Abstract
Activation of the immune system is beneficial in defending against pathogens, but may also have costly side effects on an organism’s fitness. In this study we examine the fitness consequences of immune challenge in female Drosophila melanogaster by examining both direct (within generation) and indirect (between generations) costs and benefits of immune challenge. Though passing immunity to offspring has been studied in mammals for many years, only recently have researchers found evidence for a cross-generational priming response in invertebrates. By examining both potential fitness costs and benefits in the next generation, we were able to determine what effect immune challenge has on fitness. In agreement with other studies, we found a direct cost to infection, where immune challenged females laid fewer eggs than unchallenged females in two of the three lines we examined. In addition, we found some evidence for indirect costs. Offspring from immune challenged mothers had shorter lifespans than those from unchallenged mothers in two of the three lines. Interestingly, we do not see any effect of maternal immune challenge on offspring’s ability to overcome an infection, nor do we see an effect on other fitness traits measured, including egg size, egg-adult viability and offspring resistance to oxidative stress. While previous studies in bumblebees and beetles have demonstrated cross-generation priming, our results suggest that it may not be a general phenomenon, and more work is needed to determine how widespread it is.
Keywords: Drosophila melanogaster, immunity, maternal effects, cross-generational priming
Introduction
Over the last few decades, dramatic advances have been made in the study of immunity in insects. We now know that insects have quite a sophisticated innate immune response, consisting of humoral (IMD and Toll), cellular and melanization defences.1–4 Even though insects do not have what is typically thought of as an ‘adaptive’ immune response, there is some evidence that invertebrates challenged with a low dose of an infectious agent garner increased resistance to a subsequent infection with the same pathogen, a phenomenon known as ‘priming’. This has been demonstrated in copepods,5 D. melanogaster,6 and mealworm beetles initially challenged with lipopolysaccharides (LPS).7 In addition, recent evidence suggests that there may also be ‘cross-generational priming’, where offspring from mothers that were immune challenged have a greater immune response in the next generation than offspring from un-challenged mothers. Cross-generational priming has been demonstrated in Daphnia,8 bumblebees9 and beetles.10 This evidence suggests there may be many factors that contribute to immunocompetence and ultimately to offspring fitness in the next generation.
The fitness of an organism is made up of several components, including age-specific survival and reproduction.11 Reproductive fitness can be defined not only by the number of offspring produced, but also by their quality. Limited maternal resources may lead to changes in resource allocation between reproduction and other physiological traits. For example, if a female has a limited amount of resources available, investing more in reproduction can come at a cost to her survival.12 While this trade-off occurs within the mother, these costs can also have consequences for her offspring. The trade-off between egg number and size is an oft-cited example,13,14 where the increased fecundity in the female may come at a cost of decreased per-offspring resources. These so-called ‘maternal effects’ occur when factors that affect the mother influence offspring quality, independent of the genes handed down from mother to offspring.15 These factors could include the mother’s physiological state. For example, the age of the mother has been found to alter longevity of the offspring,16,17 as well as pupal survival.18 These factors can also be genetic. Allelic variation and gene expression patterns in the mother can influence offspring development independent of the offspring’s genotype (e.g., maternal bicoid and hunchback expression influences the patterning of zygotes in D. melanogaster).19,20 Finally, external environmental factors experienced by the mother can also alter offspring fitness. For example, temperature has been shown to affect egg size, viability, offspring longevity, and overall offspring fitness in flies.21
Here we focus on the relationship between immune function and maternal effects, using the fruit fly, D. melanogaster, as a model system. While the innate immune system can provide rapid and often complete protection for a host, only recently have researchers begun to consider the impact of infection and immune gene expression on the host’s reproduction and offspring. For example, recent work has suggested that survival from an infection is not straightforward as previously thought. Differences in an organism’s ability to tolerate an infection versus actually resist an infection point to a complex relationship between immune system function and infection.22,23 This added complexity may be similar to the relationship between reproduction and immunity. In D. melanogaster, researchers have found that immune-challenged individuals exhibit a decrease in female fecundity.24 In addition, there seems to be a cost to immune system upregulation and maintenance. For example, in fruit flies, male courtship of females leads to a reduction in immune function.25 It has also been found that the act of mating can induce immune system upregulation in female flies,26,27 but the females have a decreased ability to overcome a bacterial infection after mating.28 This evidence suggests that reproduction and immunity are intertwined. Though much work has been done in D. melanogaster on immunity, we still do not know how immune upregulation affects offspring fitness, either negatively (through reduced resources available to the offspring) or positively (through heightened offspring immune response).
Here we set out to determine if stimulation of the immune system in response to a bacterial infection has a fitness cost in the quantity or quality of the offspring produced. In addition, we wanted to determine if there were any benefits in the next generation, via cross-generational priming. We examined fecundity and egg size of infected versus non-infected mothers as well as egg to adult viability, longevity, oxidative stress resistance and pathogen resistance of offspring derived from infected and non-infected mothers. Results from this study should provide further insight into the way in which maternal infection influences offspring fitness.
Results
Two inbred strains (IR 56 and IR 57) and one outbred strain (GAo) of the fruit fly, D. melanogaster, were used in these experiments. Mothers were collected as virgins, aged for three days, and then mated for one hour in a ratio of five females to seven males to insure females were only mated once.29 Females were then anesthetized with CO2, and placed ten females per vial, where they recovered for 24 hours with a small pinch of live yeast. The next day, flies were pooled together and placed into one of four treatments. One treatment was composed of mothers that were infected with the gram-positive bacterium Lactococcus lactis (LL), and a second treatment was infected with the gram-negative bacterium Pseudomonas aeruginosa (PA). Two control treatments were used, including a sterile broth injection (sham), and a naïve treatment, in which there was no septic injury and the flies were anesthetized at the same time as the other treatments. Mothers were allowed to recover for one hour and then several different measurements were taken in order to determine the fitness consequences of immune challenge to the mothers as well as to the offspring.
