Abstract
Objective
An imbalance between maternal angiogenic/anti-angiogenic factors concentrations has been observed in preeclampsia (PE) and other obstetrical syndromes. However, the frequency of pathologic findings in the placenta and the changes in maternal plasma angiogenic/anti-angiogenic factor concentrations differ between late-and early-onset PE. The aim of this study was to determine if the maternal plasma concentrations of placental growth factor (PlGF), soluble endoglin (sEng), and soluble vascular endothelial growth factor receptor-1 and 2 (sVEGFR-1 and sVEGFR-2) are different in late-onset PE with and without placental pathologic findings consistent with maternal underperfusion.
Study design
A cross-sectional study was conducted including 64 uncomplicated women and 66 women with late-onset PE (>34 weeks) who had blood samples and placenta available for pathologic examination. Patients with late-onset PE were divided into those with and without placental histologic findings consistent with maternal underperfusion as proposed by the Society for Pediatric Pathology. Maternal plasma concentrations of PlGF, sEng, sVEGFR-1 and sVEGFR-2 were determined by ELISA. Non-parametric statistics were used for analysis.
Results
1) the prevalence of placental histological findings consistent with maternal underperfusion among women with late-onset PE was higher than that of those with an uncomplicated pregnancy (47% (31/66) vs. 7.8% (5/64) respectively; p<0.01); 2) patients with late-onset PE and histological findings consistent with maternal underperfusion had a significantly lower median plasma concentration of PlGF, plasma PlGF/sVEGFR-1 ratio and plasma PlGF/sEng ratio than those with late-onset PE without placental underperfusion lesions (each p<0.05); 3) the most common pathological findings in the placenta of patient with PE were lesions consistent with villous changes (77%, 24/31); and 4) isolated vascular lesions in the placenta were found only in 2 cases (6.5%), and the rest had a combination of villous and vascular lesions.
Conclusions
Nearly half of the patients with late-onset PE have placental lesions consistent with maternal underperfusion. These lesions are associated with an imbalance in the maternal concentration of angiogenic/anti-angiogenic factors. We propose that there is a link between maternal underperfusion and an anti-angiogenic state characterized by the changes in the concentrations of angiogenic and anti-angiogenic factors in women with late onset PE.
Keywords: Placental growth factor (PlGF), soluble endoglin (sEng), soluble vascular endothelial growth factor receptor-1 (sVEGFR-1), soluble vascular endothelial growth factor receptor-2 (sVEGFR-2), ischemic placenta
Introduction
Preeclampsia (PE) is one of the leading causes of perinatal and maternal mortality [1–6]. Despite extensive research, the pathophysiology of this syndrome is still unclear [7,8]. Accumulating evidence, however, indicates that a central feature in the pathophysiology of preeclampsia is failure of physiologic transformation of the spiral arteries [9–13]. Although the primary insults for these abnormalities remain elusive [14,15], it is postulated that the resulting poor placentation and reduced blood supply to the placenta in early pregnancy leads to the release of factors into the maternal circulation causing systemic endothelial cell dysfunction [16,17], metabolic changes [18–20], a pro-thrombotic state [21–26], complement activation [27–32], intravascular inflammation [33–38] and multiple organ damage [1,39,40]. Candidates for these unknown factors [7,41,42] are cytokines [43–45] syncytiotrophoblast microparticles [46,46,47] apoptotic products [48,49] reactive-oxygen species [50,51] activated leukocytes [52,53] angiotensin II type 1 receptor antibody [54–56], galactins [57–59], soluble vascular endothelial growth factor receptor (sVEGFR)-1 [60–64] and soluble endoglin (sEng) [65–67]. Recently, an anti-angiogenic state has been proposed to play a central role in the pathophysiology of PE [62–102].
An imbalance between circulating angiogenic factors [vascular endothelial growth factor (VEGF) and placental growth factor (PlGF)] and anti-angiogenic factors (sVEGFR-1, sVEGFR-2 and sEng) has been observed both prior to the clinical manifestation [62,67,72,73,82,86–88,90,95–97,103–106] and at the time of clinical diagnosis of PE [63,65,68,69,71,75,79,84,107,108]. The sources of the imbalance between angiogenic and antiangiogenic factors in the maternal circulation are thought to derive mainly from the ischemic or underperfused placenta [61,68,109,110]. Consistent with this hypothesis, increased impedance to blood flow in the uterine artery [75,86,87,96,111,112] or uterine artery ligation in pregnant animals [113–115] are associated with an imbalance in the concentrations of angiogenic/anti-angiogenic factors in maternal circulation.
Several investigators have proposed that early- and late-onset PE may have different pathophysiology and that these two phenotypes should be studied individually [116–118]. Early-onset PE (≤34 weeks) is associated with greater perinatal and maternal mortality and morbidity than late-onset disease (≥34 weeks) [119–121]. Thus, the former type of PE received much more attention from investigators than the latter. However, a recent study from South Africa reported a 30% rate of severe maternal complications, 13% of eclampsia and 1.9% of fetal deaths, in patients with late-onset PE [122]. Moreover, the prevalence of late-onset disease is much higher than that of early-onset PE [123]. Thus, late-onset PE remains a major cause of maternal morbidity/mortality and fetal death worldwide, especially in several developing countries [124–126].
Although the magnitude of the imbalance between angiogenic and antiangiogenic factor concentrations in maternal blood is greater in early-onset than in late-onset PE [62,71,75,90,112,127], and the presence of placental lesions such as decidual arteriolopathy, infarctions, hypermaturity of villi (which may be related to reduced uteroplacental blood flow) is more common in early-onset than in late-onset PE [128–130], a subset of patients with late-onset PE has been affected by an imbalance of angiogenic/anti-angiogenic factors in maternal circulation [71,75,97,112,131]. Interestingly, the relationship between pathologic lesions of the underperfused placenta and plasma angiogenic/anti-angiogneic factors concentrations in late-onset PE has never been studied.
To gain insight into the pathophysiology of late-onset PE, the aim of this study was to examine if there is a change in the maternal plasma concentrations of PlGF, sEng, sVEGFR-1 and sVEGFR-2 in patients with late-onset PE with and without placental lesions consistent with maternal underperfusion.
Material and Methods
This retrospective cross-sectional study was conducted by searching the clinical database and bank of biological samples of the Perinatology Research Branch of NICHD/NIH and included 66 patients with the diagnosis of late-onset PE (>34 weeks) and 64 women with uncomplicated pregnancies. All were enrolled at Hutzel Women’s Hospital in Detroit, MI, USA between June 1999 to July 2002. The inclusion criteria for women with PE (cases) were: 1) singleton gestation; 2) absence of major fetal structural or chromosomal anomalies; 3) absence of chronic hypertension; and 4) blood samples available for assay of angiogenic and anti-angiogenic factors, and placental blocks available for pathological review. Patients with PE had venipuncture upon diagnosis at enrollment. The control group consisted of women who had a venipuncture at the time of admission to Labor and Delivery (in cases of scheduled Cesarean section) or at the antenatal clinic prior to delivery or the initiation of labor.
Clinical definitions
Preeclampsia was defined as new onset hypertension that developed after 20 weeks of gestation (systolic or diastolic blood pressure ≥140 and/or ≥90 mm Hg, respectively, measured on at least two occasions, 4 hours to 1 week apart) and proteinuria (≥300 mg in a 24-hour urine collection, or two random urine specimens obtained 4 hours to 1 week apart containing ≥1+ by dipstick [132]or one dipstick demonstrating ≥2+ protein) [133]. Severe PE was diagnosed as described previously [133]. Late-onset PE was defined as diagnosis of PE after 34 weeks of gestation [116]. An SGA neonate was defined by a birthweight below the 10th percentile for gestational age using a reference range from Alexander et al [134].
A patient was considered to have an uncomplicated pregnancy if she met the following criteria: 1) singleton gestation; 2) no medical, obstetrical or surgical complications; 3) absence of labor at the time of venipuncture; and 4) delivery of a normal term infant (≥37 weeks) whose birth weight was between the 10th and 90th percentile for gestational age.
Histopathological examination of the placenta
Histopathological changes of the placenta were diagnosed according to the criteria proposed by the Perinatal Section of the Society for Pediatric Pathology [135], which included lesions consistent with maternal vascular underperfusion. Findings consistent with maternal underperfusion were classified as: 1) villous changes, further subdivided into abrupt onset (remote villous infarcts, recent villous infarcts), gradual onset with intermediate duration (increased syncytial knots, villous agglutination, increased intervillous fibrin), or gradual onset with prolonged duration (distal villous hypoplasia); and 2) vascular lesions (persistent muscularization of basal plate arteries, mural hypertrophy of decidual arterioles, acute atherosis of basal plate arteries and/or decidual arterioles). The decreased placental weight/increased fetoplacental weight ratio, which is part of the villous changes of prolonged duration in the classification, was not included because this information was not uniformly available. The presence of findings consistent with maternal underperfusion was defined by the presence of at least one pathologic lesion consistent with the above classification.
