The design of molecular systems with stimuli-sensitive properties is of great interest for applications ranging from drug delivery to gene transfection.[1] The use of these molecular systems in drug-delivery applications has been enhanced by imparting amphiphilic character to these designs, as these systems are capable of self-assembling into various supramolecular architectures such as micelles and vesicles, and thus provide interiors that can encapsulate guest molecules noncovalently.[2] In this context, significant effort has been devoted to amphiphilic polymers with stimuli-sensitive elements because they are able to 1) form stable micelles, thus providing interiors that can sequester lipophilic guest molecules noncovalently, and 2) release guest molecules in response to both external and internal stimuli such as light,[3] pH,[4] temperature,[5] and reduction/oxidation.[6] Since dendrimers can be obtained with a high degree of control over their polydispersity and size,[7] it is fundamentally interesting to investigate stimuli-sensitive characteristics in these branched macromolecules. Incorporation of stimuli-sensitive characteristics into dendrimers has been relatively underexplored.[8] In particular, amphiphilic dendrimers[8f,9] with stimuli-responsive properties would significantly expand the scope of these molecules in a variety of applications. Herein, we describe the design and syntheses of dendritic micelles that can release their guest molecules in response to a light stimulus.
Light-induced release of guest molecules is interesting, because it provides a pathway for releasing a molecule with a remote control, that is, an external physical stimulus.[3,10] Light-induced release of lipophilic guest molecules from small-molecule surfactant aggregates has been reported previously.[3c,11] While this is interesting, small-molecule-based micelles exhibit rather high critical aggregate concentrations (CACs) and low inherent stabilities. Therefore, light-induced guest release has also been performed with polymer-based micelles.[3,12] To the best of our knowledge, there is no prior report on the light-induced disassembly of dendrimer-based micelles, accompanied by the release of a lipophilic guest molecule.
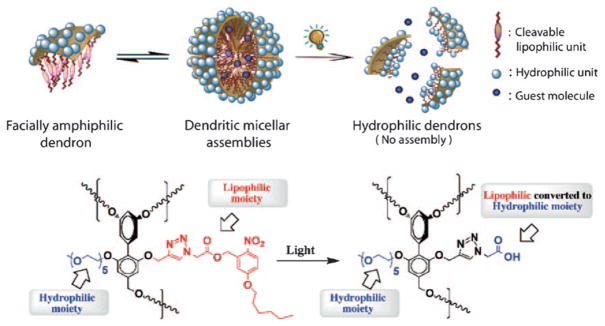
Recently, we reported a unique class of amphiphilic biaryl dendrimers in which every repeating unit in the dendritic backbone contains both lipophilic and hydrophilic functionalities.[8f,13] We have shown that these facially amphiphilic biaryl dendrimers form micelle-type aggregates in water and inverted micelle-type aggregates in apolar solvents such as toluene. The micellar aggregates from our dendrimers are formed through aggregation of several dendrimer molecules and can sequester lipophilic guest molecules. The ability of these molecules to assemble and bind guest molecules is dependent on their hydrophilic–lipophilic balance (HLB).[14] We hypothesized that a change in the HLB in response to light would result in alterations in the amphiphilic assembly, which should concurrently effect release of guest molecules (Figure 1).
Figure 1.
Schematic representation of the light-induced disassembly of dendritic micellar assembliles.
To test this hypothesis we have designed and synthesized amphiphilic dendrimers with a photolabile 2-nitrobenzyl ester moiety as the lipophilic unit (Scheme 1). 2-Nitrobenzyl esters have been widely used as photolabile groups.[15] The hydrophilic part of these facially amphiphilic dendrons is based on oligoethylene glycol units. Our molecule is designed in such a fashion that the light-induced cleavage of the nitrobenzyl ester disconnects a significant part of the lipophilic chain from the dendrimer. Moreover, the functionality—a carboxylic acid—in the product, generated on the dendron side of the molecule, is significantly hydrophilic (Figure 1). We envisaged that this transformation should result in a significant change in the HLB and thus cause the supramolecular assembly to release its guest molecules.