Stastistical analysis
For fecundity, viability and egg size, ANOVAs were conducted on each replicate separately. We then calculated an overall p-value for each trait using Fisher’s Combined Probability test. Post-hoc analyses were done using the False Discovery Rate method,30 and q-values were reported. A priori, we had decided to make four comparisons among the treatments: Naïve ≠ PA, Naïve ≠ LL, Sham ≠ PA and Sham ≠ LL. For treatment-by-strain interactions, a standard ANOVA was carried out. All survival analyses were conducted using a proportional hazards model.
Fecundity
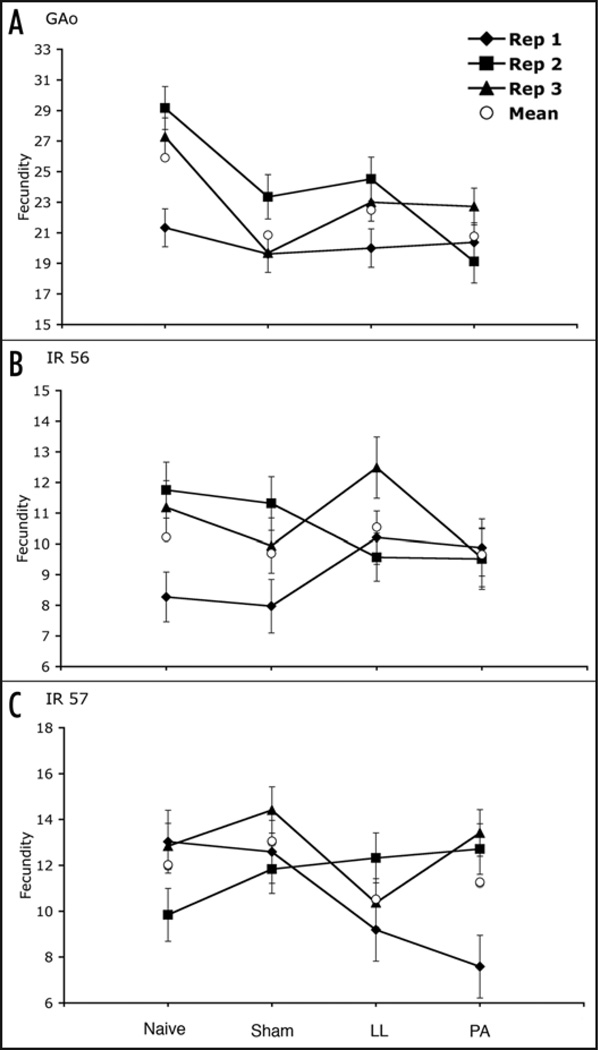
Offspring number over 24 hours was measured in all four treatments. In two of the three strains, infection appeared to reduce fecundity, though the pattern differed slightly between strains. There were significant differences between treatment in both GAo females (p < 0.0001; Fig. 1A) and IR 57 females (p = 0.0014; Fig. 1C), but not IR 56 females (p = 0.0851; Fig. 1B). Post hoc tests indicated that Naïve females produced more offspring than both LL infected (q = 0.0012) and PA infected (q = 0.0001) females in the GAo flies. For the IR 57 flies we found that Naïve females produced more offspring than LL (q = 0.0262), Sham produced more than LL (q = 0.0134) and more than PA mothers (q = 0.0134). There was a significant treatment-by-strain interaction (treatment*strain: F6, 1420 = 8.261, p < 0.0001; treatment: F3.1423 = 8.728, p < 0.0001; and strain: F2, 1424 = 405.1, p < 0.0001). Egg to adult viability was also measured. However, treatment had no significant affect on this trait (data not shown), with the average viability for each line being GAo = 87.2%, IR 56 = 86.3% and IR 57 = 69.5%. In addition, treatment-by-strain interactions were not significant for viability (treatment*strain: F6, 1420 = 0.407, p < 0.875; treatment: F3.1423 = 1.005, p = 0.3895; and strain: F2, 1424 = 100.74, p < 0.0001).
Figure 1.
Average fecundity (+1 S.E.) of singleton females after 24 hours, by replicate. Offspring number was significantly higher in naïve flies compared to LL and PA flies in the GAo lines (A), and fecundity was higher in sham-injected flies compared to PA and LL as well as higher in naïve compared to LL flies in IR 57 lines (C). No differences were seen among the treatments for the IR 56 (B) lines. Means of the three replicates are shown as open circles.
Viability
Viability (egg to adult survival) was also measured at higher densities. In the ‘Fecundity’ measurement, egg density was very low (approximately 10–30 eggs per vial, depending on line, see Fig. 1) and we wanted to determine viability at a density that may be more stressful for the larvae. In all of the following measurements eggs were collected from egg-laying chambers. Each chamber housed 30 females for 18 hours. Egg chambers included a petri dish with a 30% molasses, 10% agar medium with a dab of 0.05 g of yeast paste on which the females could feed and lay eggs. After 18 hours females were discarded and eggs were collected. Females were placed into chambers one hour after exposure to the treatment. In this ‘Viability’ measurement we placed 150 eggs from each treatment into a vial and measured the number of offspring that eclosed. We found that treatment did not have a significant affect on viability at a density of 150 eggs for GAo (p = 0.284), IR 56 (p = 0.624) or IR 57 (p = 0.112). Average viability and standard errors (SE) are given in Table 1. There were no treatment-by-strain interactions for viability (treatment*strain: F(6,106) = 0.4578, p = 0.8379; treatment: F(3,109) = 3.825, p = 0.0122; strain: F(2,110) = 53.83, p < 0.0001).
Table 1.
