Abstract
Background
Chronic iron-overload (CIO) is associated with blood disorders such as thalassemias and hemochromatosis. A major prognostic indicator of survival in patients with CIO is iron-mediated cardiomyopathy, characterized by contractile dysfunction and electrical disturbances including slow heart rate (HR; bradycardia) and heart block.
Methods and Results
We have used a mouse model of CIO to investigate the effects of iron on sinoatrial node (SAN) function. As in humans, CIO reduced HR (~20%) in conscious mice as well as in anesthetized mice with autonomic nervous system blockade and in isolated Langendorff-perfused mouse hearts, suggesting bradycardia originates from altered intrinsic SAN pacemaker function. Indeed, spontaneous action potential frequencies in SAN myocytes with CIO were reduced in association with decreased L-type Ca2+ current (ICa,L) densities and positive (rightward) voltage shifts in ICa,L activation. Pacemaker current (If) current was not affected by CIO. Since ICa,L in SAN myocytes (as well as in atrial and conducting system myocytes) activates at relatively negative potentials due to the presence of CaV1.3 channels (in addition to CaV1.2 channels), our data suggest that elevated iron preferentially suppresses CaV1.3 channel function. Consistent with this suggestion, CIO reduced CaV1.3 mRNA levels by ~40% in atrial tissue (containing SAN) and did not lower HR in CaV1.3 knockout mice. CIO also induced PR interval prolongation, heart block, and atrial fibrillation, conditions also seen in CaV1.3 knockout mice.
Conclusion
Our results demonstrate that CIO selectively reduces CaV1.3-mediated ICa,L leading to bradycardia, slowing of electrical conduction and atrial fibrillation as seen in iron-overload patients.
Keywords: ion channels, action potential, calcium current, intracardiac electrophysiology
Introduction
The accumulation of excessive amounts of iron in tissues, including the heart, occurs under conditions of primary hemochromatosis (inherited) or hemosiderosis (secondary iron-overload) and causes severe cardiac dysfunction that is a leading cause of death in iron-overload patients.1–4 Patients suffering from iron-overload cardiomyopathies have impaired systolic and diastolic function, often in conjunction with cardiac rhythm disturbances including slowed electrical conduction, heart block and increased susceptibility to atrial fibrillation.5–8 Another common feature of iron-overload patients is a marked slowing of HR (i.e. bradyarrhythmias).1,9–11 Heart rate (HR) reductions along with altered electrical conduction are also prominent features of animal models of chronic iron-overload.12–15
Although the functional changes seen with iron-overload are well documented, the molecular and physiological basis for the bradycardia and other electrical disturbances remains unknown. Since HR is a primary determinant of cardiac output, and a key parameter used to regulate the cardiovascular system, bradycardia is predicted to contribute to cardiovascular changes, such as reduced blood pressure, typically observed in iron-overload patients.16 Bardycardia in iron-overload patients could originate from factors extrinsic or intrinsic to the heart. For example, elevated iron could affect autonomic nervous system activity, possibly by impairing neuronal function17 or interfere with other elements of the baroreceptor reflex pathways external to the heart.18 Alternatively, intrinsic electrical activity of the specialized pacemaker myocytes located in the sinoatrial node (SAN)19,20 could also be disrupted by iron as a result of modulation of a number membrane currents21, Ca2+ handling22,23 or intracellular signalling.24
In the present study, we investigated the mechanism(s) by which chronic iron-overload (CIO) slows HR using iron-overloaded mice. Our data demonstrate that CIO causes pronounced HR slowing, in association with other electrical changes including P-R interval prolongation and heart block as well as impaired intrinsic firing rate of SAN myocytes and rightward shifts in ICa,L activation These results suggest bradycardia is caused by reductions in CaV1.3-based L-type Ca2+ current, which is expressed (along with Cav1.2-based channels), in SAN, atria and the cardiac conduction system, but not working ventricles.20 Indeed, CIO caused reductions in CaV1.3 mRNA but not CaV1.2 mRNA. Mice lacking CaV1.3 did not show reductions in HR or P-R interval prolongation when iron was elevated. Thus, we conclude that the slowing of HR and electrical conduction induced by iron-overload is mediated by reductions in CaV1.3 expression. Some of these data have been presented in abstract form.25
Methods
An expanded methods section is provided in the Online Data Supplement.
Animals and iron-loading protocol
Mice with CIO were generated as described previously14 and in the Online Data Supplement
Electrocardiography
Electrocardiograms (ECGs) were recorded using telemetry in conscious mice or using body surface and intracardiac ECGs in anesthetized mice. See Online Data Supplement.
Isolated SAN and right atrial myocyte electrophysiology
Single cardiomyocytes from the SAN or the right atrial appendage were isolated and used in patch-clamp studies as previously descibed27–29 See Online Data Supplement for further details.
Statistics
All summary data are presented as means±SEM. Results were considered statistically significant if P-values were below 0.05. P-values were determined using paired or unpaired Student’s t-tests, 1-way ANOVA, 1-way repeated measures ANOVA or rank sum tests (when the variance differed between groups) as appropriate. Actual P-values are provided unless they were unavailable, as occurred when 1-way repeated measures ANOVA or rank sum tests were applied to the data; thus significance is indicated as <0.05 or 0.001 for nonparametric tests.
Statement of Responsibility
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Following 4 weeks of iron loading14, iron levels in hearts of CIO mice were elevated (P=6.1×10−7) to 13.1±2.1 mg/g-dry weight (DW) compared to 0.55±0.1 mg/g-DW measured in dextrose-injected (control) mice. CIO also caused reductions in systolic function (Table S1 and Figure S1 in Online Supplement) and an increased E/A ratio. These results are identical to previous reports which established that the iron accumulates in cardiomyocytes.14 Although there were reductions in body-weights (P<0.001) and heart-weights (P<0.001) in CIO mice compared to control, there were no differences (P=0.87) in heart-weight/body-weight ratios (Table SII). Similar to previous studies 30, hepatic iron levels were elevated (P<0.001) to 73.1±6.8 mg/g-DW in CIO mice compared with 0.41+/−0.1 mg/g-DW for control in conjunction with increased liver-weights (P<0.001) and liver-weight/body-weight ratios (P<0.001).
