Abstract
Neointimal hyperplasia (NIH) in bypass conduits such as veins and prosthetic grafts is an important clinical entity that limits the long-term success of vascular interventions. Although the development of NIH in the conduits shares many of the same features of NIH that develops in native arteries after injury, vascular grafts are exposed to unique circumstances that predispose them to NIH, including surgical trauma related to vein handling, hemodynamic changes creating areas of low flow, and differences in biocompatibility between the conduit and the host environment. Multiple different approaches, including novel surgical techniques and targeted gene therapies, have been developed to target and prevent the causes of NIH. Recently, the PREVENT trials, the first molecular biology trials in vascular surgery aimed at preventing NIH, have failed to produce improved clinical outcomes, highlighting the incomplete knowledge of the pathways leading to NIH in vascular grafts. In this review, we aim to summarize the pathophysiologic pathways that underlie the formation of NIH in both vein and synthetic grafts and discuss current and potential mechanical and molecular approaches under investigation that may limit NIH in vascular grafts.
Keywords: edifoligide, fistula, gene therapy, heparin, neointimal hyperplasia, patch, vascular graft, vein
Neointimal hyperplasia (NIH) is an important clinical entity in vascular surgery because it limits the long-term effectiveness of surgical and endovascular interventions. NIH is usually defined in an artery as thickening of the intimal layer after an injury such as angioplasty, stenting or surgical repair. NIH is also used to describe the thickening of venous and prosthetic bypass grafts that leads to reduced lumen diameter and flow and, ultimately, graft occlusion and thrombosis. NIH affects all forms of vascular grafts, including both venous and prosthetic conduits used in coronary and peripheral arterial bypass, and arteriovenous fistulae (AVF) created for hemodialysis access. Since the 1970s, ‘intimal and neointimal fibrous proliferation’ has been recognized as a cause of restenosis and graft failure [1]. An estimated 30–60% of vascular grafts are complicated by clinically detectable NIH, with the incidence and clinical consequences varying depending on the type of graft. Despite the commonplace development of NIH, which is usually asymptomatic in its early forms, successful surgical bypass is associated with improved quality of life in patients with critical limb ischemia [2], potentially tempting patients to ignore surveillance of their bypass and miss the opportunity to diagnose and treat NIH in its early stages.
Although it is tempting to assume that the processes leading to NIH, and its treatment, are similar regardless of its location in the vascular system, new findings indicate this may not be true. Vascular grafts, be they venous or synthetic conduits used on peripheral bypass or AVF, are exposed to unique factors predisposing them to NIH that are not experienced by native arterial vessels. In this review, we focus on the histologic and pathophysiologic processes leading to NIH in peripheral bypass grafts, and its treatment.
Histology & pathophysiology of NIH
Arteries and veins are composed of three layers: the intima, media and adventitia; the media and adventitia are markedly thicker in arteries compared with veins. In the vein, the intima is formed by a single layer of squamous epithelium that rests atop an elastic basement membrane. The middle layer consists of a thin layer of smooth muscle cells (SMCs), whereas the outermost layer, the adventitia, is made up of connective tissue elements. In both arterial and venous NIH, there is fibroblast and SMC accumulation in the intima with extracellular matrix (ECM) deposition. The result of this excessive cellular deposition is expansion of the intimal layer, termed the ‘neointima,’ which is associated with loss of luminal area. Interestingly, the medial layer in arterial NIH tends to remain thin despite increased thickness of the intimal layer. This is in contrast to vein graft adaptation, where there is also concurrent expansion of the media [3]. The histologic changes observed in NIH are shown in Figures 1 & 2.
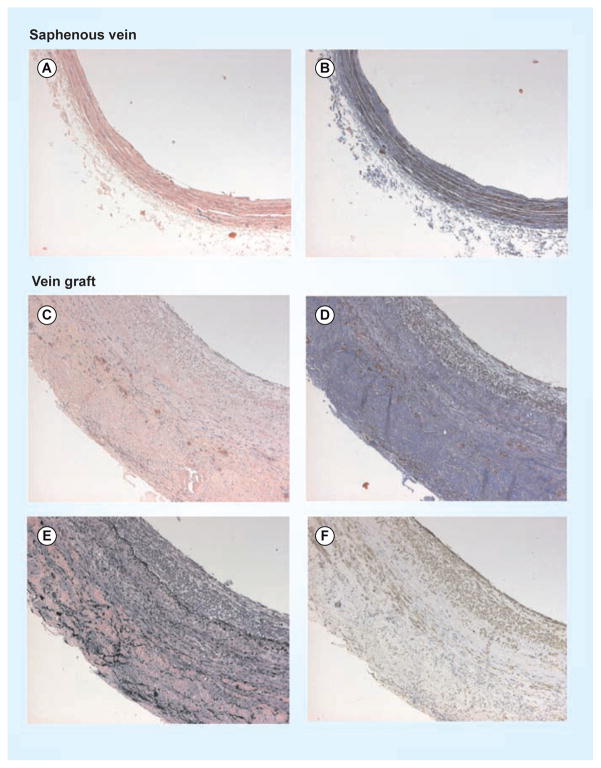
Figure 1. Representative histologic sections of human vein and vein graft.
(A) Human saphenous vein stained with hematoxylin and eosin stain and (B) Masson’s trichrome. In comparison, sections taken from a (C) representative patent human vein graft demonstrate neointimal hyperplasia shown by increased vein thickness (hematoxylin and eosin stain), (D) increased deposition of collagen (Masson’s trichrome) and (E) elastin (elastin stain), and (F) infiltration of smooth muscle cells (α-actin stain).
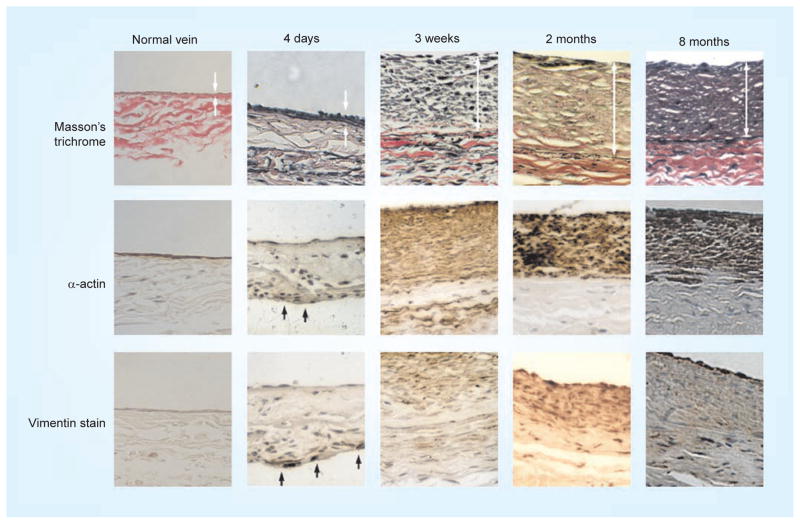
Figure 2. Time course of development of neointimal hyperplasia in a rabbit vein graft model.
Neointimal hyperplasia results in the development of a thickened intima (top row arrows) and the migration and accumulation of smooth muscle cells (middle row arrows) and fibroblasts (bottom row arrows).
The processes that regulate formation and expansion of NIH have been an intense focus of investigation. NIH lesions are the result of the individually complex processes of cell proliferation, cell migration and ECM deposition. SMCs in normal vessels are characterized by a low rate of proliferation and cell turnover, and differentiation into a mature phenotype that functions to contract and relax as needed by the function of the vessel. Under the pathologic conditions where NIH develops, the neointimal volume is composed of large numbers of SMCs, the origin of which is unclear. Several theories as to the origin of these neointimal SMCs have been proposed. First, proliferation and migration of SMCs requires a change in the SMC phenotype from a mature differentiated ‘contractile’ phenotype to a dedifferentiated ‘synthetic’ phenotype characterized by a high proliferation rate, increased cell migration and increased synthesis of cytoskeletal and contractile proteins [4]. These ‘synthetic’ dedifferentiated SMCs are one source of the active SMCs present within the NIH. The growth factors PDGF, EGF and FGF and other inflammatory cytokines such as IL-6 and IL-8 are found in endothelial cells, SMCs, macrophages and platelets, and are believed to be responsible for SMC activation, phenotype switching and proliferation, whereas IGF-1 may block this process. Vein grafts with significant NIH express increased levels of inflammatory cytokines [5,6], and Kenagy et al. reported that the response of cultured SMCs taken from vein grafts at the time of implantation to PDGF correlates with the later development of graft stenosis [7].
In addition to phenotype switching, it has also been proposed that fibroblasts residing in the adventitia are capable of transformation into ‘myofibroblasts’ that migrate through the media to become SMCs within the developing neointima [8]. A third theory is that bone marrow-derived circulating progenitor cells may hone to the site of vascular injury, incorporate into the vessel wall and differentiate into the endothelial cell, myofibroblast or synthetic SMC. In a mouse model of postangioplasty restenosis, these bone marrow-derived circulating progenitor cells contributed the majority of both endothelial cells and SMCs within the NIH lesion found after femoral artery angioplasty [9]. Other circulating cells, such as platelets and monocytes, may interact with the graft to ultimately determine the formation and composition of the neointima [6].
