Abstract
Neuraminidase (NA) plays a critical role in the life cycle of influenza virus and is a target for new therapeutic agents. A series of influenza neuraminidase inhibitors with the pyrrolidinobenzoic acid scaffold containing lipophilic side chains at the C3 position have been synthesized and evaluated for influenza neuraminidase inhibitory activity. The size and geometry of the C3 side chains have been modified in order to investigate structure-activity relationships. The results indicated that size and geometry of the C3-side chain are important for selectivity of inhibition against N1 vs N2 NA, important type A influenza variants that infect man, including the highly lethal avian influenza.
Keywords: Neuraminidase, avian influenza, pyrrolidinobenzoic acid
1. Introduction
Annual epidemics of influenza virus are responsible for considerable morbidity and mortality1, and pandemics are particularly devastating2–4. Infection is especially dangerous to the very young, elderly, and to those who have suppressed immune systems5–7. Vaccination has been the main preventive measure and has provided limited control, but vaccines must be reformulated each year in response to antigenic drift and may be ineffective against new variants of influenza viruses2, 8. The replicative cycle of influenza virus offers several virus-specific events which could be considered as targets for chemotherapeutic intervention.9 One of these is influenza neuraminidase (NA), which catalyzes the cleavage of the terminal sialic acid attached to host cell glycoproteins and glycolipids.10, 11 This process is believed to be necessary for the release of newly formed virus from infected cells and for efficient spread of virus in the respiratory tract.
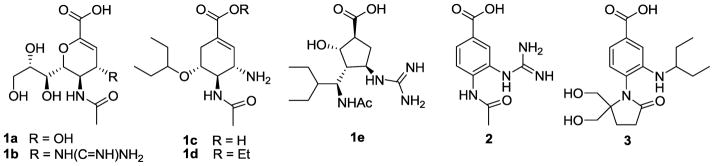
In recent years several very potent inhibitors of both influenza A and B NA have been developed.12–18 Early studies on NA inhibition revealed that 2,3-didehydro-2-deoxy-N-acetylneuraminic acid (1a) (Neu5Ac2en), an analogue of sialic acid, is a modest inhibitor with a Ki of 4 μM, and is believed to be a transition state analog due to planarity near the carboxylate. Solving the crystal structure of 1a in complex with NA was crucial in that it provided for the structure-based drug design19 of Relenza® (1b), which was followed by Tamiflu® (the prodrug 1d; 1c is the active form), and Rapiacta® (1e, peramivir), and all have been marketed as anti-influenza drugs. These drugs have in common a nonaromatic central ring containing a carboxylate and several side chains with defined stereochemistry, including the N-acetyl moiety, a strongly basic nitrogen, and (except for Relenza®) a hydrophobic group. The requirement for multiple chiral centers in some cases has resulted in high manufacturing costs.
We have developed a number of NA inhibitors containing the benzoic acid ring scaffold.20–24. The benzene ring was selected as a template because it provides planarity near the carboxylate as found in structures 1a–1d and, further, the benzene ring has no stereogenic centers at substitution sites. Such inhibitors thus potentially offered a more economical synthesis of anti-influenza drugs. Our lead benzoic acid inhibitor20 was 4-(N-acetylamino)-3-guanidinobenzoic acid (2) (BANA 113, Ki 2.5 μM). Further optimization of 2 resulted in the potent benzoic acid inhibitor 3 (1-[4-carboxy-2-(3-pentylamino)phenyl]-5,5-bis(hydroxymethyl)pyrrolidin-2-one), which is unique among potent inhibitors in that it contains a substituted pyrrolidinone ring in place of the N-acetyl functionality. Note that compound 3 is also unique in that it contains no chiral carbons and lacks a strongly basic nitrogen. Compound 3 exhibited an IC50 of 48 nM against influenza A NA (N9, an avian strain),22 but was less effective against other type A NAs (see Table 1).
Table 1.
In Vitro Inhibitory Effects of Benzoic Acid Analogues on Influenza A and B Neuraminidases
| Inhibition of Neuraminidase, IC50 (μM)
| ||||
|---|---|---|---|---|
| Compounds
|
H3N2
|
H1N1
|
B
|
|
| A/Uruguay/716/07 | A/Brisbane/59/07 | A/Solomon Islands/3/06 | B/Malaysia/5206/04 | |
| 3a | 0.49 | 79 | 271 | |
| 10a | 4000 ± 15 | >10000 | N/Ab | N/A |
| 10b | 0.20 ± 0.19 | 16 ± 2.1 | 11 ± 4.4 | 80 ± 30 |
| 10c | 0.50 ± 0.10 | 1.0 ± 0.21 | 0.80 ± 0.07 | 30 ± 1.4 |
| 10d | 40 ± 8.1 | 420 ± 45 | 400 ± 25 | N/A |
| 10e | 200 ± 95 | N/A | 1600 ± 255 | N/A |
|
| ||||
| Compounds
|
H3N2
|
H1N1
|
B
|
|
| A/Wisconsin/05 | A/Brisbane/59/07 | A/OK 3052/09 | B/Malaysia/2506/04 | |
|
| ||||
| 13a | 0.50 ± 0.15 | N/A | N/A | 190 ± 10 |
| 13b | 0.41 ± 0.070 | 2.4±0.9 | 3.4 ± 1.2 | 120 ± 16 |
| 13c | 3.0 ± 0.90c | 70 ± 7.8 | N/A | 140 ± 17 |
| 14b | 0.20 ± 0.090 | 1.6 ± 0.60 | 3.2 ± 1.3 | 32 ± 3.4 |
Data from ref 24 using A/PR/8/34 (N1), A/Udorn/72 (N2), and B/Lee/40.
N/A= data not available
A/Uruguay/716/07.
Here we describe the synthesis and evaluation of additional inhibitors related to 3, with special emphasis on determining effects on the N1 and N2 NAs of human viruses. Given the importance of the hydrophobic side chain in potent inhibitors 1c, 1e, and 3, we proposed for this study modifications to the hydrophobic side chain at the C3 position of 3 by incorporating alkyl or ether moieties.
2. Chemistry
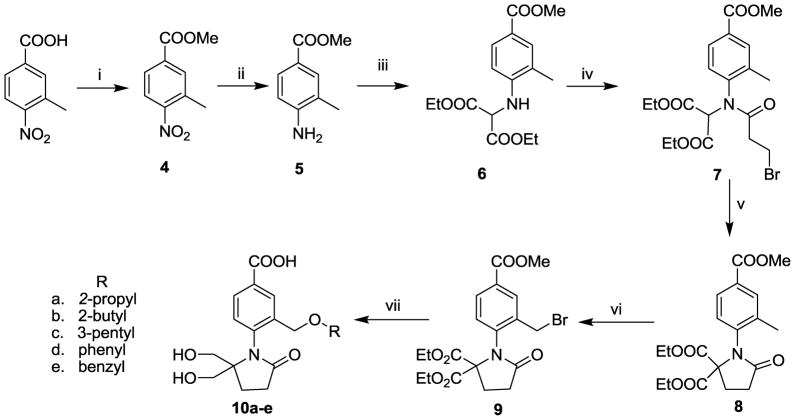
We first pursued the synthesis of analogue 10a–e, which introduced lipophilic ether side chains (similar to 1c) at C3. These were prepared as shown in Scheme 1. 3-Methyl-4-nitrobenzoic acid was esterified with MeOH to give 4, and reduced with Zn/HCl to afford methyl 3-methyl-4-aminobenzoate (5). Intermediate 5 was reacted with diethyl bromomalonate to give 6, which was N-acylated with 3-bromopropionic acid and PCl3 to provide 7. This underwent cyclization in the presence of NaH to yield lactam 8, which was treated with NBS to produce the bromo compound 9 in 18% overall yield. There were three steps involved in the conversion of compound 9 to the final acids 10a–e and intermediates were not purified. The first step was the alkylation with the appropriate alkoxide to form the corresponding ether, In this step, the methyl benzoate of 9 was partially transesterified by the alkoxide to provide a mixture of methyl benzoate and/or alkyl benzoate (for example, 2-propyl, etc.). The ethers containing mixed benzoate esters were used for the next steps without further purification. The second step was selective reduction of the ethyl malonate esters on the pyrrolidinone ring to form the diols. The third step was hydrolysis of the mixed benzoate esters using aqueous NaOH to afford the final acids 10a–e.