Figure 1 summarizes telemetry recordings in mice. Since HR fluctuates daily due to circadian influences, we measured ECGs every 4 hour over a 48 hour period each week. Figure 1A shows typical ECG recordings made during the sleeping period for mice prior to injection (i.e. baseline), as well as 2 and 4 weeks after either placebo or iron injection. As expected, HR showed circadian fluctuations (Figure 1B). Iron injection for 2 weeks reduced HRs without noticeably affecting diurnal sleep-wake fluctuations. Indeed, HRs (averaged over 48 hours) progressively decreased as the period of iron injection was extended, with HRs decreasing (P<0.05) from 605±12 beats/min at baseline to 529±14 beats/min after 4 weeks of CIO (Figure 1C). Placebo injection had no effect (P=0.91) on HR over the same four-week period (607±14 beats/min versus 591±12 beats/min at baseline). It is conceivable that these HR reductions contribute to the impaired cardiac contractility typically seen in this condition1–5, since contractility increases with elevated HRs (see Discussion).
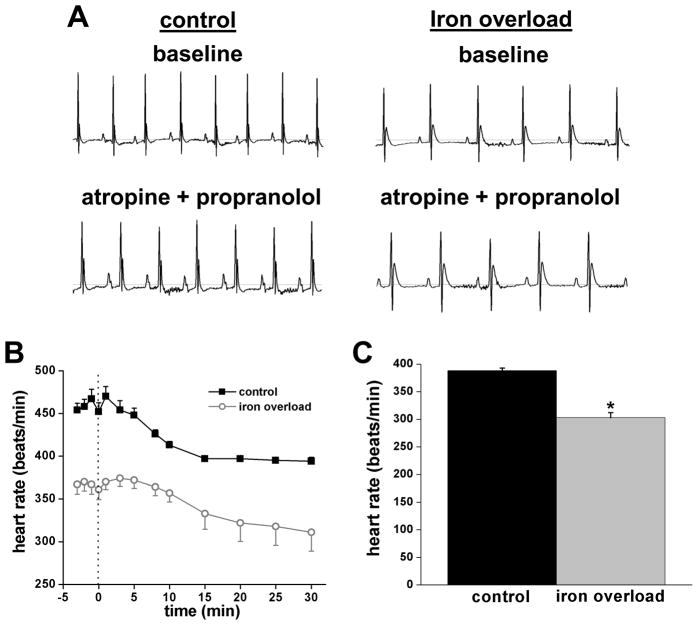
Figure 1.
Effects of chronic iron-overload on HR in conscious mice. A, Representative telemetry ECG recordings (1 second duration) before iron injection (i.e. baseline) and after 2 and 4 weeks of injection with iron or placebo. Sample ECGs were measured at 2 PM (during mouse sleeping) to control for circadian rhythms. B, HR measured every 4 hours over a consecutive 48 h period illustrating prominent circadian rhythms. C, Summary HR data averaged over a 4 week period in control conditions and following iron-overload. For panels B and C data are means±SEM; n = 5–7 mice per group. *P<0.05 compared to control at the same time point analyzed by Student’s t-test.
The alterations in HR observed in CIO mice could arise from several mechanisms. For example, iron elevations could affect autonomic nerve activity31 or directly influence properties of the SAN. To distinguish between these possibilities, ECGs were recorded in anesthetized mice with and without blockade of the autonomic nervous system, thereby allowing assessment of intrinsic SAN function.27 As illustrated in Figure 2, intraperitoneal injection of atropine (1 mg/kg) plus propranolol (10 mg/kg) caused similar relative HR reductions in placebo (i.e. from 458±9 to 394±5 beats/min) and iron-injected (i.e. from 367±12 to 311±22 beats/min) mice. Consequently, HR remained reduced (P<0.05) in the anesthetized CIO mice compared to control mice after autonomic blockade. These results establish that bradycardia associated with iron-overload results from impairment of the intrinsic properties of the SAN and further suggest that autonomic nerve function is not measurably altered by iron-overload, consistent with the absence of alterations in circadian rhythms by elevated iron. Intrinsic changes in SAN firing were confirmed (Figure 2C) in isolated Langendorff-perfused hearts which showed lower (P=1×10−7) HRs when iron loaded (303±9 beats/min) versus control (388±5 beats/min). The similarity in HR between isolated hearts and mice injected with atropine and propranolol confirms that complete autonomic blockade was achieved when mice were treated with these agents.
Figure 2.
Effects of chronic iron-overload on ECGs measured in anesthetized mice following autonomic nervous system blockade by intraperitoneal injection of atropine (1 mg/kg) and propranolol (10 mg/kg) in isolated Langendorff-perfused hearts. A, Representative ECGs (1 second recordings) in control conditions and in iron-overload at baseline (pre-injection) as well as 25 min after injection of atropine and propranolol. B, Summary data showing HR at baseline and following autonomic nervous system blockade. Data are means±SEM, n=7 mice in each group. HR was reduced (P<0.05) at all time points in iron-overload mice compared to controls (Student’s t-test). C, Summary data showing averaged HRs in isolated Langendorff-perfused hearts from control and iron-overload mice. Data are means±SEM, n=10–11 hearts per group, *P<0.05 vs. control, by Student’s t-test.
