Abstract
17β-estradiol (E2) treatment activates a set of protective response that have been found to protect cells from injury and more importantly to significantly abate the injuries associated with trauma-hemorrhage in vivo. Rapid NFκB activation has been found to be an important signaling step in E2 mediated protection in cell culture, in vivo ischemia and trauma-hemorrhage. In the current study, we investigated the signaling cascades linking E2 signaling with NFκB activation and the protective response, and compared them with the effects of two selective estrogen receptor modulators (SERMs), raloxifene and tamoxifen. Two candidate pathways, mitogen activated protein kinases (MAPK), and phosphatidylinositol-3-kinase (PI3-K) were studied. Selective inhibitors were used to identify each pathway's contribution to NFκB activation. Treatment of HCAECs with E2 activated PI3-K/Akt, p38, and JNK, all of which activated ERK 1/2 followed by NFκB activation. The combined activation of Akt, p38 and JNK was essential to activate NFκB. The two SERMs activated PI3-K and p38, which then phosphorylated ERK 1/2 and activated NFκB independent of the JNK pathway. NFkB activation by these compounds protected cells from hypoxia/reoxygenation injury. However, E2, unlike either SERM, led to modest increases in apoptosis through the JNK pathway. SERM treatment led to increased expression of the protective proteins, Mn-superoxide dismutase and endothelial nitric oxide synthase, that was not seen with E2. These results provide new insight into the pathways activating NFkB by E2 and SERMS and demonstrate that SERMs may have greater protective benefits than E2 in adult endothelial cells and potentially in vivo, as well.
Keywords: estrogen, cellular protection, trauma-hemorrhage, ischemia, NFkB
Introduction
Previously we have shown that 17β-estradiol (E2) rapidly activates nuclear factor κB (NFκB) followed by activation of heat shock factor (HSF)-1 and an increase in heat shock protein(HSP)72 expression.(1;2) This rapid activation of NFκB protects the cell from hypoxia/reoxygenation (H/R) injury independent of the activation of HSF-1 and up-regulation of HSP72. A similar protective effect for E2 has been found in vivo in trauma-hemorrhage (T-H).(3;4) Likewise, E2 treatment induced heat shock protein (HSP) expression and protected against injury from cerebral ischemia.(5) This protective rapid activation of NFκB by E2 is typical of the nongenomic response to E2 involving membrane-associated estrogen receptors (ER). However, the underlying signaling pathways linking E2 to rapid NFκB activation are unknown. In addition, whether synthetic estrogen receptor modulators (SERMs), such as the clinically available tamoxifen and raloxifene, will have protective effects similar to E2 has not been investigated.
E2 activates intracellular signaling pathways by binding to the membrane associated ER, or it can alter gene expression by binding the cytoplasmic/nuclear ER, leading to changes in gene transcription.(6) The membrane-initiated or nongenomic ER effects are rapid as they are mediated by intracellular signaling.(7;8) The best described nongenomic effect of E2 is activation of endothelial nitric oxide synthase (eNOS) and the subsequent release of nitric oxide.(9) A number of different signaling pathways have been found to be activated by E2 in different cell types. These pathways include G protein coupled receptors, phosphatidylinositol-3-kinase (PI3-K)/Akt, and the mitogen activated protein kinase (MAPK) family.
The rapidity of activation of NFκB by E2 points to a nongenomic effect. To define the pathways linking the membrane associated ER with NFκB activation and cellular protection, we investigated likely candidates, the MAPK family of kinases (p38, ERK 1/2, and JNK) and PI3-K. To further understand how selective estrogen receptor modulators (SERMs) differ in their actions from E2, comparison studies were done with raloxifene and tamoxifen, two clinically available drugs, examining both signaling pathways and the protein composition of the activated NFκB nuclear complex. Furthermore, we sought to identify whether treatment with either E2 or SERMs was associated with protection from hypoxia/reoxygenation injury through NFκB activation. Lastly, we investigated if E2 or SERM treatment led to differences in the expression of protective or inflammatory proteins.
Methods
Endothelial Cell Culture
Primary adult human coronary artery endothelial cells (HCAECs) were purchased from Clonetics (La Jolla, CA), and cultured as previously described.(2) All experiments were performed at passage 5 -7 on HCAECs from male donors, 22-34 years old.
Intracellular Signaling Protocols
70-80% confluent cells were treated with 5 nM 17β- estradiol (E2), 5 nM raloxifene (Sigma) or 5 nM tamoxifen (Sigma), or a combination of 5 nM E2 and 5 nM ICI 182,780, an inhibitor of ER. Cells were incubated with E2 or SERMs for various time points. The MEK 1 inhibitor, PD98059 (1μM, Alexis), was used to block phosphorylation of ERK1/2 (p42/44 MAPK), and SB203,580 (10 μM, Calbiochem) was used to inhibit MAPK p38. A JNK1 inhibitor (Alexis) was used at 10 nM. Two PI3-K inhibitors, Wortmannin (50 nM, Alexis) and LY 294,002 (10 μM, Alexis), were used to inhibit PI3-K. Cells were pre-incubated for 30 min with inhibitors before treatment with E2 or SERMs. The concentration of inhibitors used in this study were based on previous reports in the literature.(10;11) Inhibition studies for western blotting were performed at times of maximal activation of the respective pathways.
NFκB Activation Assay
Following treatment, cells were trypsinized and processed for nuclear isolation for the NFκB assay (Pierce Biotechnology, Rockford, IL). NFκB was assayed as previously described.(2) This assay, which utilizes a microtiter plate coated with the binding domain for NFκB, and measures binding of NFκB with an antibody for p50 or p65, allows for precise and reproducible measurement of NFκB activation. The results correlate well with EMSA, as we have previously shown.(1;2)
Western Blotting
Cells were washed and lysed in ice cold lysis buffer (1% Triton X-100, 1% deoxycholic acid, 150mM NaCl, 5mM EDTA, 10mM Tris pH 7.2, containing 2mM PMSF 10mM leupeptin, 10mM aprotinin, 1mM sodium orthovanadate, and 1nM sodium fluoride). Protein concentrations were measured using the BCA assay (Pierce) and lysates were stored at -70°C until used. Western blots were performed as previously described with some modifications for the detection of phosphorylated proteins.(12) Briefly, following transfer of proteins to the nitrocellulose membrane, membranes were blocked with 5% milk for 1 hour prior to overnight incubation at 4°C with primary antibody diluted in 5% milk or BSA. Phosphorylated proteins were detected using antibodies at 1:500 against phospho-Akt (Ser 473), phospho-p38 (Thr 180/Tyr 182), phospho-HSP 27 (Ser 87), phospho SAP/JNK (Thr 183/Tyr 185) or phospho ERK 1/2 (T202/204) (all from Cell Signaling, Beverly, MA) followed by incubation with a HRP conjugated secondary (Amersham Biosciences, Piscataway, NJ). Blots were stripped and reprobed for total protein by incubating the membranes with antibodies against total Akt, p38, HSP 27, SAP/JNK ERK 1/2, IκB-α at 1:1000 (Cell Signaling), iNOS and eNOS at 1:1000 (BD Transduction, San Diego, CA), Mn-SOD at 1:2000 (Assay Designs, Ann Arbor, MI), and VCAM-1 (Abcam, Cambridge, MA). HSP 27 phosphorylation was monitored as an index of p38 activation, as the p38 inhibitor, SB 203,580, does not inhibit p38 activation itself, but only phosphorylation events downstream of p38.(13)
Densitometry
Was performed as previously described using a Hewlett-Packard Scanjet model G3010 (Hewlett-Packard Co., Palo Alto, CA) and Un-Scan-It Gel software (Silk Scientific, Inc., Orem, UT).(12) Individual bands of the same size were measured for both phosphorylated and total proteins, as well as background. After subtracting the background pixilation, we calculated the ratios by dividing the average pixels/area for the phosphorylated to that of the total protein or the total protein to the respective β-actin. All ratios were then normalized to either a vehicle or zero time point.