Scheme 1.
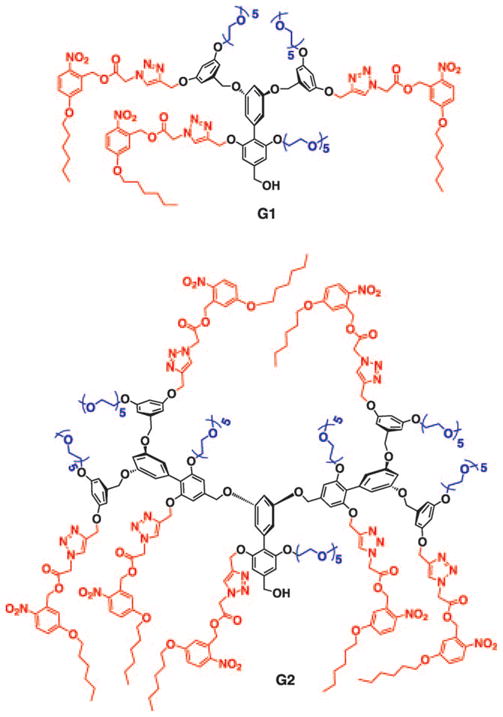
Structures of the photocleavable G1 and G2 dendrons.
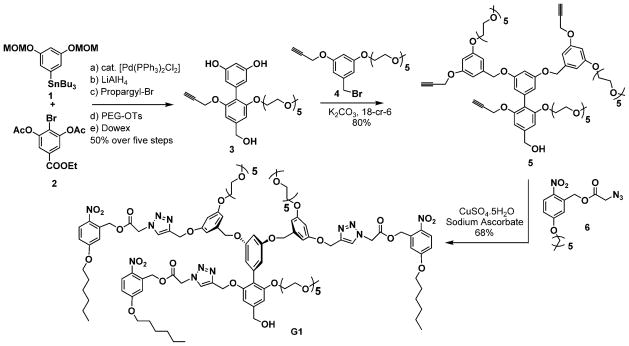
The structures of the targeted light-sensitive G1 and G2 dendrons are shown in Scheme 1. The dendrons were constructed from a biaryl monomer (3 in Scheme 2), which was synthesized from the arylstannane 1 and bromoarylester 2 by using Stille coupling as the key step (Scheme 2). Reaction between the peripheral unit 4 and the biaryl building block unit 3 in the presence of potassium carbonate afforded the dendron 5 in 80% yield. Similarly, the corresponding G2 acetylene dendron was synthesized from 3 and the brominated version of the G1 dendron 5. Attachment of the photolabile nitrobenzyl moiety 8 by a Huisgen 1,3-dipolar cycloaddition reaction (click chemistry) led to the targeted G1 and G2 dendrons.[16] All dendrons were characterized by 1H NMR and 13C NMR spectroscopy as well as MALDI-TOF mass spectrometry; details of the synthesis and characterization data are outlined in the Supporting Information.
Scheme 2.
Synthetic scheme for the photolabile G1 dendron (18-cr-6 = [18]crown-6; MOM = methoxymethyl; PEG-OTs = tosylate of pentaethlylene-glycol monomethyl ether).