Signal transduction pathways that regulate cell proliferation, such as Ras–MAPK and PI3K–Akt, are also active in NIH. The MAPK pathway is a signal-amplification pathway involving multiple cascades of protein kinases that ultimately activate transcription factors, such as Elk-1 and Sap1, to stimulate proliferation, migration and differentiation. The Akt pathway is an insulin-sensitive cascade that responds to ambient nutrition levels and signals through the mTOR complex to stimulate cell growth, survival, proliferation and cell cycling [4]. Cell migration is promoted by VEGF, PDGF, basic FGF (bFGF), TGF-β, ECM components, angiotensin-II, thrombin and endothelin-1, which regulate downstream effects such as actin polymerization, focal intercellular and matrix adhesion changes, microtubule remodeling and myosin force generation. The Rho kinase G proteins Cdc42, Rac and Rho are intimately involved in actin filament formation, whereas focal adhesion kinase and Src are key regulators of the changes in adhesion molecules [10]. In addition to cell migration and proliferation, ECM production and deposition are important to neointimal formation. The growth factors PDGF and TGF-β, and angiotensin-II, alter the ECM components synthesized and secreted by SMCs, and bFGF decreases elastin production. ECM remodeling is driven by the balance of matrix synthesis and degradation and is regulated by matrix metalloproteinases (MMPs) and the MMP inhibitors tissue inhibitors of metalloproteinases and reversion-inducing cysteine-rich protein with kazal motifs [4].
Although the processes regulating NIH have been studied for decades, they remain complex processes that are incompletely understood. In in vivo lesions, cell migration and infiltration, cell proliferation and secretion of matrix-degrading enzymes are regulated by complex and overlapping endocrine, paracrine and juxtacrine signals. Although it is tempting to assume that a single critical pathway may be targeted for the therapeutic inhibition of NIH, the failure of the PREVENT trials, as detailed below, demonstrates that additional research is required to completely understand the molecular pathways regulating NIH in native vessels.
The processes that underlie NIH leading to failure of prosthetic grafts are less well understood. NIH immediately adjacent to the venous anastomoses of AVF have been well documented and share many of the same features described earlier [11,12]; however, relatively few studies have described the characteristics of NIH in the prosthetic grafts themselves. Histologically, the NIH found within explanted prosthetic grafts shows some features similar to NIH in arteries and is characterized by fibroblasts, ECM deposition and thrombi [13]; however, important differences exist. In a recent study of 48 polytetrafluoroethylene (PTFE) grafts, Mehta et al. also noted calcification present in the neointima and within the graft material itself, with the degree of calcification varying with the duration of graft implantation [14]. Although NIH in prosthetic grafts is most prominent at the anastomosis, it may occur at any point along the graft; it is unclear whether SMCs and fibroblasts present in the neointima of prosthetic grafts are the result of cellular migration from adjacent vessels or through the graft matrix, or whether these cells are seeded and expanded from circulating progenitor cells. The pathophysiologic changes leading to NIH are summarized in Figure 3.
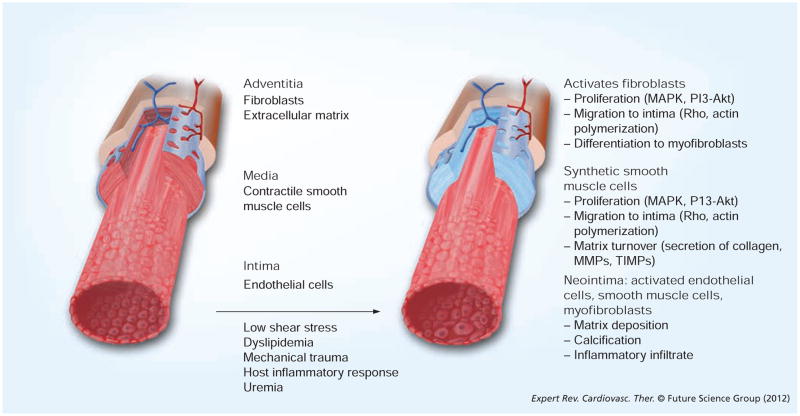
Figure 3. Summary of the cellular changes that occur in vein grafts associated with the development of neointimal hyperplasia.
MMP: Matrix metalloproteinase; TIMP: Tissue inhibitor of metalloproteinases.
NIH in vascular grafts
Although the pathophysiologic processes underlying NIH arteries after angioplasty and stenting have been well studied, there are important considerations for vascular grafts not experienced by arterial vessels that predispose these grafts to NIH. These include surgical trauma, mechanical forces at the anastomotic site and, in the case of prosthetic conduits, biocompatibility. In addition, the length and caliber of the harvested vein also affect vein graft patency, with grafts >50 cm and vein diameter <3.5 mm associated with reduced patency [15].
Vein harvesting for use as a conduit involves surgical trauma to the vein at several stages of the procedure. There is trauma directly related to handling of the vessel, a hypoxic period between the excision of vein and reperfusion after completion of the anastomoses, and loss of adventitial blood supply [16,17]. Manipulation of the vessel can lead to spasm, and resultant changes in the vessel’s responses to hemodynamic stresses probably contribute to NIH [18,19]. Ischemia time and mechanical instrumentation of the vein during harvest, especially during dilation, to check for leaks and unligated side branches results in endothelial disruption, thereby exposing the media to proinflammatory and procoagulant blood components [19]. Both warm saline and hydrostatic dilatation may be harmful to the vein, and this injury is manifested by the platelet coating of the endothelium for several days after graft preparation [20]. In addition, vein harvesting results in disruption of the adventitial blood supply and affects its nervous innervations by removal of the vein from its bed. The net result of surgical handling is vasospasm and inflammatory changes with release of cytokines that promote NIH [21]. Examination of vein grafts in a canine model showed significant changes after only 3–7 days of arterial flow, with inflammation of the subendothelium, media and adventitia, and fragmentation of myocytes with subendothelial hemorrhage and endothelial denudation [22].
Hypoxia experienced by the vein before reperfusion can also result in generation of free radicals that have the capacity to damage cell proteins, lipids and DNA via reactions with molecular oxygen. One consequence of increased free radicals is the upregulation of MMPs: MMPs degrade ECM proteins such as elastin and collagen that are present in the internal elastic lamina, thus allowing migration of SMCs from the media into the developing neointima. Conversely, the ability of MMPs to degrade the ECM may also facilitate beneficial outward remodeling and dilation of the injured arterial segment or the vein graft [23].
Hemodynamic forces, including shear stress and transmural pressure, also play an important role in the formation of NIH within vascular grafts. The creation of the new anastomosis changes the normal vessel hemodynamics because the newly created anastomosis inherently forms areas of turbulence and low shear stress, both at the site of the anastomosis and in the downstream venous segment [19]. These areas, which are similar to native vessel branch points, predispose the new anastomosis to NIH [24]. Loth et al. demonstrated an inverse relationship between wall shear stress and the development of NIH [25]. Unlike native vessels, in which the endothelium is the only vessel component directly exposed to the shear stress, the endothelium of vascular grafts is frequently denuded as described above, thereby directly exposing the underlying SMCs and fibroblasts to increased magnitudes of shear stress. In addition to the effects of shear stress, vein grafts and native vessels on the venous side of an AVF are exposed to arterial pressures and experience a ten fold increase in intramural wall stress from the increased luminal pressures [19]. Numerous studies have demonstrated that increased wall stress activates transcription factors involved in regulating PDGF and PDGF receptors and in interacting with MAPK and Akt pathways, all of which have been shown to play a role in the formation of NIH [26–29]. In the case of prosthetic grafts, these hemodynamic forces are complicated by compliance mismatch between artery and vein and the prosthetic material, which further alters hemodynamic forces and predisposes to the formation of NIH.
The interaction of prosthetic graft material with host tissue may intrinsically contribute to NIH. Histologic studies have demonstrated large numbers of inflammatory cells present near the anastomosis of polytetrafluoroethylene (PTFE) grafts, including the presence of giant cells [11,30]. In addition to expressing pro-inflammatory cytokines, these inflammatory cells have been found to express PDGF and VEGF [13]. Inflammation as a mechanism of NIH has been studied extensively in the context of maturation of AVF and arteriovenous grafts (AVG). Increased levels of inflammatory markers such as hsCRP, IL-6 and TNF-α have been found in patients with failed AVF compared with lower levels in patients with patent fistulae. The inflammatory reaction to an implanted prosthetic graft, such as PTFE or Dacron, stimulates macrophage infiltration and activation, leading to synthesis and secretion of bFGF and other inflammatory cytokines.
In addition to the above factors, grafts and fistulae created for dialysis access also experience unique conditions that predispose to NIH. Dialysis access grafts are of short length and have a wide diameter, high flow and low resistance outflow – as such, they should theoretically have improved patency compared with the typical long-length, narrow-diameter, lower-flow and high- resistance bypass used in the periphery. However, additional factors decrease the patency of dialysis access grafts. Most importantly, dialysis access grafts are placed in patients with uremia, who often also have increased circulating levels of inflammatory markers [31,32], and they are subject to frequent, repeated injury to the graft via the large-bore needlestick necessary for connection to the dialysis machine. Uremia is associated with a higher baseline level of circulating cytokines and, thus, is a state of chronic inflammation. Uremia has also been shown to be a predisposing factor for endothelial cell dysfunction and venous NIH, even before fistula creation or graft implantation [33]. It is likely that other metabolic derangements in the uremic patient also contribute to NIH. Other potential pathophysiologic mechanism for the high rate of NIH in AVG is the extremely high rate of blood flow in these grafts, resulting in turbulence and predisposition to anastomotic NIH. Compliance mismatch between the vein or artery and graft material is an additional factor that may predispose to NIH.
Although both AVF and AVG demonstrate high failure rates as a result of venous stenosis and venous NIH, they have different mechanisms of failure. AVF are also associated with perianastomotic stenosis that may lead to both early ‘failure to mature’ and late fistula failure. Venous stricture development and presence of accessory veins may also lead to AVF failure to mature [34]. In AVG, stenosis most commonly found at the graft–vein anastomosis and perianastomotic venous sites most frequently leads to graft failure [12].