Egg to adult viability at a density of 150 eggs, ± SE
| Line | Naïve | Sham | LL | PA |
|---|---|---|---|---|
| GAo | 0.697 ± 0.020 | 0.748 ± 0.026 | 0.664 ± 0.020 | 0.656 ± 0.027 |
| IR 56 | 0.642 ± 0.030 | 0.646 ± 0.023 | 0.617 ± 0.023 | 0.581 ± 0.027 |
| IR 57 | 0.536 ± 0.029 | 0.550 ± 0.023 | 0.511 ± 0.017 | 0.515 ± 0.014 |
Egg size
Eggs were collected from egg-laying chambers, photographed and egg-length and egg-width were measured using NIH Image J software. We used the volume of a prolate spheroid, , to determine egg volume. Treatment had a significant effect on egg volume in all three lines (GAo, p < 0.0001; IR 56, p < 0.0001; and IR 57 p < 0.0001). Post hoc comparisons were significant in a few cases. For IR 56: Naïve > PA (q = 0.022), Sham > PA (q < 0.0001) and Sham > LL (q < 0.0001). In addition, in IR 57 we found a difference between Naïve > PA (q < 0.0001). Though there were a few significant post hoc comparisons, the treatments were not consistent between lines and do not suggest a pattern. This is demonstrated by the highly significant treatment-by-strain interactions for egg volume (treatment*strain: F(6,106) = 17.432, p < 0.0001; treatment: F(3,109) = 14.723, p < 0.0001; strain: F(2,110) = 43.355, p < 0.0001). Average volume per replicate for each treatment in each line can be found in Table 2.
Table 2.
Mean egg volume for each replicate in mm3, ±SE
| Line | Rep | Naïve | Sham | LL | PA |
|---|---|---|---|---|---|
| GAo | 1 | 0.00839 ± 0.00014 | 0.00798 ± 0.00014 | 0.00860 ± 0.00014 | 0.00797 ± 0.00014 |
| 2 | 0.00782 ± 0.00016 | 0.00836 ± 0.00016 | 0.00858 ± 0.00016 | 0.00795 ± 0.00016 | |
| 3 | 0.00772 ± 0.00016 | 0.00831 ± 0.00016 | 0.00842 ± 0.00016 | 0.00783 ± 0.00016 | |
| IR 56 | 1 | 0.00787 ± 0.00014 | 0.00867 ± 0.00014 | 0.00787 ± 0.00014 | 0.00795 ± 0.00014 |
| 2 | 0.00808 ± 0.00015 | 0.00880 ± 0.00013 | 0.00769 ± 0.00015 | 0.00752 ± 0.00015 | |
| 3 | 0.00801 ± 0.00015 | 0.00849 ± 0.00015 | 0.00802 ± 0.00015 | 0.00770 ± 0.00015 | |
| IR 57 | 1 | 0.00888 ± 0.00015 | 0.00817 ± 0.00015 | 0.00883 ± 0.00015 | 0.00897 ± 0.00015 |
| 2 | 0.00899 ± 0.00016 | 0.00839 ± 0.00016 | 0.00882 ± 0.00016 | 0.00781 ± 0.00016 | |
| 3 | 0.00926 ± 0.00017 | 0.00825 ± 0.00017 | 0.00882 ± 0.00017 | 0.00824 ± 0.00017 |
Longevity of offspring
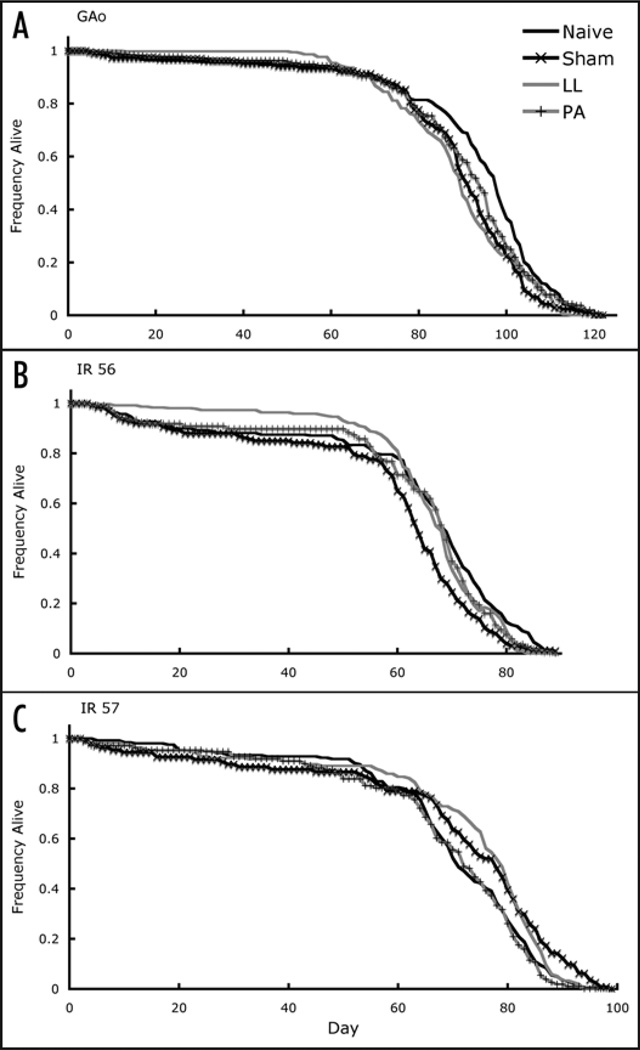
One hundred twenty (120) eggs from each maternal treatment were collected from egg-laying chambers and placed into individual vials. Virgin females were collected from those vials and placed into survival cages. We replenished the food in the cages and recorded mortality every other day in order to determine what effect maternal treatment had on the longevity of their offspring. Treatment of mothers had a significant effect on longevity of offspring (Fig. 2). Overall, our data suggest that offspring from immune-challenged mothers may pay a survival cost compared to offspring from naïve or sham injected mothers in two of the three lines, while in the third line offspring of immune-challenged mothers seemed to benefit, at least early on in life. GAo offspring showed significant differences between treatments (Cox Proportional Hazards model , p < 0.0001; Fig. 2A). Post-hoc pair-wise tests showed that offspring derived from naïve mothers lived significantly longer than those derived from LL infected mothers (q = 0.0004) and a trend was seen where Naïve > PA, (q = 0.07). Treatment of mothers also had a significant effect on offspring longevity for IR 57 (, p = 0.019; Fig. 2C). Pair-wise tests showed a significant effect for offspring derived from sham mothers, which lived longer than those derived from PA infected mothers (q = 0.038). Treatment of mothers also had a significant effect on offspring longevity for IR 56 (, p = 0.0002; Fig. 2B), and pair-wise tests suggest a marginal trend that offspring from naïve mothers died off faster than offspring from LL infected mothers in early life, but the pattern (Fig. 2B) appeared to switch later in life (q = 0.0856). In addition, there was a significant treatment-by-strain interaction (treatment*strain: , p < 0.0001; treatment: , p = 0.055; strain: , p < 0.0001).
