Abstract
The purpose of this study was to evaluate the usefulness of the California mastitis test (CMT) to detect an intramammary infection caused by a major mastitis pathogen in early lactation cows. The gold standard used for comparison was bacteriological culture of single milk samples. The sensitivity (82.4%) and specificity (80.6%) of a positive CMT were highest on the 4th day of lactation.
Intramammary infections (IMIs) found in early lactation can be the result of either IMIs that do not resolve or new IMIs that develop, during the dry period. The importance of the dry period to eliminate existing and prevent new intramammary infections is well understood (1). There has been increased interest recently in novel dry cow management strategies that would prevent new IMIs from occurring during the dry period, such as external and internal teat sealers (2). New management strategies offer promise in helping to reduce new IMIs to a greater extent than can be achieved by administering intramammary dry cow antibiotic therapy (DCT) alone. In general, DCT products do not persist late into the dry period and are ineffective against infections with gram-negative organisms in the prepartum period (1,3). Therefore, despite dry cow udder health management programs, IMIs are likely to remain from the previous lactation or develop during the dry period. Identifying infected cows in the immediate postpartum period should be an important procedure for the control of mastitis in dairy herds.
Identifying and eliminating IMIs early in lactation may have significant economic benefits. Preventing clinical mastitis in early lactation, decreasing the amount of discarded milk, and reducing the bulk milk somatic cell count (BMSCC) are some of the potential benefits (4,5). Bacteriological culture of milk samples is the standard method for identifying IMIs (6). However, the logistic and financial considerations involved with sampling all fresh cows have precluded this technique from being widely adopted (5,7). The use of individual cow somatic cell counts (SCC) to identify the presence of IMIs in early lactation cows is possible. However, practical application of this method is restricted by the Dairy Herd Improvement (DHI) test date interval being too long to identify all cows in early lactation. Previous research had suggested that SCC is normally elevated during the first 2 wk of lactation, followed by a rapid decrease (8); however, more recent studies have demonstrated that cow level SCC declines more rapidly than previously thought (5,9). Consistent and reliable methods to determine the udder health status of early lactation cows have yet to be identified.
The California mastitis test (CMT), a qualitative measurement of the somatic cell count in milk, is a screening test for subclinical mastitis that can be used easily at cowside (7). The use of the CMT to identify infected quarters has been extensively validated in cows that were not in early lactation (7). Recently, the CMT has been used to identify IMIs in the first 10 d of lactation (5). This study determined that the optimal sampling strategy to select infected quarters for bacteriological culture was to sample at 3 d postcalving. Similar work in the Netherlands supports these findings (9). Furthermore, other studies using the CMT to evaluate diagnostic and treatment protocols for early postpartum dairy cows are in progress (4). The purpose of this study was to evaluate the CMT for its ability to identify IMIs caused by major pathogens in dairy cows within the 1st wk of calving.
Two research herds associated with the University of Guelph, and 1 herd each at Kansas State University, Iowa State University, and the State University of New York at Cobleskill, participated in this study for a period of just over 1 y. Purposive selection of research herds was required to facilitate this study augmenting on-going research of a similar nature, and to have access to trained technicians. Data were collected from 325 cows that were all starting their 2nd or a subsequent lactation, and that had at least 3 functional quarters. Upon calving, all cows had a CMT performed on each functional quarter, with quarter milk samples being taken immediately afterwards. Teats were prepared aseptically, prior to sample collection, according to the National Mastitis Council (NMC) sample collection and handling guidelines (6). Milk samples were sent to the microbiological laboratory associated with each university for routine culture techniques. The CMT and culture were performed only once on each cow, as close to calving as possible. Farms were visited once per week, so for any individual cow these tests may have been performed between 1 and 7 d in milk (DIM). Each laboratory had standard operating procedures in place for the handling of samples, culture techniques, and interpretation of results consistent with NMC recommended procedures (6).
An IMI was defined as the presence of a major mastitic organism, including Staphylococcus aureus, Streptococcus dysgalactiae, Streptococcus uberis, nonspeciated streptococci, Escherichia coli, Klebsiella spp., other coliforms, Enterococcus spp., Mannheimia sp., Arcanobacterium pyogenes, Pasteurella spp., Pseudomonas spp., yeast, Serratia marcescens, Proteus spp., and Prototheca spp.. All levels of growth of coagulase-negative staphylococci and Corynebacterium bovis were considered minor pathogens. The minor pathogens and nonsignificant bacterial growth, such as gram-positive rods, were not considered to be IMIs in this dataset. This decision was based on the definitions and guidelines used in each laboratory for nonsignificant growth and on the fact that the colony forming units of minor pathogens were not reported quantitatively from each laboratory. Contaminated samples, defined as growth of 3 or more pathogens from the same sample, were excluded.