Treatment of NIH
Graft surveillance
There are no strategies that are currently approved and used widely to prevent NIH. The best that clinicians can do currently is to detect and repair NIH as early as possible before graft failure. Graft surveillance is most commonly performed with Doppler ultrasound evaluation, most frequently within the first 2 years of the surgery, which is the most prevalent time of NIH formation. Early detection of nascent stenoses before manifestation as a thrombosed graft allows the possibility of early intervention and graft life extension. As with any preventative strategy, the cost–effectiveness of graft surveillance is dependent on proper patient selection – that is, to focus on which patients are most likely to develop NIH and clinically significant stenoses that lead to graft failure. One recent study showed no improvement in outcome for surveillance of prosthetic grafts or femoral tibial vein grafts, whereas above-knee popliteal and below-knee popliteal vein grafts did benefit from graft surveillance; interestingly, prosthetic graft occlusions were often not preceded by detectable lesions on ultrasound [35]. Another study reported that only those grafts with early ultrasound abnormalities, detected within the first month, or ‘high-risk’ factors, such as war-farin therapy, composite saphenous vein conduit or redo bypasses, benefited from long-term graft surveillance [36]. As our knowledge of early molecular mediators of NIH increases, surveillance and detection of molecular markers of NIH may provide increased accuracy and information regarding optimal treatment times.
Mechanical interventions
Once NIH develops in a bypass graft, both percutaneous and mechanical interventions may be used to attempt graft salvage. Angioplasty to treat NIH within vein grafts has been used, although early studies showed high restenosis rates as early as 6 months after the procedure [37,38]. Angioplasty is believed to injure the vein grafts in a similar manner to arterial angioplasty, but the resultant lesions can be more unstable. Histologic examination of vein grafts that have been treated with angioplasty show intimal thickening with fibrocollagenous tissue, and fibrotic medial and adventitial layers, at both early and late (>1 year) time points. As is the case with native arteries, late restenotic lesions after angioplasty showed changes consistent with recurrent atherosclerosis [39]. The decision to intervene with angioplasty must be weighed against the risk of graft rupture, especially in grafts >1 year old [40], although some reports have demonstrated safety in stenotic but not occluded vein grafts [41]. Although some studies show equivalent results of angioplasty compared with traditional surgical revision of the graft [42], the majority of studies show angioplasty to be inferior to surgical repair [43,44]. Recent studies have demonstrated the superiority of stent grafts compared with angioplasty alone in the treatment of hemodialysis graft stenosis [32].
The use of stents to treat NIH in peripheral bypass grafts has not been well studied, but they have been used more extensively in coronary bypass grafts. As with stent placement in the arterial circulation, stenting in vein grafts risks inducing further NIH with subsequent vein graft restenosis and occlusion. The use of bare-metal stents (BMS) has not been associated with a survival benefit and long-term, 5-years event-free survival after BMS placement is estimated to be only approximately 30% [45,46]. The early results with drug-eluting stents in cardiac bypass grafts are mixed, with some studies showing reduced rates of restenosis but no change in the rates of myocardial infarction or survival [47]. However, more recent results from the Reduction of Restenosis In Saphenous Vein Grafts with Cypher® sirolimus-eluting stent trial, which compared long-term outcomes for stenotic vein grafts treated with either sirolimus-eluting stents or BMSs, showed increased long-term mortality compared with patients treated with BMS, mainly due to sudden in-stent thrombosis [48]. Most recently, the use of cutting-balloon angioplasty has emerged as a viable option in the treatment of restenosis in infrainguinal bypass grafts, with studies demonstrating patency equivalent to surgical repair and superior to angioplasty alone [43,49].
Open surgical revision has remained the gold standard for the treatment of stenoses in bypass grafts. Surgical options include vein patch angioplasty, interposition grafting and jump grafting. Patch angioplasty has been shown to be especially effective in the treatment of focal lesions. In a study of 108 patients undergoing revision of infrainguinal vein bypass grafts, the 5-year patency was 84% after patch angioplasty, whereas the patency rates for interposition and jump grafts were 65 and 73%, respectively [50]. Overall patency rates after surgical correction are reported to be between 80 and 85% [51,52]. However, surgical revision has significant drawbacks, including the need for additional conduit and the technical challenges associated with operating in a re-do surgical field. Revision of tibial grafts compared with popliteal grafts and the need for surgical reintervention after <1 year have been associated with poor long-term outcomes [50]. Nonetheless, the overall excellent results of surgical revision have led to the commonplace practice of tunneling the vein graft subcutaneously, rather deeper in a subfascial anatomic plane, in anticipation of future surgical revisions.
An alternative approach to the treatment of NIH has focused on reducing SMC proliferation. Directed radiation has antiproliferative effects, and both endovascular and external-beam delivery have been tested. Brachytherapy has been used extensively in coronary lesion restenosis after BMS placement, with improved results over angioplasty or additional stenting [53]. However, recent studies have shown improved outcomes with sirolimus-eluting stents compared with brachytherapy at 3 years [54]. The BRAVO-I trial tested one dose of endovascular radiation delivered to failing dialysis-access grafts and showed improvement in target lesion revascularization, but not in overall patency at 6 or 12 months [55]. Peripheral bypass intravascular brachytherapy for restenosis has been examined in a limited manner, with studies showing no improvement in ankle–brachial index, clinical symptoms or angiographic restenosis with a 5-year follow-up, despite moderate improvement at 6 months [56,57]. Future therapies that are promising include temporary absorbable stents and drug-eluting balloons that can deliver drug therapy but not leave a permanent implant.
Prevention of NIH
Mechanical prevention strategies
Multiple different strategies have been used to prevent the formation of NIH in bypass grafts, including novel techniques of anastomosis aimed at reducing flow variation at the anastomosis and pharmacologic interventions targeting the molecular pathways involved in NIH. Because NIH lesion formation most frequently occurs at points of low wall-shear stress, surgeons have reasoned that alterations in the fluid mechanics of bypass grafts may be an attractive option to prevent the formation of NIH.
To this end, various boots, patches and cuffs have been introduced to limit compliance mismatch between prosthetic grafts and native arterial vessel. A randomized study of 235 peripheral grafts showed improved patency at 3 years in below-knee popliteal bypasses using a cuff, in which a cylindrical loop of vein was interposed between the distal end of the synthetic graft and the native artery. However, the rate of limb salvage was not improved [58]. Similarly, the Linton patch uses a vein patch, which is sewn into the arteriotomy of the distal anastomosis; the patch is then incised and the prosthetic bypass graft is sutured to it. Despite promising early results, large animal models have not shown a difference in the degree of NIH that ultimately forms [59]. A variation of this technique, the Taylor patch, uses a vein patch to expand the distal end of the graft, which is then sutured to an extended arteriotomy. Although some large animal studies have not shown a change in graft NIH [59], other animal and human studies have shown favorable results with increased patency rates at 2 years compared with absence of the patch [60,61]. The shapes of these patches with an expanded, trumpeted distal anastamosis led to the design and construction of the precuffed graft (Bard Peripheral Vascular, AZ, USA) [62]. Early results for popliteal and tibial bypasses with this graft have shown patency and limb salvage results at 1–2 years comparable to those of PTFE with the hand-sewn vein patch and improved results compared with unmodified PTFE grafts [63,64]. Somewhat paradoxically, other studies showed limited NIH in animal studies using nitinol meshes to constrict vein diameter, thereby limiting size mismatch between the vein and the vessel [65].
Creation of a distal arteriovenous anastomosis has also been attempted to improve outcomes of distal bypasses by increasing outflow and increasing shear stress, minimizing areas of low shear stress. Multiple techniques, including the common ostium, proximal or distal vein piggyback, vein interposition and remote and proximal side-to-side anastomosis variants, have been described [66–69]. Creation of distal AVF may provide improved patency and limb salvage compared with unmodified prosthetic grafts, but autogenous vein remains the best option for distal revascularization [70]. It is also possible that AVF may increase flow and turbulence at the anastomosis, paradoxically leading to late development of NIH.
Pharmacologic prevention strategies
No mechanical approaches to prevent NIH have adequately addressed the clinical problem. Thus, pharmacologic prevention of NIH has become an area of research of increasing interest. Antiplatelet agents have been shown to improve outcomes, including aspirin, dipyridamole, thromboxane A-synthase inhibitors, thienopyridines such as ticlopidine and clopidogrel, and glycoprotein IIb/IIIa inhibitors such as abciximab, eptifibatide and tirofiban. Aspirin and dipyridamole therapy significantly reduced graft occlusion rates at 1 year after coronary artery bypass surgery (CABG) in a randomized controlled trial [71], whereas dipyridamole, aspirin and clopidogrel have all been shown to improve patency in hemodialysis grafts [72,73].
Other anticoagulants, such as warfarin, heparin, and the heparinoids, have also been shown to improve patency. In the Dutch Bypass Oral Anticoagulants or Aspirin study, 2690 patients were randomized to receive aspirin or oral anticoagulation to an international normalized ratio (INR) of 3–4.5. The study found a 50% reduction in the incidence of vein graft occlusion in patients treated with oral anticoagulants, but a higher incidence of occlusion of prosthetic or composite grafts compared with patients treated with aspirin [74]. Patients treated with oral anticoagulants were also more likely to experience a significant hemorrhage with age >75 years, systolic blood pressure >140 mmHg and diabetes mellitus being the greatest risk factors for hemorrhage [75,76]. Subsequent analysis compared patients receiving oral anticoagulants at high risk of bleeding with patients with the same characteristics taking aspirin and found that aspirin treatment reduced the number of hemorrhages, but this benefit was outweighed by an increase in graft occlusion [76]. A low-molecular-weight heparin, dalteparin, was recently examined as a potential adjunctive therapy to femoral popliteal angioplasty and showed less restenosis at 6 months in patients with critical limb ischemia, although not in patients with claudication [77].