DNA Affinity Immunoassay (DAI)
In order to identify proteins in the activated NFκB binding complex in the nucleus we the performed DAI assay, which allows qualitative analysis of the proteins in an activated transcription factor complex. The DAI assay was executed according to the methods of Liu et al. (14) Briefly, 50ug of nuclear lysate was incubated with a 5′end labeled biotin-consensus NFκB oligo (5′ AGTTGAGGGGACTTTCCCAGGC 3′) in 400 ul of DNA binding buffer (20 mM Tris-HCl (pH 7.2), 1 mM EDTA, 0.1% Triton X-100, and 4% glycerol). The binding buffer was then supplemented with 10nM of probe, 2ug of poly (dI.dC), 5mM DTT, and 0.15 mg of streptavidin magnetic coated beads (Promega, Madison, WI) and incubated at 4°C for 1 hr on a rocking platform. The protein-bead complex was captured using a magnetic stand (Dynal, Norway), and the beads were washed 3 times with DNA binding buffer. Samples were eluted in 20ul of 2× sample buffer and heated at 70°C for 5 minutes prior to being resolved on SDS-PAGE. Following transfer, blots were probed overnight for p105/p50, p65, Rel B (1:1000, Cell Signaling), p52/p100 (1:1000), Bcl-3 (1:500, eBioscience, San Diego, CA), c-Rel (1:1000, Calbiochem, San Diego, CA), and GRIP-1 (1:1000, Lab Vision, Fremont, CA) followed by incubation by an HRP conjugated secondary Ab (Amersham). The procedure was repeated using five different sample sets with similar results each time.
siRNA Transfection
Cells were transfected at 60-70% confluency with a mixture of 25nM of p42MAPK siRNA and p44MAPK siRNA (Sigma-Aldrich, St. Louis, MO) using HiPerfect transfection reagent (Qiagen, Valencia, CA) for 72 hours according to the manufacturer's instructions. Control transfections were performed with 25nM of fluorescein conjugated negative control siRNA (Qiagen).
Hypoxia-reoxygenation
H/R was done using a hypoxia workstation (Forma), which produces near-zero oxygen. The medium was changed to DMEM base (no glucose, glutamine, or phenol red to prevent switching to glycolysis), and the cells were subjected to hypoxia for 12 hr in an anaerobic workstation (model 1025, Forma Scientific, Marietta, OH; 4.8% CO2, 10.3% H2, and 84.9% N2) as previously described.(15) After hypoxia, cells were returned to normoxic conditions for an additional 2 hrs. Supernatant was assayed for LDH release using a commercially available kit according to the manufacturer's instructions (Biovision, Mountain View, CA).
TUNEL Assay
HCAECs were treated with E2, R, or T for 24 hours. A subset of E2 treated cells were pre-treated with a JNK inhibitor. Cells undergoing apoptosis were detected by labeling for DNA strand breaks by terminyl deoxynecleotidyl transferase (TdT)- mediated dUTP-nick end labeling (TUNEL) using a commercially available kit (Roche Applied Science, Indianapolis, IN).
NFκB Decoy Experiments
Were conducted as previously described.(2) Briefly, phosphorothioate dimers of the NFκB DNA binding domain were added to the cardiac myocytes in culture at a concentration of 2 μmol/L. Cells were treated for 6hrs with the NFκB decoy, and then the media was changed and the cells treated with E2, raloxifene, or tamoxifen. The decoys essentially act as a cytosolic sponge for activated NFκB, preventing nuclear translocation and binding to target promoters. A second nonspecific phosphorothioate dimer (Scramble) was used to control for the addition of phosphorothioate.
Statistical Analyses
The results are presented as the mean ± SEM of at least three separate experiments. Data was analyzed by a one way ANOVA or ANOVA on Ranks, where appropriate, followed by the Student-Newman-Keuls or Dunn's test (Sigma Stat). A p < 0.05 was considered significant.
Results
E2 and SERMS rapidly activate Akt and MAPK
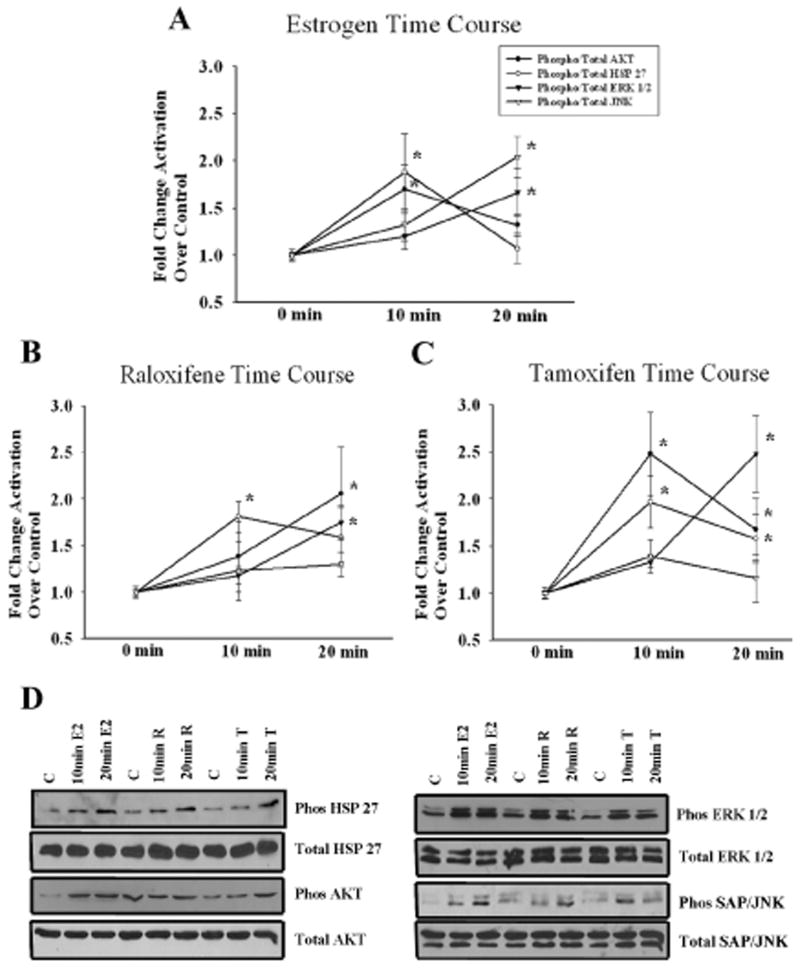
To investigate the role of the MAPK and PI3-K/Akt in NFκB activation, a critical step in E2-mediated protection, we treated endothelial cells for 10 and 20 minutes with E2, raloxifene and tamoxifen. E2 treatment induced a transient phosphorylation of Akt and HSP 27 (an index of p38 activity) by 10 minutes that was attenuated by 20 minutes. ERK 1/2 did not become activated until 20 minutes of E2. JNK phosphorylation was detected at 20 minutes (fig 1A), but not at 15 min.(data not shown). In comparison, the effects of SERMS were similarly assessed. Raloxifene activated HSP 27 by 10 minutes and both Akt and ERK 1/2 by 20 minutes. JNK phosphorylation was not significant (fig 1B). Tamoxifen's effects on Akt and HSP 27 phosphorylation in comparison to the other compounds were more sustained. Both Akt and HSP 27 were activated by 10 minutes of treatment and remained activated at 20 minutes. ERK 1/2 was phosphorylated after 20 minutes of tamoxifen treatment. Similar to raloxifene, JNK activation was not significant (fig 1C). Representative western blots for E2, raloxifene and tamoxifen are shown in fig 1D. Thus, these experiments demonstrate sequential activation of first Akt, p38 and JNK followed by ERK 1/2.
Figure 1.
Time Course for Signaling Pathway Activation by E2, raloxifene, and tamoxifen (10nM) for 10 or 20 minutes. Cells were analyzed for phosphorylation of Akt, HSP 27, ERK 1/2, and SAP/JNK. The membranes were stripped and reprobed for the respective total proteins. Graphs summarize time course for Akt and MAPK/JNK activation by E2 (A), raloxifene (B), and tamoxifen (C). D) Representative blots showing phosphorylation of Akt, HSP 27, ERK 1/2, and JNK for each compound following 10 and 20 minutes of treatment. n= 5-7. *p<0.05 vs. Ctl. C= Ctl, R= Raloxifene, & T= Tamoxifen.
