been documented and are dependent upon the dose, route of administration, duration of treatment, and chemical form used (24,25,27). The mechanisms of action of glucocorticoids on thyroid function appear complex and may include inhibition of the hypothalamic-pituitary-thyroid axis, decreased binding of T4 to transport proteins, and decreased peripheral deiodination of T4 to T3.
Stresses induced by nonthyroidal illnesses may increase endogenous glucocorticoid production and alter thyroid hormone concentrations (24). Although purely hypothetical, chronic pain induced by severe osteoarthritis could potentially induce enough stress to alter thyroid function tests, hence the goal of the present study.
The results of the present study revealed no significant difference in serum TT4 concentrations among groups. Conversely, serum FT4 concentrations of osteoarthritic dogs, regardless of the severity of their condition, were significantly higher than those of the control group. However, this difference has limited clinical implications, because results usually remained within the reference range for euthyroid dogs, except for 1 control and 4 osteoarthritic dogs with mildly elevated results, which would not have been misdiagnosed as hypothyroid.
Nevertheless, based on these results, it could be hypothesized that, although unlikely, a truely hypothyroid dog suffering from osteoarthritis could be misdiagnosed as being euthyroid if serum FT4 concentration were the only parameter evaluated. Further investigations of hypothyroid dogs suffering from osteoarthritis are needed to confirm this possibility. The mild increase in serum FT4 concentration might result from the putative increases in endogenous glucocorticoids that potentially could alter the binding of thyroid hormones to serum carrier proteins or reduce T3 production from T4, or both (2,24,35,37).
In the osteoarthritic group (group I) and the group of osteoarthritic dogs with the lowest cumulative score (group Ia), the serum TSHc concentrations were significantly higher than in the control group. This difference has no clinical implication and, with the exception of 1 dog, the serum TSHc concentration was within the reference range for euthyroid dogs (0 to 0.60 ng/mL). The changes related to glucocorticoid excess are more likely to induce a decrease rather than an increase of TSHc. Moreover, there was no significant difference between the control group and the group of dogs with the highest cumulative osteoarthritic score (group Ib), which probably had the highest endogenous glucocorticoids secretion. Finally, serum TSHc concentrations are more likely to remain within the reference range in dogs with nonthyroidal illnesses (15).
It has been shown that age and body size have an effect on serum TT4 concentration in dogs (38). Nursing puppies have serum TT4 concentrations higher than those of adult dogs, and geriatric dogs have slightly lower serum TT4 concentrations than do younger adults. In addition, medium-sized and large dogs have lower serum TT4 concentrations than do small breeds (2). However, the effect of age and body size on serum FT4 and TSHc concentrations in dogs is unknown. Although the mean age and mean BW of the control group were less than those of the osteoarthritic group, it is unlikely that this was the reason for the mild differences in FT4 and TSHc between the 2 groups. Nevertheless, the mild differences observed in our study may be the reflection of differences in the groups and not necessarily due to the presence of osteoarthritis in the group I dogs.
In conclusion, based on the results of the present study, basal serum TT4 concentrations do not appear to be affected by moderate or severe osteoarthritis in dogs. The mild but significant differences observed in serum FT4 and TSHc concentrations have limited clinical relevance, since none of the osteoarthritic dogs had values within the hypothyroid reference range (except for 1 mildly elevated TSHc) and none of the changes observed would have lead to a misdiagnosis of hypothyroidism. Therefore, osteoarthritis does not appear to need to be considered in dogs undergoing thyroid function evaluation. CVJ
Footnotes
This project was funded by the “Fonds du Centenaire” of the Faculté de médecine vétérinaire, Université de Montréal.
Address all correspondence to Dr. Manon Paradis.
Reprints will not be available from the authors.
References
- 1.Scott-Moncrieff JCR, Guptill-Yaoran L. Hypothyroidism. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 5th ed. Philadelphia: WB Saunders; 2000:1419–1429.
- 2.Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction, 2nd ed. Philadelphia, 1996:68–117.