Other classes of drugs have also been investigated. mRNA levels of inflammatory cytokines, such as IL-β, TNF-α, IL-6 and IL-8, and IFN-γ are all present at 6–30-times higher levels in vein grafts compared with atherosclerotic carotid arteries [5], and it is therefore not surprising that anti-inflammatory drugs have been shown to be protective against development of NIH in bypass grafts. Investigated drugs include cyclosporine, dihydroepiandrosterone, rapamycin and dexamethasone (Figure 4). Newer drugs such as growth factor inhibitors (angiopeptin, the bFGF saporin and ornithine decarboxylase inhibitors) and insulin sensitizers (thiazolidinediones and MAPK inhibitors) are currently under investigation. Patients treated with angiopeptin showed less restenosis after angioplasty compared with control patients, but administration of the drug was by continuous infusion for 5 days, which is impractical for general clinical use [78]. Pioglitazone treatment has shown benefit in preventing coronary stent restenosis in small trials in both diabetic and nondiabetic patients [79]. Imatinib mesylate, a PDGF receptor inhibitor, has been show to suppress vein graft NIH in animal models [80].
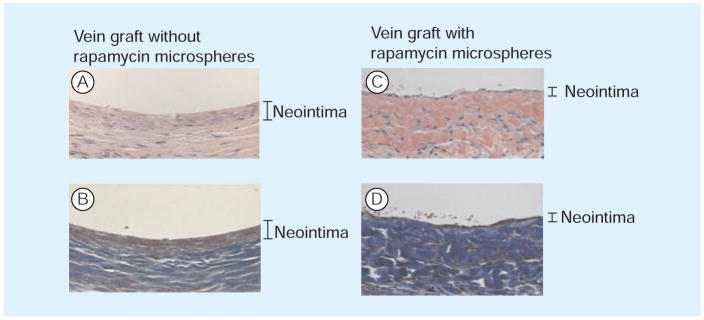
Figure 4. Rat vein grafts treated with or without rapamycin-infused microspheres.
(A & C) Hematoxylin and eosin; (B & D) Masson’s trichrome. Rapamycin significantly reduces neointimal hyperplasia in this model.
Experimental techniques in animal models have also targeted components of the inflammatory cascade to limit NIH. Inhibition of monocyte chemotactic protein-1 with antisense-laden nanoparticles significantly limited restenosis in carotid artery models [81]. Overexpression of the inhibitory peptide Nogo-B has been shown to limit SMC proliferation and reduced NIH formation in a porcine model [82]. Pharmacologic inhibition of cell proliferation and migration has also yielded promising results for limiting NIH in vein grafts. Treatment with FK778, an immunosuppressant that limits SMC proliferation, was found to limit vein graft adaptation in animal models [83]. Similarly, treatment of vein grafts with adenovirus leading to overexpression of phosphate and tensin homolog, a downstream effector of the PI3K pathway and an important regulator of cell proliferation, resulted in reduced intima–media ratio in canine coronary bypass grafts [84]. Similarly, the use of DNAzymes to inhibit downstream components of the Erk1/2 pathway, including c-Jun, also attenuated NIH [85].
Anti-thrombotic agents, such as heparin, hirudin and iloprost, have been used as potentially useful agents to reduce NIH in stents. Heparin-coated PTFE grafts (Gore, AZ, USA) are currently available for peripheral bypass, with initial studies showing excellent patency at 1–2 years [86]. Heparin-coated PTFE grafts have shown promise in dialysis access and with reduced graft thrombosis at 1 year compared with unmodified expanded PTFE grafts [87].
MMP inhibitors, including statins, have been associated with a decrease in NIH in both animal and in vitro models; other agents studied include collagen and ECM synthesis inhibitors such as halofuginone. Angiopeptin, an analog of somatostatin that has antiproliferative effects on SMCs, has been examined in drug-eluting stent form in animal models and early human studies, with mixed results [88,89].
Recently, interest in nitric oxide (NO) as a regulator of vein graft adaptation has led to attempts to increase NO production to limit NIH. Work in animal models has demonstrated that increased NO production can significantly limit vein graft thickening [90]. Trials using NO to limit NIH are currently underway, including the PATENT trial, which uses vein grafts bathed in a solution of nona-L-arginine to provide a sustained reservoir of L-arginine, the substrate for production of NO, thereby increasing NO production [91]. Finally, multiple studies on the use of dietary supplements, including fish oils and omega-3 fatty acids, have demonstrated improvements in blood flow and graft survival [92].
Gene therapies
Over the past decade, multiple gene therapies have been developed to target and inhibit cellular processes leading to the development of NIH, especially SMC proliferation and migration. Specific targets include the cell cycle inhibitors p53 and p21. There has also been intensive research on gene therapy to increase NO production to promote endothelialization and decrease NIH. In vitro and animal models have used adenoviral delivery of both endothelial NO synthase and VEGF-C to this effect. VEGF-C triggers NO and prostacyclin release, potentially reducing angioplasty-induced restenosis [93]. Liposomes and adenoviruses have been used as an alternate delivery mechanism of inducible NO synthase in some studies and have significantly limited NIH in animal models [90].
A unique characteristic of vein grafts is an obligate ex vivo period after harvesting, but before implantation, which allows time for treatment in the operating room. Ex vivo treatment of vein grafts is an attractive means to pharmacologically treat the vein in isolation from the rest of the patient, thus avoiding side effects associated with systemic administration; this method of treatment was used to deliver the E2F-peptide decoy in the PREVENT trials. Ex vivo topical inhibition of Erk1/2–MAPK with U0126 limits inflammatory infiltrates and vein graft thickening in animal models [94], whereas application of suramin, a PDGF receptor blocker, similarly inhibited Erk activation and limited SMC migration and proliferation [95]. Vein grafts pre-treated with adenoviruses are capable of locally overexpressing selected proteins to allow for selective activation or inhibition of key signaling pathways. Overexpression of TIMP-3, an inhibitor of MMPs, is able to limit SMC migration and NIH in large animal models [96]. Interestingly, ex vivo incubation of vein grafts with adenovirus encoding tissue plasminogen activator resulted in decreased incidence of flow-restricting thrombi without producing significant systemic bleeding [97]. Similarly, adenoviral overexpression of Nogo-B, which is capable of inducing SMC apoptosis via activation of the JNK/p38/MAPK pathway, may also limit SMC proliferation to prevent NIH [98].
The most well-studied ex vivo treatments involve the use of decoys for transcription factors involved in SMC proliferation and inflammation. Molecules investigated include E2F, NF-κb, AP-1, shear stress responsive element (SSRE), midkine and nuclear factor of activated T-cells (NF-AT). These decoys consist of synthesized short double-stranded deoxynucleotides that bear a specific consensus binding site for the targeted transcription factor. Once delivered into cells, these nucleotides serve as a competitive inhibitor to the transcription factor, blocking the activation of the transcription factor and transcription of its specific downstream genes. The PREVENT trials were the first molecular biology trials in vascular surgery testing the E2F transcription factor decoy, edifoligide. E2F coordinates the expression of several genes that regulate cell cycle progression, and inhibition of E2F prevents SMC proliferation. PREVENT III was a prospective, double-blind randomized controlled trial that randomized 1404 patients with critical limb ischemia to either surgical bypass with edifoligide-= or placebo-treated veins. However, edifoligide treatment did not confer any advantage in protection from reintervention for graft failure at 1 year [99]. PREVENT IV, a similar trial of edifoligide treatment for coronary artery bypass grafts, randomized 3000 patients but demonstrated no advantage for edifoligide [100]. Similar studies using edifoligide treatment for the prevention of NIH in dialysis grafts have been terminated after poor early results. These trial failures bring into question the prevention of NIH by inhibition of SMC proliferation alone and reinforce the complexity of NIH; the mechanisms involved in the formation of NIH may be activated by multiple pathways requiring various transcription factors, many of which have not yet been identified. In addition, it is possible that the single-dose application may not have delivered enough activity to be clinically useful.
Heme-oxygenase-1 is another potential target being considered for prevention of NIH and restenosis. Heme-oxygenase-1 is an inducible stress protein that metabolizes heme into carbon monoxide, biliverdin and free iron. Heme-oxygenase-1 serves as a ‘protective’ gene by virtue of the anti-inflammatory, antiapoptotic and antiproliferative actions of these products [101,102]. Angiogenesis and increased endothelialization have also been proposed as targets of gene therapy, with specific molecules such as prostacyclin-2, NO and C-type natriuretic peptide currently under examination. Angiogenic factors being studied include VEGF, bFGF, HGF and hypoxia-inducible factor. Endothelialization may also involve endothelial progenitor cell mobilization from the bone marrow and is another possible mechanism for intervention and reduction of NIH. VEGF, statins, erythropoietin, granulocyte colony-stimulating factor and MMP-9 have all been implicated in endothelial progenitor cell mobilization [103].
Delivery of new potential therapies
An older strategy to prevent NIH in bypass grafts by increasing endothelialization is direct endothelial cell transplantation onto the luminal surface of grafts [104]. In vitro coating of PTFE grafts with autologous endothelial cells has been performed with primary patency of 62.8% at 7 years in 6-mm grafts [105]. However, the techniques involved for this treatment have proven difficult and time consuming, with a complex cell culture process and months of advance planning preventing routine clinical use. Another newer strategy has been to apply endothelial cells to the adventitial surface of grafts, capitalizing on the ability of endothelial cells to secrete multiple growth regulatory proteins, such as FGF-2 and heparan sulfate, to prevent abnormal SMC proliferation. Endothelial cell-loaded gel foam wraps for grafts have been trialed in animal studies [106,107], and early clinical trials are underway with positive results [108].