Maternal plasma concentrations of angiogenic and anti-angiogenic factors
After venipuncture was performed, the blood was collected into tubes containing EDTA, centrifuged and stored at −70°C. Maternal plasma concentrations of PlGF, sEng, sVEGFR-1 and sVEGFR-2 were determined by sensitive and specific immunoassays obtained from R&D Systems (Minneapolis, MN). All four immunoassays utilized the quantitative sandwich enzyme immunoassay technique and their concentrations in maternal plasma were determined by interpolation from the standard curves. The results of plasma PlGF, sEng, sVEGFR-1 and sVEGFR-2 concentrations have been previously reported in a study that evaluated the relationship between angiogenic/antiangiogenic factors and Doppler studies [62,111,112]. The inter- and intra-assay coefficients of variation obtained were: PlGF: 6.02% and 4.8%, respectively; sEng: 2.3% and 4.6% respectively; sVEGFR-1: 1.4% and 3.9%, respectively and sVEGFR-2: 2% and 4%, respectively. The sensitivity of the assays was: PlGF: 9.52 pg/ml, sEng: 0.08 ng/ml, sVEGFR-1: 16.97 pg/ml and sVEGFR-2: 19.01 pg/ml.
Placental histopathologic examinations
Placental tissue samples were taken by systematic random sampling [136], and subsequently fixed in 10% neutral-buffered formalin overnight, and embedded in paraffin. Five 3m-thick paraffin sections were stained with hematoxylin and eosin, and examined using bright-field light microscopy. Histopathologic examinations were performed by anatomic pathologists who were blinded to the clinical diagnosis.
Patients were divided into those with and without placental histologic findings consistent with maternal underperfusion [135]. The maternal plasma concentrations of PlGF, sEng, sVEGFR-1 and -2, the ratios of PlGFs/VEGFR-1 and PlGF/sEng in late-onset PE were compared between patients with and without these lesions.
All women provided written informed consent prior to the collection of samples. The collection of samples was approved by the Human Investigation Committees and its utilization for research purposes by the Institutional Review Boards of both, Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Statistical Analysis
The normality of the data was tested using the Shapiro-Wilktest. Kruskal Wallis and post-hoc Mann-Whitney U tests were utilized for comparison of continuous variables among and between groups. Adjustment for multiple comparisons was performed with the Holm method. The Pearson’s chi-square test was used to compare the proportion of placental lesions in the two groups. Analysis was conducted with SPSS V.15 (SPSS Inc., Chicago, IL). A p value <0.05 was considered significant.
Results
The clinical and obstetrical characteristics of women with an uncomplicated pregnancy (control) and that of those with late-onset PE, with and without underperfused placental lesions, are displayed in Table I. Patients with late-onset PE (with or without evidence of underperfused placental lesions) had a lower median gestational age at delivery and birthweight than normal pregnant women (all p<0.01; Table I). There were no significant differences in the demographic characteristics between patients with late-onset PE with and without evidence of underperfused placental lesions. As expected, women with late-onset PE and evidence of underperfused placental lesions delivered a higher proportion of SGA neonates than those without evidence of these lesions (64.5% vs. 37.1%; p<0.05). There was no difference in the proportion of severe PE among patients with late-onset PE with and without underperfused placental lesions (83.9% vs. 88.6%; p>0.05).
Table I.
Clinical and obstetrical characteristics of the study population
| Uncomplicated Pregnancy (n = 64) | Preeclampsia without placental lesions of underperfusion (n = 35) | Preeclampsia with placental lesions of underperfusion (n = 31) | |
|---|---|---|---|
|
| |||
| Maternal age (y) | 27 (22 – 30) | 21 (18 – 27)* | 24 (20–30) |
| Nulliparity | 11 (17.2) | 21 (60) | 20 (64.5) |
| Smoking | 14 (21.9) | 5 (14.3) | 6 (19.4) |
| Gestational age at venipuncture (wks) | 39.1 (38.3 – 39.4) | 37.7 (36.5 – 39)* | 38.4 (36 – 39.8) |
| Severe preeclampsia | 0 | 31 (88.6) | 26 (83.9) |
| Gestational age at delivery (wks) | 39.1 (38.8 – 39.8) | 37.8 (36.5 – 39)* | 38.5 (36 – 39.8)* |
| Birthweight (g) | 3342 (3152 – 3622) | 2790 (2380 – 3210) *μ | 2650 (2060 – 2880) *μ |
| SGA (BW <10%) | 0 | 13 (37.1) | 20 (64.5) |
| Male neonates | 35 (54.7) | 18 (51.4) | 13 (41.9) |
Values expressed as median (interquartile range) or number (percent)
p<0.05 when compared to normal pregnancy
p<0.05 when compared to late-onset preeclampsia without underperfusion placental lesions
SGA: Small for gestational age; BW: Birthweight
Placental histologic findings
Patients with late-onset PE had a higher rate of placental lesions consistent with maternal underperfusion than those with uncomplicated pregnancies [47% (31/66) vs. 7.8% (5/64); p<0.01). Among patients with late-onset PE with underperfused placental lesions, 77.5% (24/31) had lesions consistent with villous changes, 6.5% (2/31) had isolated vascular lesions and 16% (5/31) had a combination of villous and vascular lesions. In contrast, all of the underperfused placental lesions observed in the control group consisted of villous changes.
To exclude the potential contribution of SGA to the frequency of underperfused placental lesions, a sub-analysis was performed excluding the women with PE who delivered an SGA neonate in the late-onset PE group. There were 33 patients with late-onset PE without SGA and 33% (11/33) of these cases had histologic lesions consistent with maternal underperfusion. After exclusion of patients with PE and SGA, the frequency of underperfused placental lesions was still more common in late-onset PE than in women with uncomplicated pregnancies [33.3% (11/33) vs. 7.8% (5/64); p<0.05].
Late-onset PE was associated with an imbalance of angiogenic/anti-angiogenic factors in maternal circulation
Women with late-onset PE, (either with or without underperfused placental lesions), had a higher median plasma concentration of sEng and sVEGFR-1 and lower median plasma concentrations of PlGF, PlGF/sVEGFR-1 ratio and PlGF/sEng ratio than women with an uncomplicated pregnancy (all p<0.001; see Figures 1–5 and Table II). In addition, the median plasma concentration of sVEGFR-2 was significantly lower in women with PE without underperfused placental lesions than that of uncomplicated pregnancies (p=0.001; see Figure 6 and Table II).
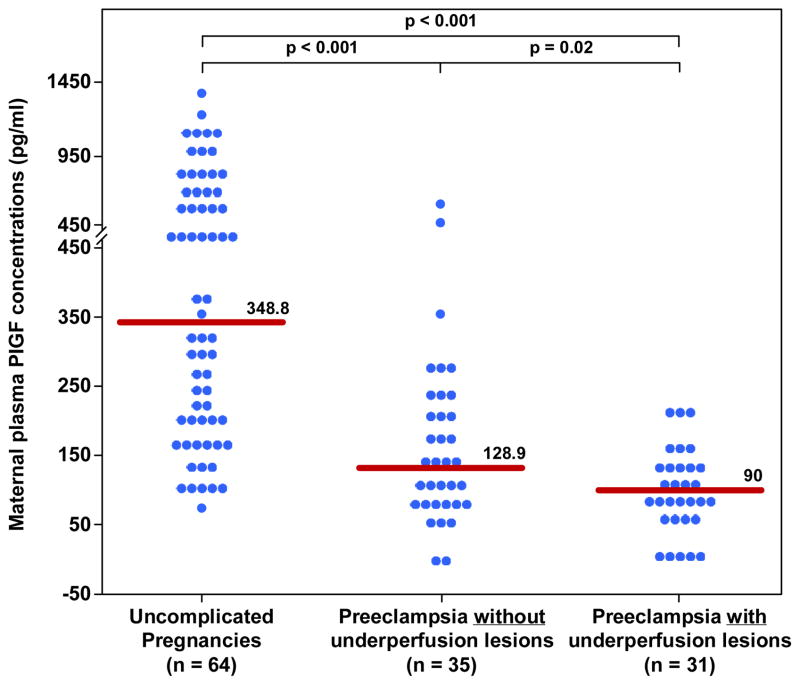
Figure 1. Maternal plasma concentration of PlGF between women with an uncomplicated pregnancy and women with late-onset PE with and without underperfused placental lesions.
Women with late-onset PE without underperfused placental lesions had a lower median plasma PlGF concentration than women with uncomplicated pregnancies. Similarly, women with late-onset PE with underperfused placental lesions had a lower median plasma PlGF concentration than women with uncomplicated pregnancies. Among women with late-onset PE those who had underperfused placental lesions had a lower median plasma PlGF concentration than those without these lesions.