- 3.Scott DW, Miller WH, Griffin CE. Endocrine and metabolic diseases. In: Muller and Kirk's Small Animal Dermatology. 6th ed. Philadelphia: WB Saunders, 2001:851–865.
- 4.Graham PA, Nachreiner RF, Refsal KR, Provencher-Bolliger AL. Lymphocytic thyroiditis. Vet Clin North Am Small Anim Pract 2001;31:915–933. [DOI] [PubMed]
- 5.Dixon RM, Reid SWJ, Mooney CT. Epidemiological, clinical haematological and biochemical characteristics of canine hypothyroidism. Vet Rec 1999;145:481–487. [DOI] [PubMed]
- 6.Peterson ME, Melian C, Nichols R. Measurement of serum total thyroxine, triiodothyronine, free thyroxine, and thyrotropin concentrations for diagnosis of hypothyroidism in dogs. J Am Vet Med Assoc 1997;211:1396–1402. [PubMed]
- 7.Kemppainen RJ, Behrend EN. Diagnosis of canine hypothyroidism. Perspectives from a testing laboratory. Vet Clin North Am Small Anim Pract 2001;31:951–962. [DOI] [PubMed]
- 8.Panciera DL. Developing a rational approach to diagnosing and treating canine hypothyroidism. Vet Med 1997:43–67.
- 9.Jensen AL, Iversen L, Hoier R, Kristensen F, Henriksen P. Evaluation of an immunoradiometric assay for thyrotropin in serum and plasma samples of dogs with primary hypothyroidism. J Comp Pathol 1996;114:339–346. [DOI] [PubMed]
- 10.Kooistra HS, Diaz-Espineira M, Mol JA, van den Brom WE, Rijnberk A. Secretion pattern of thyroid-stimulating hormone in dogs during euthyroidism and hypothyroidism. Domest Anim Endocrinol 2000;18:19–29. [DOI] [PubMed]
- 11.Dixon RM, Graham PA, Mooney CT. Serum thyrotropin concentrations: a new diagnostic test for canine hypothyroidism. Vet Rec 1996;138:594–595. [DOI] [PubMed]
- 12.Williams DA, Scott-Moncrieff JCR, Bruner J, et al. Validation of an immunoassay for canine thyroid-stimulating hormone and changes in serum concentration following induction of hypothyroidism in dogs. J Am Vet Med Assoc 1996;209:1730–1732. [PubMed]
- 13.Nachreiner RF, Refsal KR, Graham PA, Hauptman J. Prevalence of autoantibodies to thyroglobulin in dogs with non thyroidal illness. Am J Vet Res 1998;59:951–955. [PubMed]
- 14.Dixon RM, Mooney CCT. Canine serum thyroglobulin autoantibodies in health, hypothyroidism, and non-thyroidal illness. Res Vet Sci 1999;66:243–246. [DOI] [PubMed]
- 15.Kantrowitz LB, Peterson ME, Melian C, Nichols R. Serum total thyroxine, total triiodothyronine, free thyroxine, and thyrotropin concentrations in dogs with nonthyroidal disease. J Am Vet Med Assoc 2001;219:765–769. [DOI] [PubMed]
- 16.Scott-Moncrieff JCR, Nelson RW, Bruner JM, Williams DA. Comparison of serum concentrations of thyroid-stimulating hormone in healthy dogs, hypothyroid dogs, and euthyroid dogs with concurrent diseases. J Am Vet Med Assoc 1998;212:387–391. [PubMed]
- 17.Ramsey IK, Evans H, Herrtage ME. Thyroid-stimulating hormone and total thyroxine concentrations in euthyroid, sick euthyroid and hypothyroid dogs. J Small Anim Pract 1997;38: 540–545. [DOI] [PubMed]
- 18.Ramsey I, Herrtage M. Distinguishing normal, sick, and hypothyroid dogs using total thyroxine and thyrotropin concentrations. Canine Pract 1997;22:43–44.
- 19.Ferguson DC. Euthyroid sick syndrome. Canine Pract 1997; 22:49–51.