Scheme 1a.
aReagents and conditions: (i) MeOH, H2SO4, reflux, 15h; (ii) Zn/HCl, EtOAc, 0–20 °C 15h; (iii) diethyl bromomalonate, 120 °C, 22h; (iv) 3-bromopropionic acid, PCl3, toluene, 100 °C, 22h; (v) NaH, 0–20 °C, 4h; (vi) CCl4, NBS, AIBN, 80 °C, 3h; (vii) a, BuLi, ROH, −10–20 °C 15h; b, NaBH4, MeOH, THF, −21h; c, NaOH, MeOH, 20 °C, 1–2h.
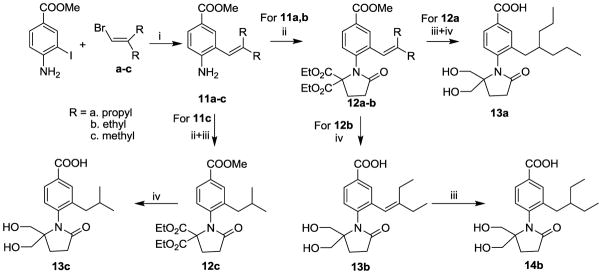
Next, compounds 13a, 13c, and 14b were designed as analogues of 3 that contain an alkyl side chain in place of the aliphatic amino side chain at the C3 position. The preparations of 13a, 14b and 13c are described in Scheme 2. Compounds 11a–c were synthesized through a palladium-catalyzed cross-coupling reaction between methyl 4-amino-3-iodobenzoate and the corresponding vinyl bromide (a–c). While the syntheses of intermediates 12a–c from the intermediate 11a–c each involved three major steps (in one “pot”), only the purified intermediates are shown in Scheme 2: The first step was the introduction of the pyrrolidinone ring, which was accomplished by reductive amination of the aniline with diethyl ketomalonate, N-alkylation with 3-bromopionic aid in the presence of PCl3, and cyclization in the presence of NaH. We found that overall yields were highest when these reactions were carried out without purifying the intermediates. The alkenes 12a and 12b were purified at this stage, while alkane 12c was obtained after further hydrogenation in the presence of Pd/C. Final compounds 13a and 13c were prepared by catalytic hydrogenation of the alkene, selective reduction of the malonate esters to diols using NaBH4, and saponification of the benzoates to the final acids. Compounds 13a and 13c were synthesized following the same sequence except that the intermediate were purified at different stage. Compound 13b was prepared in a similar fashion, but without the catalytic hydrogenation step, to allow the alkene to be preserved in the larger side chain of final product 13b, and the catalytic hydrogenation of 13b then afforded 14b.
Scheme 2a.
aReagents and conditions: : (i) a, vinyl bromide, dioxane, Et3N, Pd(PPh3)4, pinacolborane, 80 °C, 1h; b, methyl 4-amino-3-iodobenzoate, Ba(OH)2·8H2O, water, 90°C, 8h; (ii) a, p-TSA, toluene, diethyl ketomalonate, reflux, 19h; b, NaBH4, DEC, AcOH, 15h; c, 3-bromopropionic acid, PCl3, toluene, 100°C, 20h; d, NaH, 0–20 °C, 4h; (iii) Pd/C, H2, MeOH, 25 °C; (iv) a, NaBH4, MeOH/THF, 2–5h; b, NaOH, MeOH, 20 °C, 1–2h.
3. Biological Assays
All compounds were evaluated for in vitro inhibitory activity against NA using intact purified virions of influenza virus A (current vaccine strains of H1N1 and H3N2 subtypes) and influenza virus B using the fluorogenic substrate 2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid 32, and IC50 values were determined using Prism software. The results are contained in Table 1.
4. Results and Discussion
The numerous crystal structures that have been reported for a variety of type A and B neuraminidases (NAs) demonstrate that this site is highly conserved and, essentially, structurally identical irregardless of the source. Thus it is not surprising to find that inhibitors often show similar effects for all subtypes. For example, with oseltamivir carboxylate (1c; the active form of Tamiflu®), inhibition (IC50) of N1 NAs ranged from 0.30–0.80 nM, N2 NAs ranged from 0.68–1.78 nM, and type B NAs ranged from 5.00–24.3 nM28. One recent exception to this structural conservation among NA binding sites is the observation that type A NAs can be grouped into two structural classes. The Group 1 NAs include N1, which in some crystal structures (but not all) shows an additional cavity next to the active site that is not present in N2 (a member of Group 2 NAs). The cavity is created by movement of the “150 loop” (residues 147-152) and has been suggested to provide a new target for drug design.29 While, as mentioned previously, selectivity of inhibition for N1 vs N2 NAs is not observed for the potent clinically used inhibitors, any observed selectivity might be rationalized based on this observation.
As shown in Table 1, the parent compound for the present study, pyrrolidinone 3, indeed exhibited significant selectivity for N2 vs N1 NA. The much poorer inhibition seen for type B NA is commonly observed for certain inhibitors containing a hydrophobic side chain, and has been rationalized based on an energetically unfavorable conformational change in Glu276 upon ligand binding.30,31 Type A influenza causes more serious disease in humans and is the major target of clinically useful NA inhibitors. Since pyrrolidinone 3 exhibited good activity against N2 NA (relatively rigid binding site), but poor activity against N1 NA (may contain extra binding cavity), here we proposed to determine if smaller or larger side chains of different presentation might improve N1 activity without decreasing N2 activity. Particular choices for these side chains were limited by synthetic accessibility and resources but attempted to vary side chain length, presentation, and branching size. For this study we chose to measure inhibition against NAs of recently circulating H3N2 and H1N1 viruses, using the current vaccine strains in most cases.
In evaluating the analogs of 3 described in Table 1, we were therefore interested in two trends- changes in potency of inhibition and changes in selectivity for N1 vs N2 NA. Analogs of 3 included in this study were those containing aliphatic side chains with the same branching position(13a–c, and 14b), relative to the benzene ring, as found in 3, and those containing ether side chains branched one atom further from the benzene ring (10a–e).
For the latter series, the most effective inhibitors against N1 and N2 NA are 10b and 10c. If the hydrophobic chain is too small (10a) or too large (10d–e) potency is lost. Further, 10c is less selective than 10b for N2 vs N1 NA. While 10b and 10c exhibit nearly the same IC50 against N2, 10c is much more effective against N1, and is the most potent N1 NA inhibitor of this series, with dramatically improved (80–100 fold) N1 activity compared to the parent 3. The activities of 10b and 10c for N2 NA are similar to that for the parent 3.
For the former series (13a–c, 14b), all but 13c are nM inhibitors against N2 NA that exhibit similar potency to each other and to the parent 3. Within this series, compounds 13b and 14b are also the least selective for N2 vs N1 NA and exhibit a 30–40 fold increase in N1 NA inhibition compared to the parent 3.
As discussed above, for the series of compounds presented, N2 activity is always better than N1 activity, suggesting that any involvement of the 150-loop for binding of these inhibitors results in a penalty in all but the most potent N1 inhibitors such as 10c.
5. Conclusions
In summary, none of these analogs resulted in significantly enhanced potency, relative to 3, against N2 NA. However, relatively subtle changes in the nature of the hydrophobic group did yield up to 100-fold improvements against N1 NA. In this regard the best compounds (10c, 13b, and 14b) all contain the same 3-pentyl grouping, but this was contained in different structures with either a one or two atom spacer to the benzene ring. It is interesting that this spacer length made little difference in activity.