To explore the cellular basis for the iron-dependent changes in intrinsic SAN function, spontaneous APs were measured (Figure 3A) in isolated SAN myocytes (22–23°C). The frequency of spontaneous APs (Figure 3B) was reduced (P=3.0×10−5) in SAN myocytes from CIO mice (91±5 beats/min) compared to control (138±6 beats/min). Detailed analysis of the AP profiles revealed that the slope of the diastolic depolarization (DD), a major determinant of firing frequency, was decreased (P=3.1×10−5) in SAN myocytes from CIO mice (Figure 3B and Table SIII). Elevated iron also reduced (P=0.04) the action potential overshoot (OS) as well as action potential durations, at both 50% (P=0.01) and 90% (P=0.02) repolarization times. On the other hand, the maximum diastolic potential and AP upstroke velocity (Vmax) were not affected by iron (Table SIII).
Figure 3.
A, representative spontaneous action potentials recorded from isolated SAN myocytes in control condition and following iron-overload. B, Summary data illustrating the effects of iron loading on spontaneous AP frequency, diastolic depolarization slope and APD90. Data are means±SEM, n=10 myocytes in each group, *P<0.05 vs. control by Student’s t-test. See also Supplemental Table SIII.
Because the DD slope is a major determinant of spontaneous firing rate, we measured selected ionic currents expected to be active during the diastolic period (i.e. hyperpolarization-activated “pacemaker” current (If) and L-type Ca2+ currents (ICa,L)).20 The density of If, measured at the end of 2 s voltage-clamp steps, did not differ (P=0.87) between control and CIO SAN myocytes regardless of the voltage-step used (Figure 4). This lack of effect of iron loading on If density between the groups is consistent with the absence of differences (P=0.84) in the. maximum diastolic potentials (Table SIII) between CIO SAN myocytes (−68.1±1.6 mV) and control (−67.7±1.5 mV) during spontaneous AP firing. Next, we used voltage-clamp protocols designed to identify the two components of ICa,L (i.e. CaV1.2 and CaV1.3-based current) observed in myocytes of SAN, atria and conducting systems.32 Peak ICa,L density (Figure 5) was reduced in CIO compared to control, particularly at negative membrane potentials. Specifically, ICa,L densities in control SAN myocytes have the largest magnitude (−5.1±0.9 pA/pF) at about −10 mV. By contrast, the maximum ICa,L magnitude (−2.6±0.2 pA/pF) was shifted to ~ +10 mV and was reduced (P=0.006) in CIO SAN myocytes compared to control. This voltage where ICa,L peaks in CIO SAN myocytes is similar to voltages typically observed in ventricular myocytes (~10 mV), which only express CaV1.2-based ICa,L.32 These observations suggest that iron preferentially reduces the more negatively activating CaV1.3-based ICa,L. To further quantify the changes in ICa,L induced by iron, the steady-state conductance was calculated (Figure 5C). Maximum ICa,L conductance (Gmax) in CIO SAN myocytes was reduced (P=0.004) to 71.7±2.3 pS/pF from 118.9±7.3 pS/pF in control SAN myocytes. Furthermore, the voltage required for 50% channel activation (V1/2) was shifted (P=0.002) from −21.9±3.1 mV in controls to −6.2±2.6 mV in CIO myocytes. Preferential reductions in ICa,L at negative potentials induced by elevated iron readily explain the DD slope reductions observed during measurements of spontaneous AP firing.
Figure 4.
Properties of the hyperpolarization-activated current, If, in SAN myocytes from CIO mice. A, Representative If recordings in SAN myocytes. Voltage-clamp protocol is shown in the inset. B, Summary If I-V curves showing no significant difference between control and iron-overload conditions. Data are means±SEM, n=5 control myocytes and n=6 iron-overload myocytes.
Figure 5.
Effects of CIO on ICa,L in SAN and right atrial myocytes. CaV1.2 and CaV1.3-dependent ICa,L was measured simultaneously (see Methods and inset in panel A). A, Representative ICa,L recordings in SAN myocytes. B, ICa,L I-V curves in sinoatrial node myocytes. Data are means±SEM, n=8 myocytes for each group; P<0.05 compared to control at the same membrane potential analyzed by Student’s t-test. C, ICa,L activation curves in SAN myocytes. D, Representative ICa,L recordings in working right atrial myocytes. E, ICa,L I-V curves in working right atrial myocytes. Data are means±SEM, n=7 atrial myocytes for control and n=11 atrial myocytes for iron-overload; *P<0.05 compared to control at the same membrane potential analyzed by Student’s t-test. F, ICa,L activation curves in working right atrial myocytes.
Since CaV1.3 channels activate more negatively than CaV1.2 channels32, the positive shifts in V1/2 of ICa,L induced by iron suggest a preferential reduction in CaV1.3-mediated ICa,L. To test this suggestion, we measured ICa,L in right atrial myocytes (Figure 5D) which also express CaV1.3. Similar to SAN myocytes, but unlike ventricular myocytes14, peak ICa,L was reduced (P=0.022) to −1.4±0.1 pA/pF in CIO right atrial myocytes from −2.5±0.3 pA/pF in control while the voltage at which the maximal ICa,L amplitude occurred was rightward shifted in CIO (Figure 5E). Indeed, Gmax for ICa,L (Figure 5F) was reduced (P=0.006) from 70.6±4.4 pS/pF in control atrial myocytes to 43.9±2.3 pS/pF in CIO myocytes while the V1/2 was right-shifted (P=0.034) from −19.5±1.8 mV (control) to −13.6±1.6 mV (CIO).