Figure 2.
Survival curves for GAo (A), IR 56 (B) and IR 57 (C) offspring derived from females exposed to each of the four treatments. A proportional hazards model showed significant differences between treatments for all three lines. After pair-wise comparisons, we find that offspring from naïve females live significantly longer than offspring from LL infected females for GAo and a marginally significant effect for IR 57, where offspring derived from sham mothers survived longer than offspring derived form PA infected mothers. We find the opposite effect in IR 56, where flies from LL mothers live longer than those from naïve mothers.
Oxidative stress of offspring
We also measured the offspring’s ability to resist oxidative stress by exposing three-day old virgin offspring to hydrogen peroxide. Treatment of mothers was not found to have any significant effect on offspring’s ability to resist hydrogen peroxide oxidative stress (Cox Proportional Hazards model, GAo: , p = 0.714; IR 56: , p = 0.294; IR 57: , p = 0.101). There was not a significant treatment-by-strain interaction (treatment*strain: , p = 0.665); treatment: , p = 0.103; strain: , p < 0.0001).
Immune ability (priming) of offspring
Finally in order to determine whether offspring from immune challenged females had improved ability to defend against pathogens in the next generation, immune ability of the offspring was measured. One hundred twenty (120) eggs from each maternal treatment were collected and placed into individual vials. Virgin female offspring were collected from those vials and then were allowed to age for three days. Offspring were then exposed to immune challenge. Flies derived from L. lactis infected mothers were challenged with L. lactis (LL-LL), flies from P. aeruginosa mothers were challenged with P. aeruginosa (PA-PA), and a subset of flies from naïve and sham mothers were challenged either with L. lactis (Naïve-LL, Sham-LL) or P. aeruginosa (Naïve-PA, Sham-PA). Treatment of mothers had no effect on offspring’s ability to overcome an immune challenge (survival curves not shown). When infected with PA, survival rate of offspring derived from PA infected mothers was not significantly different than offspring from sham or naïve mothers for GAo (, p = 0.244), IR 56 (, p = 0.720) or IR 57 (, p = 0.871). In addition, when infected with LL, survival rate of offspring derived from LL infected mothers also did not differ significantly from the survival rate of offspring from naïve or sham mothers for GAo (, p = 0.605), IR 56 (, p = 0.537) or IR 57 (, p = 0.093). There was not a significant treatment-by-strain effect for either LL (treatment*strain: , p = 0.394; treatment: , p = 0.349; strain: , p < 0.0001) or for PA (treatment*strain: , p = 0.483; treatment: , p = 0.779; strain: , p < 0.0001).
Discussion
Maternal effects can play an important role in determining offspring fitness of invertebrates.17,18,21,31 However, only recently have researchers begun to use invertebrates to study how an immune challenge to the mother can affect the fitness of her offspring. In the last few years, researchers have found that offspring derived from mothers challenged with a particular pathogen may be better at resisting that pathogen in the next generation.9,10 In this study, we took a more comprehensive approach by examining both the potential costs as well as benefits that we might see in the next generation after maternal immune challenge. This study allowed us to obtain a more complete understanding of the evolutionary forces acting on immunity in insects.
Our results point to both direct fitness costs of infection in the host mother as well as indirect fitness costs to the offspring through a maternal effect in two of the three lines examined. In terms of direct effects to the host, we see a fecundity cost of infection in immune challenged females (Fig. 1), in agreement with previous studies.24,32,33 However, we only see this effect in two of the three lines. In addition, in the GAo line, we do not see a difference between infected and sham females (only between naïve and infected females). Previous studies have shown that piercing the fly causes the immune system to be upregulated.34 So even though it is only the sterile jab that is causing the effect in this line, it is still relevant to determining the effect that immune upregulation has on the next generation. The variability of these results might also be due to genetic variation for immune-mediated maternal effects. Priest et al.17 found a similar pattern—genotype background influenced role of parental age on offspring fitness. Previous studies have established that there is abundant variation for immune ability in flies ranging from Africa to the northern United States.35 Moreover, researchers have also observed genotype-by-environment interactions in the cost of deploying the immune system in flies.36 In light of these studies and our own findings, if future studies find genetic variation for cross generational effects to be consistent and robust, subsequent studies on a larger scale could help us to identify specific loci that influence immune-mediated maternal effects.