E2 and SERMS activate NFκB through p50/p65
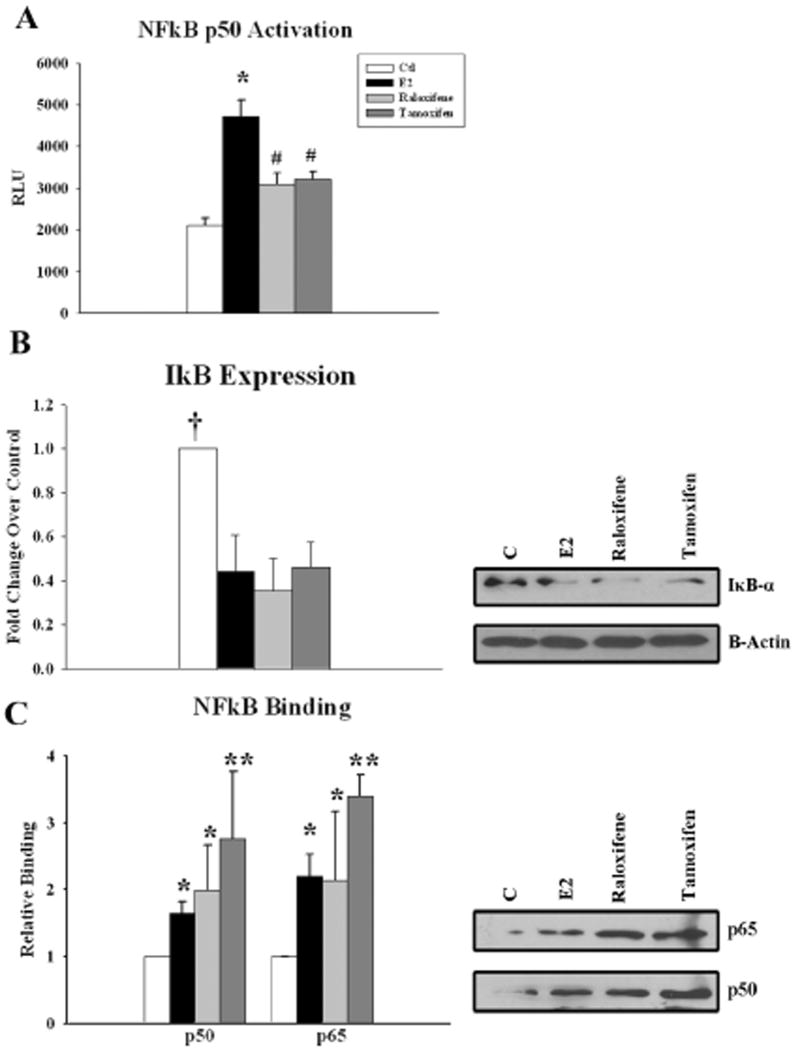
E2, raloxifene, and tamoxifen treatment all led to p50 NFκB activation after 20 minutes of treatment. NFκB activation by E2 was greater than both raloxifene and tamoxifen, neither of which activated JNK, unlike E2 (fig 2A). IκB degradation was also detected with E2 and both SERMs after 20 minutes of treatment (fig 2B). We investigated whether there was a difference in the NFkB complex components binding in the nucleus after E2, raloxifene and tamoxifen treatment. To address this question, we used the DAI assay to identify the proteins in the activated NFκB nuclear complex. P50 and p65 were both part of this complex for E2 as well as SERM treated HCAEC (fig 2C). E2, raloxifene, and tamoxifen all displayed increased binding of p50 and p65; however, no significant differences were detected between E2 and the SERMs. The NFkB binding subunits, Rel b, c-Rel, and p52/p100, along with the co-factors Bcl-3 and GRIP-1 were not detected.
Figure 2.
Effect of E2 and SERMs on NFκB- Cells were treated with E2, raloxifene, or tamoxifen for 20 minutes. A) Graphs summarizing increased p50 NFκB activation following 20 minutes of treatment with E2 and both SERMs, and the corresponding decrease in IκB (B). C) Graphs and representative blots showing increased nuclear translocation and association of p50 and p65 with the NFκB consensus sequence upon treatment with E2, raloxifene, or tamoxifen. The assay was repeated five times with similar results. n=5-7. * p<0.05 vs. Ctl, # p<0.05 vs. Ctl and E2, † p<0.05 vs. all other treatments, **p<0.01 vs. Ctl.
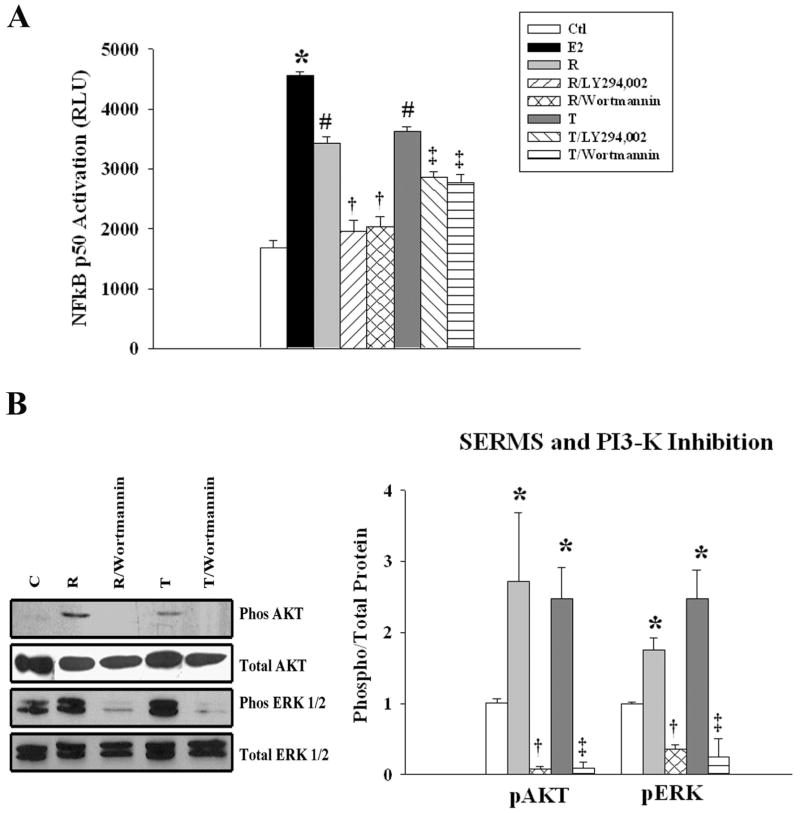
Inhibiting the PI3K/Akt pathway blocks E2 activation of NFκB
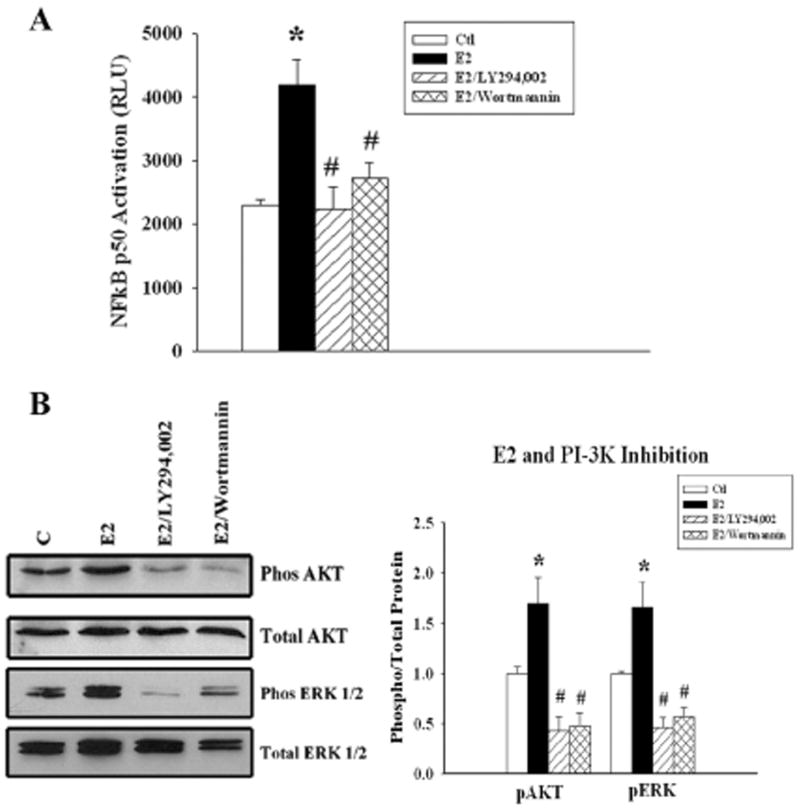
As shown in figure 3A, E2 more than doubled activation of NFκB, and this was blocked by the PI3-K inhibitors, Wortmannin and LY 294,002 (fig 3A). Pre-incubation with either inhibitor abolished the E2 induced phosphorylation of Akt. Inhibition of the PI3-K pathway also inhibited E2 induced ERK 1/2 phosphorylation (fig 3B).