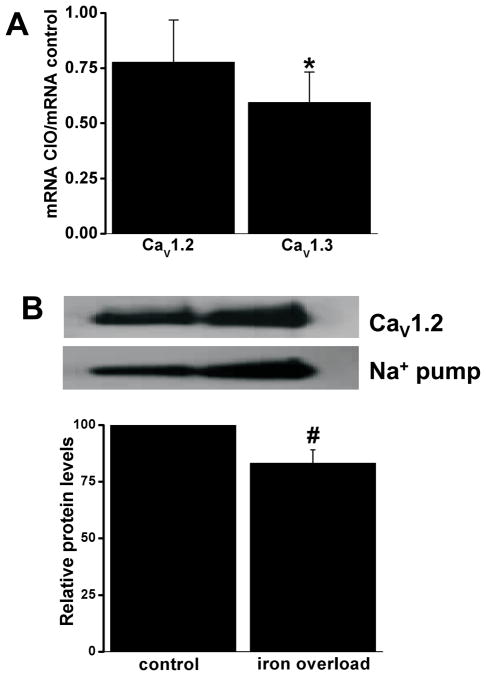
Overall, these changes in CIO resemble closely those seen in CaV1.3-deficient mice33, supporting the conclusion that CIO preferentially reduces CaV1.3-mediated ICa,L compared to Cav1.2 channels (see below). This conclusion is bolstered by our previous results showing that CIO had only minor effects on ICa,L density in ventricular myocytes14 which, unlike SAN and atrial myocytes, do not express CaV1.3.32–34 To interrogate this conclusion further, CaV1.2 and CaV1.3 expression levels (mRNA and protein) were measured in homogenates from the right atrium (including the SAN). Consistent with the iron-induced ICa,L changes, CaV1.3 mRNA levels were more than 40% lower (P=0.018) in CIO compared to control (Figure 6). We tried to confirm these changes at the protein level but these attempts failed (See Online Supplement). By contrast, CaV1.2 mRNA trended towards reductions (P=0.336) while CaV1.2 protein was modestly reduced by ~20% (Figure 6). Thus, we conclude that iron-overload preferentially reduces CaV1.3-mediated currents, leading to reduced spontaneous firing rates of SAN myocytes.
Figure 6.
A, Real-time PCR measurements of mRNA level for the CaV1.2 and CaV1.3 alpha subunits of the L-type Ca2+ channels. The results show the ratio of mRNA in iron-loaded atria versus placebo control for CaV1.2 and CaV1.3. n=5 for all groups. *P<0.05 compared to 1 using the Student’s t-test. B, Representative Western Blot for CaV1.2 and Na+/K+ ATPase (loading control) in atria isolated (top) along with the relative protein expression levels of CaV1.2 normalized by the level of Na+ pump expression for control and iron-overload atria. n=3 for control and iron-overload. #P<0.05 relative to control, Student’s t-test.
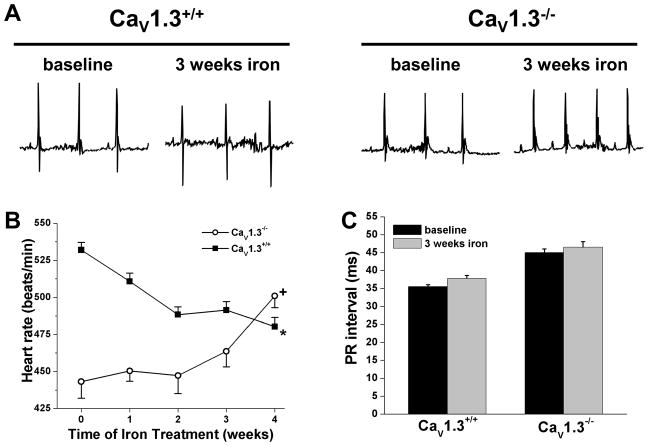
If down-regulation of CaV1.3-based ICa,L is responsible for the observed effects of CIO, then HR reductions should not occur in mice lacking the CaV1.3 channel subunit. Consistent with previous reports33,35, HRs in mice lacking CaV1.3 are lower (P<0.001) than in wild-type littermates (Figure 7). More important, iron-overload caused HR increases (P<0.05) in CaV1.3−/− mice which caused HRs convergence of HRs between the CaV1.3−/− and wild-type littermates during the iron injection period. Data beyond 3 weeks of iron injection are not shown because of relatively high mortality in both cohorts after 3 weeks of iron injection, which appears to be related to strain differences in response to iron. These findings support the conclusion that decreased CaV1.3-based channel expression is the dominant factor responsible for the HR reductions in iron-overload.
Figure 7.
Effects of iron treatment in conscious CaV1.3 knockout mice. A, Representative telemetry ECGs (0.5 second recordings) before (baseline) and after (3 weeks) iron injection in Cav1.3 knockout and littermate wild-type mice. All sample ECGs were measured at 2 PM (sleeptime). B, HRs averaged over a 48 hour period (measured every 4 hours) for Cav1.3 knockout and littermate wild-type mice showing increases in HR for Cav1.3 knockout mice but decreases in wild-type littermate controls. C, Summary of PR-intervals after 3 weeks of iron injection in Cav1.3 knockout and littermate wild-type mice. For panels B and C data are means±SEM; n>7 mice per group. +P<0.001 compared to baseline (time = 0) analyzed by Kruskal-Wallis test with Dunn’s posthoc analysis. *P<0.05 compared to wild-type littermates at the same time point using Student’s t-test.
In addition to being critical in setting spontaneous firing rates of SAN myocytes32,34, CaV1.3 alpha subunits also regulate electrical conduction in hearts33,36 which was assessed in anesthetized mice using surface ECGs (with and without autonomic blockade), octapolar electrophysiology catheters, and isolated Langandorff-perfused hearts. As summarized in Table 1, elevated iron altered several ECG parameters, similar to iron-overload patients4 and gerbils.12,13 For example, the durations of the P- and QRS-signals were prolonged in intracardiac recordings, possibly as a result of reductions in voltage-gated Na+ currents (data not shown), as seen in gerbils.21 More important, PR intervals were prolonged by iron-overload in anesthetized mice and isolated hearts (Figure 8), indicating 1st degree heart block. Intracardiac PR intervals were also prolonged (P=0.035) in CIO mice (37.2 +/− 1.0 ms) compared to control (47.4 +/− 0.7 ms) in conjunction with longer AH intervals (Table 1), establishing slowing through the AV node and HIS bundle, as reported previously.12 In addition, 2:1 AV-node block and sinus pauses (Figure 8) as well as prolonged (P=0.006) SAN recovery time (Table 1) were also observed (Figure 8) in anesthetized CIO mice, and with increased frequency following autonomic blockade (data not shown). None of these conduction disturbances were observed in control mice. Similar conduction and ECG changes have been documented in CaV1.3 knockout mice33,36 and elevated iron did not affect the PR interval in Cav1.3 knockout mice (Figure 7), supporting further the conclusion that the electrical disturbances induced by iron-overload arise from reductions in CaV1.3-based ICa,L.