At the same time, our data point to a small cost to survival in the next generation. Longevity is reduced in offspring derived from immune challenged females in both GAo and IR 57. This effect, along with the fact that we see higher fecundity in the control flies of these lines, suggests that mothers have to choose between allocating resources from a finite pool either to reproduction or to immune function. This trade-off between reproduction and immunity has been seen in several different studies on insects.25,37,38 These observations further support the hypothesis that infection is costly in the current generation, but can also have effects on offspring quality in the next generation. However, in the IR 56 line, we see a trend that offspring from LL infected mothers have lower early-age mortality but higher late-age mortality than offspring derived from naïve mothers, suggesting a potential trade-off between early-age and late-age fitness influenced by immune-mediated maternal effects. Interestingly, IR 56 did not show any direct costs of infection on number of eggs produced, so it might be that the fitness cost of infection is lowest in this line. The three genetic variants we used in this study gave us different results. Although the differences among genetic variants seen here complicates our interpretation, it is important to examine several different genetic lines when trying to answer both evolutionary and molecular based questions to determine the generality of any results. Even though we see different outcomes among lines, we can still observe a general pattern, namely that maternal condition influences offspring fitness traits. Thus, in any study that sets out to determine the overall fitness consequences of infection, one should consider effects both within and between generations.
Although our data point to costly maternal effects after infection, many aspects of fitness that we measured did not show an effect. For example, while egg volume varied among treatments, there was no consistent difference between infected and uninfected mothers across strains. There was evidence for eggs from sham mothers being larger than the LL or PA mothers in IR 56. However, the pattern was not consistent between lines and naïve females did not demonstrate a strong difference in egg size from infected mothers. We chose to measure egg volume due to the fact it has been used in previous studies to measure egg size.31 However, a measurement like weight may be more accurate for examining differences in investment in egg size. Or perhaps there is no selection for differential investment in egg size while infected with a pathogen in our flies, and the differences we see are just due to random variation, or due to the fact most egg development occurs several days before they are laid.
We also failed to find an effect of maternal infection on viability of the eggs (Table 1). This may have been due in part to the fact that females in this study had abundant resources. In previous studies of trade-offs in invertebrates, researchers have often found that fitness costs are only apparent under conditions of limited resources and/or high levels of competition,12,39 and the costs of immune system deployment are exaggerated with limited resources.36 Alternatively, infection in mothers may simply not lead to maternal effects on egg viability. However, that seems unlikely, since viability costs are seen at later stages of development in offspring and in many other maternal effects.16,18,31
In this study, we examined many different aspects of fitness. It is perhaps not surprising that we only found an effect of maternal condition on a few of the offspring fitness components. Even if trade-offs constrain fitness optima, it is still possible that while some traits will correlate negatively with each other, others will show no correlation, or even positive correlations. As noted earlier, non-negative fitness correlations could arise if females had abundant resources, as they did in the laying chambers. While we did try to carry out experiments in which females in egg-laying chambers had limited yeast, the females would not then lay eggs. We note that females in the egg laying chambers were exposed to media with substantially higher sugar content than in the normal media (30% molasses versus 10% molasses). Again, abundant (albeit temporary) resources may have mitigated against finding trade-offs between some fitness traits.
Finally, we did not see any maternal effects on resistance to infection in the next generation. This result is in contrast to Moret10 and Sadd et al.,9 both of whom found that offspring of infected mothers had greater immune upregulation in the next generation. This difference between our study and previous work may be due to the fact that our infection protocol did not stimulate the priming mechanism, or at least not to a detectible degree. A recent study by Pham et al.6 suggested that phagocytes may be the critical component of priming in insect immunity. If we were to challenge flies with another type of pathogen, such as the eggs of a parasitoid wasp, we might see an effect of priming. In our own study, we examined priming in D. melanogaster using a lethal pathogen. In previous studies that showed cross-generational priming, the authors used heat killed bacteria and LPS in bumblebees and beetles.9,10 In addition, these previous studies measured the offspring immune ability by examining phenoloxidase (PO) levels, which they obtained by extracting hemolymph. D. melanogaster is significantly smaller than both bumblebees and beetles, and it is extremely difficult to extract a significant amount of hemolymph out of adult flies. Since the authors used an inert immune stimulus, the functional ability to overcome an infection was not demonstrated. Previous studies have shown discrepancies in measures of immunocompetence. For example, pathogen load (number of colonies) did not correlate with ability to overcome a bacterial infection with P. aeruginosa in D. melanogaster.40 Furthermore, measuring actual survival in response to infection is arguably more directly relevant to fitness than measures of PO levels. In a series of pilot studies in our lab, we challenged mothers with LPS in order to see if that immune challenge would demonstrate a priming response as in Moret10 and Sadd et al.9 or show costs of immune challenge, but were not able to find a concentration that effectively and consistently showed any effects (Linder JE, unpublished data).
It is unlikely that our negative results are due to insufficient statistical power. We used relatively large sample sizes, with 150 flies for each treatment and each type of bacteria, which were spread across three independent replicates. This size is adequate for Drosophila and larger than both of the prior studies that found cross-generational priming. In addition, neither Moret10 nor Sadd et al.9 considered the effect of genotypic variation. We examined the effect in three genetically distinct populations, whereas all of the bumblebees measured in each treatment from Sadd et al.9 were derived from one queen. And although Moret10 started with multiple cultures of beetles, they originated from the same original population. Perhaps some of our variation in results comes from looking at many different populations of flies, as is demonstrated by significant treatment-by-strain results in the fecundity, egg size and longevity treatments.
Interestingly, a study was just published in the Yellow Fever mosquito, which also failed to find cross-generational priming. Voordouw et al.41 examined viability, age at emergence, body size and melanization in offspring of mosquitoes that had been challenged with an injection of sephadex beads. They found no difference between offspring from challenged mothers and offspring from sham or naïve mothers. These results, taken together with our own findings, suggest that cross-generation priming is not universal among insects. In this light, to better understand the relative importance of cross-generational priming, it would be of benefit not only to replicate this and previous studies, but also to examine other types of insects for evidence of cross-generation priming.
Our results may also have been limited by the timing of our infections. We chose to measure fitness traits shortly after infection in order avoid females dying before we could collect eggs. However, egg development in flies actually starts approximately seven days before the egg is laid, so even though we see an effect of treatment on some traits (like egg size and offspring longevity) we are not examining how infection influences the egg from the first stage of development. Future work should examine more closely the effect that infection has on the different stages of egg development, in order to better understand the potential impact of infection and maternal affects.