- 20.Ferguson DC, Peterson ME. Serum free and total iodothyronine concentrations in dogs with hyperadrenocorticism. Am J Vet Res 1992;53:1636–1640. [PubMed]
- 21.Vail DM, Panciera DL, Ogilvie GK. Thyroid hormone concentrations in dogs with chronic weight loss, with special reference to cancer cachexia. J Vet Intern Med 1994;8:122–127. [DOI] [PubMed]
- 22.Yu AA, Kemppainen RJ, MacDonald JM. Effect of endotoxin on hormonal responses to thyrotropin and thyrotropin-releasing hormone in dogs. Am J Vet Res 1998;59:186–191. [PubMed]
- 23.Paradis M, Pagé N, Larivière N, Fontaine M. Serum free thyroxine concentration measured by chemiluminescence assay before and after thyrotropin administration in healthy dogs, hypothyroid dogs and euthyroid dogs with dermatopathies. Can Vet J 1996;37: 289–294. [PMC free article] [PubMed]
- 24.Kaptein EM, Moore GE, Ferguson DC, Hoenig M. Effects of prednisone on thyroxine and 3,5,3'-triiodothyronine metabolism in normal dogs. Endocrinology 1992;130:1669–1679. [DOI] [PubMed]
- 25.Daminet S, Paradis M, Refsal KR, Price C. Short term influence of prednisone and phenobarbital on thyroid function in euthyroid dogs. Can Vet J 1999;40:411–415. [PMC free article] [PubMed]
- 26.Moore GE, Ferguson DC, Hoening M. Effects of oral administration of anti-inflammatory doses of prednisone on thyroid hormone response to thyrotropin-releasing hormone and thyrotropin in clinically normal dogs. Am J Vet Res 1993;54:130–135. [PubMed]
- 27.Torres SMF, McKeever PJ, Johnston SD. Effect of oral administration of prednisolone on thyroid function in dogs. Am J Vet Res 1991;52:416–421. [PubMed]
- 28.Moore GE, Ferguson DC, Hoenig M. Effects of oral administration of antiinflammatory doses of prednisone on thyroid hormone response to thyrotropin-releasing hormone and thyrotropin in clinically normal dogs. Am J Vet Res 1993;54:130–135. [PubMed]
- 29.Rubello D, Sonino N, Casara D, et al. Acute and chronic effects of high glucocorticoid levels on hypothalamic-pituitary-thyroid axis in man. J Endocrinol Invest 1992;15:437–441. [DOI] [PubMed]
- 30.Johnston SA. Osteoarthritis. Vet Clin North Am Small Anim Pract 1997;27:699–720. [DOI] [PubMed]
- 31.McLaughin R. Management of chronic osteoarthritic pain. Vet Clin North Am Small Anim Pract 2000;30:933–949. [DOI] [PubMed]
- 32.Vaughan-Scott T, Taylor JH. The pathophysiology and medical management of canine osteoarthritis. J S Afr Vet Assoc 1997;68: 21–25. [DOI] [PubMed]
- 33.Martinez SA. Medical management of osteoarthritis in companion animals. Symposium on articular, cartilage and joint health. Vet Orthop Soc 27th Annu Conf, Val D'Isère, France, March 2000:24–29.
- 34.Moreau M, Dupuis J, Bonneau NH, Desnoyers M. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs afflicted with osteoarthritis. Vet Record. In press. [DOI] [PubMed]
- 35.Ferguson DC. The effect of nonthyroidal factors on thyroid function tests in dogs. Compend Contin Educ Pract Vet 1988;10:1365–1377.
- 36.Curran P, Degroot L. The effect of hepatic enzyme-inducing drugs on thyroid hormones and the thyroid gland. Endocr Rev 1991;12:135. [DOI] [PubMed]
- 37.Surks MI, Sievert R. Drugs and thyroid function. New Eng J M 1995;333:1688–1694. [DOI] [PubMed]
- 38.Reimers TJ, Lawler DF, Sutaria PM, Correa MT, Erb HN. Effects of age, sex, and body size on serum concentrations of thyroid and adrenocortical hormones in dogs. Am J Vet Res 1990;51:454–457. [PubMed]