Figure 3.
Effect of PI3-K/Akt inhibition on E2 activation of NFκB - Cells were pretreated with LY 294,002 or wortmannin followed by E2 for 20 min. A). Graph summarizes results of treatment with E2, or E2 plus PI3-K/Akt inhibitors, LY294,002 or wortmannin. B) Parallel cell cultures were used to detect phosphorylated Akt (top panel) and phosphorylated ERK 1/2. The membranes were stripped and re-probed for total Akt and total ERK 1/2. n=5-7. * p < 0.05 vs. Ctl, #p<0.05 vs. E2. C= Ctl.
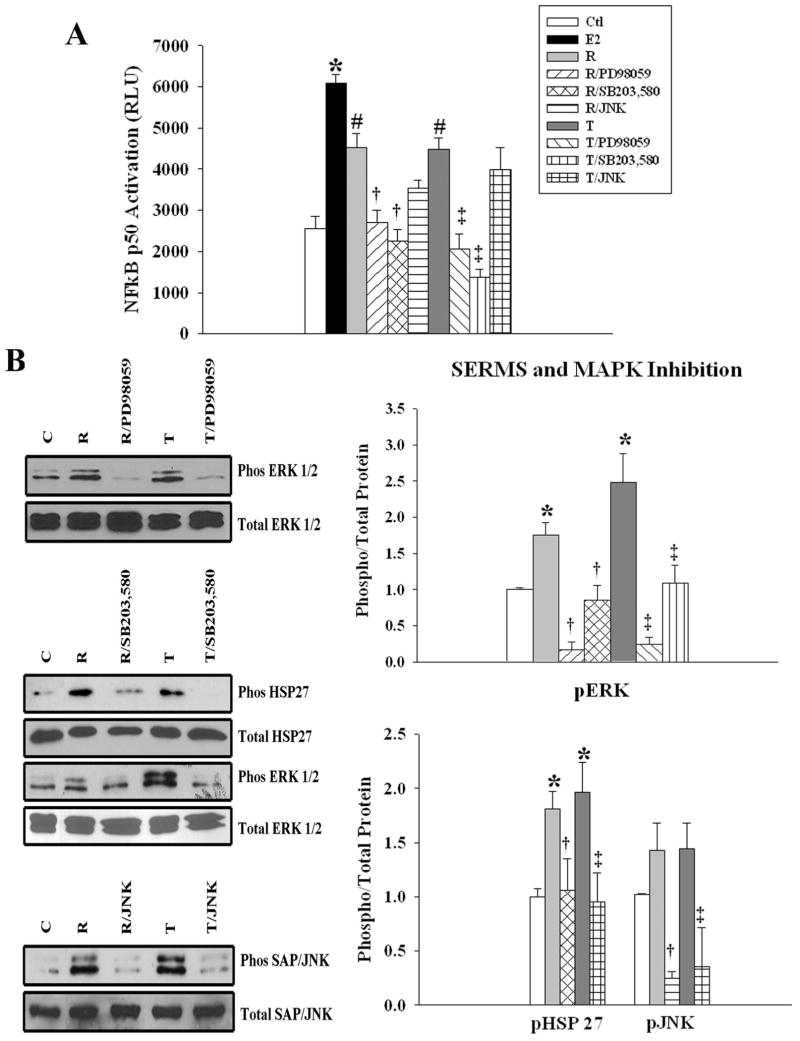
Inhibiting the MAPK pathway blocks E2 activation of NFκB
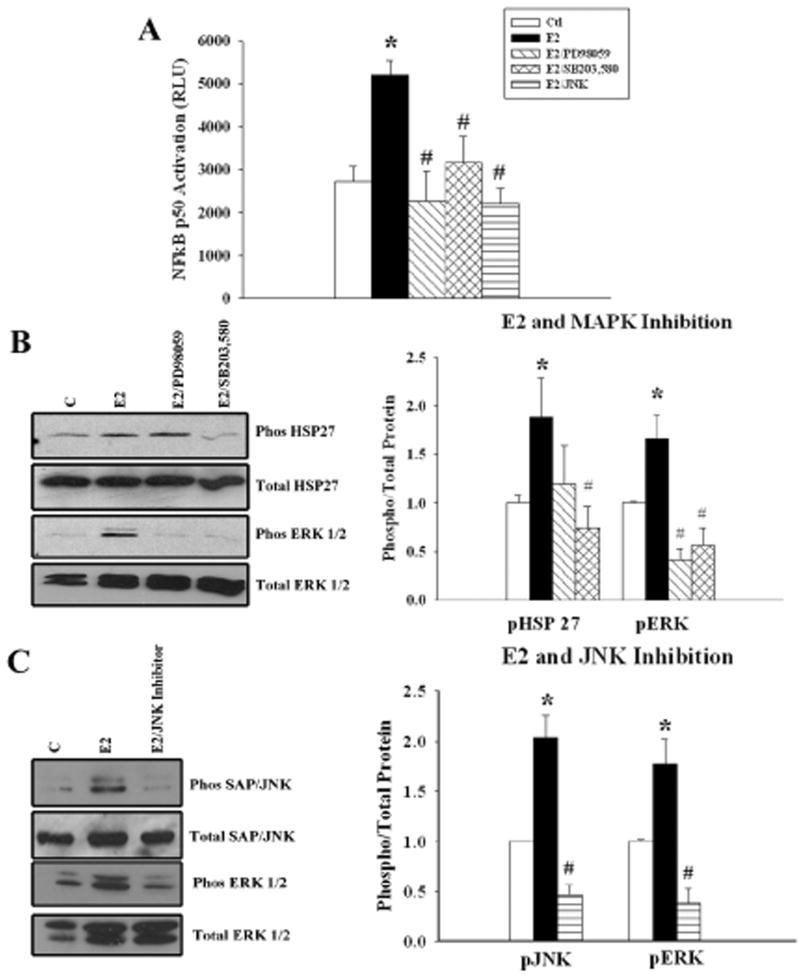
To determine whether the MAPK pathway is involved in the activation of NFκB, we examined the effect of MAPK inhibition on NFκB activation, using a MEK1 inhibitor (PD98059), p38 inhibitor (SB 203,580) and JNK1 inhibitor. Inhibition of either MAPK prevented NFκB activation by E2. There was no significant difference in the ability of the ERK1/2, p38, or JNK inhibitor to block E2-induced NFκB activation. (fig. 4A). Phosphorylation of HSP 27 could be inhibited by the p38 inhibitor, SB203,580 but not by the ERK inhibitor, PD98059. ERK 1/2 phosphorylation was inhibited by both PD98059 and SB203,580 (fig 4B). JNK inhibition attenuated the phosphorylation of SAP/JNK as well as ERK 1/2 (fig 4C). ERK 1/2 inhibition did not affect JNK activation by E2 (data not shown). Thus, p38 and JNK both activated ERK 1/2, which was phosphorylated later than Akt, p38 or JNK (fig. 1).
Figure 4.
Effect of MAPK inhibition on E2-induced NFκB activation. A) Graph summarizes the results of treatment with E2, or E2 plus ERK1/2 (PD98059), p38 MAPK (SB203,580), and JNK1 pathway inhibitors. B) Representative blots and a summary graph for data from parallel cell cultures used to detect phosphorylated and total HSP 27 (an index of p38 activation) and ERK 1/2. C) Representative blots and a summary graph for data from parallel cell cultures used to detect phosphorylated and total JNK and ERK 1/2 in cells pre-treated with a JNK1 inhibitor. n= 5-7. * p< 0.05 vs. Ctl, # p<0.05 vs. E2. C= Ctl.
SERMS activate NFκB through PI3-K
SERMS exhibit differences in their effects and have tissue and cell specific differences compared to E2.(16) However, little is known about the mechanisms responsible for these differences. To determine whether the signaling pathways involved in NFκB activation are the same as seen with E2, we examined the effect of PI3-K inhibitors on SERM activation of NFκB. Both raloxifene and tamoxifen treatment activated NFκB (fig 5A). The PI3-K inhibitors, LY294,002 and Wortmannin blocked NFκB activation mediated by either raloxifene or tamoxifen (fig.5A). Both SERMS activated Akt and ERK 1/2. The PI3-K inhibitor Wortmannin blocked Akt and ERK 1/2 phosphorylation (fig 5B).