It is important to note that 3, and the analogs in Table 1, can only undergo three subsite interactions with NA, unlike the more potent inhibitors 1b, 1c, and 1e, which all contain four interactions (for example 1c contains substituents that occupy subsites for the carboxylate, N-acetyl, amino, and hydrophobic groups). Thus we anticipate that introduction of the missing basic group to provide a fourth interaction on pyrrolidinobenoic acids should also provide highly potent inhibitors. Current studies are pursuing such targets.
6. Experimental Section
6.1. Neuraminidase inhibition assays
Influenza virus strains A/Uruguay/716/07 (H3N2), A/Brisbane/59/07 (H1N1), A/Solomon Islands/3/06 (H1N1), A/Wisconsin/67/05 (H3N2), A/OK/3052/09 (H1N1pdm09), and B/Malaysia/5206/04 were grown in embryonated chicken eggs. IC50 determinations were carried out using the fluorogenic substrate 2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid32 adapted to a 96-well plate. The reactions included neuraminidase in the form of purified intact virions, preincubated for 30 minutes at room temperature with two-fold dilutions of inhibitor in 50 mM sodium acetate, pH 6.0, 100 μM CaCl2, 320 μM MgCl2 and 60 mM NaCl, before adding 100 μM of the substrate (Sigma). The reaction mixtures were incubated for 15 minutes at 37 °C, after which the reaction was stopped with 5 volumes of 0.2 M glycine-NaOH, pH 11. The IC50s were determined by Prism software using the log (inhibitor) versus normalized response option and cross-checked by plotting the enzyme fractional activity against the inhibitor concentration, and the IC50 was determined from the linear region of the dose-response curve.
6.2. General experimental procedures
NMR data were recorded on Bruker ARX 300 or 400 MHz spectrometer. Mass spectra were obtained using electrospray ionization on a MicroMass Platform LCZ LC/MS with HP1100HPLC/autoinjector and diode array detector. Flash column chromatography was carried out using silica gel (40 μm, Silicycle, Inc.). TLC was performed on silica gel (25 μm platesWhatman), and spots were visualized with UV light (UV-254 nm). Melting points were determined on a Mel-Temp electrothermal melting point apparatus (Laboratory Devices) and are uncorrected.
Methyl 3-Methyl-4-nitro-benzoate (4)27
To a solution of 3-methyl-4-nitrobenzoic acid (25.0 g, 138 mmol) in anhydrous methanol (400 mL) was added conc. H2SO4 (2 mL), at room temperature. The resulting solution was refluxed for 15h. The reaction mixture was concentrated. The solid was filtered and washed with cold methanol and dried to obtain the ester 4 (24 g, 89%) as a colorless solid. mp 78–80 °C.
Methyl 4-Amino-3-methylbenzoate (5)27
Methyl 3-methyl-4-nitrobenzoate (23 g, 117.84 mmol) was dissolved in ethyl acetate (400 mL) and conc.HCl (58 mL). Then the solution was cooled to 0 °C and Zn powder (46.2 g, 707 mmol) was added in portions over 20 minutes with stirring. The mixture was allowed to warm up to room temperature and stirring was continued for 15h. The reaction mixture was passed through a bed of Celite and washed with ethyl acetate (100 mL). The filtrate was washed with sat.NaHCO3 solution (4 × 100 mL), water (3 × 100 mL) and brine (2 × 100 mL). The organic layer was dried over anhydrous Na2SO4, filtered and concentrated to dryness to obtain the amino compound 5 (17.4 g, 90.0%) as a colorless solid. mp 111–113 °C; 1H NMR (300 MHz, CDCl3) δ 2.18 (s, 3H), 3.85 (s, 3H), 4.02 (s, br, 2H), 6.65 (d, J = 8.1Hz, 1H), 7.76-7.72 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 17.2, 51.7, 113.8, 119.5, 121.1, 129.4, 132.3, 149.5, 167.6; MS (ES) m/z 166 (M+1).
Diethyl 2-(4-Methoxycarbonyl-2-methyl-phenylamino)malonate (6)
A mixture of methyl 4-amino-3-methylbenzoate 5 (10.0 g, 60.5 mmol) and diethylbromomalonate (7.90 g, 33.3 mmol) was heated at 120 °C for 22h. The mixture was then cooled to room temperature and triturated with ethyl acetate (100 mL). The separated solid was filtered and washed with ethyl acetate (50 mL). The filtrate was concentrated and the crude product was purified by flash silica chromatography (75% hexane in EtOAc) to give a pale yellow solid (5.53 g, 56.0%). mp 40–42 °C; 1H NMR (300 MHz, CDCl3) δ 1.30 (t, J = 7.1 Hz, 6H), 2.27 (s, 3H), 3.85 (s, 3H), 4.30 (q, J = 7.1 Hz, 4H), 4.82 (d, J = 6.8 Hz, 1H), 5.17 (d, J = 6.8 Hz, 1H), 6.50 (d, J = 8.3 Hz, 1H), 7.77–7.81 (m, 2H); 13C NMR (100 MHz, Acetone-d6) δ 14.3, 17.2, 51.6, 60.6, 63.0, 110.3, 120.3, 122.8, 130.1, 132.3, 148.7, 167.1, 168.0; MS (ES) m/z 324 (M+1).
Diethyl 2-[(3-Bromo-propionyl)-(4-methoxycarbonyl-2-methyl-phenyl)-amino]malonate (7)
To a solution of 3-bromopropionic acid (2.44 g, 16.0 mmol) in toluene (25 mL), PCl3 (3.49 g, 25.4 mmol) was added and the mixture was heated at 110 °C for 2h. The mixture was cooled to room temperature, and a solution of 6 (2.35 g, 7.26 mmol) in toluene (10 mL) was added dropwise. Heating was continued at 100 °C for an additional 20h. The reaction mixture was concentrated and diluted with EtOAc (100 mL), washed with sat.NaHCO3 solution (2 × 75 mL), water (2 × 75 mL) and brine (2 × 75 mL). The organic layer was dried over anhydrous Na2SO4, evaporated and the crude product was purified by flash silica chromatography (25% EtOAc in hexanes; Rf 0.5) to afford 7 as a colorless solid (2.86 g, 85.0%). mp 104–106°C; 1H NMR (300 MHz, CDCl3) δ; 1.12 (t, J = 6 Hz, 3H), 1.31 (t, J = 7.2 Hz, 3H), 2.43 (s, 3H), 2.49–2.70 (m, 2H), 3.49–3.63 (m, 2H), 3.93 (s, 3H), 4.00–4.18 (m, 2H), 4.30 (q, J = 7.2 Hz, 2H), 5.05 (s, 1H), 7.58 (d, J = 8.4 Hz, 1H), 7.90 (dd, J = 8.4 Hz, 1.5 Hz, 1H), 8.00 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 13.9, 14.1, 18.1, 26.1, 37.3, 52.5, 62.4, 65.2, 128.7, 129.7, 131.2, 132.9, 137.7, 142.9, 165.2, 165.6, 166.3, 170.1; MS (ES) m/z 458, 460 (M+1).