Table 1.
Intracardiac ECG parameters from control and iron-overload mice.
| Control | Iron-overload | P-value | |
|---|---|---|---|
| RR | 124±5 | 158±7** | 0.0032 |
| PR | 39.8±1.0 | 43.6±1.2* | 0.0346 |
| Pwidth | 16.4±0.5 | 20.3±0.8** | 0.0012 |
| AH | 25.7±0.7 | 29.2±1.4* | 0.0397 |
| HV | 12.3±0.5 | 12.00±0.5 | 0.633 |
| QRS | 10.8±0.5 | 11.3±0.5 | 0.493 |
| QTC | 41.9±0.9 | 48.2±1.4** | 0.0027 |
| cSNRT | 26.4±4.7 | 62.0±10.3** | 0.0059 |
| AVNERP | 60.3±2.6 | 63.4±7.7 | 0.6694 |
| AVW | 78.4±2.3 | 88.8±5.5 | 0.0815 |
| VERP | 17.9±2.8 | 44.4±4.9*** | 0.0005 |
RR, R-R interval; PR, P-R interval; Pwidth, P wave width; AH, interval from the start of P wave to the start of the HIS bundle signal (i.e. H signal); HV interval; QTc, corrected QT interval; cSNRT, corrected sinus node recovery time; AVNERP, AVN effective refractory period; AVW, induction of Wenckebach AVN block; VERP, ventricular effective refractory period. Data are means±SEM; n=7 control and 5 iron-overload mice. Data were analyzed with Student’s T-test;
p<0.05,
p<0.01,
p<0.001 vs. control.
Figure 8.
A. Representative ECG recordings illustrating conduction disturbances observed in anesthetized CIO mice after 3 weeks of iron injection: top trace sinus pause, middle PR interval prolongation (1st degree ANV block) and bottom trace 2:1 AVN block. No conduction disturbances were observed in control mice. B. Average PR-intervals at baseline (before iron injection) and after 3 weeks of iron injection in anesthetized mice and hearts excised from iron-overload mice.
Discussion
Our mouse model of secondary iron-overload reproduces the electrical changes observed in patients suffering from iron-overload.1,9–11 This model has been used by us14 and others12,13,15, and leads to iron elevations in the liver and heart similar to humans with advanced iron-overload. Our investigations revealed a pronounced bradycardia in CIO mice, as seen in iron-overload patients.1,9–11 Bradycardia could contribute to the impaired cardiac contractility seen with CIO4,5,8,14 (independent of iron’s effects on myocardial contractility and cardiac fibrosis4,5,8,14), as a consequence of the force-frequency relationship (FFR), which varies between mouse strain.37 Regardless, our results show that the bradycardia in CIO results from slowing of the spontaneous activity of the SAN in association with changes AP properties. These electrical changes were tightly linked (see below) to ICa,L reductions and rightward shifts in ICa,L activation consistent with preferential reduction in CaV1.3-, versus CaV1.2-dependent, L-type Ca2+ currents. CIO did not cause changes in If current. These observations support the conclusion that the reductions in HR as well as the PR-interval prolongation, heart block and conduction deficits seen in iron-overload result from selective reductions in CaV1.3-mediated ICa,L. Indeed, CIO did not cause HR reductions or PR-interval prolongation in mice lacking Cav1.3. In addition, lower HRs observed in Cav1.3−/− mice compared to wild-type littermates26,32 were abolished by iron treatment, supporting the conclusion that Cav1.3 reductions are, indeed, the primary cause of HR reductions in iron-overload.