Figure 5.
Effect of PI3-K on SERM activation of NFκB- Cells were pretreated with inhibitors followed by raloxifene (R) or tamoxifen (T) for 20 minutes. A) Graph summarizes the effect of raloxifene and tamoxifen, with or with the PI 3-K inhibitors, LY294002 and wortmannin, on NFκB activation. B) Parallel cell cultures were used to detect phosphorylated Akt and ERK 1/2. The membranes were stripped and re-probed for the total Akt and ERK. n=5. * p < 0 .05 vs. Ctl, # p<0.05 vs. Ctl and E2, † p<0.05 vs. R, ‡ p<0.05 vs. T. C= Ctl.
SERMS activate NFκB through p38 and ERK 1/2
Inhibition of ERK 1/2 or p38 blocked raloxifene and tamoxifen induced NFκB activation, similar to the effects seen with E2. JNK inhibition had no effect on NFκB activation by either raloxifene or tamoxifen (fig. 6A). The p38 inhibitor SB203,580 blocked HSP 27 and ERK 1/2 phosphorylation by raloxifene and tamoxifen, while MEK inhibition blocked ERK 1/2 activation (6B). Neither SERM had increased JNK phosphorylation over control, however JNK inhibition did reduce basal JNK activation (fig 6B).
Figure 6.
Effect of MAPK on SERM activation of NFκB. A) Graph summarizes the effect of raloxifene (R) and tamoxifen (T), with or without the MAPK/JNK inhibitors, PD98059, SB203,580, or JNK1 on NFκB activation B) Representative blots and graphs from parallel cell cultures were used to detect phosphorylated total HSP27 and ERK 1/2. C) Representative blots and graphs from parallel cell cultures were used to detect phosphorylated and total JNK in cells pre-treated with a JNK1 inhibitor. n=5-7. *p<0.05 vs. Ctl, # p<0.05 vs. Ctl and E2, † p<0.05 vs. R, ‡ p<0.05 vs. T. C= Ctl.
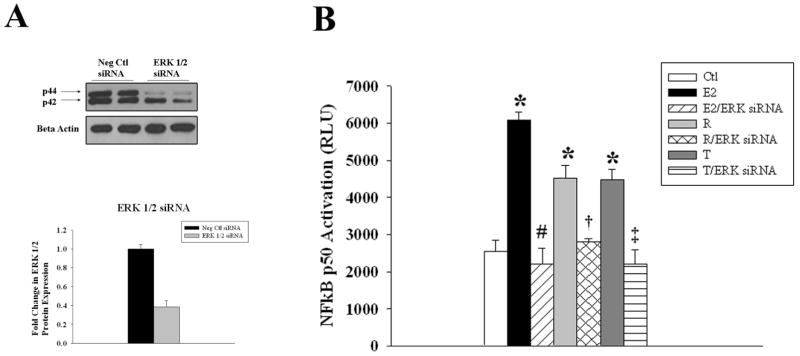
ERK 1/2 knockdown abolishes NFκB activation by E2 and SERMs
As inhibition of p38, JNK, or Akt inhibited ERK 1/2, we investigated whether the pathways converged on ERK 1/2 to activate NFκB. Treatment of HCAEC with 25nM of p42/44 siRNA resulted in approximately a 60% knockdown of ERK 1/2 protein expression, compared to negative control siRNA treated cells (fig 7A). In addition, knockdown of ERK 1/2 inhibited NFκB activation by E2, raloxifene or tamoxifen (fig 7B), supporting that ERK 1/2 phosphorylation is the convergence point for the upstream signaling pathways, PI3/Akt, p38 and JNK for NFκB activation by E2 and both SERMS (except no JNK activation by SERMs).
Figure 7.
Effect of ERK 1/2 siRNA on NFκB activation by E2 and SERMs. A) Representative blots and corresponding graph from cells transfected with negative control (Ctrl siRNA) or ERK 1/2 siRNA. β-Actin served as a protein-loading control. B) Graph summarizes the effect of ERK 1/2 siRNA on NFκB activation by E2, raloxifene (R), and tamoxifen (T). n=3-4. *p<0.05 vs. Ctl, #p<0.05 vs. E2, †p<0.05 vs. R, ‡p<0.05 vs. T. C= Ctl.
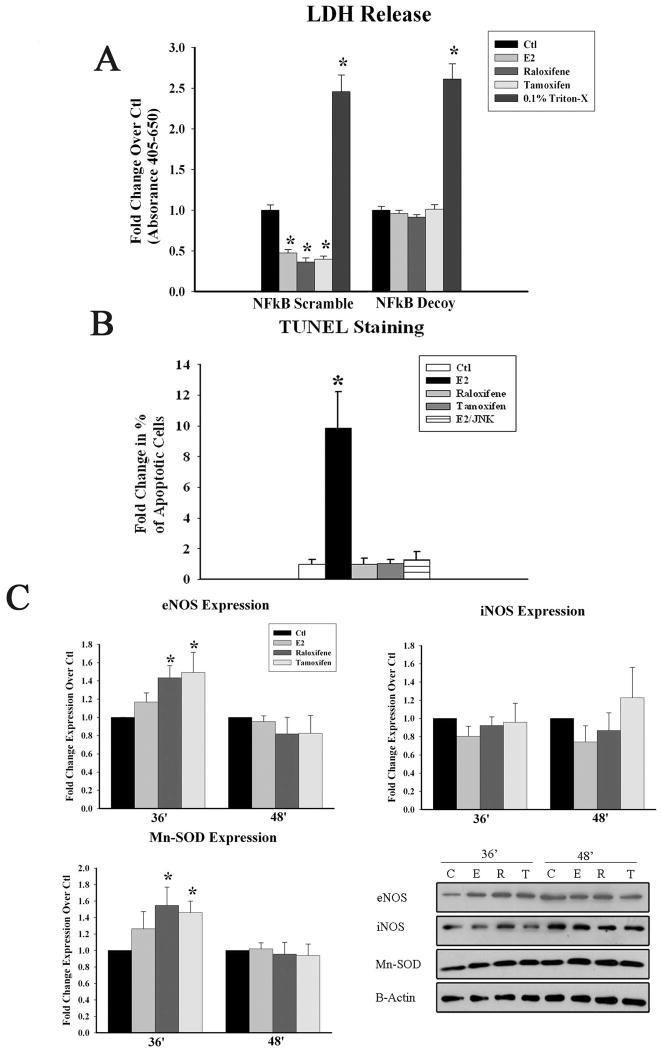
E2 and SERMs protect against H/R injury
We have previously shown that E2's protection against H/R injury is dependant on NFκB activation. In the current study, we tested whether the same is true with both raloxifene and tamoxifen. We found that pre-treatment with E2 or either SERM at 5nM for 36 hours prior to H/R injury reduced cell necrosis, as measured by LDH release. The protection against H/R injury was lost when NFκB activation was blocked using phosphorothioate DNA binding decoys to the NFκB consensus sequence, but not with the non-specific scramble treatment. (fig 8a).
Figure 8.
Downstream Effects of E2 and SERMs. A) Graph summarizes the effects of inhibiting NFkB activation using either scramble control or NFκB decoy activation and its attenuation of E2 or SERM induced protection against hypoxia/reoxygenation (H/R) injury B) Graph summarizes the effect of E2, raloxifene (R), tamoxifen (T), and E2 with JNK inhibition on apoptosis after 24 hours of treatment. C) Graph summarizes the results of the effect of E2, raloxifene, and tamoxifen treatment for 36 and 48 hours on the expression of eNOS, iNOS, and Mn-SOD after normalization to GAPDH and to Ctl. Representative western blots are shown below. * p<0.05. C=Ctl.
E2 induces apoptosis through the JNK pathway
As JNK activation is associated with pro-apoptotic cascades and 20 minutes of E2 treatment led to a significant increase in JNK phosphorylation, whereas neither SERM did, we investigated whether E2 treatment led to apoptosis through JNK activation. E2 but not, raloxifene or tamoxifen, increased apoptosis in HCAEC at 24h. Pre-treatment with a JNK1 inhibitor reduced E2 mediated increased apoptosis (fig 8b).