5,5-Bis(ethoxycarbonyl)-1-(4-methoxycarbonyl-2-methylphenyl)pyrrolidin-2-one (8)
To a suspension of NaH (0.300 g, 12.6 mmol) in anhydrous DMF (35 mL) was added a solution of 7 (5.26 g, 11.5 mmol) in anhydrous DMF (20 mL) dropwise for a period of 15 min at 0 °C. The mixture was stirred at room temperature for 4h. The reaction mixture was diluted with EtOAc (200 mL) and washed with water (4 × 150 mL) and brine (2 × 150 mL). The organic layer was dried over anhydrous Na2SO4, evaporated and the crude product was purified by flash silica chromatography (25% EtOAc in hexanes, Rf 0.4) to afford pure 8 as a colorless solid (4.0 g, 92%); mp 68–70 °C; 1H NMR (400 MHz, CDCl3) δ 7.93 (s, 1H), 7.85 (d, J = 8.3 Hz, 1H), 7.52 (d, J = 8.3 Hz, 1H), 4.34 (m, 2H), 3.93 (m, 2H), 3.90 (s, 3H), 2.98 (m, 1H), 2.65 (m, 2H), 2.52 (m, 1H), 2.17 (s, 3H), 1.30 (t, J = 7.1 Hz, 6H), 0.88 (t, J = 7.1 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 13.8, 14.4, 18.5, 29.3, 29.7, 52.6, 62.7, 63.1, 74.6, 128.2, 128.6, 130.3, 132.2, 138.4, 140.6, 166.8, 167.0, 1170.2, 174.6; MS (ES) m/z 378(M+1).
Diethyl 1-(2-(Bromomethyl)-4-(methoxycarbonyl)phenyl)-5-oxopyrrolidine-2,2-dicarboxylate (9)
To a solution of 8 (1.6 g, 4.2 mmol) and AIBN (70 mg, 0.40 mmol) in CCl4 (36 mL), NBS (0.80 g, 4.6 mmol) was added. The mixture was refluxed for 3 h. The solid was filtered and the filtrate was concentrated to dryness. The obtained residue was chromatographed on a flash silica gel column (hexane/EtOAc, 2:1 v/v) to yield 9 (1.0 g, 52%) as a white solid. mp 58–60 °C; TLC Rf 0.4, (hexane/EtOAc, 1:1 v/v). 1H NMR (400 MHz, CDCl3) δ 0.86 (t, J=7.1 Hz, 3H), 0.95 (t, J=7.1 Hz, 3H), 2.53 (m, 1H), 2.67 (m, 2H), 2.96 (m, 1H), 3.93 (s, 3H), 4.01 (m, 2H), 4.34 (m, 2H), 4.35 (d, J = 11.4 Hz, 1H), 4.40 (d, J = 11.4 Hz, 1H), 7.48 (d, J = 8.4 Hz, 1H), 7.97 (dd, J = 1.9, 8.4 Hz, 1H), 8.21 (d, J = 1.9 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 13.7, 14.4, 29.3, 29.4, 29.8, 52.8, 62.8, 63.4, 74.6, 128.4, 130.5, 130.7, 132.9, 137.1, 140.5, 166.3, 166.4, 170.1, 175.2; MS (ES) m/z 456, 458(M+1).
Note: In the following conversion of 9 to 10a–e, overall yields were highest when intermediates were not isolated and purified.
4-(2,2-Bis(hydroxymethyl)-5-oxopyrrolidin-1-yl)-3-(alkoxymethyl)benzoic Acid (10a–e)
General procedure
After the alcohol was treated with 4 mL of butyllithium (1.6 M) at −10 °C, compound 9 (1 mmol) was added at room temperature. The resulting mixture was stirred for 15 h at room temperature. The reaction mixture was diluted with ethyl acetate (50 mL), and washed with saturated NaHCO3 (3 × 20 mL), water (2 × 20 mL) and brine (2 × 20 mL). The organic layer was dried (Na2SO4) and concentrated. The residue was chromatographed on a flash silica gel column to afford an oil. The oil was dissolved in dry MeOH (3 mL) and THF (3 mL) and cooled to 0°C under an argon atmosphere. Then NaBH4 (189 mg, 5.00 mmol) was added in portions over 20 min. The mixture was allowed to warm to room temperature, stirred for 1–2 h, and quenched by adding cold water (5 mL), This mixture was then extracted with EtOAc (3 × 10 mL), and the combined organic extracts were washed with water (1 × 5 mL) and brine (1 × 5 mL), dried (Na2SO4) and evaporated to dryness. The residue was chromatographed on a flash silica gel column to afford a semisolid. The semisolid in MeOH (5 mL) was treated with a 1M solution of NaOH (5 mL) and the solution was stirred at room temperature for 1–2h. The mixture was neutralized by using 1N HCl. The solvent was evaporated and the residue was purified by flash chromatography to obtain the benzoic acids 10a–e. The three steps overall yields were calculated from bromo compounds 9 to the benzoic acids 10a–e.
4-(2,2-Bis(hydroxymethyl)-5-oxopyrrolidin-1-yl)-3-(isopropoxymethyl)benzoic Acid (10a)
Colorless solid: mp 146–148 °C; yield 20%; TLC Rf 0.3, (EtOAc/MeOH, 4:1, v/v). 1H NMR(400 MHz, CD3COCD3) δ 1.01(d, J=6.1 Hz, 6H), 2.12(m, 2H), 2.39(m, 2H), 3.09(d, J=11.8 Hz, 1H), 3.14(d, J=11.8 Hz, 1H), 3.40(d, J=11.7 Hz, 1H), 3.46(d, J=11.7 Hz, 1H), 3.55(m, 1H), 4.18(d, J=12.9 Hz, 1H), 4.54(d, J=12.9 Hz, 1H), 7.25(d, J=8.3 Hz, 1H), 7.78(dd, J=8.3, 2 Hz, 1H), 8.04(d, J=2 Hz, 1H); 13C NMR (100 MHz, CD3COCD3) δ 21.3, 21.5, 25.1, 30.4, 63.0, 63.8, 65.8, 72.2, 72.4, 129.1, 129.4, 130.6, 131.0, 138.5, 139.9, 168.3, 178.1; Purity by HPLC 100%; MS (ES) m/z 338 (M+1).
4-(2,2-Bis(hydroxymethyl)-5-oxopyrrolidin-1-yl)-3-(2-butoxymethyl)benzoic Acid (10b)
Colorless solid: mp 156–158 °C; yield 29%; TLC Rf 0.3, (AcOEt/MeOH, 4:1, v/v). 1H NMR(400 MHz, CD3COCD3) δ 0.76–0.81(m, 3H), 1.01–1.08(m, 3H), 1.35–1.52(m, 2H), 2.18(m, 2H), 2.38(m, 2H), 3.19–3.28(m, 2H), 3.41(m, 1H), 3.53–3.61(m, 2H), 4.23(d, J=12.7 Hz, 0.5H), 4.33(d, J=12.7 Hz, 0.5H), 4.64(d, J=12.6 Hz, 0.5H), 4.70(d, J=12.6 Hz, 0.5H), 7.38(d, J=8.2 Hz, 1H), 7.81(m, 1H), 8.14(m, 1H); 13C NMR (100 MHz, CD3COCD3) δ 10.4, 10.6, 19.8, 20.0, 31.3, 64.3, 64.4, 65.1, 65.2, 67.1, 67.3, 72.2, 72.3, 77.9, 78.2, 130.0, 130.1, 131.1, 131.2, 131.4, 131.8, 140.6, 140.8, 140.9, 141.2, 167.8, 167.9, 176.8, 176.9; Purity by HPLC 96%; MS (ES) m/z 352 (M+1).
4-(2,2-Bis(hydroxymethyl)-3-((3-pentyloxy)methyl)-5-oxopyrrolidin-1-yl)benzoic Acid (10c)
Colorless solid: mp 177–179 °C; yield 20%; TLC Rf 0.3, (EtOAc/MeOH, 4:1, v/v). 1H NMR(400 MHz, CD3COCD3) δ 0.67(d, J=7.5 Hz, 6H), 1.33(m, 2H), 2.08(m, 2H), 2.29(m, 2H), 3.09–3.17(m, 3H), 3.44(d, J=11.5 Hz, 1H), 3.48(d, J=11.5 Hz, 1H), 4.18(d, J=12.9 Hz, 1H), 4.54(d, J=12.9 Hz, 1H), 7.28(d, J=8.2 Hz, 1H), 7.71(dd, J=8.2, 1.9 Hz, 1H), 8.07(d, J=1.9 Hz, 1H); 13C NMR (100 MHz, CD3COCD3) δ 10.2, 10.4, 14.8, 23.7, 26.1, 26.7, 26.9, 31.3, 64.3, 65.1, 67.6, 72.2, 83.4, 130.0, 130.4, 131.1, 1231.5, 140.5, 141.2, 167.8, 176.8; Purity by HPLC 98%; MS (ES) m/z 366 (M+1).