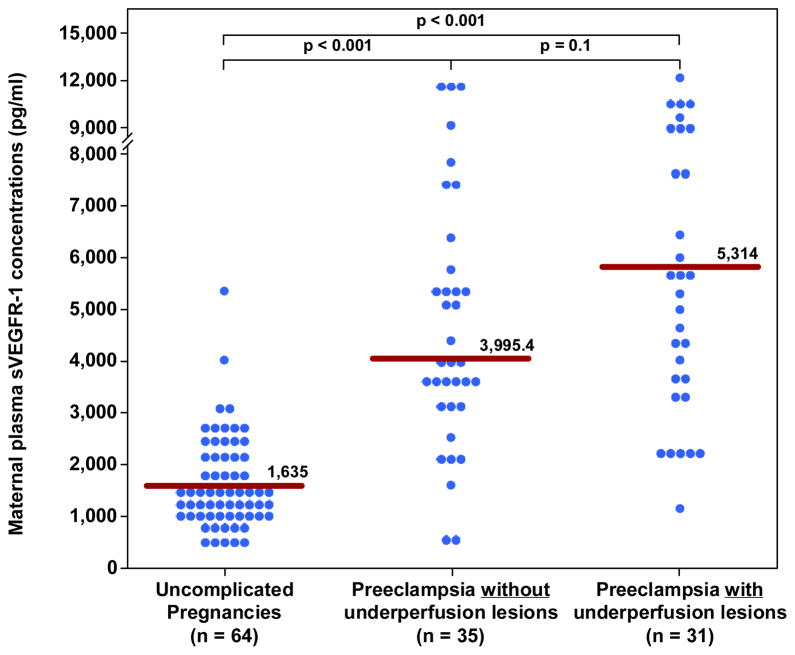
Figure 5. Maternal median plasma concentration of sVEGFR-1 between women with an uncomplicated pregnancy and women with late-onset PE with and without underperfused placental lesions.
Women with late-onset PE without underperfused placental lesions had a higher median plasma sVEGFR-1 than women with uncomplicated pregnancies. Similarly, women with late-onset PE with underperfused placental lesions had higher median plasma sVEGFR-1 than women with uncomplicated pregnancies. In contrast, there was no difference in the median plasma sVEGFR-1 between women with late-onset PE with or without underperfused placental lesion.
Table II.
Maternal plasma concentration of angiongenic and antiangiogenic factors in the study population
| Uncomplicated Pregnancy (n=64) | Preeclampsia without placental lesions of underperfusion (n= 35) | Preeclampsia with placental lesions of underperfusion (n=31) | |
|---|---|---|---|
|
| |||
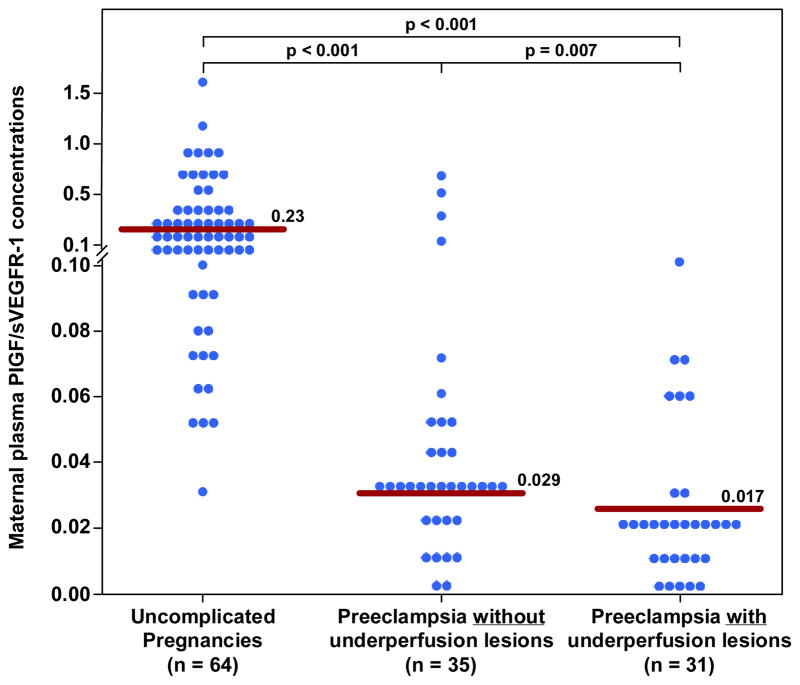
| PlGF(pg/ml) | 348.8 (196.3–729.3) | 128.9 (85–212)† | 90 (68–137)*† |
| PlGF/sVEGFR-1 | 0.23 (0.11–0.43) | 0.029 (0.020–0.048)† | 0.017 (0.10–0.26)*† |
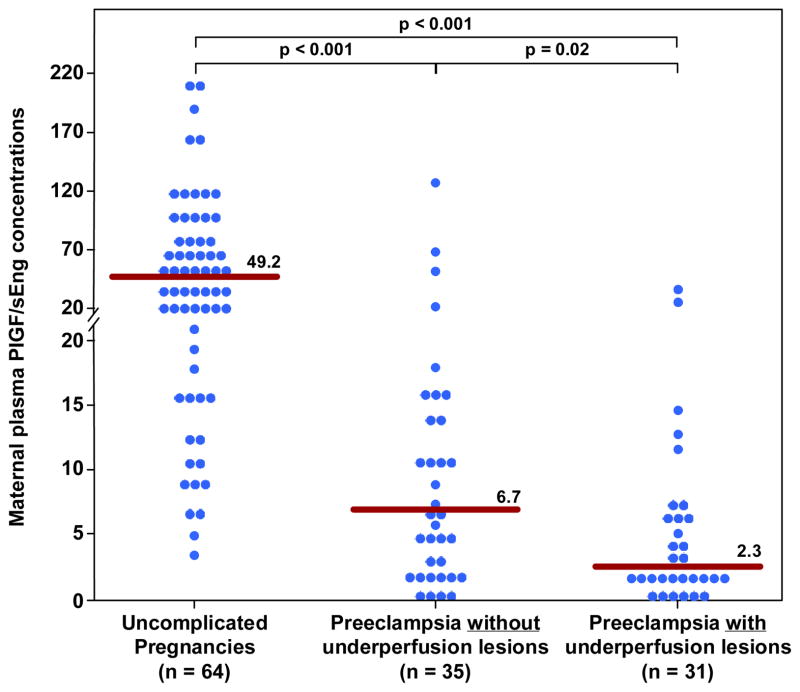
| PlGF/sEng | 49.2 (17.2–96) | 6.7 (1.9–13.1)† | 2.3 (1.5–6)*† |
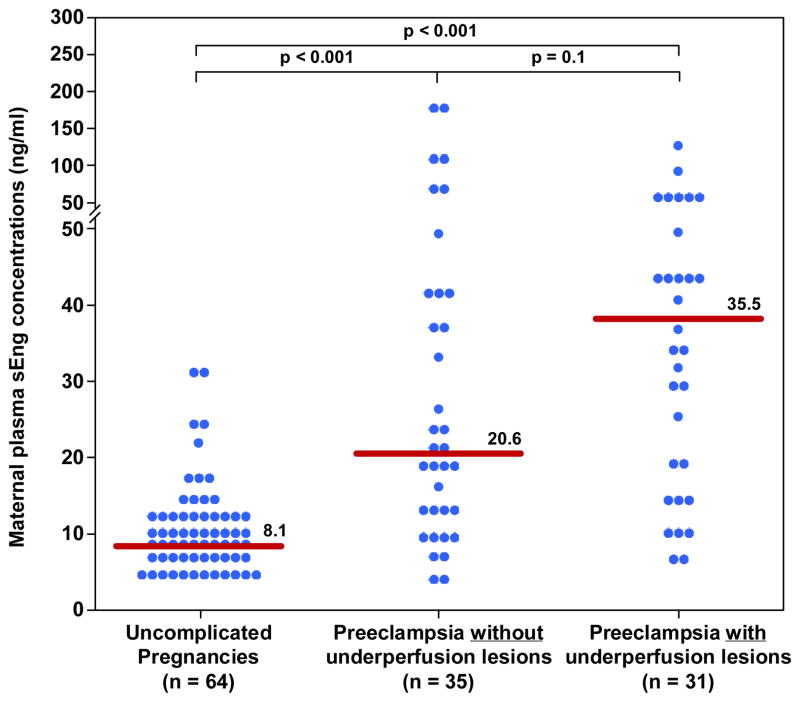
| sEng(ng/ml) | 8.1 (6.1–12.5) | 20.6 (12–40.7)† | 35.5 (15–49.2)† |
| sVEGFR-1(pg/ml) | 1635 (1150–2138) | 3995 (3058–5679)† | 5314 (3404–8231)† |
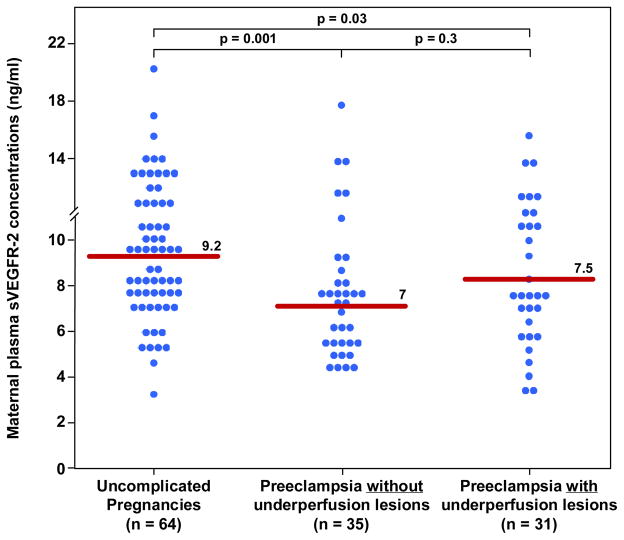
| sVEGFR-2(ng/ml) | 9.2 (7.4–11.2) | 7 (5.4–8.5)† | 7.5 (5.6–10.5) |
Values expressed as median (interquartile range)
PE: Preeclampsia; PlGF: placental growth factor; sEng: soluble endoglin;
sVEGFR-1: soluble vascular endothelial growth factor receptor 1
sVEGFR-2: soluble vascular endothelial growth factor receptor 2
p-value <0.05 when compared to late-onset PE without placental lesions of underperfusion.