The PTFE graft itself is another possible delivery platform for various compounds, as noted earlier regarding heparin-bonded grafts. Another interesting example is NO-releasing grafts [109]. Use of NO as a strategy to prevent NIH has been facilitated by delivery of NO via nanoparticle gels that release NO for up to 4 days. Animal studies with one of these gels, PROLI/NO, placed onto carotid arteries resulted in a 77% decrease in NIH compared with control arteries and decreased vessel inflammation [110]. In a similar concept, vein grafts coated with pluronic gel containing siRNA against c-myc [111] and Toll-like receptor 4 [112] successfully limited SMC proliferation and NIH formation in small animal models.
The use of an external drug-impregnated mesh wrap may also represent a potential new mechanism for localized drug delivery. Early large animal models in which a paclitaxel-impregnated biodegradable mesh was wrapped around the distal anastomosis of a PTFE arteriovenous fistula significantly reduced NIH [113]. Multicenter human trials using a similar system were initiated but terminated early owing to increased infection in the treatment group compared with patients receiving a PTFE arteriovenous fistula alone.
Conclusion
NIH in peripheral bypass grafts limits their long-term effectiveness. NIH in vein grafts shares many of the same histologic and pathophysiologic characteristics as NIH in native arteries. However, vascular grafts are exposed to unique factors, such as surgical trauma, hemodynamic disturbances, and differences in biocompatibility between the graft and the native vessel. In many cases, the site of NIH is predictable, occurring at the site of the anastomosis and areas of low shear stress. Although our understanding of the pathophysiologic pathways is improving, therapies that specifically target these pathways to prevent NIH, such as the PREVENT trial, have yielded disappointing results. Despite these early disappointing results, a number of new therapies targeting multiple pathways of NIH, delivered through new technologies, are currently underway.
Expert commentary
NIH in vascular grafts remains a challenging clinical problem that limits the long-term success of surgical revascularization. Surgeons have creatively devised several mechanical strategies, such as the use of patches and cuffs or the creation of AVF, to delay NIH, but these strategies are limited because they seem to only delay, not prevent, clinical disease. Treatment of NIH with surgical revision of the graft and minimally invasive options, such as angioplasty and stenting, have their own morbidity and secondary incidence of NIH – that is, restenosis. Therefore, there is intense interest in the development of pharmacologic strategies to prevent NIH in vascular grafts. The disappointing failure of the PREVENT trials, which used ex vivo treatment of preimplantation veins with edifoligide, an E2F transcription factor decoy, shows that our knowledge of the relevant pathophysiology is incomplete.
Five-year view
Clinical translation of current basic science research will probably continue to use delivery systems that capitalize on the surgical time in the operating room, after vein harvest but before implantation, during which high-dose treatment can be delivered locally to the vein without systemic effects. Targeting a single downstream pathway, as done in the failed PREVENT trials, is likely to achieve temporary results at best, because the host response to the vascular graft, whether vein or prosthetic, is likely to induce secondary pathways that ultimately promote NIH. Targeting multiple pathways, or more upstream ‘master’ pathways, may be another strategy, although it is unclear whether this strategy is superior to others. Identification of patient-specific factors that predict graft failure, probably based on individual genetic variation, may provide therapeutic strategies based on personalized medicine.
Key issues.
N eointimal hyperplasia (NIH) is the result of the individually complex processes of cell migration, cell proliferation and extracellular matrix deposition. These processes are regulated by overlapping endocrine, paracrine and juxtacrine signals and cytokines.
Many of the underlying processes leading to the formation of NIH in vascular grafts are similar to NIH that occurs after arterial injury.
Unique factors that predispose vascular grafts to NIH include trauma to the vein as a result of vein harvesting and loss of adventitial blood supply, and ischemia before implantation that may damage the venous endothelium. Veins implanted into the arterial circulation experience new hemodynamic forces, including increased shear stress and wall stress. Interaction of prosthetic foreign graft material with host tissue also contributes to NIH. Currently unknown factors, such as host genetic contributions, are likely to also play a role.
Mechanical strategies to prevent and treat NIH include the use of patches and cuffs to limit compliance mismatch between the native vessel and the conduit, and the creation of distal arteriovenous fistulae to improve flow. The use of angioplasty and stenting to treat NIH in vascular grafts remains controversial, with the majority of studies showing high recurrence compared with surgical revision.
As surgically harvested veins have an ex vivo period of time before implantation, there is the potential to pharmacologically treat the vein in isolation from the rest of the patient, increasing potency and minimizing side effects. Pretreatment with pharmacologic or molecular inhibitors, gene therapy with transcription inhibitors or adenoviruses, and drug-eluting meshes and conduits are all potential therapeutic options.
Recent work has focused on identifying key molecular pathways that can be manipulated to prevent NIH. However, the failure of the PREVENT trials shows that our knowledge of the mechanisms regulating NIH is still incomplete. Several promising preclinical studies have identified other potential targets that could be the focus of new trials.
Acknowledgments
The authors appreciate the help of Drs Akimasa Yamashita and Fabio Kudo in creating and staining the specimens in Figures 2 and 4.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
This work was supported by NIH grant R01-HL095498, the American Vascular Association William J von Liebig Award, and the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Imparato AM, Bracco A, Kim GE, Zeff R. Intimal and neointimal fibrous proliferation causing failure of arterial reconstructions. Surgery. 1972;72(6):1007–1017. [PubMed] [Google Scholar]
- 2.Nguyen LL, Moneta GL, Conte MS, Bandyk DF, Clowes AW, Seely BL PREVENT III Investigators. Prospective multicenter study of quality of life before and after lower extremity vein bypass in 1404 patients with critical limb ischemia. J Vasc Surg. 2006;44(5):977–983. doi: 10.1016/j.jvs.2006.07.015. discussion 983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muto A, Model L, Ziegler K, Eghbalieh SD, Dardik A. Mechanisms of vein graft adaptation to the arterial circulation: insights into the neointimal algorithm and management strategies. Circ J. 2010;74(8):1501–1512. doi: 10.1253/circj.cj-10-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muto A, Fitzgerald TN, Pimiento JM, et al. Smooth muscle cell signal transduction: implications of vascular biology for vascular surgeons. J Vasc Surg. 2007;45(Suppl A):A15–A24. doi: 10.1016/j.jvs.2007.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christiansen JF, Hartwig D, Bechtel JF, et al. Diseased vein grafts express elevated inflammatory cytokine levels compared with atherosclerotic coronary arteries. Ann Thorac Surg. 2004;77(5):1575–1579. doi: 10.1016/j.athoracsur.2003.10.107. [DOI] [PubMed] [Google Scholar]
- 6.Moreno K, Murray-Wijelath J, Yagi M, et al. Circulating inflammatory cells are associated with vein graft stenosis. J Vasc Surg. 2011;54(4):1124–1130. doi: 10.1016/j.jvs.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenagy RD, Fukai N, Min SK, Jalikis F, Kohler TR, Clowes AW. Proliferative capacity of vein graft smooth muscle cells and fibroblasts in vitro correlates with graft stenosis. J Vasc Surg. 2009;49(5):1282–1288. doi: 10.1016/j.jvs.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy-Chaudhury P, Wang Y, Krishnamoorthy M, et al. Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol Dial Transplant. 2009;24(9):2786–2791. doi: 10.1093/ndt/gfn708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sata M, Saiura A, Kunisato A, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8(4):403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 10.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res. 2007;100(5):607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- 11.Roy-Chaudhury P, Kelly BS, Miller MA, et al. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001;59(6):2325–2334. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee T, Roy-Chaudhury P. Advances and new frontiers in the pathophysiology of venous neointimal hyperplasia and dialysis access stenosis. Adv Chronic Kidney Dis. 2009;16(5):329–338. doi: 10.1053/j.ackd.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy-Chaudhury P, Arend L, Zhang J, et al. Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis. 2007;50(5):782–790. doi: 10.1053/j.ajkd.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Mehta RI, Mukherjee AK, Patterson TD, Fishbein MC. Pathology of explanted polytetrafluoroethylene vascular grafts. Cardiovasc Pathol. 2011;20(4):213–221. doi: 10.1016/j.carpath.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Schanzer A, Hevelone N, Owens CD, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg. 2007;46(6):1180–1190. doi: 10.1016/j.jvs.2007.08.033. discussion 1190. [DOI] [PubMed] [Google Scholar]