4-(2,2-Bis(hydroxymethyl)-5-oxopyrrolidin-1-yl)-3-(phenoxymethyl)benzoic Acid (10d)
Colorless solid: mp 198–200 °C; yield 20%; TLC Rf 0.5, (EtOAc/MeOH, 4:1, v/v). 1H NMR(400 MHz, CD3OD) δ 2.33(m, 2H), 2.63(m, 2H), 3.36(d, J=11.5 Hz, 1H), 3.42(d, J=11.5 Hz, 1H), 3.64(d, J=11.7 Hz, 1H), 3.70(d, J=11.7 Hz, 1H), 5.04(d, J=13.6 Hz, 1H), 5.40(d, J=13.6 Hz, 1H), 6.95(m, 1H), 7.04(d, J=7.8 Hz, 2H), 7.28(m, 2H), 7.55(d, J=8.2 Hz, 1H), 8.04(dd, J=8.2, 1.9 Hz, 1H), 8.31(d, J=1.9 Hz, 1H); 13C NMR (100 MHz, CD3OD) δ 26.6, 31.2, 64.5, 65.1, 67.2, 73.6, 116.3, 122.5, 130.8, 131.0, 132.4, 139.6, 139.9, 160.5, 169.6, 179.6; Purity by HPLC 97%; MS (ES) m/z 372 (M+1).
3-((Benzyloxy)methyl)-4-(2,2-bis (hydroxymethyl)-5-oxopyrrolidin-1-yl)benzoic Acid (10e)
Colorless solid: mp 198–200 °C; yield 20%; TLC Rf 0.5, (AcOEt/MeOH, 4:1, v/v). 1H NMR(400 MHz, CD3COCD3) δ 2.05(m, 1H), 2.15(m, 1H), 2.27(m, 1H), 2.38(m, 1H), 3.33–3.44(m, 2H), 4.13(w, 1H), 4.44–4.58(m, 5H), 7.14–7.31(m, 6H), 7.86(dd, J=8.2, 1.9 Hz, 1H), 8.14(d, J=1.9 Hz, 1H); 13C NMR (100 MHz, CD3COCD3) δ 22.6, 31.8, 62.7, 63.7, 69.7, 73.7, 128.8, 129.1, 129.6, 130.5, 130.6, 131.5, 138.7, 139.7, 142.0, 167.6, 175.5; Purity by HPLC 98%; MS (ES) m/z 386 (M+1).
General Procedure for the Palladium-Catalyzed Cross-coupling Reaction (11a–c)
To a solution of vinyl bromide a–c (6.7 mmol) in dioxane (30 mL) at 25 °C under argon were successively added Et3N (3.70 mL, 26.9 mmol), Pd (PPh3)4 (779 mg, 0.670 mmol), and pinacolborane (2.90 mL, 20.2 mmol) dropwise. The solution was heated to 80 °C for 1 h. After cooling, water (5 mL) was added dropwise followed by methyl 4-amino-3-iodobenzoate (1.5 g, 5.4 mmol) dissolved in dioxane (15 mL) and Ba(OH)2·8H2O (6.40 g, 20.2 mmol). The solution was heated to 90 °C for 8 h. After cooling, the mixture was filtered through Celite, brine was added (40 mL), and the solution was extracted with ethyl acetate (3 × 50 mL). After drying over magnesium sulfate and evaporation of the solvents under vacuum, the residue was purified by flash chromatography to obtain the benzoates
Methyl 4-Amino-3-(2-propylpent-1-ynyl)benzoate (11a)
Colorless oil, yield 76 %, TLC Rf 0.4 (hexane/EtOAc 1/1 v/v). 1H NMR(400 MHz, CDCl3) δ 0.81(t, J=7.4 Hz, 3H), 0.97(t, J=7.4 Hz, 3H), 1.40(m, 2H), 1.54(m 2H), 2.02(m, 2H), 2.17(m, 2H), 3.85(s, 3H), 4.10(w, 2H), 6.00(s, 1H), 6.64(d, J=8.4Hz, 1H),7.68(d, J=2.0 Hz, 1H), 7.74(dd, J=8.4, 2.0 Hz, 1H); 13C NMR(100 MHz, CDCl3) δ 14.4, 14.4, 21.6, 21.6, 33.1, 38.7, 52.0, 114.0, 119.5, 120.2, 123.6, 130.0, 132.5, 147.3, 149.1, 167.9; MS (ES) m/z 262 (M+1).
Methyl 4-Amino-3-(2-ethylbut-1-ynyl)benzoate (11b)
Colorless oil, yield 73 %, TLC Rf 0.4 (hexane/EtOAc 1/1 v/v). 1H NMR(400 MHz, CDCl3) δ 0.97(t, J=7.6, 3H), 1.14(t, J=7.4 Hz, 3H), 2.08(q, J=7.4 Hz, 2H), 2.23( q, J=7.6 Hz, 2H), 3.85(s, 3H),4.07(w, 2H), 5.96(s, 1H), 6.64(d, J=8.4 Hz, 1H), 7.69(d, J=2.0 Hz, 1H), 7.75(dd, J=8.4, 2.0 Hz, 1H); 13C NMR(100 MHz, CDCl3) δ 12.9, 13.0, 24.1, 28.6, 51.5, 113.6, 117.8, 119.2, 123.2, 129.7, 132.0, 148.6, 150.2, 167.4; MS (ES) m/z 234 (M+1).
Methyl 4-Amino-3-(2-methylprop-1-ynyl) benzoate (11c)
Colorless oil, yield 70 %, TLC Rf 0.4 (hexane/EtOAc 1/1 v/v). 1H NMR(400 MHz, CDCl3) δ 1.70(d, J=0.4 Hz, 3H), 1.91(d, J=0.8 Hz, 3H), 3.84(s, 3H), 4.12(w, 2H), 6.00(s, 1H), 6.64(d, J=8.4 Hz, 1H), 7.69(d, J=1.7 Hz, 1H), 7.75(dd, J=8.4, 1.7 Hz, 1H); 13C NMR(100 MHz, CDCl3) δ 19.8, 19.8, 26.3, 52.0, 114.1, 119.5, 120.2, 123.5, 132.5, 139.3, 149.1, 167.8; MS (ES) m/z 206 (M+1).
Note: In the following conversion of 11a–b to 12a–b, and for 11c to 12c, overall yields were highest when intermediates were not isolated and purified.