p-value <0.01 when compared to normal pregnancy
Figure 6. Maternal median plasma concentration of sVEGFR-2 between women with uncomplicated pregnancy and women with late-onset PE with and without underperfused placental lesions.
Women with late-onset PE without underperfused placental lesion had higher median plasma sVEGFR-2 than women with uncomplicated pregnancies. In contrast, there was no difference in the median plasma concentration of sVEGFR-2 between women with late-onset PE with underperfused placental lesions women with uncomplicated pregnancies. There was no difference in the median plasma sVEGFR-2 between women with late-onset PE with or without underperfused placental lesion.
Patients with late-onset PE with underperfused placental lesions had a higher magnitude of an imbalance of angiogenic/anti-angiogenic factors than those without these lesions
Among women with late-onset PE, those with histological findings consistent with maternal underperfusion had a lower median plasma concentration of PlGF, PlGF/sVEGFR-1 ratio and PlGF/sEng ratio than those without these lesions; (all p<0.05; Figure 1–3 and Table II). The median plasma concentration of sEng and sVEGFR-1 was higher in patients with PE and evidence of underperfused placental lesions than that of those without these lesions. However, the difference did not reach statistical significance (p=0.1 for each; Figure 4 and 5; Table II). In contrast, there was no significant difference in the median plasma concentration of sVEGR-2 between the 2 groups (p= 0.3; Figure 6 and Table II).
Figure 3. Maternal plasma PlGF/s-Eng ratio between women with an uncomplicated pregnancy and women with late-onset PE with and without underperfused placental lesions.
Women with late-onset PE without underperfused placental lesion had lower median plasma PlGF/s-Eng ratio than women with uncomplicated pregnancies. Similarly, women with late-onset PE with underperfused placental lesions had a lower median plasma PlGF/s-Eng ratio than women with uncomplicated pregnancies. Among women with late-onset PE those with underperfused placental lesions had a lower median plasma PlGF/s-Eng ratio than women with late-onset PE without evidence of underperfused placental lesions.
Figure 4. Maternal median plasma concentration of s-Eng between women with an uncomplicated pregnancy and women with late-onset PE with and without underperfused placental lesions.
Women with late-onset PE without underperfused placental lesions had a higher median plasma s-Eng than women with uncomplicated pregnancies. Similarly, women with late-onset PE with underperfused placental lesions had a higher median plasma s-Eng than women with uncomplicated pregnancies. In contrast, there was no difference in the median plasma s-Eng between women with late-onset PE with or without underperfused placental lesions.
Discussion
Principal findings of the study
1) A subset of patients with late-onset PE had an imbalance of angiogenic/anti-angiogenic factor concentrations in maternal circulation; these patients also had a higher frequency of histologic findings consistent with maternal underperfusion than that of women with a normal pregnancy; 2) among women with late-onset PE, the most common placental lesion associated with maternal underperfusion is villous changes (77%); and 3) the plasma concentrations of PlGF, PlGF/sVEGFR1 ratio and PlGF/sEng ratio, markers of angiogenic forces, were lower in patients with late-onset PE with underperfused placental lesions compared to those without these lesions.
Late-onset PE is associated with an imbalance of angiogenic/antiangiogenic factors
The finding that women with late-onset PE had significantly lower plasma concentrations of PlGF, sVEGFR-2, PlGF/sEng ratio and PlGF/sVEGFR-1 ratio and higher sVEGFR-1 and sEng compared to normal pregnant women extended our observations from a previous study in which we reported the changes of maternal plasma concentrations of angiogenic/anti-angiogenic factor in late-onset PE compared to normal pregnancy in a Hispanic population [62,67,71]. Although the mean plasma concentrations of PlGF and sVEGFR-1 has been reported to be different between Caucasians and Hispanics [137], evidence of an imbalance of angiogenic/antiangiogenic factors concentrations in PE has consistently been observed in women of Hispanic, Caucasian, Asian, African and African-American ethnic origin, and therefore, appears to be a consistent finding of the disorder [79,94,137–141]. Additional findings from the current study are that patients with late-onset PE, either with or without underperfused placental lesions, also experienced perturbation of these angiogenic/anti-angiogenic factors in their circulation.
Late-onset PE with underperfused placental lesions
The observation that patients with late-onset PE had a higher frequency of placental lesions consistent with maternal underperfusion than those with uncomplicated pregnancies is consistent with our observation in a Hispanic population [142]. A nested case-control study that included 8,307 women with singleton pregnancies reported that the prevalence of placental findings suggestive of maternal underperfusion on patients with PE decreased gradually with gestational age [142]. For example, the prevalence of histological lesions of placental underperfusion, using similar criteria, in PE decreased from approximately 75% before 32 weeks of gestation to about 50% after 34 weeks, and to 30% at term gestation [142]. In the current study, almost half of women with late-onset PE had evidence of histologic placental lesions consistent with maternal underperfusion.
Erez et al. have reported that women with PE had a higher proportion of underperfusion placental lesions than women who had an SGA neonate without PE [55.6% (65/117) vs. 32.7% (16/49)] [24]. In addition, syncytial knots was the lesion most commonly observed in women with PE [24]. Such findings are consistent with those observed in this study. In contrast, in a small histomorphometric study, placenta from women with PE at term without growth restriction were described to be similar to those from uncomplicated pregnant women at term regarding weight, parenchymal and cellular content, and surface areas of exchange between the mother and the fetus [143]. The prevalence of placental lesions indicative of underperfusion in the current study was 33% after exclusion of patients with PE and SGA, one of the most severe forms of late-onset PE. Possible explanations for these discrepant findings are the differences in histological criteria used to evaluate the placentas and the higher proportion of patients with severe PE included in the current study. While abnormal morphology of the villous tree is frequently observed in early-onset IUGR (elongated maldeveloped villi) and PE (excessive branching villi) [144–147], Egbor et al reported that late-onset PE had a minimal influence on placental villous and vascular morphology compared with gestational age-matched controls [148]. The current study did not evaluate anatomical or morphometric changes of the villous tree.
Relationship between underperfused placental lesions and an imbalance of angiogenic/antiangiogenic factors among patients with late-onset PE
Patients with late-onset PE without placental lesions of underperfusion had lower plasma concentrations of PlGF and higher sVEGFR-1, sEng than uncomplicated pregnant women. However, patients with late-onset PE who had placental lesions of underperfusion had a phigher magnitude of an imbalance of angiogenic/anti-angiogenic factors concentration than those without placental lesions as demonstrated by significantly lower PlGF, PlGF/sVEGFR-1 ratio, and PlGF/sEng ratio. This perturbation is mainly driven by lower concentrations of the angiogenic factor PlGF, rather than an increase in anti-angiogenic factors as observed in early-onset disease [63,71,75,112,131].
In fact, in vitro studies have demonstrated that hypoxia may regulate the expression of PlGF, as demonstrated in isolated human term syncytiotrophoblast under hypoxic conditions (e.g. reduced mRNA PlGF expression) [149]. Similarly, hypoxia reduced the PlGF concentrations in the supernatant of primary cytotrophoblast cultures [150]. Thus, it is possible that chronic uteroplacental ischemia may account for the low maternal plasma concentration of PlGF in this subgroup of patients. However, a primary deficiency in PlGF cannot be excluded. The changes of sVEGFR-1 and sEng, which can also be induced by hypoxia, were modest and did not reach statistical significance in this study.