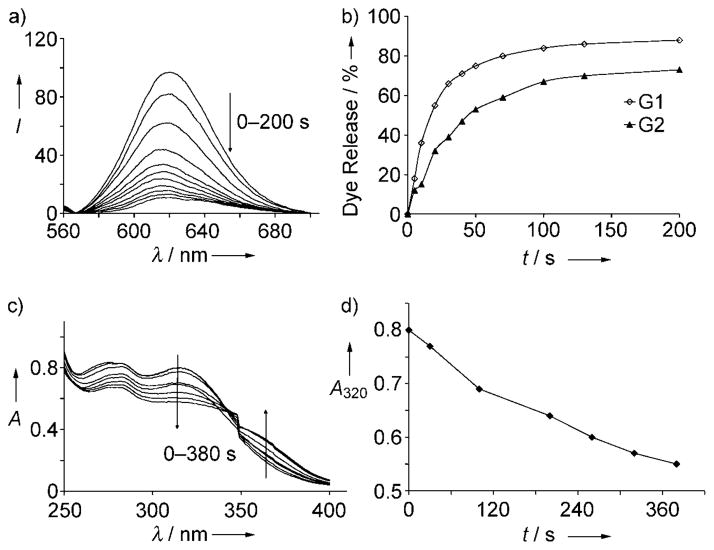
First, we investigated the micellar behavior of these dendrons in the aqueous phase by encapsulating a hydrophobic dye, Nile red. Nile red is not soluble in water, unless it is accomodated in a hydrophobic pocket of micellar aggregates. Emission spectra of Nile red in the presence of various concentrations of G1 and G2 dendrons were used to calculate the CACs of these dendrons,[16] as about 18 and 20 μM, respectively. Dynamic light-scattering (DLS) experiments further verified the formation of micellar aggregates from the G1 and G2 dendrons. The micellar aggregates formed by G1 and G2 dendrons are about 80 and 85 nm in diameter, respectively, thus indicating that these dendrons are able to form micelle-like aggregates in water. The light-triggered disassembly of dendritic micellar aggregates was first investigated by monitoring the change in the emission spectrum of Nile red. When a 55 μM solution of Nile red encapsulated G1 dendron was irradiated at a wavelength of 365 nm we observed a systematic decrease in the emission intensity of Nile red over time, thus indicating disassembly of the micelle and the concomitant release of Nile red from the interiors of the dendritic micelles (Figure 2a). The total amount of dye released, after 200 seconds, was about 88%. When a similar experiment was carried out with the G2 dendron, a 72% release of the guest molecules was observed (Figure 2b). The smaller amount of Nile red released from the G2 dendron compared to that from the G1 dendron is likely due to the more tightly packed nature of the assembly generated from the cleaved form of the higher generation G2 dendron. The difference in the slopes of the lines in Figure 2b also indicates that a generation-dependant controlled release of the guest molecules can be obtained with these dendrons. The cleavage of photolabile ester groups was further verified by UV/Vis spectroscopy. It is known that cleavage of 2-nitrobenzyl esters leads to the formation of a by-product, 2-nitrosobenzalde-hyde, which weakly absorbs at 360 nm and hence can be detected with absorption spectroscopy. Irradiation of the G1 and G2 dendrons with UV light at a wavelength of 365 nm resulted in a decrease in the intensities of the absorption at 320 nm and a concomitant increase at 360 nm over time, thus indicating cleavage of the photolabile ester bond and the formation of the by-product (Figure 2c and 2d).
Figure 2.
a) Release of Nile red from a 55 μM micellar solution of the G1 dendron upon irradiation with UV light for different time intervals (0–200 s), b) release of Nile red from the G1 and G2 dendrons upon irradiation with UV light, c) UV/Vis spectra of the G1 dendron upon irradiation with UV light for different time intervals (0–380 s), d) plot of the absorbance at 320 nm, which illustrates cleavage of the photolabile ester bond.