- 16.McCabe M, Cunningham GJ, Wyatt AP, Rothnie NG, Taylor GW. A histological and histochemical examination of autogenous vein grafts. Br J Surg. 1967;54(2):147–155. doi: 10.1002/bjs.1800540216. [DOI] [PubMed] [Google Scholar]
- 17.Wyatt AP, Taylor GW. Vein grafts: changes in the endothelium of autogenous free vein grafts used as arterial replacements. Br J Surg. 1966;53(11):943–947. doi: 10.1002/bjs.1800531107. [DOI] [PubMed] [Google Scholar]
- 18.Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006;84(2):115–124. doi: 10.1111/j.1440-1711.2005.01407.x. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Terry CM, Shiu YT, Cheung AK. Neointimal hyperplasia associated with synthetic hemodialysis grafts. Kidney Int. 2008;74(10):1247–1261. doi: 10.1038/ki.2008.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LoGerfo FW, Corson JD, Mannick JA. Improved results with femoropopliteal vein grafts for limb salvage. Arch Surg. 1977;112(5):567–570. doi: 10.1001/archsurg.1977.01370050027004. [DOI] [PubMed] [Google Scholar]
- 21.McGeachie JK, Meagher S, Prendergast FJ. Vein-to-artery grafts: the long-term development of neointimal hyperplasia and its relationship to vasa vasorum and sympathetic innervation. Aust NZ J Surg. 1989;59(1):59–65. doi: 10.1111/j.1445-2197.1989.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 22.Ramos JR, Berger K, Mansfield PB, Sauvage LR. Histologic fate and endothelial changes of distended and nondistended vein grafts. Ann Surg. 1976;183(3):205–228. doi: 10.1097/00000658-197603000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90(3):251–262. [PubMed] [Google Scholar]
- 24.Davies PF, Polacek DC, Shi C, Helmke BP. The convergence of haemodynamics, genomics, and endothelial structure in studies of the focal origin of atherosclerosis. Biorheology. 2002;39(3–4):299–306. [PMC free article] [PubMed] [Google Scholar]
- 25.Loth F, Jones SA, Zarins CK, et al. Relative contribution of wall shear stress and injury in experimental intimal thickening at PTFE end-to-side arterial anastomoses. J Biomech Eng. 2002;124(1):44–51. doi: 10.1115/1.1428554. [DOI] [PubMed] [Google Scholar]
- 26.Ma YH, Ling S, Ives HE. Mechanical strain increases PDGF-B and PDGF β receptor expression in vascular smooth muscle cells. Biochem Biophys Res Commun. 1999;265(2):606–610. doi: 10.1006/bbrc.1999.1718. [DOI] [PubMed] [Google Scholar]
- 27.Chen AH, Gortler DS, Kilaru S, Araim O, Frangos SG, Sumpio BE. Cyclic strain activates the pro-survival Akt protein kinase in bovine aortic smooth muscle cells. Surgery. 2001;130(2):378–381. doi: 10.1067/msy.2001.116668. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Hu Y, Mayr M, Xu Q. Cyclic strain stress-induced mitogen-activated protein kinase (MAPK) phosphatase 1 expression in vascular smooth muscle cells is regulated by Ras/Rac–MAPK pathways. J Biol Chem. 1999;274(36):25273–25280. doi: 10.1074/jbc.274.36.25273. [DOI] [PubMed] [Google Scholar]
- 29.Zhang BC, Zhou ZW, Li XK, Xu YW. PI-3K/AKT signal pathway modulates vascular smooth muscle cells migration under cyclic mechanical strain. VASA. 2011;40(2):109–116. doi: 10.1024/0301-1526/a000080. [DOI] [PubMed] [Google Scholar]
- 30.Swedberg SH, Brown BG, Sigley R, Wight TN, Gordon D, Nicholls SC. Intimal fibromuscular hyperplasia at the venous anastomosis of PTFE grafts in hemodialysis patients. Clinical, immunocytochemical, light and electron microscopic assessment. Circulation. 1989;80(6):1726–1736. doi: 10.1161/01.cir.80.6.1726. [DOI] [PubMed] [Google Scholar]
- 31.Wasse H, Rivera AA, Huang R, et al. Increased plasma chymase concentration and mast cell chymase expression in venous neointimal lesions of patients with CKD and ESRD. Semin Dial. 2011;24(6):688–693. doi: 10.1111/j.1525-139X.2011.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee T, Chauhan V, Krishnamoorthy M, et al. Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant. 2011;26(7):2264–2270. doi: 10.1093/ndt/gfq733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17(4):1112–1127. doi: 10.1681/ASN.2005050615. [DOI] [PubMed] [Google Scholar]
- 34.Nassar GM. Endovascular management of the ‘failing to mature’ arteriovenous fistula. Tech Vasc Interv Radiol. 2008;11(3):175–180. doi: 10.1053/j.tvir.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Carter A, Murphy MO, Halka AT, et al. The natural history of stenoses within lower limb arterial bypass grafts using a graft surveillance program. Ann Vasc Surg. 2007;21(6):695–703. doi: 10.1016/j.avsg.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Tinder CN, Chavanpun JP, Bandyk DF, et al. Efficacy of duplex ultrasound surveillance after infrainguinal vein bypass may be enhanced by identification of characteristics predictive of graft stenosis development. J Vasc Surg. 2008;48(3):613–618. doi: 10.1016/j.jvs.2008.04.053. [DOI] [PubMed] [Google Scholar]
- 37.Grüntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary-artery stenosis: percutaneous transluminal coronary angioplasty. N Engl J Med. 1979;301(2):61–68. doi: 10.1056/NEJM197907123010201. [DOI] [PubMed] [Google Scholar]
- 38.Douglas JS, Jr, Gruentzig AR, King SB, 3rd, et al. Percutaneous transluminal coronary angioplasty in patients with prior coronary bypass surgery. J Am Coll Cardiol. 1983;2(4):745–754. doi: 10.1016/s0735-1097(83)80315-5. [DOI] [PubMed] [Google Scholar]
- 39.Waller BF, Orr CM, Van Tassel J, et al. Coronary artery and saphenous vein graft remodeling: a review of histologic findings after various interventional procedures –part V. Clin Cardiol. 1997;20(1):67–74. doi: 10.1002/clc.4960200114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saber RS, Edwards WD, Bailey KR, McGovern TW, Schwartz RS, Holmes DR., Jr Coronary embolization after balloon angioplasty or thrombolytic therapy: an autopsy study of 32 cases. J Am Coll Cardiol. 1993;22(5):1283–1288. doi: 10.1016/0735-1097(93)90531-5. [DOI] [PubMed] [Google Scholar]
- 41.de Feyter PJ, van Suylen RJ, de Jaegere PP, Topol EJ, Serruys PW. Balloon angioplasty for the treatment of lesions in saphenous vein bypass grafts. J Am Coll Cardiol. 1993;21(7):1539–1549. doi: 10.1016/0735-1097(93)90366-9. [DOI] [PubMed] [Google Scholar]
- 42.Avino AJ, Bandyk DF, Gonsalves AJ, et al. Surgical and endovascular intervention for infrainguinal vein graft stenosis. J Vasc Surg. 1999;29(1):60–70. doi: 10.1016/s0741-5214(99)70361-7. discussion 70. [DOI] [PubMed] [Google Scholar]
- 43.Schneider PA, Caps MT, Nelken N. Infrainguinal vein graft stenosis: cutting balloon angioplasty as the first-line treatment of choice. J Vasc Surg. 2008;47(5):960–966. doi: 10.1016/j.jvs.2007.12.035. discussion 966. [DOI] [PubMed] [Google Scholar]
- 44.Berceli SA, Hevelone ND, Lipsitz SR, et al. Surgical and endovascular revision of infrainguinal vein bypass grafts: analysis of midterm outcomes from the PREVENT III trial. J Vasc Surg. 2007;46(6):1173–1179. doi: 10.1016/j.jvs.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 45.Keeley EC, Velez CA, O’Neill WW, Safian RD. Long-term clinical outcome and predictors of major adverse cardiac events after percutaneous interventions on saphenous vein grafts. J Am Coll Cardiol. 2001;38(3):659–665. doi: 10.1016/s0735-1097(01)01420-6. [DOI] [PubMed] [Google Scholar]
- 46.de Jaegere PP, van Domburg RT, Feyter PJ, et al. Long-term clinical outcome after stent implantation in saphenous vein grafts. J Am Coll Cardiol. 1996;28(1):89–96. doi: 10.1016/0735-1097(96)00104-0. [DOI] [PubMed] [Google Scholar]
- 47•.Brilakis ES, Lichtenwalter C, de Lemos JA, et al. A randomized controlled trial of a paclitaxel-eluting stent versus a similar bare-metal stent in saphenous vein graft lesions the SOS (Stenting of Saphenous Vein Grafts) trial. J Am Coll Cardiol. 2009;53(11):919–928. doi: 10.1016/j.jacc.2008.11.029. Important randomized controlled trial (RCT) that demonstrated the superiority of drug-eluting stents in the treatment of stenotic bypass grafts. [DOI] [PubMed] [Google Scholar]