Imbalance of angiogenic factor in patients with late-onset PE without placental lesions of underperfusion
It is noteworthy that plasma sVEGFR-2 concentrations in late-onset PE are significantly lower than that of uncomplicated, pregnant women only in patients without evidence of an underperfused placenta. We interpret our results as suggesting that this subgroup of patients may have evidence of systemic inflammation without placental involvement [33–35]. Low plasma concentrations of sVEGFR-2 have been observed in patients with systemic inflammation, such as pyelonephritis during pregnancy [151]. Non-placental sources of angiogenic/antiangiogenic factors are activated monocytes, platelets and endothelial cells which may be responsible for the increase of anti-angiogenic factors in this subgroup of patients [152]. This interpretation would be consistent with the hypothesis that other factors (i.e. maternal systemic inflammation), rather than unique placenta factors, may play a significant role in the pathogenesis of late-onset PE [34,153].
Strengths and limitations of the study
This study is the first to examine the relationship between placental histologic findings and several maternal plasma angiogenic/antiangiogenic factors implicated in the pathophysiology of late-onset PE. Moreover, the histologic criteria used in this study were derived from a research and consensus study in which the pathological findings are reproducible [135,154]. Three limitations of this study are: 1) the inclusion criteria of this study required that every patient had placenta and plasma samples collected; therefore, a limited number of patients were included; 2) the criteria used to define lesions of placenta underperfusion may be too broad and the prevalence of these lesions could be decreased if more than two subclasses of lesions were required for the diagnosis; and 3) the prevalence of severe PE was high in this study. This may represent the nature of the patients admitted to a single tertiary care center.
Conclusion
A subset of patients with late-onset PE has an imbalance of angiogenic/anti-angiogenic factors concentration in maternal plasma. These patients also have a higher frequency of placental histological findings consistent with maternal underperfusion than uncomplicated pregnancies. Of note, the imbalance between angiogenic and antiangiogenic factors is predominantly due to low concentrations of the angiogenic factor PlGF.
Since late-onset PE is more common than early-onset PE, serious efforts to understand the pathophysiology and placental pathology of this condition must be undertaken. It is often overlooked that most women with eclampsia are patients with late-onset PE. Thus, the recent focus on early-onset PE should not diminish the clinical, epidemiologic, scientific, and public health importance of late-onset PE. Our study is an effort to understand late-onset PE.
Figure 2. Maternal plasma PlGF/sVEGFR-1 ratio between women with an uncomplicated pregnancy and women with late-onset PE with and without underperfused placental lesions.
Women with late-onset PE without underperfused placental lesion had lower median plasma PlGF/sVEGFR-1 ratio than women with uncomplicated pregnancies. Similarly, women with late-onset PE with underperfused placental lesions had a lower median plasma PlGF/sVEGFR-1 ratio than women with uncomplicated pregnancies. Among women with late-onset PE those with underperfused placental lesions had a lower median plasma PlGF/sVEGFR-1 ratio than women with late- onset PE without evidence of underperfused placental lesions.
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
Presented at the 58th Annual Meeting of the Society for Gynecologic Investigation on March 16–19, 2011, Miami, FL
Declaration of interest
Tinnakorn Chaiworapongsa is a consultant in the preeclampsia advisory board of Roche Diagnostics. This study was conducted without any support from Roche Diagnostics. The immunoassays used in this study were not acquired from Roche Diagnostics.
Reference List
- 1.Romero R, Lockwood C, Oyarzun E, Hobbins JC. Toxemia: new concepts in an old disease. Semin Perinatol. 1988;12:302–23. [PubMed] [Google Scholar]
- 2.von DP, Menzies J, Magee LA. The complications of hypertension in pregnancy. Minerva Med. 2005;96:287–302. [PubMed] [Google Scholar]
- 3.Sibai BM. Hypertensive disorders of pregnancy: the United States perspective. Curr Opin Obstet Gynecol. 2008;20:102–6. doi: 10.1097/GCO.0b013e3282f73380. [DOI] [PubMed] [Google Scholar]
- 4.Berg CJ, MacKay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993–1997 and 2001–2005. Obstet Gynecol. 2009;113:1075–81. doi: 10.1097/AOG.0b013e3181a09fc0. [DOI] [PubMed] [Google Scholar]
- 5.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 6.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299–306. doi: 10.1097/AOG.0b013e3181a45b25. [DOI] [PubMed] [Google Scholar]
- 7.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 8.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–9. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 9.Robertson WB, Brosens I, Dixon G. Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspect Nephrol Hypertens. 1976;5:115–27. [PubMed] [Google Scholar]
- 10.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van Assche A. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648–55. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 11.Romero R, Kusanovic JP, Chaiworapongsa T, Hassan SS. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract Res Clin Obstet Gynaecol. 2011;25:313–27. doi: 10.1016/j.bpobgyn.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silasi M, Cohen B, Karumanchi SA, Rana S. Abnormal placentation, angiogenic factors, and the pathogenesis of preeclampsia. Obstet Gynecol Clin North Am. 2010;37:239–53. doi: 10.1016/j.ogc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts JM, Hubel CA. Placenta. 2008. The Two Stage Model of Preeclampsia: Variations on the Theme. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gammill HS, Roberts JM. Emerging concepts in preeclampsia investigation. Front Biosci. 2007;12:2403–11. doi: 10.2741/2242. [DOI] [PubMed] [Google Scholar]
- 16.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–4. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 17.Roberts JM, Taylor RN, Goldfien A. Endothelial cell activation as a pathogenetic factor in preeclampsia. Semin Perinatol. 1991;15:86–93. [PubMed] [Google Scholar]
- 18.Vaisbuch E, Romero R, Mazaki-Tovi S, Erez O, Kim SK, Chaiworapongsa T, Gotsch F, Than NG, Dong Z, Pacora P, et al. Retinol binding protein 4--a novel association with early-onset preeclampsia. J Perinat Med. 2010;38:129–39. doi: 10.1515/JPM.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Erez O, Gotsch F, Chaiworapongsa T, Than NG, Kim SK, Nhan-Chang CL, et al. Maternal serum adiponectin multimers in preeclampsia. J Perinat Med. 2009;37:349–63. doi: 10.1515/JPM.2009.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, Pineles BL, Gomez R, Edwin S, Mazor M, et al. Adiponectin in severe preeclampsia. J Perinat Med. 2007;35:503–12. doi: 10.1515/JPM.2007.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: a mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in the placenta. J Matern Fetal Med. 1998;7:277–86. doi: 10.1002/(SICI)1520-6661(199811/12)7:6<277::AID-MFM5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Chaiworapongsa T, Yoshimatsu J, Espinoza J, Kim YM, Berman S, Edwin S, Yoon BH, Romero R. Evidence of in vivo generation of thrombin in patients with small-for-gestational-age fetuses and pre-eclampsia. J Matern Fetal Neonatal Med. 2002;11:362–7. doi: 10.1080/jmf.11.6.362.367. [DOI] [PubMed] [Google Scholar]