SERMS induce greater expression of Mn-SOD and eNOS than E2
As activation of NFκB by E2 and SERMS occurred through different pathways, we were also interested in whether any of the compounds differentially affected the expression of protective or inflammatory genes. Therefore we investigated the expression of several protective and inflammatory genes, Mn-SOD, eNOS, inducible nitric oxide synthase (iNOS), and vascular cell adhesion molecule-1 (VCAM-1). As shown in figure 8c, both raloxifene and tamoxifen, but not E2, increased expression of eNOS and Mn-SOD by 36 hours of treatment. Expression was not increased over control prior to 36 hours and was lost by 48 hours. The expression of the inflammatory protein iNOS did not change with E2 or SERM treatment, while VCAM-1 expression could not be detected.
Discussion
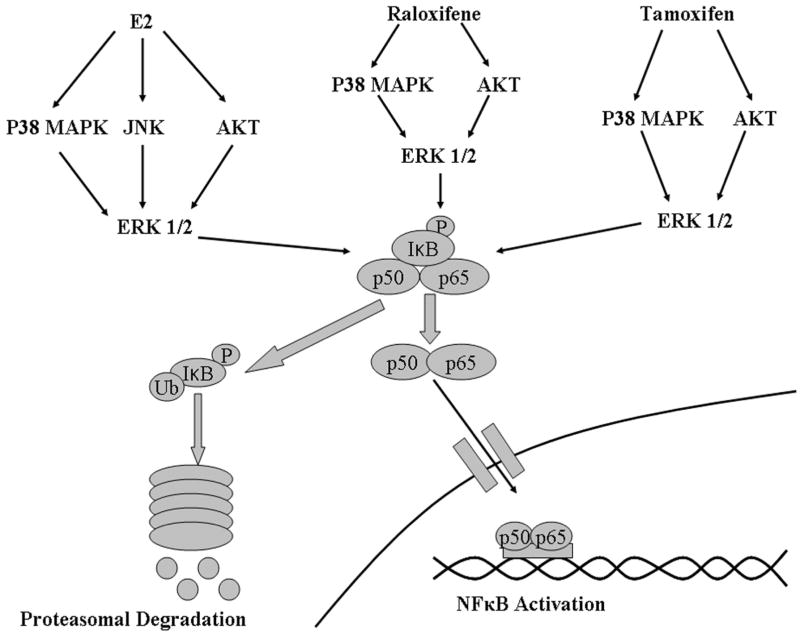
Treatment with E2 has been demonstrated in a number of studies to protect against injury both in cells as well as in in vivo models of trauma and ischemic injury, but the underlying mechanisms have not been fully defined.(1-3;5) The current experiments demonstrate that E2 activates PI3-K, and the MAP kinases p38, ERK1/2, and JNK, resulting in NFκB activation. For the first time it is demonstrated that these events occur in a sequential order over a short period of time. Inhibition of Akt, p38, or JNK blocked activation of ERK 1/2 and prevented activation of NFκB. Thus, E2 mediated activation of NFkB is like a 3 legged stool, dependent equally on 3 separate signaling pathways in order to have effect (fig 9). Inhibiting any one of these 3 pathways, Akt, p38 or JNK, is sufficient to block NFκB activation. Although the SERMs raloxifene and tamoxifen induced NFκB activation, the response to the two SERMs and the involved pathways differed somewhat from E2. Raloxifene and tamoxifen were dependent on dual activation of PI3-K/Akt, and p38 to phosphorylate ERK 1/2 and activate NFκB, whereas the JNK kinase pathway was not involved. All three different treatments activated NFκB through the p50/p65 heterodimer complex based on the DAI assay. E2 and the SERMs exhibited differences in their protective properties. Pretreatment with E2, raloxifene and tamoxifen reduced LDH release upon H/R injury and this effect was lost with NFκB inhibition. However, the activation of the JNK pathway by E2 led to a modest increase in apoptosis that was not seen with either SERM. In addition, raloxifene and tamoxifen, but not E2, increased expression of eNOS and Mn-SOD. Thus, the key finding is that the protective activation of NFκB by E2 in primary adult endothelial cells relies on the activation of three separate pathways and involves a different combination of pathways than raloxifene and tamoxifen. All treatments activated NFκB through convergence on ERK 1/2. SERMs may have greater therapeutic applications as they induced protective genes to a greater extent and did not induce apoptosis, as seen with E2 treatment.
Figure 9.
Diagram summarizing activation of NFkB by E2, raloxifene, and tamoxifen. E2 activates NFκB through activation of p38, Akt, and JNK which converge of ERK 1/2. Raloxifene and tamoxifen activate NFkB through p38 MAPK and Akt, which converge on ERK 1/2 MAPK. Activation by all three compounds lead to IκB degradation and p50/p65 nuclear translocation.
Although some aspects of the interactions between NFκB and genomic E2 signaling have been characterized, the link between membrane initiated E2 signaling and NFκB has not been well defined.(17;18) E2 has a dual role in the regulation of NFκB. Several studies have shown that E2 can activate NFkB and that this activation is protective both in vitro and in vivo.(2;11;19-21) In MCF-7 breast cancer cells, E2 rapidly activated ERK 1/2 followed by NFκB activation and increased the expression of the anti-oxidant proteins, Mn-SOD and glutathione peroxidase.(19) Here we show that E2, raloxifene and tamoxifen rapidly activated NFκB in HCAECs and protects against H/R injury. We also found that raloxifene and tamoxifen, but not E2, induced the expression of Mn-SOD and eNOS. E2 also has been shown to activate NFκB in B-cells and splenocytes through p50/p50 homodimers with the recruitment of the bcl-3 co-factor leading to expression of Bcl-2 and resistance to irradiation induced apoptosis.(20;22) In a microarray study using the immature HUVECs, 10nM E2 treatment activated NFκB non-genomically though the PI3-K/Akt pathway, leading to cyclooxygenase 2 (Cox-2) activation and prostacyclin release.(11) The induction of prostacyclins through Cox-2 has been shown to protect female mice from atherosclerotic development.(23) The study in HUVECS by Pedram et al. only investigated the PI3-K/Akt pathway; however, simultaneous activation of the MAPK signaling cascade may also occur here and be necessary for NFκB activation.(11) In the current study, activation of NFκB in primary HCAECs was more complex than in HUVECS and involved the simultaneous activation of multiple pathways; inhibition of either the PI3-K/Akt or MAPK pathway was sufficient to abolish NFκB activation by E2. Thus, the activation of NFkB by E2 is highly regulated and requires coordination of multiple pathways to induce the protective effects of acute in vivo E2 treatment.
In contrast to studies that demonstrate that E2 activates NFκB, a large body of work has shown an inhibitory effect of prolonged E2 treatment on NFκB activation and a suppression of its pro-inflammatory effects. For example, pre-treatment with E2 for 48 hours in HUVECs inhibits LPS-induced IKK activation.(24) In addition, overexpression of ER decreases IL-6 production in osteoclasts and stromal cells,(25;26) and increases IkB-α expression upon E2 treatment in HeLa cells.(27) The discrepancy between E2's activation vs. inhibition of NFκB is likely due to the duration of treatment and mode of action. While activation of NFκB occurs rapidly through membrane initiated signaling, the inhibitory effect occurs with more prolonged E2 treatment. The inhibitory effects of E2 on NFκB are most likely due to: 1) genomic E2 signaling, and 2) secondary effects on E2 on other cellular responses, such as heat shock protein synthesis, as reviewed elsewhere.(17;18) Overall, the effect of E2 in its regulation of NFκB is protective through the induction of acute stressor responses and the prevention of chronic inflammation and cellular damage that can accrue from unchecked NFκB activation.
Rapid NFκB activation by E2 and the SERMs in endothelial cells protected against injury (hypoxia/reoxygenation) in the current study. In vivo in settings of trauma and ischemia, rapid E2/SERM mediated protection could help preserve vascular integrity and perfusion of injured tissues through protection of the endothelial cells and through increased expression of MnSOD and eNOS (SERM treatment). Thus, this distinct signaling pathway has the potential to be beneficial in vivo, and a number of studies in rats have demonstrated that treatment with a single dose of E2 protects in the setting of T-H as well as in neural injury.(3-5;28;29)
A number of studies have investigated the benefit of E2 treatment, primarily in models of T-H and neural injury. In T-H models, reduction in organ neutrophil infiltration, and improved cardiac function with a single treatment of E2 have been found.(4;28) Similarly, female rats in proestrus/estrus, when estrogen levels would be high, had less red blood cell damage and less lipid peroxidation compared to males and low estrogen females after T-H-hemorrhage.(5) E2 just prior to cerebral ischemia increased heat shock protein expression in brain arteries, as well as glia and neurons.(5) Others found no difference in a spinal cord contusion model between females at low and high estrogen levels;(30) however these studies were based on vaginal smears as indicator of estrogen level and compared proestrus and estrus female rats. Depending on the exact hour given the 4 day rat cycle, there may not have been striking differences in the estrogen levels, which were not measured, between groups.