The CMT was performed on quarter foremilk samples at cowside, and the test results were read and recorded by 1 trained technician within each herd. The CMT reaction of each quarter was recorded in an ordered scale as either, 0, 1, 2, or 3, with 0 indicating no reaction, and 1 being a trace and a slight positive reaction. All data were sent to the University of Guelph, where they were stored in a database and then imported into software for analysis (Microsoft Access; Microsoft 2002 Corporation, Redman, Washington USA and SAS version 8.11; SAS Systems, Cary, North Carolina USA). Various cutoff points of the CMT scores were used to define a positive CMT reaction. Calculations of diagnostic test characteristics were performed by using the single milk bacteriological culture result as the gold standard. The sensitivity, specificity, and the predictive values of the CMT results were calculated by using standard 2-by-2 contingency tables. A 95% confidence interval was calculated for the sensitivity and specificity of the CMT, both overall and on various days in milk (EpiScope 2.0; EpiVetNet, Massey University, Palmerston North, New Zealand).
A total of 1283 quarter CMT and culture results were available for analysis. Overall, the prevalence of IMIs in early lactation in these data was 10.0% of quarters. The prevalence among each of the 5 herds was 7.6% (n = 484), 9.5% (n = 231), 9.6% (n = 135), 12.6% (n = 261), and 13.4% (n = 172). The predominant major mastitis organisms isolated in early lactation in these herds were environmental Streptococci spp. and Escherichia coli. The proportions of specific pathogens identified in early lactation were 14.8%, 3.9%, 10.2%, 15.6%, 8.6%, and 17.9% for Staphylococcus aureus, Streptococcus dysgalactiae, Streptococcus uberis, E. coli, Klebsiella, and other major pathogens, respectively.
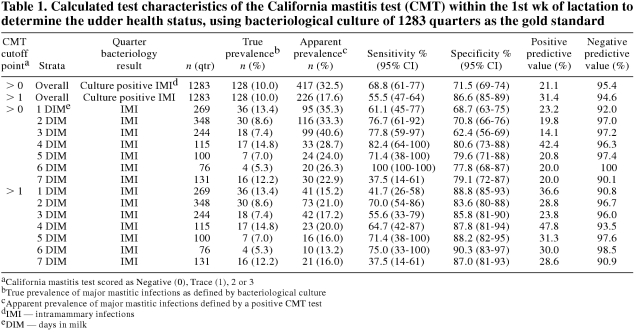
The calculated sensitivity, specificity, and predictive values of the CMT are shown in Table 1. When any level of positive CMT reaction was considered to be indicative of an IMI, with a prevalence of 10% IMIs, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 68.8%, 71.5%, 21.1%, and 95.4%, respectively. By increasing the cutoff point at which a CMT was considered positive to those reactions that were greater than a score of 1, the sensitivity and NPV decreased (55.0% and 94.6%, respectively) while the specificity and PPV increased (86.6% and 31.4%, respectively). When the sensitivity and specificity of the CMT were stratified by the DIM on which the test was performed, the sensitivity of the CMT was generally increased up to day 4 after calving at a cutoff point of greater than 0 (Table 1). The highest specificity was also calculated to be on DIM 4. The same trend in sensitivity was not seen when a cutoff point > 1 was used, although the specificity remained higher and the apparent prevalences were lower.
Table 1.
The sensitivity of the CMT is the ability to detect the presence of an IMI, and it is calculated as the proportion of quarters that had an IMI and a positive CMT. The specificity of the CMT is the ability to detect quarters that did not have an IMI, and it is calculated as the proportion of noninfected quarters that had a negative CMT reaction. In combination, these 2 test characteristics describe how well the CMT can discriminate between noninfected and infected quarters (10). Generally, as the sensitivity of a test increases, the specificity will decrease. This was not demonstrated clearly in these data, because different populations of cows were tested on different days, each having different distributions of organisms and varying abilities to induce a high SCC. The predictive values of the CMT reflect how the test results could be interpreted in the field. The PPV indicates the likelihood that a quarter with a positive CMT reaction is infected with a mastitic pathogen. Conversely, the NPV is the likelihood that a quarter with a negative CMT reaction is indeed not infected. It is not appropriate to select a test simply based on reported predictive values alone, as the predictive value of any test is influenced not only by the test sensitivity and specificity, but also by the prevalence of disease within the population in which it is used (10).