Diethyl 1-(-2-Alkenyl-4-methoxycarbonylphenyl)-5-oxopyrrolidine-2,2-dicarboxylate (12a–b)
General procedure
To a solution of methyl 3-alkenyl-4-aminobenzoate (11a–b) (3.83 mmol) and diethylketomalonate (1.33 g, 7.66 mmol) in toluene (40 mL), p-toluene sulfonic acid (73 mg, 0.38 mmol) and molecular sieves (4 g) were added. The reaction mixture was refluxed 15 h, then transferred to a separatory funnel and washed with saturated NaHCO3 (3×40mL) and brine (2×40mL). The organic layer was dried (Na2SO4), filtered, and evaporated to dryness. The residue was chromatographed on a flash silica gel column to afford an oil. To a solution of the oil in 1, 2-dichloroethane (8 mL), NaBH4 (144 mg, 3.80 mmol) was added followed by slow addition of glacial acetic acid (0.3 mL). After the mixture was stirred 15 h at r.t., the reaction was quenched by adding cold water (1 mL). The mixture was washed with saturated NaHCO3 (2×10mL) and brine (2×10mL), and the organic layer was dried (Na2SO4), filtered, and evaporated to dryness. The residue was chromatographed on a flash silica gel column to afford an oil. To a solution of the oil in toluene (5 mL), phosphorus trichloride (0.40 mL, 4.6 mmol) was added. The mixture was refluxed 15 h. The cooled reaction mixture was washed with water (2×5mL). The combined aqueous layers were extracted with ethyl acetate (2×10mL). The organic layers ware combined, and washed with saturated NaHCO3 (2×10mL) and brine (2×10mL), dried (Na2SO4), filtered, and evaporated to dryness. The residue was chromatographed on a flash silica gel column to afford an oil. A solution of the oil in dry DMF (4 mL) was added dropwise to a stirred suspension of NaH (16 mg, 0.68 mmol) in dry DMF (4 mL) over 10 minutes under Ar. The mixture was stirred at room temperature for 1h. The NaH was quenched with water (0.1 mL), and the mixture was diluted with ethyl acetate (20 mL). This was washed with NaHCO3 (2×10mL) and brine (4×10mL), dried (Na2SO4), filtered, and concentrated. The obtained residue was chromatographed on a flash silica gel column to obtain the benzoic acids 12a–b. The four step overall yields are given below.
Diethyl 1-(4-(Methoxycarbonyl)-2-(2-propylpent-1-enyl) phenyl)-5-oxopyrrolidine-2,2-dicarboxylate (12a)
Colorless solid: mp 88–90 °C, yield 17 %, TLC Rf 0.59 (hexane/EtOAc 1/1 v/v). 1H NMR(400 MHz, CDCl3) δ 0.86(m, 3H), 0.93(t, J=7.3 Hz, 3H), 0.94(t, J=7.3 Hz, 3H), 1.30(m, 3H), 1.53(m 4H), 2.09(m, 4H), 2.42(m, 1H), 2.58(m, 2H), 2.98(m, 1H), 3.90(m, 1H), 3.91(s, 3H), 3.93(m, 1H), 5.90(s, 1H), 4.32(m, 2H), 7.58(d, J=8.3 Hz, 1H), 7.90(dd, J=8.3, 2.0 Hz, 1H), 7.94(d, J=2.0 Hz, 1H); 13C NMR(100 MHz, CDCl3) δ 14.3, 14.7, 21.4, 22.0, 29.3, 29.4, 33.5, 39.0, 52.6, 62.9, 74.5, 120.3, 128.6, 129.1, 130.0, 131.8, 139.1, 140.3, 147.2, 167.1, 174.6, MS (ES) m/z 474 (M+1).
Diethyl 1-(2-(2-Ethylbut-1-enyl)-4-(methoxycarbonyl) phenyl)-5-oxopyrrolidine-2,2-dicarboxylate (12b)
Colorless solid: mp 85–87°C, yield 20 %, TLC Rf 0.53 (hexane/EtOAc 1/1 v/v). 1H NMR(300 MHz, CDCl3) δ 0.91(m, 3H), 1.04(m, 6H), 1.26(m, 3H), 2.14(m, 4H), 2.58(m, 2H), 3.89(s, 3H), 4.44-3.8(m, 4H), 5.90(s, 1H), 7.59(d, J=8.3 Hz, 1H), 7.88(dd, J=8.3, 2.0 Hz, 1H), 7.92(d, J=2.0 Hz, 1H); 13C NMR(75 MHz, CDCl3) δ 12.6, 12.8, 14.2, 24.2, 28.7, 28.8, 29.0, 52.2, 62.4, 74.1, 118.3, 128.2, 128.9, 129.6, 131.2, 138.7, 139.9, 149.6, 166.7, 174.3; MS (ES) m/z 446 (M+1).
Diethyl 1-(4-(Methoxycarbonyl)-2-(2-methylprop-1-enyl) phenyl)-5-oxopyrrolidine-2,2-dicarboxylate (12c)
To a solution of 11c (2.60 g, 12.6 mmol) and diethyl ketomalonate (6.6 g, 38 mmol) in toluene (130 mL), p-toluene sulfonic acid (242 mg, 1.30 mmol) and molecular sieves (13g) were added. The reaction mixture was refluxed overnight. The cooled reaction mixture was washed with saturated NaHCO3 (3×80mL) and brine (2×80mL). The organic layer was dried (Na2SO4), filtered, and evaporated to dryness. The residue was chromatographed on a flash silica gel column to afford an oil. To a solution of the oil in 1, 2-dichloroethane (30 mL), NaBH4 (630 mg, 33.2 mmol) was added followed by the slow addition of glacial acetic acid (2.5 mL). After the mixture was stirred overnight at r.t., the reaction was quenched by adding cold water (2 mL) and diluted with ethyl acetate (90 mL). The mixture was washed with saturated NaHCO3 (2×30mL) and brine (2×30mL), and the organic layer was dried (Na2SO4), filtered, and evaporated to dryness. The residue was chromatographed on a flash silica gel column to afford an oil. To a solution of the oil in toluene (20 mL), phosphorus trichloride (3.60 mL, 28.7 mmol) was added. The mixture was refluxed overnight. After quenching with water (50mL), the reaction mixture was diluted with ethyl acetate (100 mL). The combined aqueous layers were extracted with ethyl acetate (2×50mL). The organic layers were combined, washed with saturated NaHCO3 (2×60mL) and brine (2×60mL), dried (Na2SO4), filtered and evaporated to dryness. The residue was chromatographed on a flash silica gel column to afford an oil. A solution of the oil in dry DMF (20 mL) was added dropwise to a stirred suspension of NaH (128 mg, 3.2 mmol) in dry DMF (20 mL) over 10 minutes under Ar. The mixture was stirred at room temperature for 2h. The NaH was quenched with water (0.5 mL), and the mixture was diluted with ethyl acetate (100 mL). The mixture was washed with NaHCO3 (2×50mL) and brine (4×50mL), dried (Na2SO4), filtered, and evaporated to dryness. The residue was chromatographed on a flash silica gel column to afford a colorless semisolid. The semisolid was dissolved in THF (6 mL). Palladium on carbon (30 mg, 5%) was added. The mixture was stirred under a hydrogen atmosphere (approximately one atmosphere from an attached balloon reservoir) for 4 h. The mixture was filtered and concentrated to yield 12c (0.6 g, 11% for 5 steps, TLC Rf 0.50 (hexane/EtOAc 1/1 v/v) as a colorless solid; m.p. 73–75 °C. 1H NMR(300 MHz, CDCl3) δ 0.96-0.90(m, 9H), 1.29(t, J=5.3 Hz, 3H), 1.98(m, 1H), 2.20(dd, J=5.9, 11.9 Hz, 1H), 2.36(dd, J=5.0, 11.9 Hz, 1H), 2.44(m, 1H), 2.65(m, 2H), 3.05(m, 1H), 3.84(m, 1H), 3.90(s, 3H), 4.01(m, 1H), 4.29–4.35(m, 2H), 7.55(d, J=8.2 Hz, 1H), 7.87(dd, J=8.2, 2.0 Hz, 1H), 7.94(d, J=2.0 Hz, 1H); 13C NMR(75 MHz, CDCl3) δ 13.2, 13.9, 22.3, 22.9, 27.6, 28.8, 29.0, 39.8, 62.2, 62.6, 74.2, 127. 6, 128.8, 129.7, 130.6, 140.3, 141.0, 165.9, 166.6, 169, 7, 174.7; MS (ES) m/z 420 (M+1).