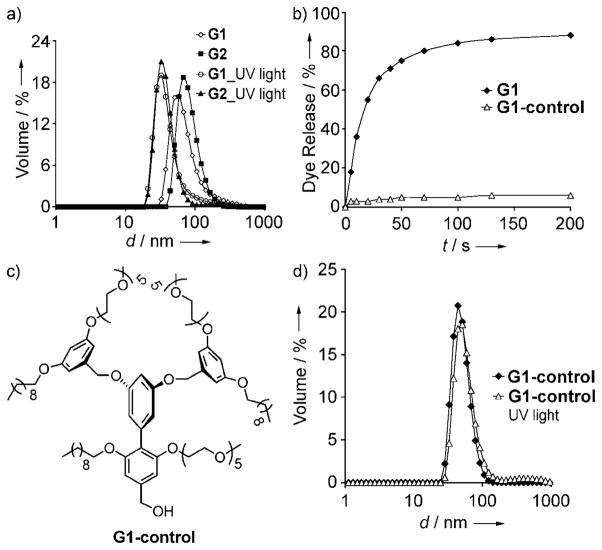
Next, we were interested in evaluating the size evolution of the dendritic micellar aggregates by using DLS (Figure 3a). The size of the aggregates was found to decrease from about 80 to 37 nm upon irradiation. This result indicates that there is some residual nanoscale assembly in the aqueous phase, even after the photochemical reaction. The change in size, however, shows that there is certainly a change in the nature of the supramolecular assembly. The DLS data, combined with the fact that we have effected a significant release of lipophilic guest molecules, suggest that the dendrimer has been converted from an amphiphilic into a significantly hydrophilic structure. Double hydrophilic macromolecules have been observed previously to assemble into core–shell structures and vesicles.[17] The 37 nm diameter of the residual hydrophilic dendrimer can be rationalized on the basis of similar arguments. However, the precise nature of the assembly could not be readily discerned at this time.
Figure 3.
a) Size evolution of 55 μM solutions of the G1 and G2 dendrons upon irradiation with UV light, b) comparision of dye release with the photolabile G1 and G1-control dendrons, c) structure of the G1-control dendron, d) sizes of the G1-control dendron before and after UV irradiation.
Ultimately, we were interested in testing whether the decrease in the emission intensity of Nile red is solely due to the release of the dye molecules from the micellar interior upon exposure to light. For this purpose, we synthesized a first-generation control dendrimer, G1-control, which lacks the photocleavable functionalities (Figure 3c). We hypothesized that there should not be any change in the fluorescence of Nile red upon irradiation with UV light at a wavelength of 365 nm if the light does not have any effect on the electronic properties of the dye molecule that cause a change in the emission spectrum. We were gratified to find that exposure of a 55 μM solution of Nile red containing G1-control dendron to UV light of a wavelength of 365 nm caused less than 5% guest release, compared to 88% release with the photolabile G1 dendron (Figure 3b). This finding supports our hypothesis that the decrease in fluorescence obtained with the photo-labile G1 and G2 dendrons is indeed due to release of Nile red. Moreover, we carried out DLS studies with the G1-control dendron to determine whether UV light has any effect on the size of the aggregate, which may result in leakage of the guest molecule. We indeed found that exposure of the G1-control dendron to UV light did not cause any change in the size of the aggregate (Figure 3d), which indicates that UV light does not cause our biaryl dendrimers to undergo any structural change.
In summary, we have designed and synthesized light-sensitive facially amphiphilic dendrimers that can form micellar aggregates in water. The hydrophobic part of these dendrons consists of photolabile ester groups, which are susceptible to cleavage by UV light. We have shown that light-induced cleavage of the hydrophobic ester groups caused the dendrimers to lose their HLB, thereby resulting in dissociation of the micellar aggregates. Since the facially amphiphilic dendrimers provide the opportunity to sequester lipophilic guest molecules noncovalently, the light-induced supramolecular disassembly provides an opportunity to demonstrate photosensitive release of noncovalently sequestered guest molecules from supramolecular aggregates.
Supplementary Material
Footnotes
We thank the NIGMS of the National Institutes of Health, U.S Army Research Office, and NSF-MRSEC for support.
Dedicated to Professor Peter Beak on the occasion of his 75th birthday.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201006193.