- 48•.Vermeersch P, Agostoni P, Verheye S, et al. DELAYED RRISC (Death and Events at Long-term follow-up AnalYsis: Extended Duration of the Reduction of Restenosis In Saphenous vein grafts with Cypher stent) Investigators. Increased late mortality after sirolimus-eluting stents versus bare-metal stents in diseased saphenous vein grafts: results from the randomized DELAYED RRISC Trial. J Am Coll Cardiol. 2007;50(3):261–267. doi: 10.1016/j.jacc.2007.05.010. RCT showing bare metal stents were associated with lower long-term mortality compared with drug-eluting stents, but that the early reduction in repeated revascularization procedures seen with drug-eluting stents was lost at longer-term follow-up. [DOI] [PubMed] [Google Scholar]
- 49.Vikram R, Ross RA, Bhat R, et al. Cutting balloon angioplasty versus standard balloon angioplasty for failing infra-inguinal vein grafts: comparative study of short- and mid-term primary patency rates. Cardiovasc Intervent Radiol. 2007;30(4):607–610. doi: 10.1007/s00270-007-9005-x. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen LL, Conte MS, Menard MT, et al. Infrainguinal vein bypass graft revision: factors affecting long-term outcome. J Vasc Surg. 2004;40(5):916–923. doi: 10.1016/j.jvs.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 51.Landry GJ, Moneta GL, Taylor LM, Jr, Edwards JM, Yeager RA, Porter JM. Long-term outcome of revised lower-extremity bypass grafts. J Vasc Surg. 2002;35(1):56–62. discussion 62. [PubMed] [Google Scholar]
- 52••.Bandyk DF, Bergamini TM, Towne JB, Schmitt DD, Seabrook GR. Durability of vein graft revision: the outcome of secondary procedures. J Vasc Surg. 1991;13(2):200–208. doi: 10.1067/mva.1991.26224. discussion 209. Original paper describing the techniques and outcomes for revision of stenosed vein grafts. [DOI] [PubMed] [Google Scholar]
- 53.Waksman R, Ajani AE, White RL, et al. Intravascular gamma radiation for in-stent restenosis in saphenous-vein bypass grafts. N Engl J Med. 2002;346(16):1194–1199. doi: 10.1056/NEJMoa012579. [DOI] [PubMed] [Google Scholar]
- 54.Holmes DR, Jr, Teirstein PS, Satler L, et al. SISR Investigators. 3-year follow-up of the SISR (Sirolimus-Eluting Stents Versus Vascular Brachytherapy for In-Stent Restenosis) trial. JACC Cardiovasc Interv. 2008;1(4):439–448. doi: 10.1016/j.jcin.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 55.Misra S, Bonan R, Pflederer T, Roy-Chaudhury P BRAVO I Investigators. BRAVO I: a pilot study of vascular brachytherapy in polytetrafluoroethylene dialysis access grafts. Kidney Int. 2006;70(11):2006–2013. doi: 10.1038/sj.ki.5001869. [DOI] [PubMed] [Google Scholar]
- 56.Hansrani M, Overbeck K, Smout J, Stansby G. Intravascular brachytherapy for peripheral vascular disease. Cochrane Database Syst Rev. 2002;4:CD003504. doi: 10.1002/14651858.CD003504. [DOI] [PubMed] [Google Scholar]
- 57.Diehm N, Silvestro A, Do DD, et al. Endovascular brachytherapy after femoropopliteal balloon angioplasty fails to show robust clinical benefit over time. J Endovasc Ther. 2005;12(6):723–730. doi: 10.1583/05-1583MR.1. [DOI] [PubMed] [Google Scholar]
- 58.Griffiths GD, Nagy J, Black D, Stonebridge PA. Randomized clinical trial of distal anastomotic interposition vein cuff in infrainguinal polytetrafluoroethylene bypass grafting. Br J Surg. 2004;91(5):560–562. doi: 10.1002/bjs.4501. [DOI] [PubMed] [Google Scholar]
- 59.Trubel W, Schima H, Czerny M, Perktold K, Schimek MG, Polterauer P. Experimental comparison of four methods of end-to-side anastomosis with expanded polytetrafluoroethylene. Br J Surg. 2004;91(2):159–167. doi: 10.1002/bjs.4388. [DOI] [PubMed] [Google Scholar]
- 60.Gentile AT, Mills JL, Gooden MA, et al. Vein patching reduces neointimal thickening associated with prosthetic graft implantation. Am J Surg. 1998;176(6):601–607. doi: 10.1016/s0002-9610(98)00286-4. [DOI] [PubMed] [Google Scholar]
- 61.Yeung KK, Mills JL, Sr, Hughes JD, Berman SS, Gentile AT, Westerband A. Improved patency of infrainguinal polytetrafluoroethylene bypass grafts using a distal Taylor vein patch. Am J Surg. 2001;182(6):578–583. doi: 10.1016/s0002-9610(01)00791-7. [DOI] [PubMed] [Google Scholar]
- 62.Tsoulfas G, Hertl M, Ko DS, et al. Long-term outcome of a cuffed expanded PTFE graft for hemodialysis vascular access. World J Surg. 2008;32(8):1827–1831. doi: 10.1007/s00268-008-9514-z. [DOI] [PubMed] [Google Scholar]
- 63.Panneton JM, Hollier LH, Hofer JM. Multicenter randomized prospective trial comparing a pre-cuffed polytetrafluoroethylene graft to a vein cuffed polytetrafluoroethylene graft for infragenicular arterial bypass. Ann Vasc Surg. 2004;18(2):199–206. doi: 10.1007/s10016-004-0012-y. [DOI] [PubMed] [Google Scholar]
- 64.Gulkarov I, Malik R, Yakubov R, et al. Early results for below-knee bypasses using Distaflo. Vasc Endovascular Surg. 2008;42(6):561–566. doi: 10.1177/1538574408322659. [DOI] [PubMed] [Google Scholar]
- 65.Zilla P, Human P, Wolf M, et al. Constrictive external nitinol meshes inhibit vein graft intimal hyperplasia in nonhuman primates. J Thorac Cardiovasc Surg. 2008;136(3):717–725. doi: 10.1016/j.jtcvs.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 66•.Ibrahim IM, Sussman B, Dardik I, et al. Adjunctive arteriovenous fistula with tibial and peroneal reconstruction for limb salvage. Am J Surg. 1980;140(2):246–251. doi: 10.1016/0002-9610(80)90016-1. Description of the benefits of improved outflow through the creation of a distal arteriovenous fistula. [DOI] [PubMed] [Google Scholar]
- 67.Dardik H, Silvestri F, Alasio T, et al. Improved method to create the common ostium variant of the distal arteriovenous fistula for enhancing crural prosthetic graft patency. J Vasc Surg. 1996;24(2):240–248. doi: 10.1016/s0741-5214(96)70099-x. [DOI] [PubMed] [Google Scholar]
- 68.Dardik H, Sussman B, Ibrahim IM, et al. Distal arteriovenous fistula as an adjunct to maintaining arterial and graft patency for limb salvage. Surgery. 1983;94(3):478–486. [PubMed] [Google Scholar]
- 69.Miller N, Dardik H, Wolodiger F, Sussman B, Kahn M, Ibrahim IM. Dual function of the distal arteriovenous fistula for maintenance of arterial and venous prosthetic graft patency in the lower extremity. J Cardiovasc Surg. 1989;30(2):225–229. [PubMed] [Google Scholar]
- 70.Kreienberg PB, Darling RC, 3rd, Chang BB, Paty PS, Lloyd WE, Shah DM. Adjunctive techniques to improve patency of distal prosthetic bypass grafts: polytetrafluoroethylene with remote arteriovenous fistulae versus vein cuffs. J Vasc Surg. 2000;31(4):696–701. doi: 10.1067/mva.2000.104597. [DOI] [PubMed] [Google Scholar]
- 71.Fuster V, Chesebro JH. Role of platelets and platelet inhibitors in aortocoronary artery vein-graft disease. Circulation. 1986;73(2):227–232. doi: 10.1161/01.cir.73.2.227. [DOI] [PubMed] [Google Scholar]
- 72.Dixon BS, Beck GJ, Vazquez MA, et al. DAC Study Group. Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med. 2009;360(21):2191–2201. doi: 10.1056/NEJMoa0805840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dember LM, Beck GJ, Allon M, et al. Dialysis Access Consortium Study Group. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA. 2008;299(18):2164–2171. doi: 10.1001/jama.299.18.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74••.Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral Anticoagulants or Aspirin Study): a randomised trial. Lancet. 2000;355(9201):346–351. RCT describing the benefits of coumadin therapy to maintain graft patency in postoperative patients. [PubMed] [Google Scholar]
- 75.Anand SS. Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (the Dutch Bypass Oral anticoagulants or Aspirin study) Lancet. 2000;355:346–351. [PubMed] [Google Scholar]
- 76.Ariesen MJ, Tangelder MJ, Lawson JA, Eikelboom BC, Grobbee DE, Algra A Dutch Bypass Oral Anticoagulants or Aspirin Study Group. Risk of major haemorrhage in patients after infrainguinal venous bypass surgery: therapeutic consequences? The Dutch BOA (Bypass Oral Anticoagulants or Aspirin) Study. Eur J Vasc Endovasc Surg. 2005;30(2):154–159. doi: 10.1016/j.ejvs.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 77.Koppensteiner R, Spring S, Amann-Vesti BR, et al. Low-molecular-weight heparin for prevention of restenosis after femoropopliteal percutaneous transluminal angioplasty: a randomized controlled trial. J Vasc Surg. 2006;44(6):1247–1253. doi: 10.1016/j.jvs.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 78.Eriksen UH, Amtorp O, Bagger JP, et al. Randomized double-blind Scandinavian trial of angiopeptin versus placebo for the prevention of clinical events and restenosis after coronary balloon angioplasty. Am Heart J. 1995;130(1):1–8. doi: 10.1016/0002-8703(95)90227-9. [DOI] [PubMed] [Google Scholar]
- 79.Katayama T, Ueba H, Tsuboi K, et al. Reduction of neointimal hyperplasia after coronary stenting by pioglitazone in nondiabetic patients with metabolic syndrome. Am Heart J. 2007;153(5):762.e1–762.e7. doi: 10.1016/j.ahj.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 80.Kimura S, Egashira K, Nakano K, et al. Local delivery of imatinib mesylate (STI571)-incorporated nanoparticle ex vivo suppresses vein graft neointima formation. Circulation. 2008;118(14 Suppl):S65–S70. doi: 10.1161/CIRCULATIONAHA.107.740613. [DOI] [PubMed] [Google Scholar]