4-(2,2-Bis (hydroxymethyl)-5-oxopyrrolidin-1-yl)-3-(2-propylpentyl)benzoic Acid (13a)
Compound 12a (0.30 mmol) was dissolved in THF (3 mL). Palladium on carbon (14 mg, 5%) was added. The mixture was stirred under a hydrogen atmosphere (approximately one atmosphere from an attached balloon reservoir) for 5 h. The mixture was filtered and evaporated to dryness. The residue was dissolved in dry MeOH (1 mL) and THF (1 mL) and cooled to 0 °C under argon. NaBH4 (54.0 mg, 1.46 mmol) was added. The mixture was warmed to room temperature, stirred for 5 h, and quenched by adding cold water (0.1 mL). The mixture was evaporated to dryness leaving a solid which was dissolved in dichloromethane (10 mL). The organic layer was washed with brine (2×5mL), dried (Na2SO4), filtered, and evaporated to dryness. The residue was dissolved in MeOH (2 mL) and 1M NaOH (2 mL) was added. After the mixture was stirred for 2 h at room temperature, the pH was adjusted to 2 with 1 M HCl, and the mixture was evaporated to dryness, leaving a solid which was dissolved in methanol (3 mL). The organic layer was concentrated. The obtained residue was chromatographed on a flash silica gel column to obtain the benzoic acids 13a. Colorless solid: mp 203–204 °C, yield 42 % for three steps, TLC Rf 0.38 (AcOEt/MeOH/AcOH 9:1:0.5, v/v/v). 1H NMR(400 MHz, CD3OD) δ 0.78(m, 6H), 1.33-1.11(m, 8H), 1.76(m, 1H), 2.20(m, 2H), 2.44-2.32(m, 2H), 2.54(m, 1H), 2.73(m, 1H), 3.24(d, J=11.2 Hz, 1H), 3.34(d, J=11.2 Hz, 1H), 3.50(d, J=11.6 Hz, 1H),3.54(d, J=11.6 Hz, 1H), 7.33(d, J=8.1 Hz, 1H), 7.78(d, J=8.1 Hz, 1H), 7.90(s, 1H); 13C NMR(100 MHz, CD3OD) δ 15.2, 21.3, 21.4, 27.2, 32.0, 37.0, 37.3, 37.7, 38.3, 65.0, 65.4, 73.3, 129.0, 131.5, 132.3, 132.6, 141.5, 143.8, 170.2, 179.8; Purity by HPLC 98%; MS (ES) m/z 378 (M+1).
4-(2,2-Bis (hydroxymethyl)-5-oxopyrrolidin-1-yl)-3-(2-ethylbut-1-enyl)benzoic Acid (13b)
Compound 12b (168 mg, 0.380 mmol) was dissolved in dry MeOH (1.2 mL) and THF (1.2 mL) and cooled to 0°C under argon. NaBH4 (71.0 mg, 1.89 mmol) was added. The mixture was warmed to room temperature, stirred for 5 h, and quenched by adding cold water (0.1 mL). The mixture was evaporated to dryness leaving a solid which was dissolved in dichloromethane (10 mL). The organic layer was washed with brine (2×5mL), dried (Na2SO4), filtered, and evaporated to dryness. The residue was dissolved in MeOH (3 mL) and 1M NaOH (3 mL) was added. After the mixture was stirred for 1 h at room temperature, the pH was adjusted to 2 with 1 M HCl, and the mixture was evaporated to dryness, leaving a solid which was dissolved in methanol (2 mL). The organic layer was concentrated. The obtained residue was chromatographed on silca gel (EtOAc/MeOH/AcOH=40:2:1, Rf=0.48) to yield acid 13b (47 mg, 36% in two steps) as a colorless solid: m.p. 189–191 °C; 1H NMR(300 MHz, CD3OD) δ 1.15-1.09(m, 6H), 2.45-2.15(m, 6H), 2.66-2.50(m, 2H), 3.22(d, J=11.2 Hz, 1H), 3.43(d, J=11.2 Hz, 1H), 3.62(d, J=11.8 Hz, 1H), 3.73(d, J=11.8 Hz, 1H), 6.24(d, J=8.0 Hz, 1H), 7.46(d, J=8.0 Hz, 1H), 7.94(d, J=8.0 Hz, 1H), 7.96(s, 1H); 13C NMR(75 MHz, CD3OD) δ 11.6, 11.8, 23.7, 25.3, 28.2, 29.3, 29.8, 63.0, 64.0, 71.3, 78.0, 120.0, 128.3, 129.4, 131.8, 138.8, 138.9, 148.5, 177.1; MS (ES) m/z 348 (M+1).
4-(2,2-Bis (hydroxymethyl)-5-oxopyrrolidin-1-yl)-3-isobutylbenzoic Acid (13c)
Compound 12c (419 mg, 1.00 mmol) was dissolved in dry MeOH (3 mL) and THF (3 mL) and cooled to 0 °C under argon. NaBH4 (190 mg, 5.00 mmol) was added. The mixture was warmed to room temperature, stirred for 2 h, and quenched by adding cold water (0.5 mL). The mixture was evaporated to dryness leaving a solid which was dissolved in dichloromethane (20 mL). The organic layer was washed brine (2×10mL), dried (Na2SO4), filtered, and evaporated to dryness. The residue was dissolved in MeOH (2 mL) and 1M NaOH (2 mL) was added. After the mixture was stirred for 2 h at room temperature, the pH was adjusted to 2 with 1 M HCl, and the mixture was evaporated to dryness, leaving a solid which was dissolved in methanol (3 mL). The organic layer was concentrated. The obtained residue was chromatographed on silca gel (EtOAc/MeOH/AcOH=40:2:1, Rf=0.57) to yield acid 13c (99 mg, 31% in two steps) as a colorless solid. m.p. 168–170 °C: 1H NMR(300 MHz, CD3OD) δ 1.10(d, J=2.5 Hz, 3H), 1.12(d, J=2.5 Hz, 3H), 2.31(m, 1H), 2.46–2.50(m, 2H), 2.60–2.88(m, 4H), 3.52(d, J=11.2Hz, 1H), 3.62(d, J=11.2Hz, 1H), 2.69–2.42(m, 3H), 2.85(m, 1H), 3.35(d, J=11.1 Hz, 1H), 3.46(d, J=11.1 Hz, 1H), 3.80(d, J=11.7 Hz, 1H), 3.83(d, J=11.7 Hz, 1H), 7.62(d, J=8.2 Hz, 1H), 8.06(dd, J=8.2, 2.0 Hz, 1H), 8.20(d, J=2.0 Hz, 1H); 13C NMR(100 MHz, CD3OD) δ 21.2, 21.9, 25.3, 27.4, 30.0, 39.1, 63.2, 63.6, 71.4, 127.3, 129.6, 130.2, 130.5, 139.6, 141.8, 168.2, 177.9; Purity by HPLC 97%; MS (ES) m/z 322 (M+1).
4-(2,2-Bis (hydroxymethyl)-5-oxopyrrolidin-1-yl)-3-(2-ethylbutyl)benzoic Acid (14b)
Compound 13b (20.0 mg, 0.058 mmol) was dissolved in THF (0.5 mL). Palladium on carbon (2 mg, 5%) was added. The mixture was stirred under a hydrogen atmosphere (approximately one atmosphere from an attached balloon reservoir) for 5 h. The mixture was filtered and concentrated to yield 14b (20 mg, 99%) as a colorless solid. m. p. 168–170 °C; 1H NMR(300 MHz, CD3OD) δ 0.91(t, J=7.3 Hz, 6H), 1.36(m, 4H), 1.70(m, 1H), 2.30(m, 2H), 2.69-2.42(m, 3H), 2.85(m, 1H), 3.35(d, J=11.1 Hz, 1H), 3.46(d, J=11.1 Hz, 1H), 3.61(d, J=11.7 Hz, 1H), 3.66(d, J=11.7 Hz, 1H), 7.43(d, J=8.2 Hz, 1H), 7.89(d, J=8.2 Hz, 1H), 8.01(s, 1H); 13C NMR(75 MHz, CD3OD) δ 10.0, 10.1, 25.0, 25.2, 25.4, 30.2, 34.4, 40.2, 63.3, 63.6, 71.5, 127.2, 129.7, 130.6, 130.8, 139.7, 142.0, 168.4, 178.0. MS (ES) m/z 350 (M+1).