References
- 1.a) Han G, Mokari T, Franklin CA, Cohen BE. J Am Chem Soc. 2008;130:15811–15813. doi: 10.1021/ja804948s. [DOI] [PubMed] [Google Scholar]; b) Han G, You CC, Kim BJ, Turingan RS, Forbes NS, Martin CT, Rotello VM. Angew Chem. 2006;118:3237–3271. doi: 10.1002/anie.200600214. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:3165–3169. doi: 10.1002/anie.200600214. [DOI] [PubMed] [Google Scholar]; c) Zhao YL, Li Z, Kabehie S, Botros YY, Stoddart JF, Zink JI. J Am Chem Soc. 2010;132:13016–13025. doi: 10.1021/ja105371u. [DOI] [PubMed] [Google Scholar]; d) Climent E, Bernardos A, Manez RM, Maquieira A, Marcos MD, Navarro NP, Puchades R, Sancenon F, Soto J, Amoros P. J Am Chem Soc. 2009;131:14075–14080. doi: 10.1021/ja904456d. [DOI] [PubMed] [Google Scholar]
- 2.a) Brunsveld L, Folmer JB, Meijer EW, Sijbesma RP. Chem Rev. 2001;101:4071–4097. doi: 10.1021/cr990125q. [DOI] [PubMed] [Google Scholar]; b) Andresen TL, Jensen SS, Kent J. Prog Lipid Res. 2005;44:68–97. doi: 10.1016/j.plipres.2004.12.001. [DOI] [PubMed] [Google Scholar]; c) Eliaz RE, Nir S, Marty C, Szoka FC. Cancer Res. 2004;64:711–718. doi: 10.1158/0008-5472.can-03-0654. [DOI] [PubMed] [Google Scholar]; d) Torchilin VP. Nat Rev Drug Discovery. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]; e) Duncan R. Nat Rev Drug Discovery. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]; f) Davis ME, Chen Z, Shin DM. Nat Rev Drug Discovery. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]; g) Savic R, Laibin L, Eisenberg A, Maysinger D. Science. 2003;300:615–618. doi: 10.1126/science.1078192. [DOI] [PubMed] [Google Scholar]; h) Discher DE, Eisenberg A. Science. 2002;297:967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 3.a) Jiang J, Tong X, Morris D, Zhao Y. Macromolecules. 2006;39:4633–4640. [Google Scholar]; b) Jiang J, Tong X, Zhao Y. J Am Chem Soc. 2005;127:8290–8291. doi: 10.1021/ja0521019. [DOI] [PubMed] [Google Scholar]; c) Goodwin AP, Mynar JL, Ma Y, Feleming GR, Fréchet JMJ. J Am Chem Soc. 2005;127:9952–9953. doi: 10.1021/ja0523035. [DOI] [PubMed] [Google Scholar]; d) Babin J, Pelletier M, Lepage M, Allard JF, Morris D, Zhao Y. Angew Chem. 2009;121:3379–3382. doi: 10.1002/anie.200900255. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:3329–3332. doi: 10.1002/anie.200900255. [DOI] [PubMed] [Google Scholar]
- 4.a) Klaikherd A, Nagamani C, Thayumanavan S. J Am Chem Soc. 2009;131:4830–4838. doi: 10.1021/ja809475a. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gillies ER, Jonsson TB, Fréchet JMJ. J Am Chem Soc. 2004;126:11936–11943. doi: 10.1021/ja0463738. [DOI] [PubMed] [Google Scholar]; c) Gillies ER, Fréchet JMJ. Chem Commun. 2003:1640–1641. doi: 10.1039/b304251k. [DOI] [PubMed] [Google Scholar]; d) Jung J, Lee IH, Lee E, Park J, Jon S. Biomacromolecules. 2008;8:3401–3407. doi: 10.1021/bm700517z. [DOI] [PubMed] [Google Scholar]; e) Gohy JF, Willet N, Varshney S, Zhang JX, Jerome R. Angew Chem. 2001;113:3314. doi: 10.1002/1521-3773(20010903)40:17<3214::AID-ANIE3214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2001;40:3214–3216. doi: 10.1002/1521-3773(20010903)40:17<3214::AID-ANIE3214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.a) Yotaro M. Angew Chem. 2007;119:1392–1394. [Google Scholar]; Angew Chem Int Ed. 2007;46:1370–1372. doi: 10.1002/anie.200603405. [DOI] [PubMed] [Google Scholar]; b) You YZ, Oupicky D. Biomacromolecules. 2007;8:98–105. doi: 10.1021/bm060635b. [DOI] [PubMed] [Google Scholar]; c) Zhang Q, Clark CG, Wang M, Remsen EE, Wooley KL. Nano Lett. 2002;2:1051–1054. [Google Scholar]