The reductions of ICa,L in SAN and atrial myocytes induced by CIO were initially somewhat surprising because ICa,L density is not measurably altered in ventricular myocytes from CIO mice.14 However, ICa,L in SAN and atria myocytes arises from both CaV1.2 and CaV1.3 L-type channels, whereas ventricular myocytes only express CaV1.2 subunits32–34, suggesting that the ICa,L reductions observed in iron-loaded SAN and atrial myocytes may result from differential reduction in CaV1.3-mediated ICa,L. Consistent with this suggestion, the mRNA levels of CaV1.3 were reduced by ~40% in right atria. Because whole atria included the SA node, we infer that reductions in CaV1.3 occur in both atrial and SAN myocytes following iron loading. On the other hand, CaV1.2 mRNA levels were unaffected by iron, although protein was slightly reduced. The connection between preferential reductions in CaV1.3-based ICa,L is further supported by the large rightward shiftsin the V1/2 for ICa,L activation and in the voltage of the maximal ICa,L amplitude in myocytes from SAN and atria since CaV1.3 channels activate at more negative potentials (~−50 mV) than CaV1.2-mediated ICa,L (~−30 mV).32 Moreover, SAN and AVN myocytes express relatively more CaV1.3 channels than atrial cells which, given the differential effects of iron on CaV1.3 channels, explains the larger effects of iron-overload on ICa,L in our SAN myocytes versus atrial myocytes. Selective loss of CaV1.3-mediated ICa,L is further supported by the observation that the voltage-dependence of ICa,L in atrial and SAN myocytes after iron are similar to that typically seen ventricular myocytes.38
Selective reductions in CaV1.3-dependent ICa,L readily explains reduced spontaneous firing rates of SAN myocytes and, thereby, HR slowing of in vivo and isolated hearts with iron-overload. Indeed, we found that mice lacking CaV1.3 channels have reduced HRs and slow spontaneous firing rates in isolated SAN-atrial preparations compared to wild-type littermates, as reported previously.32,34 Moreover, HRs actually increased in iron-overload Cav1.3 knockout mice, possibly as a result of impairment of heart function and the cardiomyopathy induced by CIO4,14 which is expected to induce compensatory changes in autonomic nerve activity. Presumably excessive iron causes reductions in diastolic depolarization slope because of reduced CaV1.3-dependent ICa,L currents, particularly at voltages between −50 mV and −20 mV where CaV1.3 channel activate. These reduced slopes delay the time required for membrane potentials to reach the threshold for CaV1.2-dependent ICa,L thereby depressing spontaneous firing rates. Consistent with this conclusion, CaV1.3 knockout mice also showed lower DD slopes in association with reduced HRs.32
Based on previous findings in SAN myocytes39, the ICa,L changes can also explain reduced AP amplitudes and durations in SAN myocytes. While is conceivable that changes in other currents also occur in iron-overload such as the changes in Na+ current (INa) and transient outward potassium current (Ito) seen in ventricular myocytes of gerbils21, the relevance of these changes is unclear since SAN myocytes have relatively low densities of INa and Ito.39,40
In addition to bradycardia, iron-overload also causes other electrical disturbances.5,7,8,41 In our CIO mice, we reproducibly observed sinus pauses, prolonged PR intervals and second degree heart block. Interestingly, these conduction disturbances are also seen in CaV1.3 knockout mice.33,34,36,42 PR-interval prolongation and 2nd degree heart block are because CaV1.3 channels are expressed in the AV node32,43 where they are critical for electrical conduction.36,42 In fact, disturbances of atrio-ventricular conduction and heart block are seen both in CaV1.3 knockout mice33,36 and in congenital heart block, a condition linked to elevated maternal antibodies to CaV1.3 channels which block CaV1.3-dependent ICa,L.43
Previous studies have established that decreased ICa,L density occurs in atrial myocytes from humans44 and animals models45 with atrial fibrillation with reductions in both CaV1.2 and CaV1.3 expression being linked to atrial fibrillation in humans.46 A reduction in CaV1.3 channels seems particularly relevant because CaV1.3 knockout mice are susceptible to atrial fibrillation42. Atrial fibrillation has also been associated with CIO. 35 Our results, therefore, suggest that atrial fibrillation in iron-overload patients could arise from a reduction in Cav1.3-based ICa,L. Indeed, although atrial fibrillation was not seen in conscious telemetric implanted CIO mice, stable rotors could be induced in 2 of 10 isolated atrial preparations obtained from iron-overload mice while the sensitivity of isolated atria to AF induction in the presence of the parasympathomimetic, carbachol, was shifted to lower concentrations by iron-overload (Figure S2). Clearly, additional studies will be required to more fully assess possible connections between iron-induced changes of CaV1.3-mediated ICa,L and atrial fibrillation in iron-overload patients.
The precise mechanism(s) by which iron-overload inhibits ICa,L in the SAN and atria are not clear. The CaV1.3 gene does not have an iron response element in its promoter.4 On the other hand, iron-overload induces oxidative damage and increases fibrosis4 which could contribute to alterations in HRs and electrical conduction. Elevated iron could also conceivably alter channel properties like permeation and gating properties, as we reported previously.38 The fact that some currents are reduced (ICa,L in SAN and atrial myocytes, INa in ventricular myocytes21) whereas others are unaffected (ICa,L in ventricular myocytes14) or even increased (Ito in ventricular myocytes21) suggests that non-specific oxidative damage to ion channel proteins is not the only factor involved in the iron-induced remodelling that occurs with iron-overload.
In conclusion, our results establish that iron-overload in mice leads to decreased CaV1.3-based ICa,L in SAN and atrial myocytes. These changes explain the development of sinus bradycardia, slowed electrical conduction and increased susceptibility to atrial fibrillation in mice as well as human iron-overload patients. Future studies will be needed to assess whether preventing reductions in CaV1.3 channel proteins alleviates the electrical remodelling and increased susceptibility to arrhythmias seen in iron-overload patients.
Supplementary Material
Acknowledgments
We thank Wallace Yang for technical assistance.
Sources of Funding
This study was supported by Canadian Institutes for Health Research (MOP 79460) to PHB. PHB is a Career Investigator of the Heart and Stroke Foundation of Ontario. GGM acknowledges funding from National Institute on Aging R01AG028488. SH is holds a Canada Research Chair. CC is the recipient of a CIHR Graduate Scholarship, RAR is a CIHR New Investigator supported by operating grants from the CIHR (MOP 93718) and the Heart and Stroke Foundation of Nova Scotia.
Footnotes
Disclosures
None.