Overall, the most surprising result of our study is that we failed to find cross-generational priming. This suggests that the phenomenon may not be widespread. In addition, this study provides further support that there are reproductive costs of immunity in D. melanogaster.24 Based on these findings, it would be appropriate to use molecular approaches to determine differences in gene expression in offspring derived from immune challenged versus naïve females. Investigating the genes involved in the differences in offspring survival could elucidate the relationship between longevity and immunity. It may also be worth developing models of host-parasite interactions that incorporate maternal effects. It would be of particular interest to determine whether maternal effects might influence the evolution of virulence.42,43 The tradeoffs that we observed here could also have important implications for the biological control of insects. Pathogens released to control insect pests might also have addition control effects by reducing fitness in the next generation.44
Methods
Fly stocks and maintenance
Flies were cultured on standard cornmeal-molasses-agar media and maintained with 14-day non-overlapping generations, with a 12-hour L:D cycle, in a manner similar to Linder et al.45 The outbred strain of flies used in this experiment (GAo) was the same as in Linder et al.45 Two inbred lines (IR 56 and IR 57) were collected from the University of Georgia horticultural farm in Athens, GA in 2003. They were maintained with full sib mating until September of 2007, at which time they were expanded using random mating to approximately 75 male/female pairs (in each line) for approximately ten generations in order to create enough flies for the experiments. Flies were cultured in vials containing seven ml of food and five to seven male/female pairs. Eggs were trimmed to a density of 120 per vial in order to control for density effects during the experiment. Trimming was carried out on all lines for vials that were to give rise to the maternal generation in the experiment.
Bacterial stocks
To test for evidence of cross-generational priming, we exposed female flies to one of four treatments. Naïve flies received no immune challenge or injury of any kind, sham flies received a sterile broth inoculation, LL flies were exposed to gram-positive bacteria, Lactococcus lactis, and finally PA flies were exposed to gram-negative bacteria, Pseudomonas aeruginosa. Both bacterial strains were originally obtained from Brian Lazzaro at Cornell University. L. lactis was derived from a natural fly population, and P. aeruginosa was derived from a laboratory culture (strain PA01). Bacteria were grown up overnight in liquid LB-broth at 37°C. Bacteria where then standardized in a spectrophotometer to an optical density (OD) of 0.8 nm at 600 Å and then diluted. An OD of 0.005 nm (for both bacteria) was used for the maternal infections, and an OD of 0.01 nm (for P. aeruginosa) and an OD of 0.06 nm (for L. lactis) was used for priming infections of the offspring.
Maternal generation
Mothers were pooled together and randomly assigned to one of the four treatments (Naïve, Sham, LL or PA). Flies were anesthetized under light CO2 and then infected by piercing the thorax with the tip of a 0.1 mm stainless steel pin dipped in the bacterial suspension. To challenge the mothers, we used a dose of bacteria that led to the death of half of the flies over the course of three days (LD50), determined prior to the actual experiments. Immune gene activation has been shown to take place rapidly after infection,26,27 so females were placed into egg laying chambers one hour after inoculation and allowed to lay for 18 hours. For the ‘fecundity’ measurement, females were placed into individual vials (see below). Separate mothers were used for each of the following measurements.
Measurements
Fecundity
Egg number and offspring number were counted for individual females. One hour after treatment, females were placed in individual vials for 24 hours. Eggs were then counted and the number of offspring that eclosed were counted 14 days later to determine egg to adult viability. Average number of eggs per vial ranged from 10–25 depending on the strain, so we also determined viability at a higher density (see ‘viability’ section below). In each of lines IR 56 and IR 57, we measured 145 females across three independent replicates for each treatment (so each replicate had ~50 Naïve, Sham, LL and PA females, and this was independently carried out three times), while 155 females across three replicates (again ~50 per replicate for each treatment) were measured for GAo.
Viability
We also determined egg to adult viability at a high density with three independent replicates. For each replicate two egg laying chambers were used for each maternal treatment. Each chamber had 30 females that had been exposed to their specific treatment. Females were placed in the chambers one hour after being exposed to the treatments. Eighteen hours later, one or two sets of 150 eggs were collected from each chamber (depending on how many eggs were laid) and placed in a vial (up to four vials of 150 eggs for each treatment could be collected for each replicate, depending on how many eggs were collected from the chambers). We recorded the number of offspring that eclosed after 14 days. Three independent experiments were carried out, in which two chambers were used for each treatment and each line. A total of 12 vials of 150 GAo eggs, 10 vials of 150 IR 57 eggs and seven vials of 150 IR 56 eggs were measured for each treatment (Naïve, Sham, LL and PA).
Egg size
Three independent replicates were carried out. Eggs were collected as in the ‘Viability’ measurement, but with only one egg-laying chamber per independent replicate (for each treatment) and 50 eggs collected from each chamber. Approximately 150 eggs in total (spread across the three independent replicates) for each treatment and each line were photographed using a Q-Imaging photo-capture system on a dissection microscope. The length and width of each egg was measured using NIH Image J software. The entire set of eggs was measured three times and the average for each egg was used to reduce the measurement error (we found a repeatability of r2 = 0.87 for length and r2 = 0.40 for width).
Longevity of offspring
After exposure to infection treatments, females from each treatment were placed in egg laying chambers for 18 hours (two chambers per replicate for each treatment; three independent replicates). Eggs were then collected and 120 eggs transferred to each vial. Virgin females were then collected from these vials nine days later and placed into survival cages (approximately 25–35 females per cage). In each replicate (three independent replicates total) there were two survival cages per treatment (to give a total of six survival cages per treatment). For the GAo line, each cage contained approximately 35 females, giving a total of approximately 200 females spread across the six cages. Approximately 130 females across six cages were measured for each treatment for IR 56 and IR 57 (in these lines there were only about 25 females per cage).