- 81.Yang J, Zeng Y, Li Y, et al. Intravascular site-specific delivery of a therapeutic antisense for the inhibition of restenosis. Eur J Pharm Sci. 2008;35(5):427–434. doi: 10.1016/j.ejps.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 82.Kritz AB, Yu J, Wright PL, et al. In vivo modulation of Nogo-B attenuates neointima formation. Mol Ther. 2008;16(11):1798–1804. doi: 10.1038/mt.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Graaf R, Kloppenburg G, Tintu A, et al. The new immunosuppressive agent FK778 attenuates neointima formation in an experimental venous bypass graft model. Vascul Pharmacol. 2009;50(3–4):83–88. doi: 10.1016/j.vph.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 84.Hata JA, Petrofski JA, Schroder JN, et al. Modulation of phosphatidylinositol 3-kinase signaling reduces intimal hyperplasia in aortocoronary saphenous vein grafts. J Thorac Cardiovasc Surg. 2005;129(6):1405–1413. doi: 10.1016/j.jtcvs.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 85.Ni J, Waldman A, Khachigian LM. c-Jun regulates shear- and injury-inducible Egr-1 expression, vein graft stenosis after autologous end-to-side transplantation in rabbits, and intimal hyperplasia in human saphenous veins. J Biol Chem. 2010;285(6):4038–4048. doi: 10.1074/jbc.M109.078345. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Daenens K, Schepers S, Fourneau I, Houthoofd S, Nevelsteen A. Heparin-bonded ePTFE grafts compared with vein grafts in femoropopliteal and femorocrural bypasses: 1- and 2-year results. J Vasc Surg. 2009;49(5):1210–1216. doi: 10.1016/j.jvs.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 87.Davidson I, Hackerman C, Kapadia A, Minhajuddib A. Heparin bonded hemodialysis e-PTFE grafts result in 20% clot free survival benefit. J Vasc Access. 2009;10(3):153–156. doi: 10.1177/112972980901000303. [DOI] [PubMed] [Google Scholar]
- 88.Armstrong J, Gunn J, Arnold N, et al. Angiopeptin-eluting stents: observations in human vessels and pig coronary arteries. J Invasive Cardiol. 2002;14(5):230–238. [PubMed] [Google Scholar]
- 89.Kwok OH, Chow WH, Law TC, et al. First human experience with angiopeptin-eluting stent: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Catheter Cardiovasc Interv. 2005;66(4):541–546. doi: 10.1002/ccd.20558. [DOI] [PubMed] [Google Scholar]
- 90.Kibbe MR, Tzeng E, Gleixner SL, et al. Adenovirus-mediated gene transfer of human inducible nitric oxide synthase in porcine vein grafts inhibits intimal hyperplasia. J Vasc Surg. 2001;34(1):156–165. doi: 10.1067/mva.2001.113983. [DOI] [PubMed] [Google Scholar]
- 91.Jewell CM, Fuchs SM, Flessner RM, Raines RT, Lynn DM. Multilayered films fabricated from an oligoarginine-conjugated protein promote efficient surface-mediated protein transduction. Biomacromolecules. 2007;8(3):857–863. doi: 10.1021/bm0609442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bowden RG, Wilson RL, Gentile M, Ounpraseuth S, Moore P, Leutholtz BC. Effects of omega-3 fatty acid supplementation on vascular access thrombosis in polytetrafluorethylene grafts. J Ren Nutr. 2007;17(2):126–131. doi: 10.1053/j.jrn.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 93.Hiltunen MO, Laitinen M, Turunen MP, et al. Intravascular adenovirus-mediated VEGF-C gene transfer reduces neointima formation in balloon-denuded rabbit aorta. Circulation. 2000;102(18):2262–2268. doi: 10.1161/01.cir.102.18.2262. [DOI] [PubMed] [Google Scholar]
- 94.Pintucci G, Saunders PC, Gulkarov I, et al. Anti-proliferative and anti-inflammatory effects of topical MAPK inhibition in arterialized vein grafts. FASEB J. 2006;20(2):398–400. doi: 10.1096/fj.05-4114fje. [DOI] [PubMed] [Google Scholar]
- 95.Hu Y, Zou Y, Dietrich H, Wick G, Xu Q. Inhibition of neointima hyperplasia of mouse vein grafts by locally applied suramin. Circulation. 1999;100(8):861–868. doi: 10.1161/01.cir.100.8.861. [DOI] [PubMed] [Google Scholar]
- 96.George SJ, Wan S, Hu J, MacDonald R, Johnson JL, Baker AH. Sustained reduction of vein graft neointima formation by ex vivo TIMP-3 gene therapy. Circulation. 2011;124(11 Suppl):S135–S142. doi: 10.1161/CIRCULATIONAHA.110.012732. [DOI] [PubMed] [Google Scholar]
- 97.Thomas AC, Wyatt MJ, Newby AC. Reduction of early vein graft thrombosis by tissue plasminogen activator gene transfer. Thromb Haemost. 2009;102(1):145–152. doi: 10.1160/TH08-11-0772. [DOI] [PubMed] [Google Scholar]
- 98.Zheng H, Xue S, Lian F, Wang YY. A novel promising therapy for vein graft restenosis: overexpressed Nogo-B induces vascular smooth muscle cell apoptosis by activation of the JNK/p38 MAPK signaling pathway. Med Hypotheses. 2011;77(2):278–281. doi: 10.1016/j.mehy.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 99••.Conte MS, Bandyk DF, Clowes AW, et al. PREVENT III Investigators. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43(4):742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 751. Trial that found treatment with a single upstream inhibitor of neointimal hyperplasia failed to protect from graft failure. [DOI] [PubMed] [Google Scholar]
- 100.Alexander JH, Hafley G, Harrington RA, et al. PREVENT IV Investigators. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294(19):2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 101.Du D, Chang S, Chen B, Zhou H, Chen ZK. Adenovirus-mediated heme oxygenase transfer inhibits graft arteriosclerosis in rat aortic transplants. Transplant Proc. 2007;39(10):3446–3448. doi: 10.1016/j.transproceed.2007.03.114. [DOI] [PubMed] [Google Scholar]
- 102.Durante W, Lin CC. HOming in on arteriovenous fistula survival. Kidney Int. 2008;74(1):9–11. doi: 10.1038/ki.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Szmitko PE, Fedak PW, Weisel RD, Stewart DJ, Kutryk MJ, Verma S. Endothelial progenitor cells: new hope for a broken heart. Circulation. 2003;107(24):3093–3100. doi: 10.1161/01.CIR.0000074242.66719.4A. [DOI] [PubMed] [Google Scholar]
- 104.Dardik A, Liu A, Ballermann BJ. Chronic in vitro shear stress stimulates endothelial cell retention on prosthetic vascular grafts and reduces subsequent in vivo neointimal thickness. J Vasc Surg. 1999;29(1):157–167. doi: 10.1016/s0741-5214(99)70357-5. [DOI] [PubMed] [Google Scholar]
- 105.Meinhart JG, Deutsch M, Fischlein T, Howanietz N, Fröschl A, Zilla P. Clinical autologous in vitro endothelialization of 153 infrainguinal ePTFE grafts. Ann Thorac Surg. 2001;71(Suppl 5):S327–S331. doi: 10.1016/s0003-4975(01)02555-3. [DOI] [PubMed] [Google Scholar]
- 106.Nugent HM, Sjin RT, White D, et al. Adventitial endothelial implants reduce matrix metalloproteinase-2 expression and increase luminal diameter in porcine arteriovenous grafts. J Vasc Surg. 2007;46(3):548–556. doi: 10.1016/j.jvs.2007.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nugent HM, Groothuis A, Seifert P, et al. Perivascular endothelial implants inhibit intimal hyperplasia in a model of arteriovenous fistulae: a safety and efficacy study in the pig. J Vasc Res. 2002;39(6):524–533. doi: 10.1159/000067207. [DOI] [PubMed] [Google Scholar]
- 108.Conte MS, Nugent HM, Gaccione P, Guleria I, Roy-Chaudhury P, Lawson JH. Multicenter Phase I/II trial of the safety of allogeneic endothelial cell implants after the creation of arteriovenous access for hemodialysis use: the V-HEALTH study. J Vasc Surg. 2009;50(6):1359–1368.e1. doi: 10.1016/j.jvs.2009.07.108. [DOI] [PubMed] [Google Scholar]
- 109.Taite LJ, Yang P, Jun HW, West JL. Nitric oxide-releasing polyurethane–PEG copolymer containing the YIGSR peptide promotes endothelialization with decreased platelet adhesion. J Biomed Mater Res B Appl Biomater. 2008;84(1):108–116. doi: 10.1002/jbm.b.30850. [DOI] [PubMed] [Google Scholar]
- 110.Kapadia MR, Chow LW, Tsihlis ND, et al. Nitric oxide and nanotechnology: a novel approach to inhibit neointimal hyperplasia. J Vasc Surg. 2008;47(1):173–182. doi: 10.1016/j.jvs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J, Liu K, Shen L, Wu H, Jing H. Small interfering RNA to c-myc inhibits vein graft restenosis in a rat vein graft model. J Surg Res. 2011;169(1):e85–e91. doi: 10.1016/j.jss.2011.03.060. [DOI] [PubMed] [Google Scholar]
- 112.Karper JC, de Vries MR, van den Brand BT, et al. Toll-like receptor 4 is involved in human and mouse vein graft remodeling, and local gene silencing reduces vein graft disease in hypercholesterolemic APOE*3Leiden mice. Arterioscler Thromb Vasc Biol. 2011;31(5):1033–1040. doi: 10.1161/ATVBAHA.111.223271. [DOI] [PubMed] [Google Scholar]
- 113.Kohler TR, Toleikis PM, Gravett DM, Avelar RL. Inhibition of neointimal hyperplasia in a sheep model of dialysis access failure with the bioabsorbable Vascular Wrap paclitaxel-eluting mesh. J Vasc Surg. 2007;45(5):1029–1037. doi: 10.1016/j.jvs.2007.01.057. discussion 1037. [DOI] [PubMed] [Google Scholar]