References
- 1.Buja LM, Roberts WC. Iron in the heart. Etiology and clinical significance. Am J Med. 1971;51:209–221. doi: 10.1016/0002-9343(71)90240-3. [DOI] [PubMed] [Google Scholar]
- 2.Engle MA, Erlandson M, Smith CH. Late cardiac complications of chronic, severe, refractory anemia with hemochromatosis. Circulation. 1964;30:698–705. doi: 10.1161/01.cir.30.5.698. [DOI] [PubMed] [Google Scholar]
- 3.Liu P, Olivieri N. Iron overload cardiomyopathies: new insights into an old disease. Cardiovasc Drugs Ther. 1994;8:101–110. doi: 10.1007/BF00877096. [DOI] [PubMed] [Google Scholar]
- 4.Oudit GY, Trivieri MG, Khaper N, Liu PP, Backx PH. Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J Mol Med. 2006;84:349–364. doi: 10.1007/s00109-005-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aessopos A, Farmakis D, Karagiorga M, Voskaridou E, Loutradi A, Hatziliami A, Joussef J, Rombos J, Loukopoulos D. Cardiac involvement in thalassemia intermedia: a multicenter study. Blood. 2001;97:3411–3416. doi: 10.1182/blood.v97.11.3411. [DOI] [PubMed] [Google Scholar]
- 6.Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Gui P, Hill MA, Wilson E. Regulation of ion channels by protein tyrosine phosphorylation. Am J Physiol. 2001;281:H1835–H1862. doi: 10.1152/ajpheart.2001.281.5.H1835. [DOI] [PubMed] [Google Scholar]
- 7.Lombardo T, Tamburino C, Bartoloni G, Morrone ML, Frontini V, Italia F, Cordaro S, Privitera A, Calvi V. Cardiac iron overload in thalassemic patients: an endomyocardial biopsy study. Ann Hematol. 1995;71:135–141. doi: 10.1007/BF01702649. [DOI] [PubMed] [Google Scholar]
- 8.Schellhammer PF, Engle MA, Hagstrom JW. Histochemical studies of the myocardium and conduction system in acquired iron-storage disease. Circulation. 1967;35:631–637. doi: 10.1161/01.cir.35.4.631. [DOI] [PubMed] [Google Scholar]
- 9.Horwitz LD, Rosenthal EA. Iron-mediated cardiovascular injury. Vasc Med. 1999;4:93–99. doi: 10.1177/1358836X9900400207. [DOI] [PubMed] [Google Scholar]
- 10.Rosenqvist M, Hultcrantz R. Prevalence of a haemochromatosis among men with clinically significant bradyarrhythmias. Eur Heart J. 1989;10:473–478. doi: 10.1093/oxfordjournals.eurheartj.a059512. [DOI] [PubMed] [Google Scholar]
- 11.Wang TL, Chen WJ, Liau CS, Lee YT. Sick sinus syndrome as the early manifestation of cardiac hemochromatosis. J Electrocardiol. 1994;27:91–96. doi: 10.1016/s0022-0736(05)80114-1. [DOI] [PubMed] [Google Scholar]
- 12.Laurita KR, Chuck ET, Yang T, Dong WQ, Kuryshev YA, Brittenham GM, Rosenbaum DS, Brown AM. Optical mapping reveals conduction slowing and impulse block in iron-overload cardiomyopathy. J Lab Clin Med. 2003;142:83–89. doi: 10.1016/S0022-2143(03)00060-X. [DOI] [PubMed] [Google Scholar]
- 13.Obejero-Paz CA, Yang T, Dong WQ, Levy MN, Brittenham GM, Kuryshev YA, Brown AM. Deferoxamine promotes survival and prevents electrocardiographic abnormalities in the gerbil model of iron-overload cardiomyopathy. J Lab Clin Med. 2003;141:121–130. doi: 10.1067/mlc.2003.18. [DOI] [PubMed] [Google Scholar]
- 14.Oudit GY, Sun H, Trivieri MG, Koch SE, Dawood F, Ackerley C, Yazdanpanah M, Wilson GJ, Schwartz A, Liu PP, Backx PH. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med. 2003;9:1187–1194. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz KA, Li Z, Schwartz DE, Cooper TG, Braselton WE. Earliest cardiac toxicity induced by iron overload selectively inhibits electrical conduction. J Appl Physiol. 2002;93:746–751. doi: 10.1152/japplphysiol.01144.2001. [DOI] [PubMed] [Google Scholar]
- 16.Veglio F, Melchio R, Rabbia F, Molino P, Genova GC, Martini G, Schiavone D, Piga A, Chiandussi L. Blood pressure and HR in young thalassemia major patients. Am J Hypertens. 1998;11:539–547. doi: 10.1016/s0895-7061(97)00263-x. [DOI] [PubMed] [Google Scholar]
- 17.Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu Rev Neurosci. 2007;30:317–337. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- 18.Cardoso LM, Pedrosa ML, Silva ME, Moraes MF, Colombari E, Chianca DA., Jr Baroreflex function in conscious rats submitted to iron overload. Braz J Med Biol Res. 2005;38:205–214. doi: 10.1590/s0100-879x2005000200008. [DOI] [PubMed] [Google Scholar]
- 19.Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- 20.Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008;88:919–982. doi: 10.1152/physrev.00018.2007. [DOI] [PubMed] [Google Scholar]
- 21.Kuryshev YA, Brittenham GM, Fujioka H, Kannan P, Shieh CC, Cohen SA, Brown AM. Decreased sodium and increased transient outward potassium currents in iron-loaded cardiac myocytes. Implications for the arrhythmogenesis of human siderotic heart disease. Circulation. 1999;100:675–683. doi: 10.1161/01.cir.100.6.675. [DOI] [PubMed] [Google Scholar]
- 22.Kim E, Giri SN, Pessah IN. Iron(II) is a modulator of ryanodine-sensitive calcium channels of cardiac muscle sarcoplasmic reticulum. Toxicol Appl Pharmacol. 1995;130:57–66. doi: 10.1006/taap.1995.1008. [DOI] [PubMed] [Google Scholar]
- 23.Stoyanovsky DA, Salama G, Kagan VE. Ascorbate/iron activates Ca(2+)-release channels of skeletal sarcoplasmic reticulum vesicles reconstituted in lipid bilayers. Arch Biochem Biophys. 1994;308:214–221. doi: 10.1006/abbi.1994.1030. [DOI] [PubMed] [Google Scholar]