As a screening test used to detect IMIs in early lactation cows, a high sensitivity (no false-negative CMT reactions) would be ideal. A high sensitivity would enable the CMT to detect the majority of quarters that had an IMI. However, it is also desirable to limit the number of false-positive reactions with a high specificity, or else the test would be no better than sampling all fresh cows anyway. False positive and false negative reactions will still occur with the CMT, and due to the subjectiveness of this particular test, there will be misclassification biases as well. False positives, associated with a low specificity, would occur when somatic cells are present in the milk with bacteria not being isolated. False negative reactions, associated with a low sensitivity, would occur when bacteria are indeed present in the gland but somatic cells are not. The highest calculated sensitivity and PPV were on DIM 4 at a cutoff point of > 0 in this study. Since samples were not taken from every cow on every day in this study design, it cannot be concluded that DIM 4 is the best day to perform the CMT. However, the findings in our study are consistent with what has been reported previously, in a study that did perform the CMT on consecutive days (5). When a CMT was scored as having no reaction with a relatively high and consistent NPV across different prevalence levels of IMI, there was a high likelihood that no IMI was present. Because of the less than ideal sensitivity and specificity of the CMT, its use for decisions on individual cows may be limited; it may be of more use in establishing the occurrence of IMIs in groups of cows for monitoring herd-level udder health, especially in dry cow management programs.
An important consideration of this study is that all CMT reactions were scored by trained technicians in research dairy herds. The performance of the CMT, due to its subjective nature, may be very different when used on commercial dairy farms by farm workers (4). Also, our gold standard was a single milk sample. Duplicate or multiple milk samples would have been a more appropriate gold standard (6). Nevertheless, a valuable addition to a fresh cow program would be a rapid, cost-effective, cow-side test that would identify IMIs. The results of this current study concur with other recent trials that the CMT has the potential to be a rapid, accurate, and economically feasible test for fresh cows. There remains a definite need for cow-side test procedures that could be used on CMT positive quarters to identify specific pathogens. CVJ
Footnotes
Dr. Dingwell's current address is Mo Dhaicdh Farms Ltd., Morell, Prince Edward Island C0A 1S0.
Address all correspondence and reprint requests to Dr. Randy T. Dingwell; e-mail: dingwellhill@hotmail.com
References
- 1.Eberhart RJ. Management of dry cows to reduce mastitis. J Dairy Sci 1986;69:1721–1732. [DOI] [PubMed]
- 2.Huxley JN, Green MJ, Green LE, Bradley AJ. Evaluation of the efficacy of an internal teat sealer during the dry period. J Dairy Sci 2002;85:551–561. [DOI] [PubMed]
- 3.Bradley AJ, Green MJ. An investigation of the impact of intramammary antibiotic dry cow therapy on clinical coliform mastitis. J Dairy Sci 2001;84:1632–1639. [DOI] [PubMed]
- 4.Wallace JA, Leslie KE, Dingwell RT, Schukken YH, Baillargeon P. An evaluation of a diagnostic and treatment protocol for intramammary infections in early postpartum dairy cows. Proc Annu Meet National Mastitis Council 2002:159–160.
- 5.Sargeant JM, Leslie KE, Shirley JE, Pulkrabek BJ, Lim GH. Sensitivity and specificity of somatic cell count and California Mastitis Test for identifying intramammary infection in early lactation. J Dairy Sci 2001;84:2018–2024. [DOI] [PubMed]
- 6.National Mastitis Council. Laboratory Handbook on Bovine Mastitis, revised ed. Madison, Wisconsin: Natl Mastitis Counc, Inc., 1999:1–30.
- 7.Leslie KE, Jansen JT, Lim GH. Opportunities and implications for improved on-farm cowside diagnostics. Proc DeLaval Hygiene Symp 2002:147–160.
- 8.Dohoo IR. An evaluation of the validity of individual cow somatic cell counts from cows in early lactation. Prev Vet Med 1993;16:103–110.
- 9.Barkema HW, Deluyker HA, Schukken YH, Lam TJGM. Quarter milk somatic cell count at calving and at the first six milkings after calving. Prev Vet Med 1999;38:1–9. [DOI] [PubMed]
- 10.Martin SW, Meek AH, Willeberg P. Veterinary Epidemiology: Principles and Methods. 1st ed. Ames, Iowa: Iowa State Univ Pr, 1987:62–73.