- 23.Erez O, Hoppensteadt D, Romero R, Espinoza J, Goncalves L, Nien JK, Kusanovic JP, Fareed J, Gotsch F, Pineles B, et al. Preeclampsia is associated with low concentrations of protein Z. J Matern Fetal Neonatal Med. 2007;20:661–7. doi: 10.1080/14767050701495011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erez O, Romero R, Hoppensteadt D, Than NG, Fareed J, Mazaki-Tovi S, Espinoza J, Chaiworapongsa T, Kim SS, Yoon BH, et al. Tissue factor and its natural inhibitor in pre-eclampsia and SGA. J Matern Fetal Neonatal Med. 2008;21:855–69. doi: 10.1080/14767050802361872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erez O, Romero R, Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Chaiworapongsa T, Than NG, Gotsch F, Kim CJ, Mittal P, et al. Maternal anti-protein Z antibodies in pregnancies complicated by pre-eclampsia, SGA and fetal death. J Matern Fetal Neonatal Med. 2009;22:662–71. doi: 10.1080/14767050902801751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erez O, Romero R, Kim SS, Kim JS, Kim YM, Wildman DE, Than NG, Mazaki-Tovi S, Gotsch F, Pineles B, et al. Over-expression of the thrombin receptor (PAR-1) in the placenta in preeclampsia: a mechanism for the intersection of coagulation and inflammation. J Matern Fetal Neonatal Med. 2008;21:345–55. doi: 10.1080/14767050802034859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soto E, Romero R, Richani K, Espinoza J, Chaiworapongsa T, Nien JK, Edwin SS, Kim YM, Hong JS, Goncalves LF, et al. Preeclampsia and pregnancies with small-for-gestational age neonates have different profiles of complement split products. J Matern Fetal Neonatal Med. 2010;23:646–57. doi: 10.3109/14767050903301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch AM, Salmon JE. Dysregulated complement activation as a common pathway of injury in preeclampsia and other pregnancy complications. Placenta. 2010;31:561–7. doi: 10.1016/j.placenta.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derzsy Z, Prohaszka Z, Rigo J, Jr, Fust G, Molvarec A. Activation of the complement system in normal pregnancy and preeclampsia. Mol Immunol. 2010;47:1500–6. doi: 10.1016/j.molimm.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Lynch AM, Murphy JR, Gibbs RS, Levine RJ, Giclas PC, Salmon JE, Holers VM. The interrelationship of complement-activation fragments and angiogenesis-related factors in early pregnancy and their association with pre-eclampsia. BJOG. 2010;117:456–62. doi: 10.1111/j.1471-0528.2009.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rampersad R, Barton A, Sadovsky Y, Nelson DM. The C5b-9 membrane attack complex of complement activation localizes to villous trophoblast injury in vivo and modulates human trophoblast function in vitro. Placenta. 2008;29:855–61. doi: 10.1016/j.placenta.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch AM, Murphy JR, Byers T, Gibbs RS, Neville MC, Giclas PC, Salmon JE, Holers VM. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol. 2008;198:385–9. doi: 10.1016/j.ajog.2007.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185:792–7. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 34.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 35.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–6. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 36.Than NG, Romero R, Erez O, Kusanovic JP, Tarca AL, Edwin SS, Kim JS, Hassan SS, Espinoza J, Mittal P, et al. A role for mannose-binding lectin, a component of the innate immune system in pre-eclampsia. Am J Reprod Immunol. 2008;60:333–45. doi: 10.1111/j.1600-0897.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim YM, Romero R, Oh SY, Kim CJ, Kilburn BA, Armant DR, Nien JK, Gomez R, Mazor M, Saito S, et al. Toll-like receptor 4: a potential link between “danger signals,” the innate immune system, and preeclampsia? Am J Obstet Gynecol. 2005;193:921–7. doi: 10.1016/j.ajog.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 38.Chaiworapongsa T, Romero R, Yoshimatsu J, Espinoza J, Kim YM, Park K, Kalache K, Edwin S, Bujold E, Gomez R. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J Matern Fetal Neonatal Med. 2002;12:19–27. doi: 10.1080/jmf.12.1.19.27. [DOI] [PubMed] [Google Scholar]
- 39.Romero R, Vizoso J, Emamian M, Duffy T, Riely C, Halford T, Oyarzun E, Naftolin F, Hobbins JC. Clinical significance of liver dysfunction in pregnancy-induced hypertension. Am J Perinatol. 1988;5:146–51. doi: 10.1055/s-2007-999675. [DOI] [PubMed] [Google Scholar]
- 40.Romero R, Mazor M, Lockwood CJ, Emamian M, Belanger KP, Hobbins JC, Duffy T. Clinical significance, prevalence, and natural history of thrombocytopenia in pregnancy-induced hypertension. Am J Perinatol. 1989;6:32–8. doi: 10.1055/s-2007-999540. [DOI] [PubMed] [Google Scholar]
- 41.Luttun A, Carmeliet P. Soluble VEGF receptor Flt1: the elusive preeclampsia factor discovered? J Clin Invest. 2003;111:600–2. doi: 10.1172/JCI18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myatt L. Role of placenta in preeclampsia. Endocrine. 2002;19:103–11. doi: 10.1385/ENDO:19:1:103. [DOI] [PubMed] [Google Scholar]
- 43.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–9. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 44.Sibai B, Romero R, Klebanoff MA, Rice MM, Caritis S, Lindheimer MD, Van Dorsten JP, Landon M, Miodovnik M, Dombrowski M, et al. Maternal plasma concentrations of the soluble tumor necrosis factor receptor 2 are increased prior to the diagnosis of preeclampsia. Am J Obstet Gynecol. 2009;200:630–8. doi: 10.1016/j.ajog.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haeger M, Unander M, Andersson B, Tarkowski A, Arnestad JP, Bengtsson A. Increased release of tumor necrosis factor-alpha and interleukin-6 in women with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Acta Obstet Gynecol Scand. 1996;75:695–701. doi: 10.3109/00016349609065729. [DOI] [PubMed] [Google Scholar]
- 46.Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent IL, von DP. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27:56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Sargent IL, Germain SJ, Sacks GP, Kumar S, Redman CW. Trophoblast deportation and the maternal inflammatory response in pre-eclampsia. J Reprod Immunol. 2003;59:153–60. doi: 10.1016/s0165-0378(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 48.Huppertz B, Frank HG, Reister F, Kingdom J, Korr H, Kaufmann P. Apoptosis cascade progresses during turnover of human trophoblast: analysis of villous cytotrophoblast and syncytial fragments in vitro. Lab Invest. 1999;79:1687–702. [PubMed] [Google Scholar]
- 49.Huppertz B, Kingdom J, Caniggia I, Desoye G, Black S, Korr H, Kaufmann P. Hypoxia favours necrotic versus apoptotic shedding of placental syncytiotrophoblast into the maternal circulation. Placenta. 2003;24:181–90. doi: 10.1053/plac.2002.0903. [DOI] [PubMed] [Google Scholar]
- 50.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens. 2008;21:1152–6. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raijmakers MT, Peters WH, Steegers EA, Poston L. NAD(P)H oxidase associated superoxide production in human placenta from normotensive and pre-eclamptic women. Placenta. 2004;25 (Suppl A):S85–S89. doi: 10.1016/j.placenta.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Mellembakken JR, Aukrust P, Olafsen MK, Ueland T, Hestdal K, Videm V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension. 2002;39:155–60. doi: 10.1161/hy0102.100778. [DOI] [PubMed] [Google Scholar]
- 53.Clark CJ, Boswell F, Greer IA, Lyall F. Treatment of endothelial cells with serum from women with preeclampsia: effect on neutrophil adhesion. J Soc Gynecol Investig. 1997;4:27–33. doi: 10.1016/S1071-5576(96)00057-3. [DOI] [PubMed] [Google Scholar]
- 54.Walther T, Wallukat G, Jank A, Bartel S, Schultheiss HP, Faber R, Stepan H. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension. 2005;46:1275–9. doi: 10.1161/01.HYP.0000190040.66563.04. [DOI] [PubMed] [Google Scholar]
- 55.Parrish MR, Wallace K, Tam Tam KB, Herse F, Weimer A, Wenzel K, Wallukat G, Ray LF, Arany M, Cockrell K, et al. Hypertension in Response to AT1-AA: Role of Reactive Oxygen Species in Pregnancy-Induced Hypertension. Am J Hypertens. 2011 doi: 10.1038/ajh.2011.62. [DOI] [PubMed] [Google Scholar]
- 56.Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brasen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, et al. Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension. 2005;45:742–6. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- 57.Than NG, Abdul RO, Magenheim R, Nagy B, Fule T, Hargitai B, Sammar M, Hupuczi P, Tarca AL, Szabo G, et al. Placental protein 13 (galectin-13) has decreased placental expression but increased shedding and maternal serum concentrations in patients presenting with preterm pre-eclampsia and HELLP syndrome. Virchows Arch. 2008;453:387–400. doi: 10.1007/s00428-008-0658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Than NG, Erez O, Wildman DE, Tarca AL, Edwin SS, Abbas A, Hotra J, Kusanovic JP, Gotsch F, Hassan SS, et al. Severe preeclampsia is characterized by increased placental expression of galectin-1. J Matern Fetal Neonatal Med. 2008;21:429–42. doi: 10.1080/14767050802041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romero R, Kusanovic JP, Than NG, Erez O, Gotsch F, Espinoza J, Edwin S, Chefetz I, Gomez R, Nien JK, et al. First-trimester maternal serum PP13 in the risk assessment for preeclampsia. Am J Obstet Gynecol. 2008;199:122. doi: 10.1016/j.ajog.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–91. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 61.Bujold E, Chaiworapongsa T, Kim YM, Espinoza J, Goncalves L, Edwin S, Romero R. Plasma soluble vascular endothelial growth factor receptor 1 is elevated in uterine vein of patients with preeclampsia. Journal of Mat, Fet and Neonat Med. 2004 doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 62.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 63.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res. 2005;57:1R–7R. doi: 10.1203/01.PDR.0000159567.85157.B7. [DOI] [PubMed] [Google Scholar]
- 65.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 66.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 67.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmed A, Whittle MJ, Khaliq A. Differential expression of placenta growth factor (PlGF) and vascular endothelial growth factor (VEGF) in abnormal placentation. J Soc Gynecol Investig. 1997:4. [Google Scholar]