Oxidative stress of offspring
Offspring were collected as virgins as in the ‘Longevity’ measurement. Females were aged for three days and then exposed to a 3% H2O2 + 5% sucrose solution. Two ml of the solution were added to a filter paper and 15 females were placed in each vial. Number of adults surviving was recorded approximately every eight hours over 120 hours, by which time >90% of the flies had died. Approximately 200 females across three independent replicates (~50 to 75 per replicate) were analyzed in each treatment for GAo, 130 females for IR 56, and 160 females for IR 57. Control vials were filled with two ml of a 5% sucrose solution.
Immune ability (priming) of offspring
Female offspring were collected as virgins as in the ‘Longevity’ measurement and aged for three days, after which flies were infected with a lethal dose of bacteria. Survival was then measured for 48 hours by which time over 80% of the flies had died. Our previous work has shown that by 48 h, any fly that was going to succumb to infections at these concentrations would have done so.28 Fifty females were infected and measured for each treatment (Naïve-PA, Sham-PA and PA-PA; or Naïve-LL, Sham-LL and LL-LL) in three independent replicates, giving a total of approximately 150 females per treatment in each line. Infections with P. aeruginosa were conducted separately from those with L. lactis.
Statistics
We used a proportional hazards model to analyze survival data.46,47 If there was a significant effect of treatment on survival based on the proportional hazards model, pair-wise comparisons of the treatments were then carried out. Post-hoc analyses were done using a False Discovery Rate (FDR) method, and q-values are reported. A priori, we had decided make four comparisons among the treatments: Naïve ≠ PA, Naïve ≠ LL, Sham ≠ PA and Sham ≠ LL. ANOVAs were conducted on treatment-by-strain interactions. For analysis within a strain ANOVAs were conducted on each replicate separately, and then post hoc comparisons were conducted using FDR. We then calculated an overall p-value using a Fisher’s Combined Probability test. All data were analyzed using JMP 7.0.48
Acknowledgements
We would like to thank the Promislow lab and Kelly Dyer for comments on early versions of this manuscript. We would like to thank Bill Rice and David Moeller for statistical advice. We would also like to thank several organizations for supporting Jodell E. Linder. during the completion of this project: the American Association of University Women for a dissertation completion grant, the Achievement Rewards for College Scientists (ARCS) fellowship foundation, and the Kirby and Jan Alton Fellowship through the University of Georgia Genetics department.
References
- 1.Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- 2.Hultmark D. Drosophila immunity: Paths and patterns. Curr Opin Immunol. 2003;15:12–19. doi: 10.1016/s0952-7915(02)00005-5. [DOI] [PubMed] [Google Scholar]
- 3.Strand MR. The insect cellular immune response. Insect Science. 2008;15:1–14. [Google Scholar]
- 4.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Ann Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 5.Kurtz J, Franz K. Evidence for memory in invertebrate immunity. Nature. 2003;425:37–38. doi: 10.1038/425037a. [DOI] [PubMed] [Google Scholar]
- 6.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. Akt and Foxo dysregulation contribute to infection-induced wasting in Drosophila. Curr Biol. 2006;16:1977–1985. doi: 10.1016/j.cub.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 7.Moret Y, Siva-Jothy MT. Adaptive innate immunity? Responsive-mode prophylaxis in the mealworm beetle, Tenebrio molitor. Proc R Soc London B Bio. 2003;270:2475–2480. doi: 10.1098/rspb.2003.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Little TJ, O’Connor B, Colegrave N, Watt K, Read AF. Maternal transfer of strain-specific immunity in an invertebrate. Curr Biol. 2003;13:489–492. doi: 10.1016/s0960-9822(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 9.Sadd BM, Kleinlogel Y, Schmid-Hempel R, Schmid-Hempel P. Trans-generational immune priming in a social insect. Biol Lett. 2005;1:386–388. doi: 10.1098/rsbl.2005.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moret Y. ‘Trans-generational immune priming’: Specific enhancement of the antimicrobial immune response in the mealworm beetle, Tenebrio molitor. Proc R Soc B Biol Sci. 2006;273:1399–1405. doi: 10.1098/rspb.2006.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlesworth B. Patterns of age-specific means and genetic variances of mortality rates predicted by the mutation-accumulation theory of ageing. J Theor Biol. 2001;210:47–65. doi: 10.1006/jtbi.2001.2296. [DOI] [PubMed] [Google Scholar]
- 12.Tatar M, Carey JR. Nutrition mediates reproductive trade-offs with age-specific mortality in the beetle Callosobruchus maculatus. Ecology. 1995;76:2066–2073. [Google Scholar]
- 13.Smith CC, Fretwell SD. Optimal balance between size and number of offspring. Am Nat. 1974;108:499–506. [Google Scholar]
- 14.Fox CW, Czesak ME. Evolutionary ecology of progeny size in arthropods. Ann Rev Entomol. 2000;45:341–369. doi: 10.1146/annurev.ento.45.1.341. [DOI] [PubMed] [Google Scholar]
- 15.Mousseau TA, Fox CW, editors. Maternal Effects as Adaptations. Oxford: Oxford University Press; 1998. [Google Scholar]
- 16.Kern S, Ackermann M, Stearns SC, Kawecki TJ. Decline in offspring viability as a manifestation of aging in Drosophila melanogaster. Evolution. 2001;55:1822–1831. doi: 10.1111/j.0014-3820.2001.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 17.Priest NK, Mackowiak B, Promislow DEL. The role of parental age effects on the evolution of aging. Evolution. 2002;56:927–935. doi: 10.1111/j.0014-3820.2002.tb01405.x. [DOI] [PubMed] [Google Scholar]