Many different endpoints have been examined as possibly involved in E2 mediated protection. ERK 1/2 activation and Akt activation have been studied with Akt mediating protective affects in the heart with increased HO-1 expression.(31) Bao et al. found E2 treatment led to early activation of Akt and ERK1/2 in a study of the cortical peri-contusional zone after cerebral contusion.(29) In contrast ERK 1/2 activation was associated with greater lung injury after T-H.(32) However, there was sustained activation of ERK1/2 with activation evident 2 h after T-H. In contrast, we show that E2 treatment leads to activation of ERK1/2, and this activation in very transient, disappearing within minutes. One of the strengths of the current study is the careful dissection of the involved signaling pathways, and the provocative finding that the sum of Akt, p38 and JNK activation are necessary to activation NfκB and the downstream protective cascade.
A key strength of this study is the comparison between E2 and the SERMs, raloxifene and tamoxifen. In contrast to E2, the actions of SERMs vary depending on the cell type, thereby granting the possibility to selectively inhibit or stimulate estrogen-like actions in various tissues.(16) The differences in NFκB activation by E2 compared to raloxifene and tamoxifen could be due to mixed agonist/antagonistic effects and/or higher bioactivity of E2 compared to the SERMs. Although E2 and both SERMs activated NFκB, they did so by different mechanisms. E2 activation of NFκB occurred partially through the pro-apoptotic JNK pathway, whereas neither SERM led to a significant activation of JNK. E2 and SERM treatment protected against H/R injury, but we also found that activation of the JNK pathway by E2 led to a modest increase in apoptosis in HCAEC following prolonged E2 treatment, which was not seen with raloxifene or tamoxifen. The mild induction of apoptosis by E2 illustrates how complex the actions of this powerful hormone are. In addition, raloxifene and tamoxifen induced increases in the protective proteins eNOS and Mn-SOD, which were not seen with E2. The rapid differential effects of E2 and SERMs on JNK, eNOS and Mn-SOD suggest a possible greater protective effect for the SERMs, given the absence of apoptosis and the increased expression on eNOS and MnSOD; however, these results in endothelial cells need to be investigated as to whether a beneficial effect for SERMs is seen in relevant in vivo models such as ischemia and T-H. As the response to E2 and SERMs is complex, the apparent benefits of SERMs vs. E2 may not translate into better outcomes in vivo. Certainly for studies of chronic use of E2 vs. SERMs, E2 has been much more potent that the SERMs for endpoints such as prevention of osteoporosis.
SERMs have beneficial cardiovascular effects, such as lowering of cholesterol levels, improved endothelial function, and reduced vascular smooth muscle tone, however most clinical studies have found these agents to be much less potent than E2.(33) Here we have demonstrated that both SERMs activated NFκB independent of the JNK pathway and did not lead to increased apoptosis. In addition SERM pre-treatment was able to attenuate LDH release following H/R injury through NFκB, and increase the expression of the protective genes eNOS and Mn-SOD. With two SERMs clinically available, they provide a possible alternative to E2 in translating the benefits of E2 in T-H and other injury to the critical care unit.
Model Choice
Many signaling studies have used human umbilical vein endothelial cells (HUVECs), as an endothelial cell model. The high availability and ease of isolation of these cells make them an attractive cell to use to study the endothelium. However, these venous endothelial cells are problematic as they are immature and differ in their responses from adult endothelial cells.(34;35) As diseases of the vasculature, such as atherosclerosis and hypertension, arise during aging it is important to use adult arterial cells, which were not available in the past, to better understand vascular biology and the potential therapeutic effect of treatments.
Limitations
The current study uses adult human coronary artery endothelial cells to compare the effects of E2, raloxifene and tamoxifen on the rapid induction of a protective pathway including the activation of NFκB. Cultured cells are a model, and not identical to the in vivo vasculature. The immediate effects of E2 and the SERMs are distinct from the effects of chronic administration of E2 and the SERMs. Although, as discussed, there is a substantial literature suggesting that immediate E2 treatment would be beneficial in the setting of acute trauma, no clinical trials have been published investigating the effects of immediate E2 or SERMs on morbidity and mortality post-trauma. A second limitation may be the use of p50 activation as an index of NFκB activation leading to gene transcription. P50 can form homodimers, which inhibit rather than activate gene transcription. When p50 binds p65, the resulting complex activates transcription. Previously we have shown that DNA binding decoys for NFκB block the protective effects of E2 treatment, including the increase in heat shock protein(HSP) 72 expression.(1) These results suggest that expression of genes rather than inhibition of gene expression is occurring with E2 mediated NFκB activation.
In the current study we sought to identify the pathways by which E2 rapidly activates NFκB in endothelial cells. In summary, we demonstrate that three different signaling pathways converge to activate of NFκB with E2 treatment. Two different SERMs had different patterns of signaling than E2, but also activated NFκB. ERK 1/2 phosphorylation was necessary for NFκB activation by E2 and both SERMs. In addition, simultaneous activation of multiple kinases are involved in the activation of NFκB. To the best of our knowledge, this is the first study to evaluate the signaling cascades leading to NFκB activation in HCAEC in response to physiological doses of E2, and equivalent doses of SERMs. Further work needs to be done to identify differences in signaling cascades induced by E2 and SERMS. Understanding the signaling cascades activated by estrogen and SERMs is critical, as elucidation of the molecular actions will lead to better comprehension of the clinical effects of these compounds, all of which are used in patients.
Acknowledgments
Funding: Supported by a grant from the Treadwell Foundation (AAK), the NIH AG19327 (AAK), and the American Heart Western States Affiliate (JPS).