- 69.Lindheimer MD, Romero R. Emerging roles of antiangiogenic and angiogenic proteins in pathogenesis and prediction of preeclampsia. Hypertension. 2007;50:35–6. doi: 10.1161/HYPERTENSIONAHA.107.089045. [DOI] [PubMed] [Google Scholar]
- 70.Bdolah Y, Karumanchi SA, Sachs BP. Recent advances in understanding of preeclampsia. Croat Med J. 2005;46:728–36. [PubMed] [Google Scholar]
- 71.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–7. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 72.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 73.Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001;184:1267–72. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 74.Aggarwal PK, Jain V, Sakhuja V, Karumanchi SA, Jha V. Low urinary placental growth factor is a marker of pre-eclampsia. Kidney Int. 2006;69:621–4. doi: 10.1038/sj.ki.5000075. [DOI] [PubMed] [Google Scholar]
- 75.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195:201–7. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 76.Levine RJ, Qian C, Maynard SE, Yu KF, Epstein FH, Karumanchi SA. Serum sFlt1 concentration during preeclampsia and mid trimester blood pressure in healthy nulliparous women. Am J Obstet Gynecol. 2006;194:1034–41. doi: 10.1016/j.ajog.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 77.Muller PR, James AH, Murtha AP, Yonish B, Jamison MG, Dekker G. Circulating angiogenic factors and abnormal uterine artery Doppler velocimetry in the second trimester. Hypertens Pregnancy. 2006;25:183–92. doi: 10.1080/10641950600912968. [DOI] [PubMed] [Google Scholar]
- 78.Wathen KA, Tuutti E, Stenman UH, Alfthan H, Halmesmaki E, Finne P, Ylikorkala O, Vuorela P. Maternal serum-soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in preeclampsia or intrauterine growth retardation. J Clin Endocrinol Metab. 2006;91:180–4. doi: 10.1210/jc.2005-1076. [DOI] [PubMed] [Google Scholar]
- 79.Masuyama H, Suwaki N, Nakatsukasa H, Masumoto A, Tateishi Y, Hiramatrsu Y. Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am J Obstet Gynecol. 2006;194:551–6. doi: 10.1016/j.ajog.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 80.Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol. 2006;195:255–9. doi: 10.1016/j.ajog.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 81.Robinson CJ, Johnson DD. Soluble endoglin as a second-trimester marker for preeclampsia. Am J Obstet Gynecol. 2007;197:174–5. doi: 10.1016/j.ajog.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 82.Espinoza J, Romero R, Nien JK, Gomez R, Kusanovic JP, Goncalves LF, Medina L, Edwin S, Hassan S, Carstens M, et al. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol. 2007;196:326–13. doi: 10.1016/j.ajog.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moore Simas TA, Crawford SL, Solitro MJ, Frost SC, Meyer BA, Maynard SE. Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol. 2007;197:244–8. doi: 10.1016/j.ajog.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 84.Staff AC, Braekke K, Johnsen GM, Karumanchi SA, Harsem NK. Circulating concentrations of soluble endoglin (CD105) in fetal and maternal serum and in amniotic fluid in preeclampsia. Am J Obstet Gynecol. 2007;197:176. doi: 10.1016/j.ajog.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 85.Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, Thadhani R. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007;50:137–42. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- 86.Stepan H, Unversucht A, Wessel N, Faber R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension. 2007;49:818–24. doi: 10.1161/01.HYP.0000258404.21552.a3. [DOI] [PubMed] [Google Scholar]
- 87.Schlembach D, Wallner W, Sengenberger R, Stiegler E, Mortl M, Beckmann MW, Lang U. Angiogenic growth factor levels in maternal and fetal blood: correlation with Doppler ultrasound parameters in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2007;29:407–13. doi: 10.1002/uog.3930. [DOI] [PubMed] [Google Scholar]
- 88.Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol. 2007;196:239–6. doi: 10.1016/j.ajog.2006.10.909. [DOI] [PubMed] [Google Scholar]
- 89.Unal ER, Robinson CJ, Johnson DD, Chang EY. Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia. Am J Obstet Gynecol. 2007;197:211–4. doi: 10.1016/j.ajog.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 90.Crispi F, Llurba E, Dominguez C, Martin-Gallan P, Cabero L, Gratacos E. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;31:303–9. doi: 10.1002/uog.5184. [DOI] [PubMed] [Google Scholar]
- 91.De Vivo A, Baviera G, Giordano D, Todarello G, Corrado F, D’anna R. Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87:837–42. doi: 10.1080/00016340802253759. [DOI] [PubMed] [Google Scholar]
- 92.Diab AE, El-Behery MM, Ebrahiem MA, Shehata AE. Angiogenic factors for the prediction of pre-eclampsia in women with abnormal midtrimester uterine artery Doppler velocimetry. Int J Gynaecol Obstet. 2008;102:146–51. doi: 10.1016/j.ijgo.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 93.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, Gotsch F, Edwin S, Nien JK, Chaiworapongsa T, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–87. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lim JH, Kim SY, Park SY, Yang JH, Kim MY, Ryu HM. Effective prediction of preeclampsia by a combined ratio of angiogenesis-related factors. Obstet Gynecol. 2008;111:1403–9. doi: 10.1097/AOG.0b013e3181719b7a. [DOI] [PubMed] [Google Scholar]
- 95.Sibai BM, Koch MA, Freire S, Pinto e Silva JL, Rudge MV, Martins-Costa S, Bartz J, de Barros SC, Cecatti JG, Costa R, et al. Serum inhibin A and angiogenic factor levels in pregnancies with previous preeclampsia and/or chronic hypertension: are they useful markers for prediction of subsequent preeclampsia? Am J Obstet Gynecol. 2008;199:268–9. doi: 10.1016/j.ajog.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 96.Stepan H, Geipel A, Schwarz F, Kramer T, Wessel N, Faber R. Circulatory soluble endoglin and its predictive value for preeclampsia in second-trimester pregnancies with abnormal uterine perfusion. Am J Obstet Gynecol. 2008;198:175–6. doi: 10.1016/j.ajog.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 97.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Edwin SS, Gomez R, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–38. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda) 2009;24:147–58. doi: 10.1152/physiol.00043.2008. [DOI] [PubMed] [Google Scholar]
- 99.Chedraui P, Lockwood CJ, Schatz F, Buchwalder LF, Schwager G, Guerrero C, Escobar GS, Hidalgo L. Increased plasma soluble fms-like tyrosine kinase 1 and endoglin levels in pregnancies complicated with preeclampsia. J Matern Fetal Neonatal Med. 2009;22:565–70. doi: 10.1080/14767050902801769. [DOI] [PubMed] [Google Scholar]
- 100.Powers RW, Jeyabalan A, Clifton RG, Van Dorsten P, Hauth JC, Klebanoff MA, Lindheimer MD, Sibai B, Landon M, Miodovnik M. Soluble fms-Like tyrosine kinase 1 (sFlt1), endoglin and placental growth factor (PlGF) in preeclampsia among high risk pregnancies. PLoS One. 2010;5:e13263. doi: 10.1371/journal.pone.0013263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aggarwal PK, Chandel N, Jain V, Jha V. The relationship between circulating endothelin-1, soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J Hum Hypertens. 2011 Mar 31; doi: 10.1038/jhh.2011.29. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 102.Haddad R, Romero R, Gould BR, Tromp G, Gotsch F, Edwin SS, Zingg HH. Angiogenesis gene expression in mouse uterus during the common pathway of parturition. Am J Obstet Gynecol. 2008;198:539–8. doi: 10.1016/j.ajog.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 103.Chaiworapongsa T, Romero R, Tarca AL, Kusanovic JP, Gotsch F, Mittal P, Kim SK, Vaisbuch E, Mazaki-Tovi S, Erez O, et al. A decrease in maternal plasma concentrations of sVEGFR-2 precedes the clinical diagnosis of preeclampsia. Am J Obstet Gynecol. 2010;202:550–10. doi: 10.1016/j.ajog.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Akolekar R, Zaragoza E, Poon LC, Pepes S, Nicolaides KH. Maternal serum placental growth factor at 11 + 0 to 13 + 6 weeks of gestation in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32:732–9. doi: 10.1002/uog.6244. [DOI] [PubMed] [Google Scholar]
- 105.Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11–13 weeks. Prenat Diagn. 2011;31:66–74. doi: 10.1002/pd.2660. [DOI] [PubMed] [Google Scholar]
- 106.Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–5. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 107.Teixeira PG, Cabral AC, Andrade SP, Reis ZS, da Cruz LP, Pereira JB, Martins BO, Rezende CA. Placental growth factor (PlGF) is a surrogate marker in preeclamptic hypertension. Hypertens Pregnancy. 2008;27:65–73. doi: 10.1080/10641950701825937. [DOI] [PubMed] [Google Scholar]