- 24.Lesnefsky EJ, Ye J. Exogenous intracellular, but not extracellular, iron augments myocardial reperfusion injury. Am J Physiol. 1994;266:H384–H392. doi: 10.1152/ajpheart.1994.266.2.H384. [DOI] [PubMed] [Google Scholar]
- 25.Sellan M, Rose RA, Ciffeli C, Heximer SP, Backx PH. Chronic iron-overload causes sinus bradycardia by altering electrical activity in sinoatrial node myocytes. Biophys J. 2009;96(suppl 1):259a–260a. [Google Scholar]
- 26.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 27.Rose RA, Kabir MG, Backx PH. Altered HR and sinoatrial node function in mice lacking the cAMP regulator phosphoinositide 3-kinase-gamma. Circ Res. 2007;101:1274–1282. doi: 10.1161/CIRCRESAHA.107.158428. [DOI] [PubMed] [Google Scholar]
- 28.Rose RA, Lomax AE, Kondo CS, Anand-Srivastava MB, Giles WR. Effects of C-type natriuretic peptide on ionic currents in mouse sinoatrial node: a role for the NPR-C receptor. Am J Physiol. 2004;286:H1970–H1977. doi: 10.1152/ajpheart.00893.2003. [DOI] [PubMed] [Google Scholar]
- 29.Cifelli C, Rose RA, Zhang H, Voigtlaender-Bolz J, Bolz SS, Backx PH, Heximer SP. RGS4 Regulates Parasympathetic Signaling and HR Control in the Sinoatrial Node. Circ Res. 2008;103:527–535. doi: 10.1161/CIRCRESAHA.108.180984. [DOI] [PubMed] [Google Scholar]
- 30.Yang T, Brittenham GM, Dong WQ, Levy MN, Obejero-Paz CA, Kuryshev YA, Brown AM. Deferoxamine prevents cardiac hypertrophy and failure in the gerbil model of iron-induced cardiomyopathy. J Lab Clin Med. 2003;142:332–340. doi: 10.1016/S0022-2143(03)00135-5. [DOI] [PubMed] [Google Scholar]
- 31.Kardelen F, Tezcan G, Akcurin G, Ertug H, Yesilipek A. HR variability in patients with thalassemia major. Pediatr Cardiol. 2008;29:935–939. doi: 10.1007/s00246-008-9240-1. [DOI] [PubMed] [Google Scholar]
- 32.Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci U S A. 2003;100:5543–5548. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, He Y, Tuteja D, Xu D, Timofeyev V, Zhang Q, Glatter KA, Xu Y, Shin HS, Low R, Chiamvimonvat N. Functional roles of Cav1.3(alpha1D) calcium channels in atria: insights gained from gene-targeted null mutant mice. Circulation. 2005;112:1936–1944. doi: 10.1161/CIRCULATIONAHA.105.540070. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Xu Y, Song H, Rodriguez J, Tuteja D, Namkung Y, Shin HS, Chiamvimonvat N. Functional Roles of Ca(v)1.3 (alpha(1D)) calcium channel in sinoatrial nodes: insight gained using gene-targeted null mutant mice. Circ Res. 2002;90:981–987. doi: 10.1161/01.res.0000018003.14304.e2. [DOI] [PubMed] [Google Scholar]
- 35.Zacharski LR, McKernan L, Metzger ME, Malone MG, Samnotra V, Bhargava A, Steiner PR, Rauwerdink CA, Ornstein DL, Cornell CJ. Remission of paroxysmal atrial fibrillation with iron reduction in haemophilia A. Haemophilia. 2010;16:726–730. doi: 10.1111/j.1365-2516.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 36.Matthes J, Yildirim L, Wietzorrek G, Reimer D, Striessnig J, Herzig S. Disturbed atrio-ventricular conduction and normal contractile function in isolated hearts from Cav1.3-knockout mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:554–562. doi: 10.1007/s00210-004-0940-7. [DOI] [PubMed] [Google Scholar]
- 37.Stull LB, Hiranandani N, Kelley MA, Leppo MK, Marban E, Janssen PM. Murine strain differences in contractile function are temperature- and frequency-dependent. Pflugers Arch. 2006;452:140–145. doi: 10.1007/s00424-005-0020-y. [DOI] [PubMed] [Google Scholar]
- 38.Tsushima RG, Wickenden AD, Bouchard RA, Oudit GY, Liu PP, Backx PH. Modulation of iron uptake in heart by L-type Ca2+ channel modifiers: possible implications in iron overload. Circ Res. 1999;84:1302–1309. doi: 10.1161/01.res.84.11.1302. [DOI] [PubMed] [Google Scholar]
- 39.Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921–1932. doi: 10.1161/CIRCULATIONAHA.106.616011. [DOI] [PubMed] [Google Scholar]
- 40.Lei M, Zhang H, Grace AA, Huang CL. SCN5A and sinoatrial node pacemaker function. Cardiovasc Res. 2007;74:356–365. doi: 10.1016/j.cardiores.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Davis BA, Porter JB. Long-term outcome of continuous 24-hour deferoxamine infusion via indwelling intravenous catheters in high-risk beta-thalassemia. Blood. 2000;95:1229–1236. [PubMed] [Google Scholar]
- 42.Mancarella S, Yue Y, Karnabi E, Qu Y, El-Sherif N, Boutjdir M. Impaired Ca2+ homeostasis is associated with atrial fibrillation in the alpha1D L-type Ca2+ channel KO mouse. Am J Physiol. 2008;295:H2017–H2024. doi: 10.1152/ajpheart.00537.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qu Y, Baroudi G, Yue Y, Boutjdir M. Novel molecular mechanism involving alpha1D (Cav1.3) L-type calcium channel in autoimmune-associated sinus bradycardia. Circulation. 2005;111:3034–3041. doi: 10.1161/CIRCULATIONAHA.104.517326. [DOI] [PubMed] [Google Scholar]
- 44.Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 45.Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- 46.Gaborit N, Steenman M, Lamirault G, Le MN, Le BS, Lande G, Leger J, Charpentier F, Christ T, Dobrev D, Escande D, Nattel S, Demolombe S. Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation. 2005;112:471–481. doi: 10.1161/CIRCULATIONAHA.104.506857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.