Footnotes
Disclosure of Interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Reference List
- 1.Hamilton KL, Gupta S, Knowlton AA. Estrogen and regulation of heat shock protein expression in female cardiomyocytes: cross-talk with NFκB signaling. Journal of Molecular and Cellular Cardiology. 2004;36(4):577–84. doi: 10.1016/j.yjmcc.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton KL, Mbai FN, Gupta S, Knowlton AA. Estrogen, Heat Shock Proteins, and NF{kappa}B in Human Vascular Endothelium. Arterioscler Thromb Vasc Biol. 2004;24(9):1628–33. doi: 10.1161/01.ATV.0000137188.76195.fb. [DOI] [PubMed] [Google Scholar]
- 3.Choudhry MA, Chaudry IH. 17beta-Estradiol: a novel hormone for improving immune and cardiovascular responses following trauma-hemorrhage. Journal of Leukocyte Biology. 2008;83:518–22. doi: 10.1189/jlb.0607369. [DOI] [PubMed] [Google Scholar]
- 4.Ba Z, Hsu J, Chen J, Kan W, Schwacha M, Chaudry IH. Systematic Analysis of the Salutary Effect of Estrogen on Cardiac Performance After Trauma-Hemorrhage. Shock. 2008;30:585–9. doi: 10.1097/SHK.0b013e31816f1a45. [DOI] [PubMed] [Google Scholar]
- 5.Lu A, Ran R, Clark J, Reilly M, Nee A, Sharp FR. 17-β-Estradiol Induces Heat Shock Proteins in Brain Arteries and Potentiates Ischemic Heat Shock Protein Induction in Glia and Neurons. Journal of Cerebral Blood Flow and Metabolism. 2002;22:183–95. doi: 10.1097/00004647-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. The American Journal of Cardiology. 2002 Jul 3;90(1):F3–F4. doi: 10.1016/s0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- 7.Razandi M, Pedram A, Park ST, Levin ER. Proximal Events in Signaling by Plasma Membrane Estrogen Receptors. Journal of Biological Chemistry. 2003 Jan 17;278(4):2701–12. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 8.Ho KJ, Liao JK. Nonnuclear Actions of Estrogen. Arterioscler Thromb Vasc Biol. 2002 Dec 1;22(12):1952–61. doi: 10.1161/01.atv.0000041200.85946.4a. [DOI] [PubMed] [Google Scholar]
- 9.Shaul PW. Regulation of Endothelial Nitric Oxide Synthase: Location, Location, Location. Annual Review of Physiology. 2002 Jan 1;64(1):749–74. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 10.Billack B, Heck DE, Mariano TM, Gardner CR, Sur R, Laskin DL, Laskin JD. Induction of cyclooxygenase-2 by heat shock protein 60 in macrophages and endothelial cells. American Journal of Physiology. 2002;283:C1267–C1277. doi: 10.1152/ajpcell.00609.2001. [DOI] [PubMed] [Google Scholar]
- 11.Pedram A, Razandi M, Aitkenhead M, Hughes CCW, Levin ER. Integration of the Non-genomic and Genomic Actions of Estrogen. Membrane-initiated Signaling by Steroid to Transcription and Cell Biology. Journal of Biological Chemistry. 2002 Dec 20;277(52):50768–75. doi: 10.1074/jbc.M210106200. [DOI] [PubMed] [Google Scholar]
- 12.Stice JP, Eiserich JP, Knowlton AA. Role of Aging vs. the Loss of Estrogens in the Reduction in Vascular Function in Female Rats. Endocrinology. 2009 Jan 1;150:212–9. doi: 10.1210/en.2008-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanno M, Bassi R, Gorog DA, Saurin AT, Jiang J, Heads RJ, Martin JL, Davis RJ, Flavell RA, Marber MS. Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation: evidence for MKK-independent activation by a TAB1-associated mechanism contributing to injury during myocardial ischemia. Circ Res. 2003;93(3):254–61. doi: 10.1161/01.RES.0000083490.43943.85. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Asch H, Kulesz-Martin MF. Functional Quantification of DNA-binding Proteins p53 and Estrogen Receptor in Cells and Tumor Tissues by DNA Affinity Immunoblotting. Cancer Research. 2001 Jul 1;61(14):5402–6. [PubMed] [Google Scholar]
- 15.Gupta S, Knowlton AA. Cytosolic HSP60, Hypoxia and Apoptosis. Circulation. 2002;106:2727–33. doi: 10.1161/01.cir.0000038112.64503.6e. [DOI] [PubMed] [Google Scholar]
- 16.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators - mechanisms of action and application to clinical practice. New England Journal of Medicine. 2003;348:618–29. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 17.De Bosscher K, Vanden Berghe W, Haegeman G. Cross-talk between nuclear receptors and nuclear factor kappa B. Oncogene. 2006;25(51):6868–86. doi: 10.1038/sj.onc.1209935. [DOI] [PubMed] [Google Scholar]
- 18.Stice JP, Knowlton AA. Estrogen, NFκB and the Heat Shock Response. Molecular Medicine. 2008;14(7-8):517–8. doi: 10.2119/2008-00026.Stice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borràs C, Gambini J, Gómez-Cabrera M, Sastre J, Pallardó F, Mann G, Viña J. 17β-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFκB cascade. Aging Cell. 2005;4:113–8. doi: 10.1111/j.1474-9726.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 20.Kurland JF, Voehringer DW, Meyn RE. The MEK/ERK Pathway Acts Upstream of NF{kappa}B1 (p50) Homodimer Activity and Bcl-2 Expression in a Murine B-Cell Lymphoma Cell Line: MEK INHIBITION RESTORES RADIATION-INDUCED APOPTOSIS. Journal of Biological Chemistry. 2003 Aug 22;278(34):32465–70. doi: 10.1074/jbc.M212919200. [DOI] [PubMed] [Google Scholar]
- 21.Yu HP, Chaudry IH. The Role of Estrogen and Receptor Agonists in Maintaining Organ Function After Trauma-Hemorrhage. Shock. 2009;31(3):1–20. doi: 10.1097/SHK.0b013e31818347e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai R, Phillips RA, Ahmed SA. Despite Inhibition of Nuclear Localization of NF-{kappa}B p65, c-Rel, and RelB, 17-beta Estradiol Up-Regulates NF-{kappa}B Signaling in Mouse Splenocytes: The Potential Role of Bcl-3. J Immunol. 2007 Aug 1;179(3):1776–83. doi: 10.4049/jimmunol.179.3.1776. [DOI] [PubMed] [Google Scholar]
- 23.Egan KM, Lawson JA, Fries S, Koller B, Rader DJ, Smyth EM, FitzGerald GA. COX-2-Derived Prostacyclin Confers Atheroprotection on Female Mice. Science. 2004 Dec 10;306(5703):1954–7. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- 24.Simoncini T, Maffei S, Basta G, Barsacchi G, Genazzani AR, Liao JK, De Caterina R. Estrogens and Glucocorticoids Inhibit Endothelial Vascular Cell Adhesion Molecule-1 Expression by Different Transcriptional Mechanisms. Circulation Research. 2000 Jul 7;87(1):19–25. doi: 10.1161/01.res.87.1.19. [DOI] [PubMed] [Google Scholar]
- 25.Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Molecular and Cellular Biology. 1995 Sep 1;15(9):4971–9. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galien R, Garcia T. Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-kappaB site. Nucleic Acids Research. 1997 Jun 15;25(12):2424–9. doi: 10.1093/nar/25.12.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodel RC, Du Y, Bales KR, Gao F, Paul SM. Sodium salicylate and 17beta-estradiol attenuate nuclear transcription factor NF-kappaB translocation in cultured rat astroglial cultures following exposure to amyloid A beta (1-40) and lipopolysaccharides. Journal of Neurochemistry. 1999;73(4):1453–60. doi: 10.1046/j.1471-4159.1999.0731453.x. [DOI] [PubMed] [Google Scholar]
- 28.Yu HP, Shimizu T, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. Tissue-specific expression of estrogen receptors and their role in the regulation of neutrophil infiltration in various organs following trauma-hemorrhage. Journal of Leukocyte Biology. 2006 May 1;79(5):963–70. doi: 10.1189/jlb.1005596. [DOI] [PubMed] [Google Scholar]
- 29.Bao Y, Li L, Li X, Wang Y. 17Beta-estradiol differentially protects cortical pericontusional zone from programmed cell death after traumatic cerebral contusion as distinct stages via non-genomic and genomic pathways. Molecular and Cellular Neuroscience. 2011;48:185–94. doi: 10.1016/j.mcn.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Baker KA, Hagg T. An Adult Rat Spinal Cord Contusion Model of Sensory Axon Degeneration: The Estrus Cycle or a Preconditioning Lesion Do Not Affect Outcome. Journal of Neurotrauma. 2005;22(4):415–28. doi: 10.1089/neu.2005.22.415. [DOI] [PubMed] [Google Scholar]
- 31.Hsu JT, Kan WH, Hsieh CH, Choudry MA, Bland KI, Chaudry IH. Mechanisms of salutary effects of estrogen on cardiac function following trauma-hemorrhage: Akt-dependent HO-1 up-regulation. Critical Care Medicine. 2009;37(8):2338–44. doi: 10.1097/CCM.0b013e3181a030ce. [DOI] [PubMed] [Google Scholar]
- 32.Hsu J, Kan W, Hsieh CH, Choudhry MA, Bland KI, Chaudry IH. Role of estracellular signal-regulated protein kinase (ERK) in 17β-estradiol-mediated attenuation of lung injury after trauma-hemorrhage. Surgery. 2009;145:226–34. doi: 10.1016/j.surg.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Leung FP, Tsang SY, Wong CM, Yung LM, Chan YC, Leung HS, Yao X, Huang Y. Raloxifene, tamoxifen and vascular tone. Clin Exp Pharmacol Physiol. 2007;34(8):809–13. doi: 10.1111/j.1440-1681.2007.04684.x. [DOI] [PubMed] [Google Scholar]
- 34.Tan PH, Chan C, Xue SA, Dong R, Ananthesayanan B, Manunta M, Kerouedan C, Cheshire NJW, Wolfe JH, Haskard DO. Phenotypic and functional differences between human saphenous vein (HSVEC) and umbilical vein (HUVEC) endothelial cells. Atherosclerosis. 2004 Apr;173(2):171–83. doi: 10.1016/j.atherosclerosis.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Rydholm H, Bostrom S, Eriksson E, Risberg B. Different fibrinolytic potentials between human umbilical vein endothelial cells and human adult vein endothelial cells. European Surgical Research. 1996;28:380–7. doi: 10.1159/000129480. [DOI] [PubMed] [Google Scholar]