- 6.a) Ghosh S, Irvin K, Thayumanavan S. Langmuir. 2007;23:7916–7919. doi: 10.1021/la700981z. [DOI] [PubMed] [Google Scholar]; b) Ryu J, Roy R, Ventura J, Thayumanavan S. Langmuir. 2010;26:7086–7092. doi: 10.1021/la904437u. [DOI] [PubMed] [Google Scholar]; c) Takae S, Miyata K, Oba M, Ishii T, Nishiyama N, Itaka K, Yamasaki Y, Koyama H, Kataoka K. J Am Chem Soc. 2008;130:6001–6009. doi: 10.1021/ja800336v. [DOI] [PubMed] [Google Scholar]; d) Li Y, Lokitz BS, Armes SP, McCormick CL. Macromolecules. 2006;39:2726–2728. [Google Scholar]
- 7.a) Bosman AW, Janssen HM, Meijer EW. Chem Rev. 1999;99:1665–1688. doi: 10.1021/cr970069y. [DOI] [PubMed] [Google Scholar]; b) Grayson SM, Fréchet JMJ. Chem Rev. 2001;101:3819–3867. doi: 10.1021/cr990116h. [DOI] [PubMed] [Google Scholar]; c) Fischer M, Vögtle F. Angew Chem. 1999;111:934–955. doi: 10.1002/(SICI)1521-3773(19990401)38:7<884::AID-ANIE884>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1999;38:884–905. doi: 10.1002/(SICI)1521-3773(19990401)38:7<884::AID-ANIE884>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]; d) Tomalia DA, Svenson S. Adv Drug Delivery Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]; e) Tomalia DA, Fréchet JMJ. J Polym Sci Part A. 2002;40:2719. [Google Scholar]; f) Menjoge AR, Kannan RM, Tomalia DA. Drug Discovery Today. 2010;15:171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]; g) Rosen BM, Wilson CJ, Wilson DA, Peterca M, Imam MR, Percec V. Chem Rev. 2009;109:6275–6540. doi: 10.1021/cr900157q. [DOI] [PubMed] [Google Scholar]
- 8.a) Jiang DL, Aida T. Nature. 1997;388:454–456. [Google Scholar]; b) Amir RJ, Pessah N, Shamis M, Shabat D. Angew Chem. 2003;115:4632–4637. doi: 10.1002/anie.200351962. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:4494–4499. doi: 10.1002/anie.200351962. [DOI] [PubMed] [Google Scholar]; c) Kojima C, Haba Y, Fukui T, Kono K, Takagishi T. Macromolecules. 2003;36:2183–2186. [Google Scholar]; d) Avital-Shmilovici M, Shabat D. Soft Matter. 2010;6:1073–1080. [Google Scholar]; e) Kostiainen MA, Kasyutich O, Cornelissen JJLM, Nolte RJM. Nat Chem. 2010;2:394–399. doi: 10.1038/nchem.592. [DOI] [PubMed] [Google Scholar]; f) Aathimanikandan SV, Savariar EN, Thayumanavan S. J Am Chem Soc. 2005;127:14922–14929. doi: 10.1021/ja054542y. [DOI] [PubMed] [Google Scholar]; g) Nishiyama N, Iriyama A, Jang WD, Miyata K, Itaka K, Inoue Y, Takahashi H, Yanagi Y, Tamaki Y, Koyama H, Kataoka K. Nat Mater. 2005;4:934–941. doi: 10.1038/nmat1524. [DOI] [PubMed] [Google Scholar]; h) Kostiainen MA, Smith DK, Ikkala O. Angew Chem. 2007;119:7744–7748. doi: 10.1002/anie.200701200. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:7600–7604. doi: 10.1002/anie.200701200. [DOI] [PubMed] [Google Scholar]
- 9.For some examples of amphiphilic dendrimers see: Percec V, Wilson DA, Leowanawat P, Wilson CJ, Hughes AD, Kaucher MS, Hammer DA, Levine DH, Kim AJ, Bates FS, Davis KP, Lodge TP, Klein ML, DeVane RH, Aqad E, Rosen BM, Argintaru AO, Sienkowska MJ, Rissanen K, Nummelin S, Ropponen J. Science. 2010;328:1009–1014. doi: 10.1126/science.1185547.Joester D, Losson M, Pugin R, Heinzelmann H, Walter E, Merkle HP, Diederich F. Angew Chem. 2003;115:1524–1528. doi: 10.1002/anie.200250284.Angew Chem Int Ed. 2003;42:1486–1490. doi: 10.1002/anie.200250284.Cooper AI, Londono JD, Wignall G, McClain JB, Samulski ET, Lin JS, Dobrynin A, Rubinstein M, Burke ALC, Fréchet JMJ, DeSimone JM. Nature. 1997;389:368–371.Newkome GR, Moorefield CN, Baker GR, Johnson AL, Behera RK. Angew Chem. 1991;103:1205–1207.Angew Chem Int Ed Engl. 1991;30:1176–1178.Hawker CJ, Wooley KL, Fréchet JMJ. J Chem Soc Perkin Trans 1. 1993:1287–1297.