Supplementary Material
Figure 1.
Structures of clinically marketed neuraminidase inhibitors compared to benzoic acid inhibitors.
Acknowledgments
This work was supported by NIAID grant AI 062950 to WJB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.World Health Organization. WHO factsheet 211: influenza. 2009 http://www.who.int/mediacentre/factsheets/fs211/en/
- 2.Webby RJ, Webster RG. Science. 2003;302:1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- 3.Kilbourne ED. Influenza pandemics of the 20th century. Emerging Infect Dis. 2006;12:9–14. doi: 10.3201/eid1201.051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, ollingsworth TD, Griffin J, Baggaley RF, Jenkins HE, Lyons EJ, Jombart T, Hinsley WR, Grassly NC, Balloux F, Ghani AC, Ferguson NM, Rambaut A, Pybus OG, Lopez-Gatell H, Apluche-Aranda CM, Chapela IB, Zavala EP, Guevara DME, Checchi F, Garcia E, Hugonnet S, Roth C. Science. 2009;324:1557–1561. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson KG. Vaccines. 2009;8(4):373–4. doi: 10.1586/erv.09.17. [DOI] [PubMed] [Google Scholar]
- 6.Sambhara S, Stephenson I. Vaccines. 2009;8(4):375–7. doi: 10.1586/erv.09.10. [DOI] [PubMed] [Google Scholar]
- 7.Trollfors B. Acta Paediatr. 2006;95(7):774–7. doi: 10.1080/08035250600729126. [DOI] [PubMed] [Google Scholar]
- 8.Carrat F, Flahault A. Vaccine. 2007;25:6852–6862. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Palese P. Nature Med. 2004;10(12 Suppl):S82–87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 10.Colman PM. Influenza Viruses: Influenza Virus Neuraminidase. In: Krug RM, editor. Enzyme and antigen. Plenum Press; New York: 1989. pp. 175–218. [Google Scholar]
- 11.Air GM, Brouillette WJ. Influenza virus antiviral targets. In: LaFemina R, editor. Antiviral Research. Washington, DC: ASM Press; 2009. pp. 187–207. [Google Scholar]
- 12.von Itzstein M, Wen-Yang W, Gaik BK, Michael SP, Jeffrey CD, Betty J, Tho VP, Mark LS, Hume FW, Stuart WO, Peter MC, Joseph NV, Michael R, Jacqueline MW, Richard CB, Vanessa JH, Janet M, Cameron JM, Charles RP. Nature. 1993;363:418–423. [Google Scholar]
- 13.Hayden FG, Treanor JJ, Betts RF, Lobo M, Esinhart JD, Hussey EK. J Amer Med Assoc. 1996;275:295–299. [PubMed] [Google Scholar]
- 14.Kim CU, Lew W, Williams MA, Liu H, Zhang L, Swaminathan S, Bischofberger N, Chen MS, Mendel DB, Tai CY, Laver WG, Stevens RC. J Am Chem Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- 15.Weixing L, Escarpe PA, Eisenberg EJ, Cundy KC, Sweet C, Jakeman K, Merson J, Lew W, Williams M, Zhang L, Kim C, Bischofberger N, Chen MS, Mendel DB. Antimicrob Agents Chemother. 1998;42:647–653. doi: 10.1128/aac.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babu YS, Chand P, Bantia S, Kotian P, Ali Dehghani El-Kattan Y, Lin T, Hutchison TL, Elliott AJ, Parker CD, Ananth SL, Horn LL, Laver GW, Montgomery JA. J Med Chem. 2000;43:3482–3486. doi: 10.1021/jm0002679. [DOI] [PubMed] [Google Scholar]
- 17.Wang GT, Chen Y, Wang S, Gentles R, Sowin T, Kati W, Muchmore S, Giranda V, Stewart K, Sham H, Kempf D, Laver WG. J Med Chem. 2001;44:1192–1201. doi: 10.1021/jm000468c. [DOI] [PubMed] [Google Scholar]
- 18.Stoll V, Stewart KD, Maring CJ, Muchmore S, Giranda V, Gu Y-gY, Wang G, Chen Y, Sun M, Zhao C, Kennedy AL, Madigan DL, Xu Y, Saldivar A, Kati W, Laver G, Sowin T, Sham HL, Greer J, Kempf D. Biochemistry. 2003;42:718–727. doi: 10.1021/bi0205449. [DOI] [PubMed] [Google Scholar]
- 19.Ward RC. Structure. 1997;5:1139–1145. [Google Scholar]
- 20.Singh S, Jedrzejas MJ, Air GM, Luo M, Laver GW, Brouillette WJ. J Med Chem. 1995;38:3217–3225. doi: 10.1021/jm00017a005. [DOI] [PubMed] [Google Scholar]
- 21.Jadrezas MJ, Singh S, Brouillette WJ, Laver WG, Air GM, Luo M. Biochemistry. 1995;34:3144. doi: 10.1021/bi00010a003. [DOI] [PubMed] [Google Scholar]
- 22.Atigadda VR, Brouillette WJ, Duarte F, Ali SM, Babu YS, Bantia S, Chand P, Chu N, Montgomery JA, Walsh DA, Sudbeck EA, Finley J, Luo M, Air GM, Laver GW. J Med Chem. 1999;42:2332–2343. doi: 10.1021/jm980707k. [DOI] [PubMed] [Google Scholar]
- 23.Brouillette WJ, Atigadda VR, Luo M, Air GM, Babu S, Bantia S. Bioorg Med Chem Lett. 1999;9:1901–1906. doi: 10.1016/s0960-894x(99)00318-2. [DOI] [PubMed] [Google Scholar]
- 24.Brouillette WJ, Bajpai SN, Ali SM, Velu SE, Atigadda VR, Lommer BS, Finley JB, Luo M, Air GM. Bioorg Med Chem. 2003;11:2739–2749. doi: 10.1016/s0968-0896(03)00271-2. [DOI] [PubMed] [Google Scholar]
- 25.Smith PW, Sollis SL, Howes PD, Cherry PC, Cobley KN, Taylor H, Whittington AR, Scicinski J, Bethell RC, Taylor N, Skarzynski AC, Singh O, Wonacott A, Varghese J, Coleman P. Bioorg Med Chem Lett. 1996;6:2931–2936. [Google Scholar]
- 26.Sollis SL, Smith PW, Howes PD, Cherry PC, Bethell RC. Bioorg Med Chem Lett. 1996;6:1805–1808. [Google Scholar]
- 27.Yang L, Qi CM, Zhang GX, Zou NZ. J Het Chem. 2003;40:1107–1112. [Google Scholar]
- 28.Bantia S, Parker D, Ananth L, Horn L, Andries K, Chand P, Kotian P, Dehghani A, Elkattan Y, Lin T, Hutchison T, Montgomery J, Kellog D, Babu Y. Antimicrobial Agents and Chemotherapy. 2001;45:1162–1167. doi: 10.1128/AAC.45.4.1162-1167.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell RJ, Haire LF, Stevens DJ, Collins PJ, Lin YP, Blackburn GM, Hay AJ, Gamblin SJ, Skehel JJ. Nature. 2006;443:45–49. doi: 10.1038/nature05114. [DOI] [PubMed] [Google Scholar]
- 30.Smith PW, Sollis SL, Howes PD, Cherry PC, Cobley KN, Taylor H, Whittington AR, Scicinski J, Bethell RC, Taylor N, Skarzynski AC, Singh O, Wonacott A, Varghese J, Coleman P. Bioorg Med Chem Lett. 1996;6:2931. [Google Scholar]
- 31.Sollis SL, Smith PW, Howes PD, Cherry PC, Bethell RC. Bioorg Med Chem Lett. 1996;6:1805. [Google Scholar]
- 32.Potier M, Mameli L, Belisle M, Dallaire L, Melancon SB. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Anal Biochem. 1979;94:287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.