- 108.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179:1539–44. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 109.Lyall F. The Placenta in Preeclampsia. 2011. pp. 246–52. 1st. [Google Scholar]
- 110.Kumazaki K, Nakayama M, Suehara N, Wada Y. Expression of vascular endothelial growth factor, placental growth factor, and their receptors Flt-1 and KDR in human placenta under pathologic conditions. Hum Pathol. 2002;33:1069–77. doi: 10.1053/hupa.2002.129420. [DOI] [PubMed] [Google Scholar]
- 111.Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, Edwin S, Kusanovic JP, Erez O, Than NG, et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med. 2008;21:25–40. doi: 10.1080/14767050701832833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chaiworapongsa T, Romero R, Kusanovic JP, Mittal P, Kim SK, Gotsch F, Than NG, Mazaki-Tovi S, Vaisbuch E, Erez O, et al. Plasma soluble endoglin concentration in pre-eclampsia is associated with an increased impedance to flow in the maternal and fetal circulations. Ultrasound Obstet Gynecol. 2010;35:155–62. doi: 10.1002/uog.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–7. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 114.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977–84. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 115.Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension. 2009;53:399–403. doi: 10.1161/HYPERTENSIONAHA.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22:143–8. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 117.Vatten LJ, Skjaerven R. Is pre-eclampsia more than one disease? BJOG. 2004;111:298–302. doi: 10.1111/j.1471-0528.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 118.Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension. 2008;52:873–80. doi: 10.1161/HYPERTENSIONAHA.108.117358. [DOI] [PubMed] [Google Scholar]
- 119.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97:533–8. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- 120.Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Preeclampsia and fetal growth. Obstet Gynecol. 2000;96:950–5. [PubMed] [Google Scholar]
- 121.Mbah AK, Alio AP, Marty PJ, Bruder K, Whiteman VE, Salihu HM. Pre-eclampsia in the first pregnancy and subsequent risk of stillbirth in black and white gravidas. Eur J Obstet Gynecol Reprod Biol. 2010;149:165–9. doi: 10.1016/j.ejogrb.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 122.Kenneth L, Hall DR, Gebhardt S, Grove D. Late onset preeclampsia is not an innocuous condition. Hypertens Pregnancy. 2010;29:262–70. doi: 10.3109/10641950902777697. [DOI] [PubMed] [Google Scholar]
- 123.Hauth JC, Ewell MG, Levine RJ, Esterlitz JR, Sibai B, Curet LB, Catalano PM, Morris CD. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol. 2000;95:24–8. doi: 10.1016/s0029-7844(99)00462-7. [DOI] [PubMed] [Google Scholar]
- 124.Tan KH, Kwek K, Yeo GS. Epidemiology of pre-eclampsia and eclampsia at the KK Women’s and Children’s Hospital, Singapore. Singapore Med J. 2006;47:48–53. [PubMed] [Google Scholar]
- 125.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011 Feb 16; doi: 10.1016/j.bpobgyn.2011.01.006. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 126.Schutte JM, Steegers EA, Schuitemaker NW, Santema JG, de Boer K, Pel M, Vermeulen G, Visser W, van Roosmalen J. Rise in maternal mortality in the Netherlands. BJOG. 2010;117:399–406. doi: 10.1111/j.1471-0528.2009.02382.x. [DOI] [PubMed] [Google Scholar]
- 127.Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Norden-Lindeberg S, Olovsson M. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet Gynecol. 2007;109:1368–74. doi: 10.1097/01.AOG.0000264552.85436.a1. [DOI] [PubMed] [Google Scholar]
- 128.Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol. 2003;189:1173–7. doi: 10.1067/s0002-9378(03)00576-3. [DOI] [PubMed] [Google Scholar]
- 129.Sebire NJ, Goldin RD, Regan L. Term preeclampsia is associated with minimal histopathological placental features regardless of clinical severity. J Obstet Gynaecol. 2005;25:117–8. doi: 10.1080/014436105400041396. [DOI] [PubMed] [Google Scholar]
- 130.van der Merwe JL, Hall DR, Wright C, Schubert P, Grove D. Are early and late preeclampsia distinct subclasses of the disease--what does the placenta reveal? Hypertens Pregnancy. 2010;29:457–67. doi: 10.3109/10641950903572282. [DOI] [PubMed] [Google Scholar]
- 131.Masuyama H, Segawa T, Sumida Y, Masumoto A, Inoue S, Akahori Y, Hiramatsu Y. Different profiles of circulating angiogenic factors and adipocytokines between early- and late-onset pre-eclampsia. BJOG. 2010;117:314–20. doi: 10.1111/j.1471-0528.2009.02453.x. [DOI] [PubMed] [Google Scholar]
- 132.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 133.Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, Goldenberg RL, Joffe G. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1997;177:1003–10. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 134.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 135.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation--a workshop report. Placenta. 2005;26 (Suppl A):S114–S117. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 136.Mayhew TM. Taking tissue samples from the placenta: an illustration of principles and strategies. Placenta. 2008;29:1–14. doi: 10.1016/j.placenta.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 137.Wolf M, Shah A, Lam C, Martinez A, Smirnakis KV, Epstein FH, Taylor RN, Ecker JL, Karumanchi SA, Thadhani R. Circulating levels of the antiangiogenic marker sFLT-1 are increased in first versus second pregnancies. Am J Obstet Gynecol. 2005;193:16–22. doi: 10.1016/j.ajog.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 138.Srinivas SK, Morrison AC, Andrela CM, Elovitz MA. Allelic variations in angiogenic pathway genes are associated with preeclampsia. Am J Obstet Gynecol. 2010;202:445–11. doi: 10.1016/j.ajog.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 139.Muy-Rivera M, Vadachkoria S, Woelk GB, Qiu C, Mahomed K, Williams MA. Maternal plasma VEGF, sVEGF-R1, and PlGF concentrations in preeclamptic and normotensive pregnant Zimbabwean women. Physiol Res. 2005;54:611–22. [PubMed] [Google Scholar]
- 140.Park CW, Park JS, Shim SS, Jun JK, Yoon BH, Romero R. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol. 2005;193:984–9. doi: 10.1016/j.ajog.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 141.Ohkuchi A, Hirashima C, Matsubara S, Suzuki H, Takahashi K, Arai F, Watanabe T, Kario K, Suzuki M. Alterations in placental growth factor levels before and after the onset of preeclampsia are more pronounced in women with early onset severe preeclampsia. Hypertens Res. 2007;30:151–9. doi: 10.1291/hypres.30.151. [DOI] [PubMed] [Google Scholar]
- 142.Ogge G, Chaiworapongsa T, Kusanovic JP, Yeo L, Kim CJ, Hassan S, Romero R. Evidence that placental lesions are more frequent in early-onset than in late-onset preeclampsia. Reproductive Science. 2011;18:124A Abstract T-188. doi: 10.1515/JPM.2011.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Teasdale F. Histomorphometry of the human placenta in maternal preeclampsia. Am J Obstet Gynecol. 1985;152:25–31. doi: 10.1016/s0002-9378(85)80170-8. [DOI] [PubMed] [Google Scholar]
- 144.Kingdom J. Adriana and Luisa Castellucci Award Lecture 1997. Placental pathology in obstetrics: adaptation or failure of the villous tree? Placenta. 1998;19:347–51. doi: 10.1016/s0143-4004(98)90073-x. [DOI] [PubMed] [Google Scholar]
- 145.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92:35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 146.Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol. 1996;175:1534–42. doi: 10.1016/s0002-9378(96)70103-5. [DOI] [PubMed] [Google Scholar]
- 147.Architecture of Normal Villous Trees. 2016. pp. 121–73. Fifth. [Google Scholar]
- 148.Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG. 2006;113:580–9. doi: 10.1111/j.1471-0528.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 149.Shore VH, Wang TH, Wang CL, Torry RJ, Caudle MR, Torry DS. Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta. 1997;18:657–65. doi: 10.1016/s0143-4004(97)90007-2. [DOI] [PubMed] [Google Scholar]
- 150.Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, Kozuma S, Taketani Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838–45. doi: 10.1210/en.2004-0533. [DOI] [PubMed] [Google Scholar]
- 151.Chaiworapongsa T, Romero R, Gotsch F, Kusanovic JP, Mittal P, Kim SK, Erez O, Vaisbuch E, Mazaki-Tovi S, Kim CJ, et al. Acute pyelonephritis during pregnancy changes the balance of angiogenic and anti-angiogenic factors in maternal plasma. J Matern Fetal Neonatal Med. 2010;23:167–78. doi: 10.3109/14767050903067378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–73. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 153.Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63:534–43. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 154.Redline RW, Boyd T, Campbell V, Hyde S, Kaplan C, Khong TY, Prashner HR, Waters BL. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7:237–49. doi: 10.1007/s10024-003-8083-2. [DOI] [PubMed] [Google Scholar]