- 10.Wang Y, Han P, Xu H, Wang Z, Zhang X, Kabanov AV. Langmuir. 2010;26:709–715. doi: 10.1021/la9023844. [DOI] [PubMed] [Google Scholar]
- 11.a) Wang Y, Ma N, Wang Z, Zhang X. Angew Chem. 2007;119:2881–2884. [Google Scholar]; Angew Chem Int Ed. 2007;46:2823–2826. doi: 10.1002/anie.200604982. [DOI] [PubMed] [Google Scholar]; b) Orihara Y, Matsumura A, Saito Y, Ogawa N, Saji T, Yamaguchi A, Sakai H, Abe M. Langmuir. 2001;17:6072–6076. [Google Scholar]
- 12.Liu X, Jiang M. Angew Chem. 2006;118:3930–3934. [Google Scholar]; Angew Chem Int Ed. 2006;45:3846–3850. doi: 10.1002/anie.200504364. [DOI] [PubMed] [Google Scholar]
- 13.a) Vutukuri DR, Basu S, Thayumanavan S. J Am Chem Soc. 2004;126:15636–15637. doi: 10.1021/ja0449628. [DOI] [PubMed] [Google Scholar]; b) Gomez-Escudero A, Azagarsamy MA, Theddu N, Vachet RW, Thayumanavan S. J Am Chem Soc. 2008;130:11156–11163. doi: 10.1021/ja803082v. [DOI] [PubMed] [Google Scholar]
- 14.a) Azagarsamy MA, Sokkalingam P, Thayumanavan S. J Am Chem Soc. 2009;131:14184–14185. doi: 10.1021/ja906162u. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Azagarsamy MA, Yesilyurt V, Thayumanavan S. J Am Chem Soc. 2010;132:4550–4551. doi: 10.1021/ja100746d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a) Mayer G, Heckel A. Angew Chem. 2006;118:5020–5042. [Google Scholar]; Angew Chem Int Ed. 2006;45:4900–4921. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]; b) Fomina N, McFearin C, Sermsakdi M, Edigin O, Almutairi A. J Am Chem Soc. 2010;132:9540–9542. doi: 10.1021/ja102595j. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.See the Supporting Information for details.
- 17.a) An Z, Shi Q, Tang W, Tsung CK, Hawker CJ, Stucky GD. J Am Chem Soc. 2007;129:14493–14499. doi: 10.1021/ja0756974. [DOI] [PubMed] [Google Scholar]; b) Pasparakis G, Alexander C. Angew Chem. 2008;120:4925–4928. doi: 10.1002/anie.200801098. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:4847–4850. doi: 10.1002/anie.200801098. [DOI] [PubMed] [Google Scholar]; c) Savariar EN, Aathimanikandan SV, Thayumanavan S. J Am Chem Soc. 2006;128:16224–16230. doi: 10.1021/ja065213o. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.