- 18.Faurby S, Kjaersgaard A, Pertoldi C, Loeschcke V. The effect of maternal and grandmaternal age in benign and high temperature environments. Exp Gerontol. 2005;40:988–996. doi: 10.1016/j.exger.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Driever W, Nussleinvolhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988;54:83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- 20.Struhl G, Johnston P, Lawrence PA. Control of Drosophila body pattern by the hunchback morphogen gradient. Cell. 1992;69:237–249. doi: 10.1016/0092-8674(92)90405-2. [DOI] [PubMed] [Google Scholar]
- 21.Gilchrist GW, Huey RB. Parental and developmental temperature effects on the thermal dependence of fitness in Drosophila melanogaster. Evolution. 2001;55:209–214. doi: 10.1111/j.0014-3820.2001.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 22.Read A, Graham A, Raberg L. Animal defenses against infectious agents: Is damage control more important than pathogen control? PLoS Biol. 2008;6:2638–2641. doi: 10.1371/journal.pbio.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayers J, Schneider DS. A signaling protease required for melanization in Drosophila affects resistance and tolerance of infections. PLoS Biol. 2008;6:2764–2773. doi: 10.1371/journal.pbio.0060305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zerofsky M, Harel E, Silverman N, Tatar M. Aging of the innate immune response in Drosophila melanogaster. Aging Cell. 2005;4:103–108. doi: 10.1111/j.1474-9728.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- 25.McKean KA, Nunney L. Increased sexual activity reduces male immune function in Drosophila melanogaster. Proc Nat Acad Sci USA. 2001;98:7904–7909. doi: 10.1073/pnas.131216398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawniczak MKN, Begun DJ. A genome-wide analysis of courting and mating responses in Drosophila melanogaster females. Genome. 2004;47:900–910. doi: 10.1139/g04-050. [DOI] [PubMed] [Google Scholar]
- 27.McGraw LA, Gibson G, Clark AG, Wolfner MF. Genes regulated by mating, sperm or seminal proteins in mated female Drosophila melanogaster. Curr Biol. 2004;14:1509–1514. doi: 10.1016/j.cub.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Fedorka KM, Linder JE, Winterhalter W, Promislow D. Post-mating disparity between potential and realized immune response in Drosophila melanogaster. Proc R Soc London B Bio. 2007;274:1211–1217. doi: 10.1098/rspb.2006.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linder JE, Rice WR. Natural selection and genetic variation for female resistance to harm from males. J Evol Biol. 2005;18:568–575. doi: 10.1111/j.1420-9101.2004.00872.x. [DOI] [PubMed] [Google Scholar]
- 30.Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 31.Azevedo RBR, French V, Partridge L. Life-history consequences of egg size in Drosophila melanogaster. Am Nat. 1997;150:250–282. doi: 10.1086/286065. [DOI] [PubMed] [Google Scholar]
- 32.Fellowes MDE, Kraaijeveld AR, Godfray HCJ. The relative fitness of Drosophila melanogaster (Diptera, Drosophilidae) that have successfully defended themselves against the parasitoid Asobara tabida (Hymenoptera, Braconidae) J Evol Biol. 1999;12:123–128. [Google Scholar]
- 33.Brandt SM, Schneider DS. Bacterial infection of fly ovaries reduces egg production and induces local hemocyte activation. Dev Comp Immunol. 2007;31:1121–1130. doi: 10.1016/j.dci.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wigby S, Domanitskaya EV, Choffat Y, Kubli E, Chapman T. The effect of mating on immunity can be masked by experimental piercing in female Drosophila melanogaster. J Insect Physiol. 2008;54:414–420. doi: 10.1016/j.jinsphys.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazzaro BP, Flores HA, Lorigan JG, Yourth CP. Genotype-by-environment interactions and adaptation to local temperature affect immunity and fecundity in Drosophila melanogaster. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKean KA, Yourth CP, Lazzaro BP, Clark AG. The evolutionary costs of immunological maintenance and deployment. BMC Evol Biol. 2008;8 doi: 10.1186/1471-2148-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fedorka KM, Mousseau TA. Immune system activation affects male sexual signal and reproductive potential in crickets. Behav Ecol. 2007;18:231–235. [Google Scholar]
- 38.Moret Y, Schmid-Hempel P. Survival for immunity: The price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins NL, McColl G, Lithgow GJ. Fitness cost of extended lifespan in Caenorhabditis elegans. Proc R Soc Lond B Bio. 2004;271:2523–2526. doi: 10.1098/rspb.2004.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corby-Harris V, Habel KE, Ali FG, Promislow DEL. Alternative measures of response to Pseudomonas aeruginosa infection in Drosophila melanogaster. J Evolutino Biol. 2007;20:526–533. doi: 10.1111/j.1420-9101.2006.01267.x. [DOI] [PubMed] [Google Scholar]
- 41.Voordouw MJ, Lambrechts L, Koella J. No maternal effects after stimulation of the melanization response in the yellow fever mosquito Aedes aegypti. Oikos. 2008;117:1269–1279. [Google Scholar]
- 42.Little T, Birch J, Vale P, Tseng M. Parasite transgenerational effects on infection. Evol Ecol Res. 2007;9:459–469. [Google Scholar]
- 43.Elliot SL, Blanford S, Horton CM, Thomas MB. Fever and phenotype: Transgenerational effect of disease on desert locust phase state. Ecol Lett. 2003;6:830–836. [Google Scholar]
- 44.McMeniman CJ, Lane RV, Cass BN, Fong AMC, Sidhu M, Wang YF, O’Neill SL. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 45.Linder JE, Owers KA, Promislow DEL. The effects of temperature on host-pathogen interactions in D. melanogaster: Who benefits? J Insect Physiol. 2008;54:297–308. doi: 10.1016/j.jinsphys.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parmar MKB, Machin D. Survival Analysis: A Practical Approach. Chichester: Wiley; 1995. [Google Scholar]
- 47.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 48.JMP. Version 7. Cary, NC: SAS Institute Inc.; 1989–2007. [Google Scholar]